Summary
G protein-coupled receptors (GPCRs) affect many physiological processes by modulating both intrinsic membrane conductances and synaptic transmission. This study describes spontaneous miniature inhibitory postsynaptic currents mediated by vesicular dopamine release acting locally on metabotropic D2 receptors leading to the activation of a G protein-coupled inwardly rectifying potassium conductance. Thus, individual exocytotic events result in spontaneous GPCR-mediated transmission similar to synaptic activation of classical ligand-gated ion channels.
Keywords: dopamine, D2 receptor, metabotropic, GIRK
Introduction
The discovery of miniature endplate potentials at the neuromuscular junction critically shaped the understanding of neurotransmitter release and synaptic transmission. Since its discovery, spontaneous neurotransmitter release has been described at many synapses in the mammalian central nervous system, but only where ligand-gated ion channels underlie the postsynaptic response. While a number of G protein-coupled receptors (GPCRs) are known to mediate inhibitory postsynaptic currents (IPSCs) by activating inwardly rectifying K+ channels (GIRKs), these are thought to result from diffusion of transmitter to extrasynaptic sites (Isaacson et al., 1993; Otis and Mody, 1992). The slow intrinsic kinetics of GPCR signaling and the temporal dispersion of activation resulting from long distance diffusion may obscure GPCR-mediated miniature synaptic events.
Electrical stimulation in the midbrain evokes vesicular dopamine release. The lack of structural evidence for axon terminals in the rat substantia nigra pars compacta (Bayer and Pickel, 1990; Groves and Linder, 1983) led to the theory that dopamine release, in this region, arises from the somatodendritic compartment. Somatodendritic release has been measured using multiple assays (Fortin et al., 2006; Heeringa and Abercrombie, 1995; Mendez et al., 2011), including whole-cell recording of a D2 receptor-dependent inhibitory postsynaptic current (IPSC) (Beckstead et al., 2004). Spontaneous, or unstimulated, somatodendritic release of dopamine has been detected by amperometry and high-performance liquid chromatography from midbrain dopamine neurons in brain slices (Jaffe et al., 1998) and cultures (Fortin et al., 2006), but there is no evidence that this spontaneous release activates a GIRK-mediated conductance. This study reports spontaneous miniature IPSCs (sIPSCs) in dopamine neurons from mouse substantia nigra pars compacta (SN). The results reveal that spontaneous vesicular dopamine release produced sIPSCs by local D2 receptor activation of GIRK channels that were not dependent on voltage-gated sodium and calcium channels or intracellular calcium stores.
Results and Discussion
Whole-cell voltage-clamp recordings were made from SN dopamine neurons in horizontal midbrain slices from wild type mice, in the presence of NMDA, AMPA, GABAA, GABAB, and nACh receptor antagonists. Spontaneous IPSCs occurred approximately 1 event/min (Figures 1A, 3A, 3D), similar to the low frequency of spontaneous amperometric events recorded from SN neurons (Jaffe et al., 1998). The sIPSCs were abolished by the D2 receptor antagonist, sulpiride (300 nM, Figure 1A). A single electrical stimulus evoked D2 receptor-mediated IPSCs (eIPSC) (Figures 1B and 1C). Blockade of GIRK conductance with Ba2+ decreases the current induced by dopamine in SN dopamine neurons (Lacey et al., 1987). Ba2+ (100 µM) eliminated both evoked and spontaneous IPSCs (P = 0.009, n = 6, Figure 1B). Thus, spontaneous IPSCs are the result of D2 receptor activation of GIRK channels.
Figure 1.
D2 receptor activation of GIRK conductance mediates spontaneous IPSCs. (A) Representative traces of sIPSCs (red stars) recorded in the absence of stimulation, blocked by the D2 receptor antagonist, sulpiride (300 nM). (B) Ba2+ blocked eIPSCs (arrows) and sIPSCs (red stars) recorded in forskolin (1 µM) and cocaine (300 nM) (See Figure 3D). Stimulation artifacts were blanked for clarity. The inward current produced by BaCl2 (42 ± 8.5 pA, n = 5 cells) was present in sulpiride (45 ± 10.3 pA, P = 0.84, n = 7 cells), thus not related to D2 receptor activation. (C) Average eIPSC and sIPSC scaled to the peak. (D) Histograms of minimal stimulation eIPSC and sIPSC amplitudes in ACSF (1 pA bins, two-sample KS test, n = eIPSCs:188 and sIPSCs:1137) along with baseline noise (checked) and Gaussian fit of eIPSC amplitudes (black line). Vertical axes have been scaled to normalize for n (See Experimental Procedures). (E) Box-and-whisker plots of eIPSC amplitudes from minimal stimulation and sIPSCs, box limits designate 25 and 75 percentiles, central line represents the median, and whisker ends show 5 and 95 percentiles (Two-tailed Mann-Whitney U test). Values from a single experiment are shown by the filled circles. ns indicates not significant and **P < 0.01.
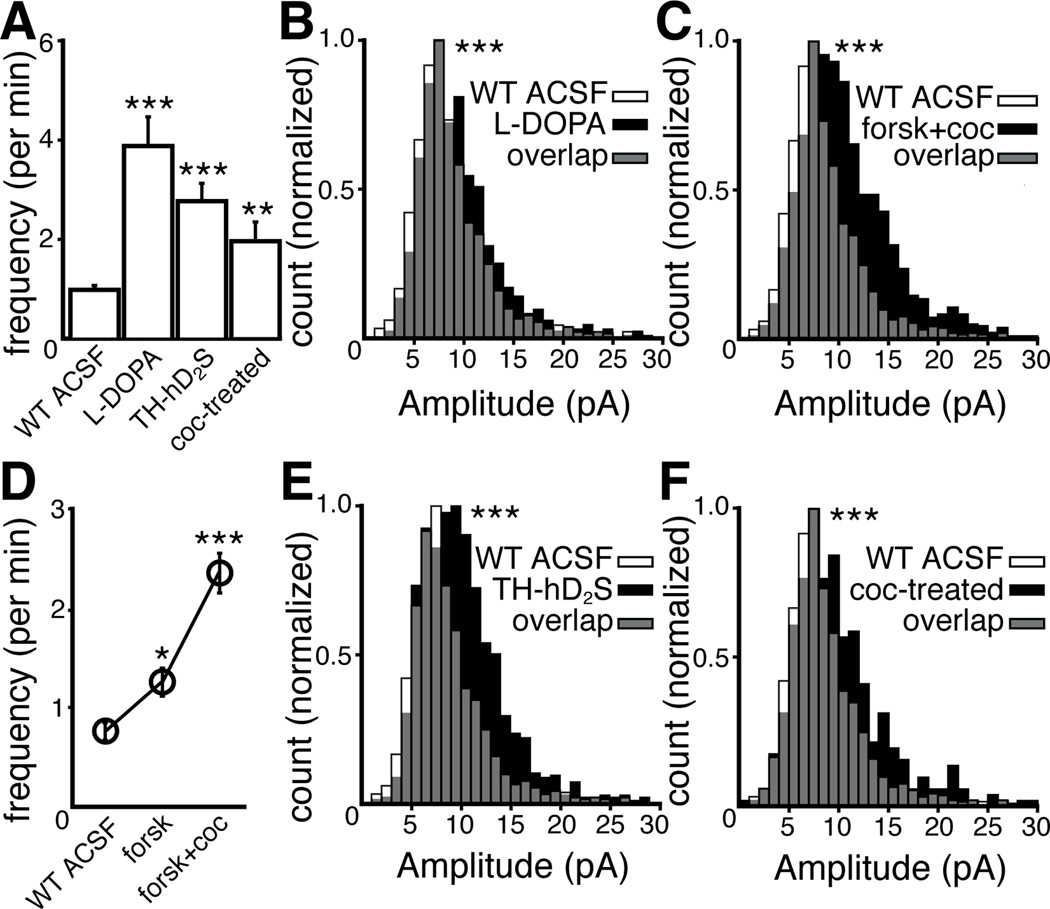
Figure 3.
The frequency and amplitude of spontaneous IPSCs are modulated by pre- and postsynaptic mechanisms and are plastic, changing after a single in vivo exposure to cocaine. (A) Frequency of sIPSCs per minute (Kruskal-Wallis test, n = cells in WT ACSF:95, L-DOPA:12, TH-hD2S: 29, and coc-treated:20) Mean ± SEM. (D) Frequency of sIPSCs per minute was increased by forskolin and the subsequent addition of cocaine (forsk+coc) (Friedman test, n = 53 cells), Mean ± SEM. (B, C, E, F) Amplitude of sIPSCs (1 pA bins, two-sample KS test, n = sIPSCs in WT ACSF:1137, L-DOPA:939, forskolin+cocaine:1605, TH-hD2S:1223, and coc-treated: 400). Vertical axes have been scaled to normalize for n (See Experimental Procedures). *P < 0.05, **P < 0.01 and ***P < 0.001.
The rise time of the sIPSCs and eIPSCs were identical (P = 0.76). However, the duration of sIPSCs, measured at 20% of the peak, was shorter (69%) than the eIPSC (P < 0.001, eIPSCs n = 77 and sIPSCs n = 76, Figure 4D). The rise time and duration of sIPSCs were similar to the current evoked using fast application of a high concentration of dopamine (≥ 10 µM) onto membrane patches (Ford et al., 2009). Thus, sIPSCs likely resulted from a sharp rise of a high concentration of dopamine, inconsistent with extended diffusion away from the release site. To compare the amplitude of sIPSCs to eIPSCs, minimal stimulation was employed to evoke eIPSCs with the smallest resolvable amplitude over baseline noise. The eIPSC amplitude distribution from minimal stimulation was normal (P = 0.4, mean of 8.8 pA, median of 8.4 pA, Figure 1D and 1 E). The amplitude distribution of sIPSCs was right-skewed (P < 0.001, mean of 9.2 pA, median of 7.9 pA, Figure 1D, 1E, 3B) such that the distributions of eIPSC and sIPSC amplitudes were statistically different (P = 0.007, n = 188 eIPSCs and 1137 sIPSCs). However, the median amplitude of sIPSCs was similar to eIPSCs evoked by minimal stimulation (P = 0.07). Although these results are suggestive of a quantal event, the slow kinetics of D2 IPSCs and low frequency of sIPSCs necessitated the combination of data from multiple cells and therefore limit further quantitative analysis. Taken together, the results suggest that, with the exception of some larger sIPSCs, the current elicited by a single resolvable release event was similar whether the release was spontaneous or evoked.
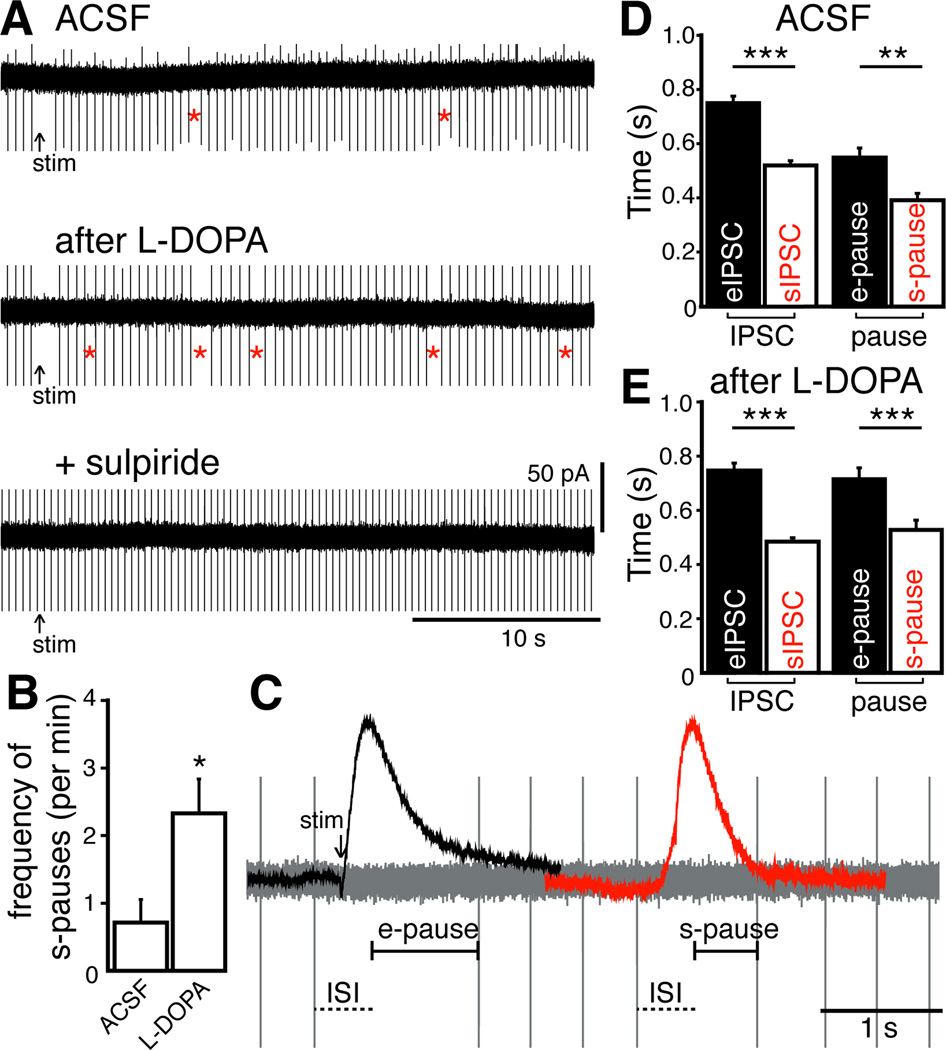
Figure 4.
Spontaneous IPSCs transiently inhibit pacemaker firing. (A) Loose cell-attached recordings from an experiment showing the pause in firing after electrical stimulation (arrow, e-pause) and spontaneous pauses (red stars, s-pause), blocked by the D2 receptor antagonist, sulpiride (600 nM). (B) Frequency of spontaneous pauses per minute increased with exposure to L-DOPA and were abolished by sulpiride (Repeated measures ANOVA, n = 13 cells) (C) Loose cell-attached recording with scaled eIPSC and sIPSC superimposed. eIPSC was positioned by aligning the time of stimulation in cell-attached and whole-cell recording (arrow). sIPSC was positioned based on alignment of eIPSC rise time with respect to ISI. (D and E) Duration of eIPSC, sIPSC (both measured at 20% peak amplitude, see Figure 1C), e-pause, and s-pause in ACSF and after exposure to L-DOPA (Kruskal-Wallis tests, ACSF n = eIPSCs:77, sIPSCs:76, e-pauses: 59, s-pauses:50; L-DOPA n = eIPSCs:41, sIPSCs:71, e-pauses:130, s-pauses:255). In traces, stimulation artifacts were blanked and action potential currents were truncated for clarity. Mean ± SEM. *P < 0.05, ** P < 0.01, and ***P < 0.001.
Next, the mechanism of spontaneous dopamine release was compared to that of electrically evoked release. Disruption of the vesicular monoamine transporter with reserpine (1 µM, > 20 min) eliminated sIPSCs (P = 0.03, Figure 2A), confirming that spontaneous dopamine release is vesicular. Application of tetrodotoxin (TTX, 600 nM) or Cd2+ (100 µM) abolished eIPSCs, but TTX failed to alter the frequency (P = 0.40, Figure 2B) or amplitude (P = 0.95, Figure 2C) of sIPSCs, demonstrating sIPSCs were not dependent on action potentials. Likewise, sIPSCs persisted in Cd2+, with no change in frequency (P = 0.35, Figure 2D), indicating sIPSCs were not dependent on calcium entry via voltage-gated calcium channels. There are conflicting reports of the role of intracellular calcium stores on the release of dopamine in the midbrain (Courtney et al., 2012; Ford et al., 2010; Patel et al., 2009). To determine if spontaneous dopamine release was dependent on intracellular calcium stores, the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor cyclopiazonic acid (CPA) was used to deplete calcium stores. The application of CPA (10 µM, > 3 min) had no effect on the frequency of sIPSCs (P = 0.41, Figures 2D and 2E), whereas previous experiments found that inositol triphosphate (IP3)-mediated SK currents in dopamine neurons were eliminated (Ford et al., 2010). Perfusion with calcium-free ACSF reduced the frequency, but did not eliminate the sIPSCs (P = 0.04, Figures 2D and 2F). While the established theory suggests that evoked and spontaneous IPSCs are the result of somatodendritic dopamine release, the site of release remains to be determined. Furthermore, the question of whether a single neuron releases dopamine onto itself has not been resolved. The experimental conditions (voltage-clamp and strong intracellular calcium buffering with BAPTA) make it unlikely that eIPSCs are induced by release from the recorded neuron. However, it remains possible that sIPSCs may result from dopamine release from the recorded neuron, since spontaneous release is TTX-insensitive and does not require voltage-gated calcium channels.
Figure 2.
Vesicular dopamine release underlying spontaneous IPSCs is resistant to TTX, inhibition of voltage-gated calcium channels, and depletion of intracellular stores of calcium. (A) Plot of spontaneous IPSC amplitude versus time from a single experiment showing the inhibition by reserpine. (B) Blocking action potentials with TTX (600 nM) did not alter the frequency (Repeated measures ANOVA, n = 5 cells) or (C) amplitude of sIPSCs (1 pA bins, two-sample KS test, n = sIPSCs in control:245 and TTX:193). Vertical axes have been scaled to normalize for n (See Experimental Procedures). (D) Cd2+ and CPA did not alter the frequency of sIPSCs. Removing extracellular calcium (0[Ca2+]o) did not eliminate the sIPSCs (paired two-tailed t-tests, n = cells in Cd2+:6, CPA:16, and 0[Ca2+]o:6. (E) Traces from a single experiment (filled circles in D) showing the average eIPSC and sIPSCs (average is red) recorded in CPA. (F) Removal of extracellular Ca2+ abolished the eIPSC but sIPSCs persisted. All recordings were made in the presence of forskolin (1 µM) and cocaine (300 nM) to increase frequency of sIPSCs (see Figure 3D). Mean ± SEM. ns indicates not significant and *P < 0.05.
The amplitude and frequency of spontaneous miniature postsynaptic currents can fluctuate due to presynaptic and postsynaptic mechanisms, including differences in transmitter content (Frerking et al., 1995), probability of release (Fatt and Katz, 1952), transmitter clearance (Liu et al., 1999), and postsynaptic receptor/channel availability (Edwards et al., 1990). Cytosolic and vesicular dopamine levels are increased after treatment with the catecholamine precursor L-DOPA (Pothos et al., 1998), thus enhancing the eIPSC (Beckstead et al., 2004). Incubation in L-DOPA (10 µM, 10 min) increased the frequency and amplitude of sIPSCs (P < 0.001, Figures 3A and 3B). L-DOPA can be taken up by 5-HT neurons, metabolized to dopamine (Arai et al., 1994), and in parkinsonian rats, released from 5-HT terminals (Carta et al., 2007). The increase in frequency of sIPSCs following treatment with L-DOPA could involve aberrant dopamine release from 5-HT terminals. This possibility was examined with the application of the selective 5-HT1 agonist, 5-CT, since activation of 5-HT1 receptors potently inhibits release from 5-HT terminals (Bobker and Williams, 1989; Carta et al., 2007). Application of 5-CT (1 µM) had no effect on the frequency of the sIPSCs (ACSF: 0.6 ± 0.2 per min, 5-CT: 1.0 ± 0.3 per min, paired two-tailed t-test, P = 0.3, n = 7 cells) even after treatment of slices with L-DOPA (Control: 0.8 ± 0.1 per min, L-DOPA: 3.7 ± 0.7 per min, Two-way ANOVA, P = 0.96, n = 7 cells). Activation of adenylyl cyclase with forskolin increased the amplitude of eIPSCs (Beckstead et al., 2007). Forskolin (1 µM) also increased the frequency (P < 0.05, Figure 3D) and amplitude of sIPSCs (P = 0.005, median of 8.7 pA). The uptake of dopamine by dopamine transporters is the primary mechanism of terminating dopamine signaling in the midbrain (Ford et al., 2010). In the presence of cocaine, a nonspecific monoamine transporter blocker, the clearance of extracellular dopamine is prolonged (Ford et al., 2010), potentiating the eIPSC (Beckstead et al., 2004; Ford et al., 2009; 2010). Cocaine (300 nM), in the presence of forskolin (1 µM), further increased the amplitude (P < 0.001, median of 10.0 pA, Figure 3C) and frequency (P < 0.001, Figure 3D) of sIPSCs.
The role of postsynaptic receptor availability on the frequency and amplitude of sIPSCs was examined using experiments with a transgenic mouse strain (TH-hD2S) that expressed a human D2 receptor (short isoform) with an amino-terminal FLAG epitope targeted to catecholamine neurons, in addition to endogenous D2 receptors (See Experimental Procedures). Functional coupling of D2 receptors to GIRK channels in TH-hD2S mice was evaluated by measuring the maximal D2 receptor-mediated outward currents evoked by iontophoretic application of dopamine onto dopamine neurons, normalized to capacitance (dopamine current density). The dopamine current density of SN neurons in wild type mice was 8.9 ± 0.4 pA/pF (n = 37), consistent with previously reported values (Gantz et al., 2011), and the dopamine current density in TH-hD2S mice was elevated (14.6 ± 1.0 pA/pF, P < 0.01, n = 32). There was no difference in current density evoked by the GABAB agonist, baclofen, in TH-hD2S (11.1 ± 0.9 pA/pF, P = 0.57, n = 14) compared to wild type mice (12.2 ± 0.8 pA/pF, n = 19). Thus, the increased expression of D2 receptors in the TH-hD2S mice did not interfere with the activation of GIRK by other GPCRs. The frequency and amplitude of sIPSCs from dopamine neurons in TH-hD2S mice was greater than those from wild type mice (P < 0.001, Figures 3A and 3E). These results suggest that the level of D2 receptor expression is factor in determining the amplitude of the IPSC, although it is not known to what extent the overexpression of D2 receptors has on other processes such as tyrosine hydroxylase expression, dopamine synthesis, or the expression of dopamine transporters. Taken together, the results indicate that the frequency and amplitude of spontaneous D2 receptor-mediated IPSCs are altered by both pre- and postsynaptic mechanisms.
Exposure to drugs of abuse causes morphological and functional changes to midbrain dopamine neurons (Heikkinen et al., 2008; Saal et al., 2003; Sarti et al., 2007). Many of these changes occur after a single exposure, including potentiated spontaneous GABA- (Melis et al., 2002) and glutamate- (Ungless et al., 2001) synaptic currents. To determine if dopamine-dependent sIPSCs were similarly plastic, mice were treated with a single dose of cocaine (20 mg/kg, i.p.). One day after treatment, the dopamine current density in SN neurons from cocaine-treated mice was elevated (12.3 ± 0.7 pA/pF, P < 0.01, n = 26), and the frequency and amplitude of sIPSCs were increased (P < 0.01, Figure 3A; P < 0.001, Figure 3F). There was no change in baclofen current density (11.3 ± 0.8 pA/pF, P = 0.57, n = 15). Therefore spontaneous dopamine transmission, like GABA- and glutamate-dependent transmission, was increased by a single exposure to cocaine.
In vitro, dopamine neurons fire action potentials in a regular, pacemaker pattern. Electrical stimulation causes a sulpiride-sensitive pause in firing, indicating eIPSCs inhibit spontaneous firing (Beckstead and Williams, 2007; Beckstead et al., 2004; Courtney et al., 2012). Loose cell-attached recordings were made from SN dopamine neurons from wild type mice to assess if sIPSCs can inhibit firing. A single electrical stimulus caused a pause (e-pause) in pacemaker firing (Figures 4A and 4C). Spontaneous pauses (s-pause) occurred approximately 1 event/min (Figures 4A and 4B). Exposure to L-DOPA (10 µM, 10 min) increased the frequency of s-pauses (P < 0.05, n = 13 cells, Figures 4A and 4B). The s-pauses were abolished by sulpiride (600 nM, P < 0.01, Figure 4A), indicating that the s-pauses were the result of D2 receptor activation. The duration of the pauses were calculated by subtracting the mean inter-spike interval (ISI) from the time between two action potentials (Figure 4C). The duration of the s-pause was shorter (71%) than the e-pause (P < 0.01, n = 59 e-pauses and 50 s-pauses, Figure 4D). It is interesting to note that the difference in duration is the same as that when comparing the evoked and spontaneous IPSCs (Figure 4D). After L-DOPA, the duration of the s-pause remained shorter than the e-pause (74% of e-pause, P < 0.001, n = 130 e-pauses and 255 s-pauses, Figure 4E). In a separate set of experiments, reserpine was used to verify that vesicular release of dopamine underlies the s-pauses. Application of reserpine (1 µM, 10 min) dramatically reduced the frequency of s-pauses (L-DOPA: 2.43 ± 0.8 per min; reserpine: 0.15 ± 0.1 per min; paired two-tailed t-test, P = 0.03, n = 8 cells). Taken together, these results indicate that sIPSCs and spontaneous pauses result from spontaneous dopamine release. Thus, spontaneous release of dopamine in brain slices influences the firing pattern of dopamine neurons.
The presence of spontaneous miniature GIRK-mediated IPSCs is the strongest evidence to date that signaling mediated by GPCRs can be similar to transmission mediated by ligand-gated ion channels. First, the results of this study demonstrate that despite the slow intrinsic signaling kinetics of GPCR activation, these receptors can signal in a point-to-point manner, where the presynaptic site of release is located very close to postsynaptic receptors. Second, as in other neurotransmitter systems, the postsynaptic currents elicited by evoked and spontaneous dopamine release are largely indistinguishable. Lastly, spontaneous D2 receptor-mediated transmission was altered by pre- and postsynaptic mechanisms and was plastic, changing after a single in vivo exposure to cocaine. Thus, the factors that regulate synaptic transmission mediated by D2 receptors and ligand-gated ion channels are similar. It is likely that spontaneous GIRK-dependent IPSCs are common, adding an unrealized role of GPCR-dependent signaling in neuronal regulation.
Experimental Procedures
Slice preparation and recording
All animals were maintained and sacrificed according to the approved protocols at Oregon Health and Science University. Male and female DBA/2J and C57BL/6J mice (> 30 days old) were used. Cocaine-treated animals received one intraperitoneal injection (20 mg/kg) 24 hrs prior to use. Horizontal midbrain slices (220 µm) were made, as previously described (Gantz et al., 2011), in ice-cold physiologically equivalent saline solution (modified Krebs' buffer) containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, and 11 D-glucose with 10 µM MK-801. Slices were incubated at 32 ºC in vials with 95/5% O2/CO2 saline with 10 µM MK-801 for at least 30 mins, before recordings. Slices once mounted on a recording chamber attached to an upright microscope (Olympus) were maintained at 36–37 ºC and perfused at a rate of 4.0 ml/min with modified Krebs' buffer. Using infrared illumination, the SN was identified visually, under 5× magnification, by location in relation to the medial terminal nucleus of the accessory optic tract and the midline.
Whole-cell patch-clamp recordings were obtained with glass electrodes (1.8–2.2 MΩ) and an internal solution containing, (in mM) 115 K-methylsulfate, 20 NaCl, 1.5 MgCl2, 2 ATP, 0.2 GTP, 10 phosphocreatine, and 10 BAPTA, pH 7.30 – 7.43, 275–288 mOsm. The cells were voltage-clamped at −60 mV with an Axopatch 200B amplifier (Molecular Devices). Loose (< 30 MΩ) cell-attached recordings were made with glass electrodes (1.4–2.0 MΩ) and an internal solution containing modified Krebs’ buffer. Dopamine neurons were identified by a large hyperpolarization-induced Ih current, the presence of spontaneous pacemaker firing of wide (~2 ms) action potentials at 1–5 Hz, and either, the presence of a D2 receptor-mediated IPSC or the sensitivity to exogenously applied dopamine. Immediately after gaining access to the cell, membrane capacitance, series resistance, and input resistance were measured with the application of 50 pulses (+2 mV for 50 ms) averaged before computation using AxoGraph (sampled at 50 kHz, filtered at 10 kHz). Dopamine release was evoked by a single electrical stimulus (0.5 ms) and pharmacologically isolated by the following receptor blockers in the external bath solution: picrotoxin (100 µM), hexamethonium (50 µM), DNQX (10 µM), and CGP 55845 (100 nM). Data were acquired using AxoGraph software (sampled at 10 kHz, filtered at 5 kHz) and Chart 5 (AD Instruments). Recordings were post-hoc filtered at 1 kHz, and visually inspected to select spontaneous IPSCs manually. Original recordings were then decimated (averaged 10 points, 1 ms). Single peak spontaneous IPSCs with amplitudes greater than 2.1 times the standard deviation of baseline noise were detected using a semi-automated sliding template detection procedure with AxoGraph X. The template was generated by averaging multiple sIPSCs. Each detected event was visually inspected. Events were discarded if the average baseline noise (> 300 ms) was greater than the peak ± 1.5 s from the peak. Peak amplitudes were determined by averaging the current ± 20 ms from the greatest upward deflection. The amplitude distribution of the baseline noise was measured by averaging the baseline current ± 20 ms from the greatest upward deflection, 220 ms after a point set to 0 pA, once every 50 s (n = 26 cells). To compare the kinetics of eIPSCs to sIPSCs, spontaneous events with a single peak were selected. Duration of eIPSCs and sIPSCs was determined by measuring the width at 20% of the peak amplitude (See Figure 1C). All drugs were applied through bath perfusion, except dopamine, which was applied by iontophoresis. Iontophoretic pipettes (70–110 MΩ) were filled with 1 M dopamine and the tip placed with 10 µm of the soma. A negative backing current (6–11 nA) prevented leakage. Dopamine was ejected with the application of positive current (2 –6 s) with an Axoclamp 2A amplifier to elicit a maximal dopamine-induced outward current.
TH-hD2S Transgenic Mice
A transgenic mouse expressing the human D2 dopamine receptor short isoform (hD2S) with a flag epitope on the amino-terminus was generated by nuclear microinjection using standard techniques. The transgene consisted of an 8.5 kb genomic fragment from the rat tyrosine hydroxylase gene (TH) containing 5’ regulatory sequences, the basal promoter, and 26 base pairs from the 5’ untranslated region in exon 1 followed by a 0.7 kb cassette containing intron 2 and splice donor/acceptor sites from the rabbit beta-globin gene (Arttamangkul et al., 2008). The hD2S construct consisted of a consensus Kozak sequence, a signal peptide from the hemagglutinin influenza followed by the sequence for the FLAG epitope (Vickery and Zastrow, 1999), a full length cDNA for the hD2S containing 1.4 kb of coding sequence and 1.0 kb of 3’ untranslated and the bovine growth hormone polyA sequence from pcDNA 3.0 (Invitrogen). After cleavage by the signal peptidase of the signal sequence during translation a hD2S protein with an amino-terminus Flag epitope is expressed in TH-expressing neurons including the dopamine neurons of the midbrain as shown by immunostaining with the M1 anti-Flag antibody (Sigma-Aldrich) using confocal microscopy on sections and two-photon microscopy on midbrain slice preparations (data not shown).
Drugs
CGP 35348 and 5-CT were obtained from Tocris Bioscience. Baclofen and sulpiride were obtained from Research Biochemical Inc. MK-801 was obtained from Abcam. Cocaine hydrochloride was obtained from National Institute on Drug Abuse-National Institutes of Health (Bethesda, MD). All other chemicals were obtained from Sigma-Aldrich.
Data analysis
Values are given as means ± SEM. All distributions with n > 30 were tested for normality with Shapiro-Wilk normality test. IPSC amplitude distributions were compared by two-sample Kolmogorov-Smirnov tests. For clarity, histograms show amplitudes ≤ 30 pA, which accounts for > 97% of all amplitudes measured in each condition. To normalize amplitude counts across conditions, the vertical axes of individual histograms have been scaled, such that the bin with the greatest count equals 1.0. Statistical significance were determined in two group comparisons by paired two-tailed t-tests or two-tailed Mann-Whitney U tests, and in more than two groups comparisons by one-way ANOVAs, one-way repeated measures ANOVAs, Kruskal-Wallis (Nonparametric ANOVA), or Friedman test (Nonparametric repeated measures ANOVA) followed, when appropriate (p < 0.05), by Dunnett’s or Bonferroni’s post hoc tests or Dunn’s multiple comparisons test. A difference of p < 0.05 was considered significant (Prism 4 and AxoGraph X).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I. Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous L-DOPA in the rat with reference to the involvement of aromatic L-amino acid decarboxylase. Brain Res. 1994;667:295–299. doi: 10.1016/0006-8993(94)91511-3. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, Zastrow, von M, Pintar J, Williams JT. Differential Activation and Trafficking of μ-Opioid Receptors in Brain Slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10:2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-Term Depression of a Dopamine IPSC. J Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Ford CP, Phillips PEM, Williams JT. Presynaptic regulation of dendrodendritic dopamine transmission. European Journal of Neuroscience. 2007;26:1479–1488. doi: 10.1111/j.1460-9568.2007.05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular Dopamine Release Elicits an Inhibitory Postsynaptic Current in Midbrain Dopamine Neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin agonists inhibit synaptic potentials in the rat locus ceruleus in vitro via 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors. J Pharmacol Exp Ther. 1989;250:37–43. [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Courtney NA, Mamaligas AA, Ford CP. Species differences in somatodendritic dopamine transmission determine D2-autoreceptor-mediated inhibition of ventral tegmental area neuron firing. Journal of Neuroscience. 2012;32:13520–13528. doi: 10.1523/JNEUROSCI.2745-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol (Lond) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol (Lond) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and Opioid Inhibition of Mesolimbic Dopamine Neurons Vary according to Target Location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Phillips PEM, Williams JT. The Time Course of Dopamine Transmission in the Ventral Tegmental Area. J Neurosci. 2009;29:13344–13352. doi: 10.1523/JNEUROSCI.3546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PEM, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau L-E. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Ford CP, Neve KA, Williams JT. Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J Neurosci. 2011;31:12629–12637. doi: 10.1523/JNEUROSCI.0684-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- Heikkinen AE, Möykkynen TP, Korpi ER. Long-lasting Modulation of Glutamatergic Transmission in VTA Dopamine Neurons after a Single Dose of Benzodiazepine Agonists. Neuropsychopharmacology. 2008;34:290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol (Lond) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Fasano C, Kortleven C, Trudeau LE. Somatodendritic Dopamine Release Requires Synaptotagmin 4 and 7 and the Participation of Voltage-gated Calcium Channels. Journal of Biological Chemistry. 2011;286:23928–23937. doi: 10.1074/jbc.M111.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992;67:227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of Calcium from Intracellular Stores Facilitates Somatodendritic Dopamine Release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. European Journal of Neuroscience. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vickery RG, Zastrow, von M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]