Abstract
Forebrain GABAergic interneurons are divided into subgroups based on their neurochemical markers, connectivity and physiological properties. Abnormal interneuron function is implicated in the pathobiology of neurological disorders such as schizophrenia, autism, and epilepsy. Studies on interneuron development and their role in disease would benefit from an efficient mechanism for the production and selection of specific interneuron subgroups. In this study, we engineered a mouse embryonic stem cell (mESC) line for doxycycline-inducible expression of Nkx2.1, a required transcription factor for cortical interneurons derived from the medial ganglionic eminence (MGE). This mESC line was modified to express GFP in Lhx6+ cells, a marker of newly postmitotic and mature MGE-derived cortical interneurons. Addition of doxycycline to differentiating ESCs efficiently induced Nkx2.1 protein and increased the production of GFP+ cells. Transplantation of GFP+ putative interneuron precursors resulted in migratory, morphological, and neurochemical features consistent with cortical interneuron fates. To test the hypothesis that Sonic hedgehog (Shh) primarily influences cortical interneuron fate determination through the induction of Nkx2.1, ESCs were grown with doxycycline and the Shh antagonist cyclopamine. We found induced Nkx2.1 renders Shh signaling dispensable for the generation of MGE-derived interneurons. These results demonstrate that inducible expression of fate determining genes in embryonic stem cells can be used to study fate determination of the developing forebrain.
Introduction
The ability to generate forebrain neurons from embryonic stem cells (ESCs) has provided a new tool with which study the complex relationships between interacting molecular systems and neuronal fate determination (Petros et al., 2011). Several studies have successfully generated cortical interneurons (cINs) from mouse (Maroof et al., 2010;Danjo et al., 2011) and human ESCs (Goulburn et al., 2011), but the derivation efficiencies and ability to produce cIN subgroups is limited. Forced expression of key fate-determining genes can promote the differentiation of ESCs into specific cell fates. There are two primary origins of cINs; those that originate in the medial ganglionic eminence (MGE) or preoptic area and require the transcription factor Nkx2.1 for their fate determination, and those that originate within the caudal ganglionic eminence and are Nkx2.1-independent. Downstream of Nkx2.1, the transcription factor Lhx6 is expressed from cell cycle exit through postnatal development (Liodis et al., 2007), and ectopic Lhx6 expression is sufficient to rescue the loss cIN subgroup markers in Nkx2.1−/− mice (Du et al., 2008). Upstream of Nkx2.1, the morphogen Sonic hedgehog (Shh) is required for the induction and maintenance of Nkx2.1 expression (Fuccillo et al., 2004;Xu et al., 2005). However, it is unknown if the only role for Shh in cIN fate determination is to induce Nkx2.1 expression, or whether Shh functions via additional factors that are required to specify cIN fate.
In this paper we engineered a mouse ESC (mESC) line to both inducibly express Nkx2.1 via doxycycline (Dox) (Kyba et al., 2002), and to express GFP under control of the Lhx6 promoter. We used this ESC line to (1) determine whether inducible Nkx2.1 expression enhances generation of GFP+ cINs, and (2) study the role of Shh signaling in cIN fate determination. Our results indicate that induced Nkx2.1 expression increases the generation of ESC-derived cINs. Additionally, forced Nkx2.1 expression is sufficient to induce cIN fates in the absence of Shh signaling, suggesting that the primary role of Shh is to maintain Nkx2.1 expression. These findings demonstrate the potential for utilizing temporally controlled gene expression in combination with fate markers to enhance the ESC-derivation of neuronal subgroups and to study the fate determination of forebrain neurons.
Results
Induced expression of Nkx2.1 enhances production of ESC-derived Lhx6+ cells
To induce Nkx2.1 expression, we obtained a mESC line (Ainv15) that constitutively expresses the reverse tetracycline transactivator (rtTA; “Dox-on”) from the Rosa26 locus and contains the tetracycline response element (TRE) in the HPRT locus (Fig. 1A) (Kyba et al., 2002). This line was modified with a bacterial artificial chromosome (BAC) in which the GFP coding sequence was inserted into the Lhx6 locus (Gong et al., 2003). This Lhx6∷GFP BAC was previously shown to accurately report Lhx6 expression in cINs in vivo (Gong et al., 2003) and in mESC-derived cINs (Maroof et al., 2010). Several clones were differentiated into cINs and a clone was selected that gave the strongest GFP signal, had a normal karyotype, and harbored a single Lhx6∷GFP insertion site (Fig. 1B). The mouse Nkx2.1 coding sequence was then inserted downstream of the TRE, resulting in the mESC line Rosa26-rTTA;TRE-Nkx2.1;Lhx6∷GFP (hereto referred to as iNkx2.1). 24-hour Dox treatment results in strong Nkx2.1 expression in iNkx2.1 ESCs (Fig. 1C).
Figure 1. Inducible Nkx2.1 expression in mESCs.
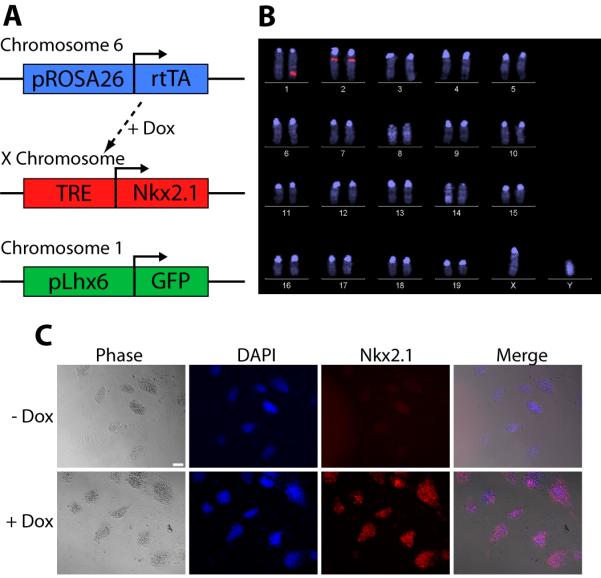
A. Schematic of the iNkx2.1 mESC line. B. Fluorescent in situ hybridization (FISH) of iNkx2.1 line demonstrating a single Lhx6∷GFP BAC insertion in chromosome 1 (with the native Lhx6 gene on chromosome 2). C. iNkx2.1 mESCs were cultured for 2 days, treated with Dox for 24 hours, and then fixed. Nearly all iNkx2.1 ESCs express Nkx2.1. Scale bar = 50 μm.
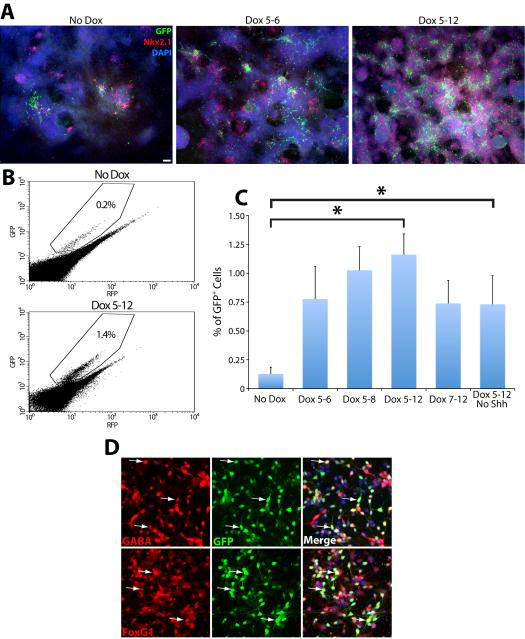
iNkx2.1 ESCs were differentiated using a modified version of our previously published cIN differentiation protocol ((Maroof et al., 2010), see Material and Methods) to determine whether forced Nkx2.1 expression increases the production of Lhx6∷GFP+ cells. Differentiation of iNkx2.1 ESCs in the absence of Dox produces a relatively small number of GFP+ cells emanating from Nkx2.1+ clusters at differentiation day 12 (DD12). Addition of Dox from DD5-6, approximately 2 days before endogenous Nkx2.1 is detected (Maroof et al., 2010), strongly enhances the number of Nkx2.1+ cell clusters and the generation of GFP+ cells (Fig. 2A). This premature Nkx2.1 expression also leads to a dramatic increase of GFP+ cells at DD8 (Supplementary Fig. 1), a time when very few GFP+ cells are otherwise observed. As the time course of Dox treatment is extended, there is a gradual increase in the number of GFP+ cells detected, with a maximal effect observed when Dox remained in the culture medium from DD5-DD12 (Fig. 2A). As expected, Nkx2.1 remains strongly expressed at DD12 in the vast majority of cells when Dox is present from DD5-12 (Fig. 2A).
Figure 2. Nkx2.1 induction enhances generation of Lhx6+ cells.
Representative examples of co-labeling for DAPI, Nkx2.1, and GFP in DD12-fixed cultures treated with doxycycline for the indicated times. A. Left, Few GFP+ cells are observed in the absence of Dox. Middle, Dox exposure on DD5–6 results in a marked increase in GFP+ cells. Right, A further increase of GFP+ (and Nkx2.1+) cells is observed when Dox is present from DD5–DD12. Scale bar = 200 μm. B. Representative examples of FACS plots depicting the percentage of GFP+ cells from cultures with no Dox and with Dox from DD5–DD12. C. FACS analysis demonstrating that induced Nkx2.1 expression increases the generation of GFP+ cells ~7 fold. ANOVA: F(5,28) = 3.03, p < .05; t test: *p < .05. D. Nearly all GFP+ cells are GABA+ and FoxG1+ (arrows), indicating that GFP+ cells are telencephalic GABAergic neurons. Scale bar = 10 μm.
To quantify these results fluorescence-activated cell sorting (FACS) was conducted at DD12 on the different Dox conditions (Fig. 2B–C). We found a nearly 5-fold increase in the percentage of GFP+ cells when Dox is present only from DD5-DD6, and a further increase when Dox remains in the culture medium from DD5-DD12. Although a trend is apparent at shorter Dox time courses, only Dox treatment from DD5-DD12 reached statistical significance after correction for multiple comparisons (ANOVA: F(5,28) = 3.03, p < .05; t test: *p < .05) (Fig. 2C). Delay of Dox treatment until DD7 produced fewer GFP+ cells compared to the DD5-12 treatment (Fig. 2C), indicating that maximizing GFP+ cells requires Dox administration prior to endogenous Nkx2.1 expression. However, Dox treatment starting at DD0 essentially eliminates all GFP+ expression (Supplementary Fig. 2), suggesting that driving Nkx2.1 expression prior to neural induction drives these cells away from a cIN fate.
To verify that GFP+ cells express markers consistent with cINs, DD5-12 Dox-treated GFP+ cells were isolated by FACS, plated onto dissociated mouse cortical cultures, then immunostained them for GABA and the telencephalic marker FoxG1 after 24 hours. Nearly all GFP+ cells express both FoxG1 (119/120, 99.2%) and GABA (111/120, 92.5%) (Fig. 2D). Additionally, DD12-fixed iNkx2.1 cultures display similar levels of FoxG1 compared to our previous J1 Lhx6∷GFP mESC line (Maroof et al., 2010) (Supplementary Fig. 3). These results indicate that iNkx2.1-derived GFP+ cells are GABAergic telencephalic cells, consistent with a cIN cell type.
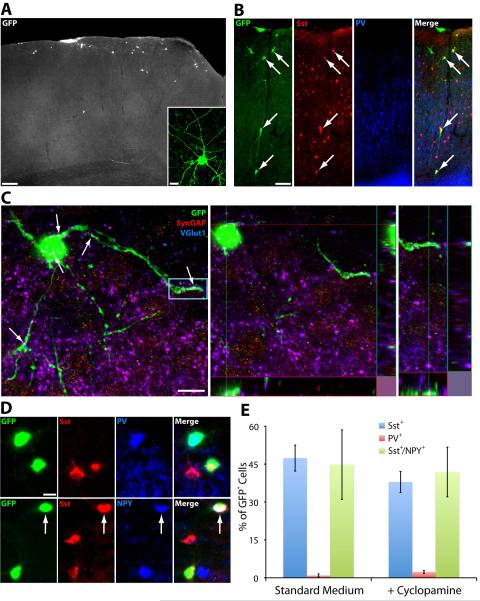
To corroborate these findings in vivo, FACS isolated GFP+ cells grown in the presence of Dox from DD5-12 were transplanted into the somatosensory cortex of early postnatal pups (P0-P3) and cell fate was examined at P30. Many GFP+ cells migrate more than 500 μm from the injection site, a characteristic for FoxG1+/GABA+/Lhx6+ cells that is strongly suggestive of cINs. In addition, the cells display an aspiny and generally multipolar morphology (Fig. 3A–B). Many GFP+ cells appear to receive excitatory synaptic input, as VGlut1+ puncta are colocalized with SynGAP+ postsynaptic sites on GFP+ cell bodies and dendrites (Fig. 3C), indicating that they have integrated into the cortical circuitry and receive excitatory synaptic input. We next used imunodetection to examine GFP+ transplanted cells for expression of parvalbumin (PV) or somatostatin (Sst), markers of non-overlapping interneuron subgroups that together comprise the majority of MGE-derived cortical interneurons (Fogarty et al., 2007). We observe a strong bias for Sst+ cells over PV+ cells in these conditions (47.4% GFP+/Sst+ vs. 1.3% GFP+/PV+) (Fig. 3D–E). Additionally, almost half of GFP+/Sst+ cINs express Neuropetptide Y (NPY, 44.8% of GFP+/Sst+ are NPY+) (Fig. 3D–E). This is substantially higher than the endogenous percentage of Sst+/NPY+ co-labeling in mouse cortex (Gonchar et al., 2007;Xu et al., 2010b). As a large portion of Sst+/NPY+ cINs arise from the Nkx6.2+ domain in the dorasl MGE (Fogarty et al., 2007), these results are consistent with the transplanted cells being biased for dorsal MGE-like cIN subgroup fates.
Figure 3. Shh signaling blockade in the presence of Nkx2.1 induction does not alter the subgroup fates of transplanted GFP+ cINs.
A. Example of GFP+ cells in P30 cortex, 4 weeks after transplantation of GFP+ FACS harvested cells at DD12. The inset shows a single cell with a multipolar and aspiny morphology typical of cortical GABAergic interneurons. Scale bar = 200 μm, inset scale bar = 10 μm. B. GFP+ transplanted cINs immunostained for somatostatin and parvalbumin. The arrows in the merged panel indicate 4 cells that co-express SST and GFP. Scale bar = 50 μm. C. A representative example of a compressed confocal stack (left) and orthogonal views (middle, right) depicting the colocalization of VGlut1+ and SynGAP+ on GFP+ cell bodies and dendrites (white arrows), indicating that transplanted GFP+ cells receive excitatory synaptic inputs. The blue box indicates the region highlighted in the right panel. Scale bar = 10 μm. D. Examples of transplanted GFP+ cells that are Sst+, PV+ and Sst+/NPY+ (arrow). Scale bar = 10 μm. E. Counts of GFP+ cells in the cortex of P30 mice indicates that inhibiting Shh signaling during the differentiation protocol does not significantly alter the relative distribution of cIN subgroup fate, with Sst+ cINs predominating in both conditions.
Blocking Shh signaling increases the percentage of GFP+ cells, but does not alter cIN fate
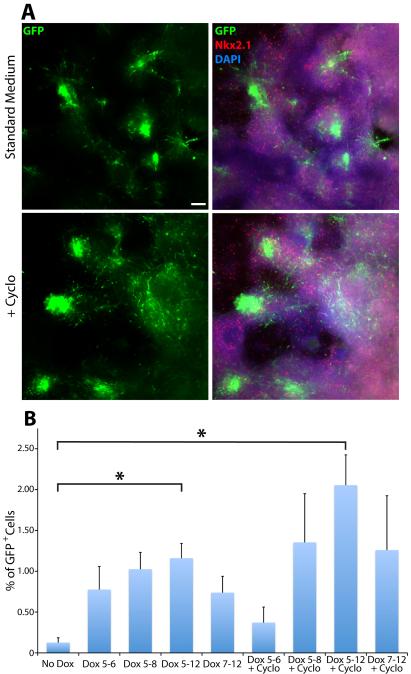
Shh is required in the MGE to maintain Nkx2.1 expression and to promote Lhx6+ interneuron fates (Xu et al., 2005). In mESC differentiations, removal of exogenous Shh from the differentiation protocol eliminates Lhx6∷GFP+ cells (A. Maroof, T. Petros, S. Anderson, unpublished data). We investigated whether Dox-induced Nkx2.1 expression might circumvent the requirement for Shh in the differentiation protocol and still generate Lhx6∷GFP+ cINs. To this end, iNkx2.1 differentiations were carried out with Dox from DD5-12 but in the absence of exogenous Shh. We observe a significant increase in GFP+ cells in Dox-treated cultures in the absence of exogenous Shh (0.13 ± 0.06% GFP+ cells without Dox in standard medium vs. 0.73 ± 0.25% GFP+ with Dox and without Shh, p < .05) (Fig. 2C). There was no statistical difference between DD5-12 Dox-treated iNkx2.1 cultures in the presence or absence of Shh. These results suggest that forced Nkx2.1 expression may eliminate the need for exogenous Shh in the differentiation protocol.
Differentiating mESC cultures produce endogenous Shh because late addition of the Shh antagonist cyclopamine at DD8 eliminates Nkx2.1 expression, and Nkx2.1 levels were similar when Shh was added from DD5-8 or DD5-12 (A. Maroof, S. Anderson, unpublished data). To eliminate Shh signaling, we differentiated iNkx2.1 ESCs in the presence of cyclopamine from DD5-12. This condition eliminates GFP+ cells in both the J1 Lhx6∷GFP mESC line used previously (Maroof et al., 2010) and in the iNkx2.1 line in the absence of Dox (data not shown). However, a large number of GFP+ cells are still observed in iNkx2.1 ESCs grown in the presence of Dox and cyclopamine from DD5-12 (Fig. 4A). Similar results were observed in cultures fixed at DD8 (Supplementary Fig. 1). FACS analysis revealed a statistically significant enhancement of GFP+ cells grown with Dox and cyclopamine compared to the no Dox or Dox DD5-12 alone conditions (ANOVA: F(3,18) = 11.26, p < .001; t test: *p < .05) (Fig. 4B) (although this difference between Dox and Dox+cyclopamine did not reach statistical significance when all conditions in Figure 3B were included due to correction for multiple comparisons). These GFP+ cells appear to be cIN progenitors even in the presence of cyclopamine, as they were FoxG1+ (119/120, 99.2%) and GABA+ (109/120, 90.8%) at DD12. Shorter Dox time courses were less effective in generating GFP+ cells, suggesting that Nkx2.1 induction of GFP+ cells in the presence of cyclopamine requires continued Nkx2.1 expression.
Figure 4. Nkx2.1 induction enhances the production of Lhx6∷GFP+ cells in the presence of Shh signaling blockade.
A. Representative images from DD12 fixed cultures immunostained for GFP and Nkx2.1. Addition of cyclopamine from DD5–12 increases the number of GFP+ cells present at DD12. Bright green regions are dense clusters of GFP+ cells. Scale bar = 200 μm. B. FACS analysis demonstrating Dox addition from DD5–12 in the presence of cyclopamine still increases the generation of GFP+ cells compared to controls without Dox and Dox alone from DD5–12. Only the 3 conditions outlined in red were used for significance testing. ANOVA: F(3,18) = 11.26, p < .001; t test: *p < .05. Inclusion of all experimental conditions in the analysis eliminates the significance between the Dox DD5–12 and Dox+cyclopamine DD5–12 due to the increase in multiple comparisons.
There is a spatial organization of fate commitment in the MGE, with a strong bias for Sst+ cINs originating from the dMGE, and a weaker bias for PV+ cINs originating from the vMGE (Flames et al., 2007;Wonders et al., 2008;Inan et al., 2012). Numerous downstream transcriptional targets of Shh are preferentially expressed in the dMGE compared to the vMGE (Wonders et al., 2008). Shh expression is enriched in the dMGE mantle zone compared to the vMGE mantle zone (Flandin et al., 2011). In addition, loss of Shh signaling has a significantly greater effect on downregulating Nkx2.1 in the dMGE than the vMGE (Xu et al., 2005), and higher Shh signaling levels drive cINs to a dMGE, Sst+ fate over a vMGE, PV+ fate (Xu et al., 2010a). Together, these results raise the possibility that higher levels of Shh signaling bias cINs to a Sst+ fate via the induction of Nkx.2.1 together with additional factors. To determine whether cyclopamine treatment in the presence of Dox alters the interneuron subgroup fates produced, we transplanted FACS harvested DD5-12 cyclopamine-treated GFP+ cells into the somatosensory cortex of P0-P3 pups and analyzed cell fate. We found that blocking Shh signaling during the differentiation protocol did not alter cIN subgroup fate compared to control conditions (37.9% GFP+/Sst+, 2.3% GFP+/PV+, and 41.9% of GFP+/Sst+ are NPY+) (Fig. 3E). These data support the notion that the primary role of Shh in the MGE is to induce Nkx2.1 expression, and that, at least in the context of forced Nkx2.1 expression, differential Shh signaling is not sufficient to bias the generation neurochemically defined cIN subgroups.
Discussion
Our findings demonstrate that induced expression of Nkx2.1 in ESCs leads to a significant increase in the generation of Lhx6∷GFP+ cINs. The ability to generate specific cell types from ESCs by inducible expression of transcription factors is becoming more common (Kyba et al., 2002;Sanchez-Danes et al., 2012;Takayama et al., 2012). Recently, one study found that dox-inducible Fezf2 expression enhanced generation of ESC-derived rostral forebrain progenitors, with late dox addition biasing cells towards a dorsal telencephalic fate at the expense of Nkx2.1+ ventral telencephalic cells (Wang et al., 2011). To our knowledge, the current study is the first demonstration of inducible gene expression being utilized to specifically enhance the generation of ESC-derived cortical interneurons. In addition, forced Nkx2.1 expression still generates Lhx6∷GFP+ cINs in the presence of cyclopamine, a Shh signaling antagonist that eliminates native Nkx2.1 expression from mitotic progenitors in these cultures. Furthermore, we previously found that Lhx6 expression can rescue the migratory, morphological, and cIN subgroup marker expression in Nkx2.1−/− mice (Du et al., 2008). Together with the current findings, this suggests that cIN fate determination involves a rather narrow lineage stream whereby Shh specifies the fates of MGE-derived interneurons primarily through the induction of Nkx2.1, which in turn induces Lhx6 expression upon cell cycle exit.
The significant increase in iNkx2.1-derived GFP+ cells in the presence of cyclopamine was intriguing. In the MGE, removal of Shh induces a loss of Nkx2.1 expression and a shift of these cells to a more LGE- or CGE-like fate (Xu et al., 2005;Butt et al., 2008;Xu et al., 2010a). Our data indicate that driving Nkx2.1 expression in the absence of Shh signaling can rescue this deficit. The increased production of GFP+ cells could be explained by an increase in cell cycle exit. In addition to its role in maintaining Nkx2.1 expression, loss of Shh signaling also reduces the expanding Nkx2.1+ progenitor population in the MGE, potentially by inducing premature cell cycle exit (Machold et al., 2003;Xu et al., 2005;Flandin et al., 2010). Thus, eliminating Shh signaling could promote differentiation of Nkx2.1+ progenitor cells in our culture, which in turn would increase production of Lhx6+ cINs. Alternatively, the high exogenous Shh levels in our differentiation protocol could be promoting other ventral telencephalic cell fates (possibly making cells in the preoptic or septal lineage were Shh levels are higher in vivo), and reducing Shh levels may bias cells towards an MGE-like fate.
One issue raised by our results is the large preponderance of Sst+ cINs observed in iNkx2.1-derived GFP+ transplants with both the standard medium and cyclopamine-treated conditions (Fig. 3E). There is evidence that higher Shh signaling levels are correlated with the production of Sst+ dMGE-derived cINs while weaker Shh signaling levels are correlated with PV+ vMGE-derived cINs (Xu et al., 2005;Wonders et al., 2008;Xu et al., 2010a). Thus, the finding that induced Nkx2.1 expression in the presence of cyclopamine continues to generate Sst+ cINs suggests that the action of Shh in cIN subgroup fate determination is immaterial in the context of Nkx2.1 overexpression. However, it seems unlikely that only role for Shh in the MGE is simply to maintain Nkx2.1 expression, and it is possible that Nkx2.1 overexpression in iNkx2.1 ESCs could mask additional roles for Shh or other signaling pathways. For example, signaling factors and molecular mechanisms that direct MGE progenitors towards a PV+ fate may be absent or silenced in our culture conditions. It also is possible that under normal conditions, high Shh signaling levels may serve to repress these factors to promote a Sst+ fate. Additionally, there is gradual decrease in the production of Sst+ cINs from E11–E15 (Cavanagh and Parnavelas, 1988;Miyoshi et al., 2007;Inan et al., 2012). Thus, it would be interesting to specifically harvest and transplant later-born GFP+ cells in our culture to see if this strategy enriches for PV+ cINs.
We observed a lower percentage of GFP+ cells under standard differentiation conditions compared to the J1 Lhx6∷GFP line (Maroof et al., 2010). We repeated the differentiations with another Lhx6∷GFP+ Ainv15-derived clone (Ainv15.16) and found similarly low numbers of GFP+ cells at DD12 in the absence of Dox. We do not observe any obvious difference in FoxG1 expression levels between iNkx2.1, Ainv15.16 or the J1 Lhx6∷GFP line (Supplementary Fig. 3), indicating that their telencephalic patterning is similar. However, there does appear to be a moderate decrease in the number of Nkx2.1+ cells in Dox-treated Ainv15-derived ESC cultures compared to the J1 Lhx6∷GFP line (Supplementary Fig. 3), which could partially explain the decreased efficiency of generating GFP+ cINs from these cells. Another possibility is that the Lhx6∷GFP BAC insertion site in the J1 line is more permissive to recapitulating endogenous Lhx6 expression compared to the Ainv15-derived ESC lines. Additionally, the iNkx2.1 and the J1 Lhx6∷GFP ESCs are generated from distinct parental cell lines, and we have observed variability in the efficiency of generating Lhx6∷GFP+ cells with different ESC lines (Q. Xu, personal communication). In the future we would like to integrate the ability to induce cIN fate-promoting genes into the more responsive J1 Lhx6∷GFP ESC line to further increase production of GFP+ cINs.
The competency by which induced Nkx2.1 drives Lhx6 expression remains unclear. Inducing Nkx2.1 expression at DD0 failed to produce GFP+ cells, but GFP+ cells were detected when Dox was administered starting at DD3, which is well before the telencephalic marker FoxG1 becomes detectable (Supplementary Fig. 2). This suggests that after a few days of differentiation, some progenitors within the culture have attained competency to respond to Nkx2.1 by inducing Lhx6. We believe that inducing high levels of Nkx2.1 expression prior to endogenous Nkx2.1 expression prematurely initiates the normal Nkx2.1 signaling pathway that drives a subset of permissible neural progenitors to an Lhx6∷GFP+ fate.
Nkx2.1+ progenitors give rise to projection neurons and interneurons of both cholinergic and GABAergic neurotransmitter subgroups. Lhx6 is expressed transiently at cell cycle exit in both subgroups, but Lhx6 is selectively maintained in those fated to GABAergic interneuron whereas Lhx8 is expressed in cholinergic neurons (Liodis et al., 2007;Fragkouli et al., 2009). Notably, we did not observe GFP+ cortical cells in our transplants that also express the cholinergic marker choline acetyltranferase (ChAT, data not shown). Future studies that identify how induced Nkx2.1 promotes Lhx6 versus Lhx8 expression could be tremendously useful for enhancing the production of ESC-derived cINs or cholinergic cells and for identifying key regulators of the fate-determining process.
Materials and Methods
Generation of the Rosa26-rtTA;TRE-Nkx2.1;Lhx6∷GFP ESC line
The Ainv15 mESC line (Kyba et al., 2002) contains the reverse tetracycline transactivator (rtTA) in the ROSA26 locus and a tetracycline response element (TRE) on the X chromosome. The Lhx6∷GFP BAC (Gong et al., 2003) was retrofitted for blasticidin selection using a slightly modified procedure described previously (Tomishima et al., 2007). 5 μg of the Lhx6∷GFP BAC was electroporated (Amaxa) into the Ainv15 ESC line. Cells were plated on gelatin-coated plates in MEF-conditioned mESC medium with 3 μg/ml blasticidin for ~10 days to screen for blasticidin-resistant colonies. Several clones were collected and the Lhx6∷GFP insertions were verified by PCR. Initial differentiations were performed on Lhx6∷GFP+ clones using our standard protocol to select the clone giving the strongest GFP signal. Karyotype and FISH analysis revealed a normal chromosomal arrangement and a single BAC integration site on chromosome 1 for the iNkx2.1 ESC line. The mouse Nkx2.1 cDNA (gift of J. Rubenstein, UCSF) was cloned into the ploxP plasmid (Kyba et al., 2002). 20 μg of ploxP-Nkx2.1 and 20 μg of a cre expression vector were electroporated into the iNkx2.1 line, and cells were selected using 350 μg/ml of G418. Proper insertion of the Nkx2.1 cDNA was confirmed in G418-resistant colonies via PCR.
ESC cultures and FACS
ESC maintenance and differentiation were performed similar to previous methods (Maroof et al., 2010). For differentiation, iNkx2.1 ESCs were dissociated with trypsin (Invitrogen) and plated on non-adherent dishes at 70,000 cells/ml in a 1:1 mixture of KSR:N2 media supplemented with either noggin (250 ng/ml, R&D systems) or LDN-193189 (500 nM, StemGent) from DD0–5. On DD5, embryoid bodies were treated with Accutase (Invitrogen) and plated as dissociated cells (20,000 cells/ml) on polylysine/laminin coated dishes in 1:1 KSR:N2 medium supplemented with Y-27632 (DD5–6, 10 μm, Sigma), FGF2 (DD5–8, 10 ng/ml, R&D systems), IGF1 (DD5–8, 20 ng/ml, R&D systems) and Shh (DD5–8, 50 ng/ml, Sonic C25II, R&D Systems). In some experiments, Shh was omitted from the protocol and instead the Shh inhibitor cyclopamine (DD5–12, 5 μM, Stemgent) was added. Media was changed on DD2, DD4, DD5, DD6, DD8, DD10 and DD12. Cultures were fixed or prepared for FACS on DD12. For FACS, cells were dissociated with trypsin, filtered and sorted on either a FACScan (Becton Dickson) for analysis or a FACS DiVa (Becton Dickson) for collection of GFP+ cells. For some experiments, GFP+ cells were sorted and re-plated on dissociated cultures from neonatal mouse cortex (Xu et al., 2004), then fixed 24 hours later and immunostained for GFP, GABA and FoxG1.
Cortical transplantation and analysis
CD1 mice were housed in a timed pregnancy breeding colony at Weill Cornell Medical College (WCMC), and care of mice was in accordance with institutional IACUC guidelines at WCMC. Transplantation into the cortex of cooling-anesthetized neonatal pups (P0–P3) was performed as previously described (Wonders et al., 2008). Briefly, ~10,000–20,000 FACS-purified GFP+ cells were injected into the somatosensory cortex of each hemisphere (~20–30 69 nl injections of ~10,000 cells/μl per hemisphere) with a nanoinjector (Nanoinject II, Drummond) at a coordinate of 1.5 mm anterior and 1.5 mm lateral from bregma, at a depth of 0.75–1.0 mm. Pups that received cell injections were sacrificed at P30 via intracardial perfusion of 4% PFA. 50 μm vibratome slices were prepared for analysis of cell fate.
Immunohistochemistry and Microscopy
The following primary antibodies were used in this study: rabbit anti-FoxG1 (1:1000, gift of E. Lai, MSKCC), -GABA (1:1000, Sigma), -GFP (1:2000, Invitrogen), - Nkx2.1 (1:2000, Epitomics), -NPY (1:2000, ImmunoStar), -parvalbumin (1:5000, Swant), -SynGAP (1:1000, Thermo Scientific), mouse anti-Nkx2.1 (1:100, Lab Vision), - parvalbumin (1:5000, Chemicon), -VGlut1 (1:500, Chemicon), chicken anti-GFP (1:2000, Abcam), rat anti-somatostatin (1:400, Chemicon), goat anti-ChAT (1:200, Chemicon). Secondary antibodies were conjugated to Alexa fluorophores (Invitrogen) or Cy5 (Jackson ImmunoResearch), and DAPI was used to as a nuclear stain.
Cell cultures were fixed in 4% PFA for 10 minutes at RT. In some instances, cells were unmasked in 1 mM EDTA in PBS at 65°C for 10 minutes prior to blocking. Cultures were blocked in 10% goat or donkey serum in PBST for ≥ 1 hour, incubated in primary antibodies in block O/N at 4°C and incubated for ≥ 2 hours in secondary antibodies at RT. 50 μm P30 vibratome slices were incubated in 10% goat or donkey serum in PBST for ≥ 5 hours, incubated in primary antibodies in block for 3 days at 4°C and incubated in secondary antibodies O/N at 4°C. Images were taken on a Nikon E800 or E400 microscope with Metamorph software, or a Zeiss LSM 510 confocal microscope with Zen software.
Analysis and statistics
For characterization of GFP+ sorted cells on cortical feeders, images were taken from a random area in 3 separate re-plated wells per condition, and a total of 40 GFP+ cells were randomly selected from each image for counting (for a total of 120 cells/condition). For FACS analysis, each data point was an average of 3 individual wells per experimental condition, with ≥ 3 different iNkx2.1 differentiation experiments per condition. All Sst and PV cell counts were performed blind to experimental conditions. 36 ≤ n ≤ 86 GFP+ cells located in layers II–VI were counted per animal, with n ≥ 6 brains for each condition taken from 3 different iNkx2.1 differentiations and transplants. For the Sst/NPY cell counts, 3 brains were used for each condition with 11 ≤ n ≤ 54 GFP+/Sst+ cells counted per brain. All graphs represent mean ± SEM. All data were analyzed using Microsoft Excel, and statistical analysis was determined using ANOVA followed by t tests with Bonferroni post hoc analysis to adjust for multiple comparisons.
Supplementary Material
Acknowledgements
We thank Dr. Michael Kyba for sharing the Ainv15 ESC line and related plasmids, the WCMC flow cytommetry core, the MSKCC molecular cytogenetics core facility for karyotyping and FISH analysis, E. Ribeiro, S. Khim and S. Milrad for technical assistance, and members of the Anderson lab for comments and critique of this project. This work was supported by F32 NS074742 (T.P), R01 MH066912 and NYSTEM C024281 (S.A.).
References
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh ME, Parnavelas JG. Development of somatostatin immunoreactive neurons in the rat occipital cortex: a combined immunocytochemical-autoradiographic study. J Comp Neurol. 1988;268:1–12. doi: 10.1002/cne.902680102. [DOI] [PubMed] [Google Scholar]
- Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci. 2011;31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JL. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron. 2011;70:939–950. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136:3841–3851. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Frontiers in neuroanatomy. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, Hatzistavrou T, Bourke J, Watmuff B, Lang RJ, Haynes JM, Pouton CW, Giudice A, Trounson AO, Anderson SA, Stanley EG, Elefanty AG. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Brown K, Shi SH, Studer L, Anderson SA. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J Neurosci. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Tyson JA, Anderson SA. Pluripotent stem cells for the study of CNS development. Frontiers in molecular neuroscience. 2011;4:30. doi: 10.3389/fnmol.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Danes A, Consiglio A, Richaud Y, Rodriguez-Piza I, Dehay B, Edel M, Bove J, Memo M, Vila M, Raya A, Izpisua Belmonte J.C. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Human gene therapy. 2012;23:56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Inamura M, Kawabata K, Sugawara M, Kikuchi K, Higuchi M, Nagamoto Y, Watanabe H, Tashiro K, Sakurai F, Hayakawa T, Furue MK, Mizuguchi H. Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1alpha transduction. J Hepatol. 2012;57:628–636. doi: 10.1016/j.jhep.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tomishima MJ, Hadjantonakis AK, Gong S, Studer L. Production of green fluorescent protein transgenic embryonic stem cells using the GENSAT bacterial artificial chromosome library. Stem Cells. 2007;25:39–45. doi: 10.1634/stemcells.2006-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Boisvert E, Zhang X, Guo M, Fashoyin A, Du ZW, Zhang SC, Li XJ. Fezf2 regulates telencephalic precursor differentiation from mouse embryonic stem cells. Cereb Cortex. 2011;21:2177–2186. doi: 10.1093/cercor/bhr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010a;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010b;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.