Summary
Molecular programs that mediate normal cell differentiation are required for oncogenesis and tumor cell survival in certain cancers. How cell lineage restricted genes specifically influence metastasis is poorly defined. In lung cancers, we uncovered a transcriptional program that is preferentially associated with distal airway epithelial differentiation and lung adenocarcinoma (ADC) progression. This program is regulated in part by the lineage transcription factors GATA6 and HOPX. These factors can cooperatively limit the metastatic competence of ADC cells, by modulating overlapping alveolar differentiation and invasogenic target genes. Thus, GATA6 and HOPX are critical nodes in a lineage-selective pathway that directly links effectors of airway epithelial specification to the inhibition of metastasis in the lung ADC subtype.
Introduction
Aberrant activation of cell lineage-restricted pathways is required for oncogenic transformation in certain malignancies (Garraway and Sellers, 2006). In contrast, the role of cell differentiation programs in constraining tumor progression and metastasis is poorly defined. Understanding how the molecular determinants of cell fate affect metastasis is particularly relevant in non-small cell lung cancers (NSCLC). NSCLC encompass therapeutically intractable and biologically diverse subtypes of tumors, including adenocarcinomas (ADC), squamous cell carcinomas (SCC), and large cell carcinomas (LCC) (Gabrielson, 2006). Each subtype harbors different genetic alterations, exhibits unique histological features, and contains epithelial cells of distinct lineages, portending major challenges in predicting their clinical outcome.
Multipotent cells from the primary lung buds differentiate into epithelial bronchiolar or alveolar progenitors of the proximal and distal airway respectively (Morrisey and Hogan, 2010). In post-natal lungs, these cells may arise from regional stem cells in the trachea or distal airways. Bronchiolar lineages include ciliated and secretory cell types whereas alveolar stem/progenitors specify into alveolar type I or type II pneumocytes that are required for proper gas exchange. Lung epithelial differentiation is coordinated by a complex network of transcription factors (TFs) whose expression and activity are lineage specific (Maeda et al., 2007). Significantly, SCC cells resemble proximal basal progenitors of the trachea and bronchi (Eramo et al., 2010). Conversely, ADCs form in the distal airways and can arise from alveolar progenitors including alveolar type II (AT2) cells (Xu et al., 2012). The distinct pathways that maintain pulmonary epithelial lineages may therefore also influence the biology of lung cancers.
Lung ADC is the most frequently diagnosed thoracic malignancy with a high incidence of metastasis and death (Jemal et al., 2008). To date, many somatic mutations have been discovered in ADCs, with most being predicted oncogenes (Weir et al., 2007). Several of these mutations are required for the survival of well-differentiated cancer cells (Singh et al., 2009; Weir et al., 2007) which can maintain features of alveolar cells (Hecht et al., 2001). However, during its clinical course, ADC can also adopt mixed histological and molecular features of squamous (Wilkerson et al., 2012) and small cell lung cancers (Alam et al., 2010), which express markers of basal and neuroendocrine cells respectively. The appearance of these alternate lineage traits in ADCs correlates with therapeutic resistance and poor prognosis, but their underlying causes and influence on metastasis are unknown.
Primary lung ADCs are biologically heterogeneous, and can be classified by gene expression profiles (Bhattacharjee et al., 2001; Wilkerson et al., 2012). Given that ADCs arise in the peripheral lungs, we hypothesized that a comprehensive analysis of genes involved in airway and/or alveolar differentiation would reveal mechanisms of ADC heterogeneity and metastasis. In the present study, we examined the molecular relationship between cell differentiation states, lung cancer subtypes, and clinical outcome, to discover a role for lineage-restricted genes in the pathogenesis of lung ADC.
Results
Identification of an alveolar-like differentiation gene module that correlates with lung ADC outcome
To stratify ADCs into biologically informative subsets, we first compiled transcriptomic alterations observed in activated embryonic stem cells (Ben-Porath et al., 2008; Wong et al., 2008), human AT2 cells differentiated from embryonic cells (Ballard et al., 2010; Gonzales et al., 2002) and mouse models of airway homeostasis (Xu et al., 2010). These gene expression patterns were analyzed across multiple cohorts of resected primary human ADCs (Figure S1A). From this, we identified a module of 249 airway and/or alveolar-like differentiation genes (Table S1) that stratifies two distinctive molecular classes of ADCs (Figure 1A). We refer to these groups here as the “Distal airway stem cell (DASC)-like” subtype and the “alveolar-like” subtype based on a number of observations.
Figure 1. Expression of an alveolar-like gene module correlates with lung ADC patient outcome.
(A) Hierarchical clustering was performed on primary ADCs from the NCI Director's Challenge Cohort (DCC; n=442) based on 249 genes that mark AT2 cell differentiation and airway homeostasis. The heatmap represents tumors clustered into “DASC-like” or “alveolar-like” subsets.
(B) Gene set enrichment analysis showing the similarities between activated genes in “DASC-like” tumors and distal airway stem cells. NES=normalized enrichment score.
(C) Frequency of poorly, moderately, or well differentiated stage I tumors within the “alveolar-like” and “DASC-like” groups.
(D) Normalized log2 expression values of epithelial lineage markers in stage I ADCs in DCC tumors. Data presented as a Box-whisker plot (5-95%). p-values calculated by Student's t-test with Bonferroni correction. ns=not significant.
(E) Kaplan-Meier curves for the overall survival of DCC patients classified as in (A). p-values calculated by log-rank test.
(F) NSCLCs from the Duke Medical Center were classified as in (A), and survival plotted for patients with ADC (green and red curves) versus SCC (black and grey curves). p-values calculated by log-rank test.
First, according to the recent characterization of human airway stem/progenitor cells (Kumar et al., 2011), the molecular features of “DASC-like” tumors are more akin to undifferentiated distal airway stem cells (DASCs) as opposed to proximal tracheal airway stem cells (TASCs) (Figures 1B and S1B). Second, many poorly differentiated ADCs are grouped in the DASC-like subset while most well differentiated tumors classify as alveolar-like (Figure 1C). Notably, even within early stage tumors, the expression of prototypical AT2 lineage markers were decreased in the DASC-like tumors, including surfactant proteins encoded by SFTPB-D (Morrisey and Hogan, 2010) and the protease that cleaves their immature form encoded by PGC (Gerson et al., 2008) (Figures 1D and S1C). These tumors also expressed lower levels of SCGB1A1 (Figure S1C), which is expressed in cells of the proximal lung and bronchioalveolar junctions. However, the expression of marker of multipotent lung progenitors ID2 (Rawlins et al., 2009) and the epithelial marker CDH1 (E-cadherin) were unchanged (Figure 1D). As such, the bulk of DASC-like tumors is partially de-differentiated relative to known airway lineages, but retains features of an epithelial cell type. In DASC-like tumors, the expression of WNT inhibitory factor 1 (WIF1), an antagonist of the canonical WNT pathway (Clevers, 2006), was suppressed (Figure S1C), consistent with hyperactive WNT/TCF signaling being a mediator of lung ADC progression and metastasis (Nguyen et al., 2009; Pacheco-Pinedo et al., 2011). Patients harboring DASC-like tumors tended to have decreased 5-year survival rates and increased incidence of metastasis or recurrence (Figures 1E-F and S1D-E; red vs. green subsets). These observations were conserved across 7 independent ADC datasets (664 tumors) and are significant based on random permutation tests (p<0.0001). However, in two SCC cohorts, this gene module was not linked to outcome (Figures 1F and S1E; grey vs. black subsets). The transcriptional control of alveolar differentiation in NSCLCs is therefore preferentially associated with ADC metastatic progression.
Expression of the lineage transcription factors GATA6 and HOPX is linked to metastatic competence in lung ADC
The transcriptome of DASC-like ADCs may reflect the activity of a molecular pathway linked to alveolar differentiation. Frequent ADC mutations include mutant EGFR, KRAS, STK11, and TP53. However, the presence of these mutations by themselves did not correlate with the lineage classification (Figure S2A). To identify mediators of this putative metastasis program, we focused on TF expression and activity, since several classes of TFs are known to balance pluripotency and pulmonary cell fate (Morrisey and Hogan, 2010). Global TF activity can be inferred from the relative enrichment of known TF binding motifs within the promoters of a given gene set. We cross-validated two independent algorithms for TF cis motif analysis across four major ADC cohorts. Motifs for 57 TFs were enriched in the transcriptome of DASC-like ADCs (Figure 2A and Table S2). The over-represented motifs were mainly for E2F family members and MYC (Figure 2A; orange), consistent with previous reports showing activation of their target genes in aggressive cancers (Rhodes et al., 2005; Sinha et al., 2008). In contrast, the under-represented motifs were targets for less-well characterized TFs that are tissue-specific. This included several conserved GATA family binding sites (Figure 2A; blue).
Figure 2. TF activity and expression in DASC-like tumors and metastatic ADC cells.
(A) Annotated cis TF motifs from TRANSFAC database were computed for enrichment in the transcriptome of ADC subsets. The heatmap depicts motifs that are significantly over (orange) or under-represented (blue) in “DASC like” ADCs from 4 independent patient cohorts. Enrichment scores based on -log10(p-value) from GSEA.
(B) Normalized log2 values for GATA6 and HOPX expression in DCC. p-values calculated by Student's t-test with Bonferroni correction.
(C) GATA6 and HOPX were measured in the indicated cell lines via real time quantitative RT-PCR (qRT-PCR). U.D=undetected. Bar charts represent the mean ± SEM.
(D) Western blot of GATA6 and HOPX in the indicated cell lines.
(E) Normalized log2 values of Gata6 and Hopx expression in non-metastatic primary (TnonMet: n=7), metastatic primary (Tmet; n=7) and metastases (Met; n=9) cell lines derived from a KrasLSL-G12D/+;p53flox/flox lung ADC mouse model. p-values calculated by Student's t-test with Bonferroni correction.
(F) Survival of DCC patients based on the median expression of GATA6 and HOPX in their primary tumors. p-values calculated by log-rank test. Hazard ratio (HR) and confidence interval calculated using patient sex, smoking status, and tumor stage as covariates.
GATAs can either activate or repress transcription and have redundant or specific target genes depending on the biological context (Molkentin, 2000). Of the 6 known family members, GATA6 is essential for endodermal differentiation and distal airway homeostasis (Keijzer et al., 2001; Liu et al., 2002b; Yang et al., 2002). It maintains proper alveolar gene expression in cooperation with other known lineage TFs including HOPX (Yin et al., 2006) and NKX2-1 (Liu et al., 2002a; Zhang et al., 2007). Interestingly, GATA6, HOPX, and NKX2-1 were part of the lineage signature (Table S1), and their expression were generally reduced in poor prognosis tumors (Figures 2B and S2B). The expression of other GATAs varied less across human ADCs (Figure S2B). HOPX lacks a DNA binding domain (Chen et al., 2002; Shin et al., 2002), explaining why it did not score in our cis motif analysis. NKX2-1 targets annotated in this analysis were not broadly over or under-represented. Based on recent ADC re-sequencing efforts, copy number variations and somatic mutations in GATA6 and HOPX were rare (Figure S2C), suggesting that perturbation of these TFs occurs primarily at the level of their expression.
To determine which lineage TFs are preferentially deregulated in metastatic ADC cells, we compared their expression in several models of ADC progression. We studied ADC metastasis generated via in vivo selection of metastatic cell sub-populations (BrM3 cells) from the human lung ADC cell lines H2030 and PC9, which carry KRAS and EGFR mutations respectively. Following transplantation in mice, BrM3 cells have enhanced metastatic predilections when compared to their parental cells (Nguyen et al., 2009). mRNA levels of GATA members were generally reduced in BrM3 cells (Figure S2D). Suppression of GATA6 and known HOPX isoforms was not entirely associated with DNA methylation (Figures S2E-F). Still, GATA6 expression was notably decreased in the metastatic H2030-BrM3 cells as compared to their indolent parental cells, while HOPX was undetectable in both H2030 populations (Figures 2C-D). GATA6 expression varied less in the PC9-BrM3 sub-population but these cells express significantly lower levels of HOPX (Figures 2C-D). Although NKX2-1 is a prognostic marker (Barletta et al., 2009), its inhibition may occur independently since its expression was already low to undetectable in these human cells and did not correlate with their metastatic predilection (Figure S2G). Murine ADC cell lines have been isolated from genetically engineered mice with NSCLC initiated by Kras and p53 mutations (Winslow et al., 2011). In this model, invasive primary tumor cells (TMet) with low Nkx2-1 and their clonally derived metastatic cells (Met) also express decreased levels of Gata6 and Hopx as compared to cells from non-metastatic ADCs (TnonMet) (Figure 2E). Altogether, the suppression of alveolar lineage TFs in a subset of primary tumors is linked to the selection of metastatic ADCs. In particular, low levels of both GATA6 and HOPX in ADCs correlated with poor outcome, even when accounting for patient smoking status, sex, and tumor stage (Figure 2F).
GATA6 and HOPX limit multi-organ metastasis
To test the functions of GATA6 and HOPX in human ADC cells, we reduced their levels via inducible shRNAs in the less aggressive H2030 and PC9 parental cells. We confirmed that doxycycline (DOX) treatment caused efficient and sustained induction of hairpin expression in disseminated tumor cells in an immuno-compromised mouse model (Figure S3A). Our functional results were validated using independent hairpins (shRNAs a and b) and siRNAs against each gene where indicated.
Based on the alveolar-like signature, H2030 cells were less differentiated than PC9 cells (data not shown) and already express low amounts of HOPX (see Figure 2D). Accordingly, we reduced GATA6 in parental H2030 cells (Figure 3A) and injected them at different dilutions into the arterial circulation of mice that were either treated with DOX or control feed. Knockdown of GATA6 in parental H2030 cells was sufficient to enhance their metastatic capabilities (Figure 3B) but had only a modest effect on subcutaneous tumorigenesis (Figure S3B). On the other hand, overexpression of GATA6 and/or HOPX in the highly metastatic H2030-BrM3 cells, which express low endogenous levels of both TFs (Figure S3C), inhibited their metastatic potential (Figure 3C). We then decreased the expression of GATA6 and HOPX individually or in combination in the more differentiated PC9 cells, which express high levels of both TFs, and achieved 71% knockdown of GATA6 and over 90% knockdown of HOPX (Figures 3D and S3D). Although HOPX is a downstream target of GATA6 in AT2 cells (Yin et al., 2006), their expressions were not inter-dependent in PC9 cells (Figure 3D). Knockdown of GATA6 together with HOPX enhanced the proportion of animals with high metastatic burden, which could be rescued by ectopic expression of shRNA resistant cDNAs (Figures 3E and S3E-F). When injected into circulation at lower cell numbers, GATA6 and HOPX knockdown increased the incidence of multi-organ metastasis (Figure 3F). Metastases were confirmed in brain, bone, and lungs (Figure 3G). GATA6 and HOPX did not significantly alter the proportion of PC9 cells expressing CD133 (Figure S3G), a putative tumor initiating cell marker, and did not affect tumorigenic potential (Figure 3H). We conclude that these TFs can cooperatively limit the metastatic competence of ADC cells independent of tumor growth.
Figure 3. The lineage TFs GATA6 and HOPX limit metastatic colonization.
(A) H2030 cells stably expressing two separate doxycycline (DOX) inducible shRNAs, (a) or (b), against GATA6 were cultured in the absence or presence of DOX. GATA6 level was measured by qRT-PCR.
(B) H2030 cells from (A) were injected at indicated cell numbers into the arterial circulation of mice fed with regular (-DOX) or DOX (+DOX) diet. Metastatic burden was measured by bioluminescent imaging. The bar chart shows the whole body bioluminescent units (BLU) collected at Day 36 (shGATA6(a)) or Day 40 (shGATA6(b)) and normalized to Day 0. Right: representative images of mice injected with H2030-shGATA6(a) cells on Day 36. p-values calculated by Mann-Whitney test.
(C) 5 × 104 H2030-BrM3 cells overexpressing GATA6, HOPX, or both were injected as in (B). The scatter plot shows the metastatic burden at Day 42. p-values calculated by Mann-Whitney test.
(D) qRT-PCR of GATA6 and HOPX in PC9 cells expressing the indicated shRNAs. shCon: a control shRNA.
(E) 5 × 104 PC9 cells expressing the indicated shRNAs were injected intracardially into DOX treated mice. The scatter plot shows the metastatic burden at Day 48. Bottom: representative images of mice with metastases. p-values calculated by Mann-Whitney test.
(F) Kaplan-Meier curve showing the metastasis free survival of mice inoculated with 2.5 × 104 PC9-shGATA6/shHOPX(b) cells as in (B) or injected with PC9-shGATA6/shHOPX(b) cells that co-express GATA6 and HOPX cDNAs (+Rescue). p-values calculated by log-rank test.
(G) Hematoxylin and eosin staining of metastases in the (a) brain, (b) bone and (c) lung of mice harvested from (E). Scale bars, 100 μm.
(H) Volumes of subcutaneous tumors formed by PC9-shGATA6/shHOPX(a) cells. Mean ± SEM. p-values calculated by Student's t-test. ns= not significant.
All bar charts represent the mean ± SEM. See also Figure S3.
GATA6 and HOPX constrain invasogenic outgrowth
Given the metastasis inhibitory functions of GATA6 and HOPX in vivo, we ascertained the cell biological mechanism of their action. Under 2-dimensional (2D) growing conditions, knockdown of GATA6 in H2030 cells did not affect cell survival even when deprived of growth factors, whereas KRAS knockdown caused a significant decrease in their viability (Figures 4A and S4A). In PC9 cells, loss of GATA6 and/or HOPX did not alter cell growth (Figure 4B), whereas reduction of GATA2 and EGFR inhibited cell viability as previously reported (Figures 4C and S4B-C) (Kumar et al., 2012; Rothenberg et al., 2008). Thus, in these ADC cells, GATA6 and HOPX are not lineage survival genes.
Figure 4. GATA6 and HOPX do not affect tumor cell survival.
(A) H2030 cells with the indicated shRNAs were cultured in media containing 0.2% serum. Cell viability was measured at 72 hr after DOX treatment.
(B) The viability of PC9 cells with the indicated shRNAs was measured in media supplemented with 10% or 0.2% FBS as in (A).
(C) The indicated PC9 cell lines were transfected with siRNA targeting GATA2 (siGATA2). Cell viability was measured in 0.2% FBS at Day 5 post-transfection.
All bar charts represent the mean ± SEM. See also Figure S4.
To study metastatic cellular phenotypes in the more differentiated PC9 cells, we optimized a 3-dimensional (3D) culture model for tumor organoid formation in extracellular matrix. Under these conditions, PC9 parental cells formed solid organoids (Figure 5A; inset). Suppression of HOPX caused an outgrowth of these organoids, and this effect was enhanced in combination with GATA6 knockdown (Figures 5A-B and S5A-B). GATA6 and/or HOPX knockdown did not alter the overall number of organoids (Figure 5C; top), even when single cells were seeded (Figure S5C), but significantly changed the morphologies of these organoids. Extending on prior characterization (Kenny et al., 2007), we termed these mass, grape-like or expansive organoids. Mass organoids maintained spherical appearance whereas grape-like and expansive organoids were disorganized. The expansive organoids were the largest multi-cellular structures and they correlated with an increase in PC9 cell outgrowth. Knockdown of GATA6 or HOPX augmented the proportion of grape-like organoids (Figure 5C; teal). HOPX suppression also moderately increased the amount of expansive organoids (Figure 5C; red). Double GATA6 and HOPX knockdown greatly increased the proportion of expansive organoids (Figure 5C), which was prevented by rescue of GATA6 and HOPX expression (Figure S5D). Moreover, GATA6 and HOPX re-induction in pre-established organoids by DOX withdrawal delayed their expansion (Figure 5D).
Figure 5. GATA6 and HOPX constrain cell invasion.
(A) PC9 cells expressing the indicated shRNAs were cultured in low attachment plates with media containing Matrigel and DOX. Phase contrast images were captured after 14 days of culture. The inset shows a cross section of a DAPI stained organoid. Scale bars, 200 μm (25 μm in insert).
(B) Organoids from (A) were harvested and cell outgrowth was measured as a function of luciferase activity.
(C) The bar chart shows the percentage of mass (black), grape-like (teal) and expansive (red) organoids. The numbers at the top of graph show the overall numbers (± SEM) of tumor organoids formed at Day 8. Representative images of indicated organoids are shown at right. Scale bars, 100 μm.
(D) After 14 days of 3D culture in the presence of DOX, PC9-shGATA6/shHOPX(b) organoids were re-plated in Matrigel with (+DOX) or without DOX (Off-Dox) for an additional 18 - 24 days. Tumor cell growth was measured at Day 32 and 38 as in (B). Representative images at Day 32 are shown. The red fluorescent protein (RFP) signal indicates shRNA expression. Scale bars, 200 μm.
(E) At Day 14, PC9-shGATA6/shHOPX(b) organoids formed in Matrigel in the presence or absence of DOX were disaggregated and subjected to invasion assay in Boyden chambers.
(F) PC9 cells expressing the indicated shRNAs were grown in monolayer with DOX for 5 days and then directly subjected to an invasion assay as in (E). Quantification was normalized to the invasion of cells expressing shCon.
(G) Invasion of additional ADC lines was determined as in (F).
All bar charts represent the mean ± SEM. p-values calculated by Student's t-test. See also Figure S5.
The morphology of the grape-like and expansive organoids suggests that they are highly invasive. To test this directly, we dissociated cells from the organoids and compared their invasive potential through Matrigel. Cells from the grape-like and/or expansive organoids were more invasive than those from the masses (Figure 5E). GATA6 and HOPX also cooperatively limit the invasion of PC9 cells cultured directly from monolayer (Figure 5F). The effect of double knockdown was consistent across 4 additional ADC cell lines that express high levels of these TFs and encompass different genetic backgrounds and tumor stages (Figures 5G and S5E). In contrast, invasion was not affected by knockdown in 2 high GATA6/HOPX expressing non-ADC cell lines (1 SCC and 1 LCC) that we tested (Figures S5F-G). GATA6 reduction was sufficient to promote invasion of the parental H2030 cells (Figure 5G; yellow), consistent with low levels of HOPX in these ADC cells. This effect was partially rescued by re-expression of HOPX (Figures S5H-I), confirming its cooperativity with GATA6. Restoration of GATA6 and HOPX in the metastatic H2030-BrM3 ADC sub-population inhibited its invasion, while over-expression of these TFs in the low GATA6/HOPX expressing SCC cell line H520 did not alter its modest baseline invasiveness (Figures S5J-K).
Activation of specific pro-invasive pathways following GATA6 and HOPX reduction
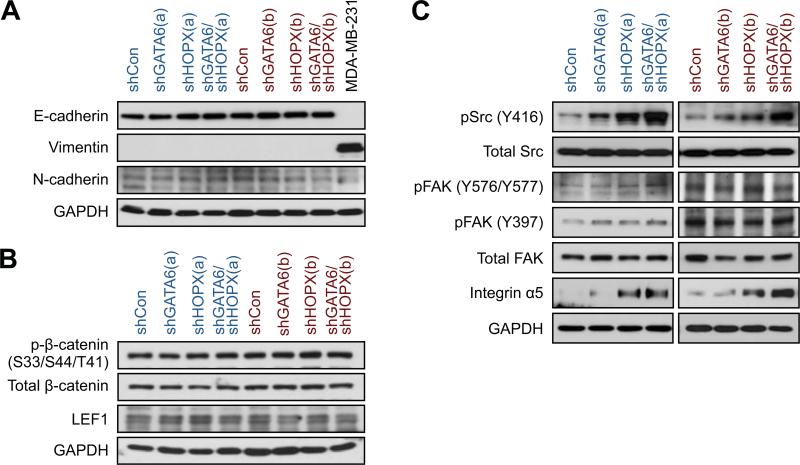
Having defined a cellular function for GATA6 and HOPX in lung ADC cells, we examined if these TFs affect pathways reported to mediate lung cancer invasion (Carretero et al., 2010; Nguyen et al., 2009; Roman et al., 2010; Saito et al., 2009). We analyzed PC9 cells with GATA6 and/or HOPX knockdown because the greatest phenotypic alterations were observed in this model. Surprisingly, the levels of epithelial-mesenchymal transition (EMT) markers, ZEB1/2, TWIST1, E-cadherin and vimentin, were not affected (Figures 6A and S6A). Activation of the canonical WNT pathway was only marginally regulated downstream of these TFs (Figures 6B and S6B). On the other hand, reduction of GATA6 and HOPX cooperatively increased Src activation but not focal adhesion kinase (FAK) (Figure 6C). Finally, we examined the steady state levels of several integrin subunits and found that GATA6 and/or HOPX knockdown led to a marked increase in integrin α5 but did not consistently affect others (Figure 6C and data not shown). Thus, GATA6 and HOPX can repress Src activity and integrin α5 expression in poorly metastatic cells.
Figure 6. Src and integrin α5 are suppressed by GATA6 and HOPX.
PC9 cells with the indicated shRNAs targeting GATA6 and/or HOPX were cultured in monolayer. After 5 days of DOX treatment, cells were harvested for western blot analysis for (A) epithelial (E-cadherin) and mesenchymal (Vimentin and N-cadherin) markers; (B) canonical WNT signaling pathway components, β-catenin and LEF1; (C) Src, FAK, and integrin α5. Lysate from MDA-MB-231 cells serves as a control for vimentin.
See also Figure S6.
GATA6 and HOPX regulate a set of common genes involved in epithelial differentiation and metastasis
To comprehensively understand how GATA6 and HOPX link lineage fate to metastatic competence, we identified their downstream target genes using genome-wide RNA sequencing. Knockdown of both TFs in PC9 cells caused transcriptomic alterations that resemble those of human DASC-like tumors (Figure 7A; orange labels). These changes also inversely correlate with the transcriptome of AT2 cells differentiated in vitro and broadly resemble those of DASCs but not TASCs (Figure S7A). To determine if the perturbation of these TFs contributes to relevant molecular and histopathological classes of human ADCs, we examined the relationship between GATA6/HOPX regulated genes and three major ADC subgroups, including bronchioid, magnoid and squamoid cancers (Wilkerson et al., 2012). The gene expression profiles of GATA6/HOPX knockdown cells correlated most with the squamoid subtype (Figure 7A; cyan labels), which includes highly invasive tumors with mixed adenosquamous features (Wilkerson et al., 2012).
Figure 7. Common regulation of airway differentiation and metastasis gene sets.
(A) Pearson correlation coefficients were calculated for each DCC tumor by comparing its mRNA transcriptome to the shGATA6/shHOPX knockdown signature generated by RNA-sequencing of PC9 cells. DCC tumors were ordered from lowest to highest correlation with shGATA6/shHOPX knockdown cells (top). Histological and molecular subtypes are annotated in the middle and bottom rows for comparisons. DASC-like samples have a higher correlation with GATA6/HOPX knockdown cells than with alveolar-like tumors (middle row; p-value by Student's t-test). Squamoid and magnoid tumors have a higher correlation than bronchioid tumors (bottom row; p-value by ANOVA).
(B) Left: the heatmap depicts 426 genes that are commonly up-regulated (red) or down-regulated (green) in shGATA6/shHOPX knockdown cells and DCC tumors. Right: top 50 up- and down-regulated genes. Genes that were validated by qRT-PCR are denoted with rectangles. Blue=controlled by HOPX. Grey=redundantly controlled by GATA6 and HOPX. Red=cooperatively controlled by GATA6 and HOPX. *=Confirmation of HOPX and GATA6 knockdown.
(C) qRT-PCR measurement of alveolar (SFTPD and PGC), basal (KRT6A and KRT6B) and neuroendocrine (SCG2) marker expression in DOX treated PC9 cells with the indicated shRNAs.
(D) qRT-PCR measurement of the expression of known metastasis genes.
For qRT-PCR data, expression was normalized to PC9-shCon cells and presented as the mean ± SEM. See also Figure S7 and Table S3.
By filtering the list of coding transcripts based on genes similarly expressed in human tumors, we identified 426 relevant mRNAs that were differentially regulated by GATA6/HOPX (Figure 7B and Table S3). We confirmed that many of these genes were redundantly or cooperatively modulated by GATA6 and HOPX (Figure 7B; grey and red rectangles), while an additional gene set was only altered by HOPX (Figure 7B; blue rectangles). The alveolar markers SFTPD and PGC were downregulated after reduction of these TFs (Figures 7C and S7B). Surprisingly, the loss of AT2 identity in tumor cells was accompanied by an increase in the expression of cytokeratins 6A (KRT6A) and 6B (KRT6B) (Figures 7C and S7B), which are normally expressed in basal cells and putative DASCs (Kumar et al., 2011). The expression of secretogranin II (SCG2), a marker of neuroendocrine cells (Feldman and Eiden, 2003), was also activated. Consistent with an epithelial lineage switch controlled by these TFs, GATA6/HOPX expression significantly correlated with AT2 markers and inversely correlated with KRT6A/B levels in a proportion of resected human ADCs (Figure S7C).
We also identified a number of target genes that are known mediators of metastatic colonization (Figures 7D and S7B). For instance, reduction of GATA6 and/or HOPX led to increased expression of IL-1B, IL-11, and EREG, which encode for secreted factors associated with organotropic metastasis (Bos et al., 2009; Gupta et al., 2007; Kang et al., 2003). Vascular and ECM remodeling genes (VEGFA and PLAU) that promote metastatic progression (Blanco et al., 2012; Weis et al., 2004) were also activated. Some of the described transcriptional changes were observed in other ADC cell lines, with KRT6A/B induction being the most consistent response (Figures S7D-E). Consequently, inhibition of GATA6 and HOPX may activate a multi-genic program that enhances dissemination as well as distant organ colonization.
GATA6 and HOPX lineage target genes control malignant cell invasion
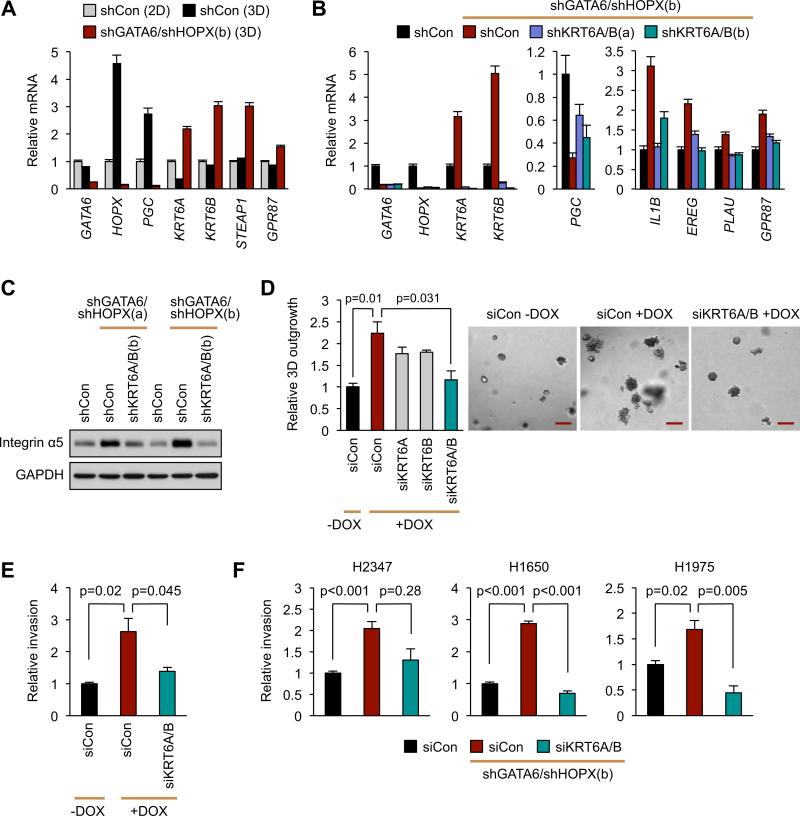
Given that GATA6/HOPX regulated genes include markers of lung differentiation and metastasis, the repression of certain metastatic functions may be intrinsic to the lineage specifying activity of these TFs. Moreover, we found that the formation of tumor organoids from 2D culture up-regulates the expression of the alveolar-like genes HOPX and PGC, but represses KRT6A expression (Figure 8A). Hence, this organoid system recapitulates extracellular conditions that enforce alveolar fate at the expense of other lineages. The expression pattern of these markers was reversed in the invasive organoids after GATA6/HOPX knockdown (Figure 8A), suggesting that this particular differentiation program may directly constrain tumor cell invasion.
Figure 8. GATA6 and HOPX link epithelial identity with invasion through KRT6A and KRT6B.
(A) PC9 cells with the indicated shRNAs were cultured in monolayer (2D) or Matrigel suspension (3D) for 5 days as in Figure 5. Expression of the indicated lung epithelial markers was detected by qRT-PCR.
(B) PC9-shGATA6/shHOPX(b) knockdown cells were super-infected with lentiviruses encoding shCon or shRNAs against KRT6A and KRT6B. Expression of the indicated GATA6/HOPX target genes upon KRT6A/B suppression was detected by qRT-PCR.
(C) Western blot showing integrin α5 protein levels in the indicated cell lines.
(D) PC9-shGATA6/shHOPX(b) cells were transfected with the indicated siRNAs and cultured in 3D. Tumor cell outgrowth was quantified and representative images captured at Day 10. Scale bar, 200 μm.
(E) PC9-shGATA6/shHOPX(b) cells were transfected with a control siRNA (siCon) or siRNAs against KRT6A and KRT6B. At 48 hours post-transfection, cells were subjected to an invasion assay.
(F) Invasion assay was performed using indicated ADC lines with the indicated shRNAs/siRNAs.
All bar charts represent the mean ± SEM. p-values calculated by Student's t-test. See also Figure S8.
To test this possibility, we focused on the function of putative epithelial lineage markers that were aberrantly activated upon GATA6 and HOPX repression, including KRT6A, KRT6B (Moll et al., 1982), STEAP1 (Gomes et al., 2012), and GPR87 (Glatt et al., 2008) (Figure 8A). We successfully inhibited their activation by shRNA and siRNAs in double GATA6/HOPX knockdown cells (Figures 8B, S8A, and data not shown). Surprisingly, reduction of both KRT6A and KRT6B partially restored the expression of the alveolar marker PGC (Figure 8B). This partial alveologenic rescue diminished the induction of several pro-metastatic genes (Figure 8B) and integrin α5 expression (Figure 8C). Functionally, reduction of both KRT6A and KRT6B, but not STEAP1 and GPR87, attenuated the organoid outgrowth initiated by GATA6/HOPX knockdown (Figures 8D, S8B, and data not shown). KRT6A/B knockdown also diminished the invasion of most of the ADC lines tested (Figures 8E-F), suggesting that airway lineage markers can directly control pathways of lung cancer cell invasion. Collectively, our data identify GATA6 and HOPX as context and lineage selective inhibitors of metastatic competence.
Discussion
Lineage markers and ADC progression
Although several genomic aberrations are associated with lung cancer progression, our understanding of the metastatic process remains incomplete. Emerging evidence indicates that the transcriptional pathways that are critical for lung morphogenesis can be disrupted or reused during chronic diseases of the airways (Whitsett et al., 2011). Differentiation and regeneration of the lung epithelium is dictated by many cooperating transcriptional effectors. Our integrated approach uncovered a transcriptional network that is preferentially active in committed airway epithelial cells and is inhibited in a subset of metastatic cancers arising from the distal lungs. Within this network, we found that the cooperative action of at least two TFs, GATA6 and HOPX, links the molecular determinants of alveologenesis to metastatic competence in the lung ADC subtype.
Lung ADCs can arise from AT2 cells and expression of alveolar markers is employed to discriminate lung ADCs from other thoracic malignancies. However, epithelial trans-differentiation may occur in ADCs. Notably some ADCs express markers of squamous (Wilkerson et al., 2012) or small cell lung cancers (Alam et al., 2010). Distinct histological subgroups correlate with relapse (Travis et al., 2011). These observations imply that the cellular composition and/or molecular determinants of airway specification vary during ADC progression and can influence clinical outcome. We found that a gene signature modulated in part by GATA6 and HOPX repression correlates with classifiers of poor prognosis. Importantly, this lineage classification is associated with metastasis in patients with ADC but not SCC.
GATA6 and HOPX as inhibitors of metastasis
We discovered that GATA6 and HOPX expression and activity are reduced in a subset of high grade ADCs and metastatic cells. Suppression of both TFs can enhance the invasion of lung ADC cell lines with different mutations but this did not have the same effect in SCC cell lines tested here. Although GATA6 with HOPX preferentially limit ADC metastatic competence, it remains to be determined under which genetic/epigenetic context(s) this activity is enforced and whether these TFs have alternate roles in other NSCLCs with particular molecular aberrations or tumor stages. In various cancers, GATA members may promote (Belaguli et al., 2010; Collisson et al., 2011; Lin et al., 2012; Yang et al., 2011) or inhibit (Cai et al., 2009; Kouros-Mehr et al., 2008; Lindholm et al., 2009) cancer. This is analogous to another context dependent TF in NSCLC, NKX2-1, which can function as an oncogene (Weir et al., 2007) or tumor suppressor (Maeda et al., 2012; Snyder et al., 2013; Winslow et al., 2011). HOPX levels correlate with tumorigenesis (Chen et al., 2007; Ooki et al., 2010) but its mechanism of action has been unknown. The biological activity of such lineage factors likely depends on their integration within a TF network that is unique to particular cellular contexts.
The lung buds are derived from the endoderm, which is specified by GATA6 (Morrisey and Hogan, 2010). GATA6 also restricts the expansion of progenitor cells in the adult airways where it is abundantly expressed (Morrisey et al., 1996; Zhang et al., 2008). To maintain AT2 homeostasis, at least 2 other TFs, NKX2-1 and HOPX, can synergize with or are regulated by GATA6 (Liu et al., 2002a; Yin et al., 2006; Zhang et al., 2007). Our data along with other studies supports a model where suppression of this TF node and divergent alveologenesis directly influence ADC progression. Our findings further suggest that inhibition of HOPX together with GATA6 in transformed ADC cells is an important determinant of invasion and metastasis initiation. This does not inherently exclude the possibility that metastasis could also originate from progenitors with low GATA6 and HOPX activity. Although we found that GATA6 and HOPX could further limit ADC metastatic colonization, overt metastasis likely requires multiple physiological or molecular perturbations and other lineage TFs may be involved. Additional insight into the diverse pathogenesis of NSCLCs will be uncovered by studying the timing, cellular context, and mechanisms by which combinations of lineage TFs are de-regulated.
GATA6 and HOPX directly link metastatic invasion to airway cell fate
GATA6 and HOPX modulated the transcription of overlapping genes, some of which were cooperatively activated or repressed. Since HOPX lacks a DNA binding domain, it must interact with other sequence specific TFs to account for its effects. Certain GATA6/HOPX regulated genes encode for cellular functions that link airway epithelial differentiation to metastasis. Indeed, suppression of these TFs in ADC cells decreased AT2 differentiation. This was not sufficient to induce EMT but caused transformed cells to display characteristics of basal epithelial cells including KRT6A/B expression. Concomitantly, these TFs inhibited pro-invasive pathways such as Src activity and integrin α5 induction while also controlling known metastasis genes. Surprisingly, KRT6A/B themselves partially mediated alveolar gene expression and ADC cell invasion. KRT6A/B are activated in epithelial tissue during wound healing (Wojcik et al., 2000) and in putative stem cells of the distal lung following infections (Kumar et al., 2011). Hence, this switch in lineage markers may represent a trans-differentiating or de-differentiating event, enabling aggressive ADC cells to adopt traits normally required for airway regeneration.
Our study highlights the distinct mechanisms of metastatic competence in different types of lung cancers. In particular, we have demonstrated that the TFs, GATA6 and HOPX, can inhibit metastasis in the lung ADC subtype. These TF factors and their target genes directly link programs of airway specification to the pathogenesis of NSCLC. Consequently, certain facets of this lineage pathway may be explored to more effectively treat patients with metastatic lung cancers.
Experimental Procedures
Additional experimental procedures are provided in Supplemental Information.
Cell culture, viability, and invasion assays
Cell lines were cultured as recommended by the American Type Culture Collection (ATCC). Cell viability and invasion were assayed as mentioned (Nguyen et al., 2009). For 3D outgrowth, cells were grown in media with 5% Matrigel in ultra-low attachment plates (Corning) at 37 °C for 8-14 days. Organoids were categorized as described (Kenny et al., 2007). Expansive organoids are a subset of grape-like organoids with diameter > 200 μm. To quantify outgrowth, cells stably expressing firefly luciferase were harvested and luciferase activity was measured by Dual-Luciferase Reporter Assay (Promega).
Gene knockdown, qPCR, and western blot
shRNA sequences, inducible lentiviral vectors, primers and antibodies were listed in Supplemental Experimental Procedures.
Animal Studies
Studies using athymic nu/nu male mice (NCI) aged between 5-7 weeks were conducted in compliance with U.S. guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Yale University..DOX was administered in chow (625 mg/kg). An IVIS Spectrum was used for bioluminescence imaging.
Microarray data analysis
Data sets for primary lung cancers, AT2/airway genes, airway stem cells as well as derivation of an “alveolar-like” signature are listed in Supplemental Experimental Procedures and Table S1. Differential gene expression and hierarchical clustering were performed in Partek© and R.
RNA sequencing
RNA samples were sequenced on a HiSeq 2000 (Illumina) and analyzed using Tophat and EdgeR.
Statistical analysis
Experimental data were presented as mean ± SEM. p-values for in vitro data and tumor volume were calculated by two-tailed Student's t-test. Survival curves and metastatic burden were analyzed by log-rank test and Mann-Whitney test respectively.
Supplementary Material
Significance.
Lung adenocarcinoma (ADC) is a deadly and heterogeneous subtype of non-small cell lung cancer. During lung ADC progression, the emergence of alternate cell lineage traits in primary tumors correlates with poor outcome. The underlying mechanisms and biological consequences of these phenomena are poorly understood. Through an integrated approach, we identified two lineage transcription factors, GATA6 and HOPX, as inhibitors of metastatic progression. Down-regulation of their expression in a subset of ADCs correlates with aberrant tumor differentiation and relapses. GATA6 and HOPX cooperatively restrict the metastatic potential of ADC cells, by modulating converging transcriptional programs of airway epithelial differentiation and malignant invasion. Our findings demonstrate that perturbation of intrinsic cell lineage pathways is a determinant of metastasis in specific lung cancers.
Highlights.
An alveolar differentiation program controls lung adenocarcinoma progression.
This pathway is modulated by the transcription factors GATA6 and HOPX.
GATA6 and HOPX cooperate to restrain lung adenocarcinoma metastasis.
Suppression of GATA6 and HOPX induces cytokeratins 6A/B and cell invasion.
Acknowledgements
We thank Mei Zhong for help with RNA sequencing and Katerina Politi for review of the manuscript. This work was funded by grants to D.X.N from Uniting Against Lung Cancer, the National Lung Cancer Partnership, ACS Institutional Grant IRG-58-012-52, and NIH/NCI grant 1R01CA166376. T.F.W. is funded by CPRIT RP120583, NIH P30 CA125123-06, and NIH CA149196. D.X.N. is a scholar of the V Foundation for Cancer Research, Yale Center for Clinical Investigation, and Young Investigator of the International Association for the Study of Lung Cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number. RNA sequencing data is deposited at GEO (GSE39121).
References
- Alam N, Gustafson KS, Ladanyi M, Zakowski MF, Kapoor A, Truskinovsky AM, Dudek AZ. Small-cell carcinoma with an epidermal growth factor receptor mutation in a never-smoker with gefitinib-responsive adenocarcinoma of the lung. Clin Lung Cancer. 2010;11:E1–4. doi: 10.3816/CLC.2010.n.046. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol. 2010;299:L36–50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta JA, Perner S, Iafrate AJ, Yeap BY, Weir BA, Johnson LA, Johnson BE, Meyerson M, Rubin MA, Travis WD, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13:1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaguli NS, Aftab M, Rigi M, Zhang M, Albo D, Berger DH. GATA6 promotes colon cancer cell invasion by regulating urokinase plasminogen activator gene expression. Neoplasia. 2010;12:856–865. doi: 10.1593/neo.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MA, Leroy G, Khan Z, Aleckovic M, Zee BM, Garcia BA, Kang Y. Global secretome analysis identifies novel mediators of bone metastasis. Cell Res. 2012 doi: 10.1038/cr.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai KQ, Caslini C, Capo-chichi CD, Slater C, Smith ER, Wu H, Klein-Szanto AJ, Godwin AK, Xu XX. Loss of GATA4 and GATA6 expression specifies ovarian cancer histological subtypes and precedes neoplastic transformation of ovarian surface epithelia. PLoS One. 2009;4:e6454. doi: 10.1371/journal.pone.0006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pacyna-Gengelbach M, Deutschmann N, Niesporek S, Petersen I. Homeobox gene HOP has a potential tumor suppressive activity in human lung cancer. International journal of cancer Journal international du cancer. 2007;121:1021–1027. doi: 10.1002/ijc.22753. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Haas TL, De Maria R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene. 2010;29:4625–4635. doi: 10.1038/onc.2010.207. [DOI] [PubMed] [Google Scholar]
- Feldman SA, Eiden LE. The chromogranins: their roles in secretion from neuroendocrine cells and as markers for neuroendocrine neoplasia. Endocr Pathol. 2003;14:3–23. doi: 10.1385/ep:14:1:3. [DOI] [PubMed] [Google Scholar]
- Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–538. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem. 2008;283:10330–10338. doi: 10.1074/jbc.M707516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt S, Halbauer D, Heindl S, Wernitznig A, Kozina D, Su KC, Puri C, Garin-Chesa P, Sommergruber W. hGPR87 contributes to viability of human tumor cells. International journal of cancer Journal international du cancer. 2008;122:2008–2016. doi: 10.1002/ijc.23349. [DOI] [PubMed] [Google Scholar]
- Gomes IM, Maia CJ, Santos CR. STEAP Proteins: From Structure to Applications in Cancer Therapy. Mol Cancer Res. 2012;10:573–587. doi: 10.1158/1541-7786.MCR-11-0281. [DOI] [PubMed] [Google Scholar]
- Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- Hecht JL, Pinkus JL, Weinstein LJ, Pinkus GS. The value of thyroid transcription factor-1 in cytologic preparations as a marker for metastatic adenocarcinoma of lung origin. Am J Clin Pathol. 2001;116:483–488. doi: 10.1309/NL4Y-FHG8-2XBC-F9XH. [DOI] [PubMed] [Google Scholar]
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development. 2001;128:503–511. doi: 10.1242/dev.128.4.503. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Hancock DC, Molina-Arcas M, Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E, Lassailly F, Matthews N, Nye E, et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Bass AJ, Lockwood WW, Wang Z, Silvers AL, Thomas DG, Chang AC, Lin J, Orringer MB, Li W, et al. Activation of GATA binding protein 6 (GATA6) sustains oncogenic lineage-survival in esophageal adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4251–4256. doi: 10.1073/pnas.1011989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm PM, Soini Y, Myllarniemi M, Knuutila S, Heikinheimo M, Kinnula VL, Salmenkivi K. Expression of GATA-6 transcription factor in pleural malignant mesothelioma and metastatic pulmonary adenocarcinoma. J Clin Pathol. 2009;62:339–344. doi: 10.1136/jcp.2008.060533. [DOI] [PubMed] [Google Scholar]
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002a;277:4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- Liu C, Morrisey EE, Whitsett JA. GATA-6 is required for maturation of the lung in late gestation. Am J Physiol Lung Cell Mol Physiol. 2002b;283:L468–475. doi: 10.1152/ajplung.00044.2002. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tsuchiya T, Hao H, Tompkins DH, Xu Y, Mucenski ML, Du L, Keiser AR, Fukazawa T, Naomoto Y, et al. Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest. 2012;122:4388–4400. doi: 10.1172/JCI64048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Kokubo K, Kobayashi H, Kim MS, Sidransky D, Watanabe M. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene. 2010;29:3263–3275. doi: 10.1038/onc.2010.76. [DOI] [PubMed] [Google Scholar]
- Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, Morrisey EE. Wnt/beta-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 2011;121:1935–1945. doi: 10.1172/JCI44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Barrette TR, Ghosh D, Chinnaiyan AM. Mining for regulatory programs in the cancer transcriptome. Nat Genet. 2005;37:579–583. doi: 10.1038/ng1578. [DOI] [PubMed] [Google Scholar]
- Roman J, Ritzenthaler JD, Roser-Page S, Sun X, Han S. alpha5beta1-integrin expression is essential for tumor progression in experimental lung cancer. Am J Respir Cell Mol Biol. 2010;43:684–691. doi: 10.1165/rcmb.2009-0375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SM, Engelman JA, Le S, Riese DJ, 2nd, Haber DA, Settleman J. Modeling oncogene addiction using RNA interference. Proc Natl Acad Sci U S A. 2008;105:12480–12484. doi: 10.1073/pnas.0803217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito RA, Watabe T, Horiguchi K, Kohyama T, Saitoh M, Nagase T, Miyazono K. Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res. 2009;69:2783–2791. doi: 10.1158/0008-5472.CAN-08-3490. [DOI] [PubMed] [Google Scholar]
- Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Adler AS, Field Y, Chang HY, Segal E. Systematic functional characterization of cis-regulatory motifs in human core promoters. Genome Res. 2008;18:477–488. doi: 10.1101/gr.6828808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EL, Watanabe H, Magendantz M, Hoersch S, Chen TA, Wang DG, Crowley D, Whittaker CA, Meyerson M, Kimura S, Jacks T. Nkx2-1 Represses a Latent Gastric Differentiation Program in Lung Adenocarcinoma. Molecular cell. 2013 doi: 10.1016/j.molcel.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Haitchi HM, Maeda Y. Intersections between pulmonary development and disease. Am J Respir Crit Care Med. 2011;184:401–406. doi: 10.1164/rccm.201103-0495PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson MD, Yin X, Walter V, Zhao N, Cabanski CR, Hayward MC, Miller CR, Socinski MA, Parsons AM, Thorne LB, et al. Differential pathogenesis of lung adenocarcinoma subtypes involving sequence mutations, copy number, chromosomal instability, and methylation. PLoS One. 2012;7:e36530. doi: 10.1371/journal.pone.0036530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, Hubbard DD, DuPage MJ, Whittaker CA, Hoersch S, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473:101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Bundman DS, Roop DR. Delayed wound healing in keratin 6a knockout mice. Mol Cell Biol. 2000;20:5248–5255. doi: 10.1128/mcb.20.14.5248-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, Onaitis MW. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang M, Wang Y, Kadambi P, Dave V, Lu LJ, Whitsett JA. A systems approach to mapping transcriptional networks controlling surfactant homeostasis. BMC Genomics. 2010;11:451. doi: 10.1186/1471-2164-11-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, et al. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121:1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, Morrisey EE. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am J Physiol Lung Cell Mol Physiol. 2006;291:L191–199. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–198. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.