Abstract
We used half-second trains of intracortical microstimulation to study the functional organization of the posterior parietal cortex (PPC) in prosimian galagos. These trains of current pulses evoked meaningful behaviors from the anterior, but not posterior, half of PPC. Stimulation of dorsal PPC caused contralateral forelimb movements, including defensive, hand-to-mouth, and reaching movements. Defensive and hand-to-mouth movement territories overlapped, although hand-to-mouth movements were usually evoked from more rostrolateral sites than defensive movements. Reaching movement sites were typically more caudal than defensive or hand-to-mouth movement sites. Stimulation of the most medial PPC sites evoked complex movements of forelimbs and hindlimbs. Ventral PPC commonly represented defensive face movements. Similar defensive movements, with the addition of widely opening the mouth to expose the teeth, were elicited from a small area in front of the PPC defensive face zone. Sometimes defensive face movements occurred with forelimb movements. Thus, subregions of PPC relate to different ethologically relevant categories of behavior. Most movements were initiated within 33–100 msec after stimulus onset. Face, eye blink, and ear movements were generally less delayed than forelimb movements. The present results in galagos, together with those obtained from macaque monkeys by Graziano and coworkers, suggest that the functional involvement of the PPC in specific types of sensorimotor behavior evolved early in the course of primate evolution and that networks for complex movements involving motor and posterior parietal areas are characteristic of all primate brains.
Keywords: long train stimulation, nonhuman primate, motor behavior, motor cortex
Electrical stimulation has been widely used to study the functional organization of the brain, especially the motor cortical areas. Most experiments on the motor cortex delivered low levels of current to the cortex through microelectrodes (Asanuma and Arnold, 1975; Asanuma et al., 1976) as single pulses or brief trains (usually less than 60 msec) of electrical pulses that evoke short muscle twitches. Recent studies of the motor cortex in monkeys, however, suggest that the twitches evoked by short trains of stimulation are just the beginnings of longer movement sequences. The stimulation of motor cortex with longer stimulus trains of about 500 msec evoked complex coordinated movements, similar to those of natural behavior (Graziano et al., 2002a). Recently, the long-stimulation-train procedure was extended to a region of intraparietal cortex with strong connections to motor cortical areas, the ventral intraparietal area, where stimulation trains evoked a constellation of defensive movements (eye closure, grimacing, protective head movements; Cooke et al., 2003), similar to those evoked from primary motor cortex (Graziano et al., 2002a). Previously, stimulation of lateral and medial intraparietal areas (parietal eye field areas, LIP and MIP) has been shown to evoke saccadic eye movements (Thier and Andersen, 1998; Mushiake et al., 1999). Thus, there are motor representations in posterior parietal cortex (PPC), and the use of long stimulation trains might help to expand our understanding of the functional organization of posterior parietal region.
Presently, the organization of PPC of primates is best understood in macaques, for which a number of different areas have been proposed within and around the intraparietal region. Proposed areas include the anterior intraparietal area (AIP; Taira et al., 1990; Sakata et al., 1995, 1998; Murata et al., 2000), the ventral intraparietal area (VIP; Maunsell and Van Essen, 1983; Ungerleider and Desimone, 1986; Preuss and Goldman-Rakic, 1991b; Colby et al., 1993; Colby and Goldberg, 1999), the medial intraparietal area (MIP; Eskandar and Assad, 1999; Cohen and Andersen, 2002), the lateral intraparietal area (LIP; Blatt et al., 1990; Barash et al., 1991a,b; Andersen et al., 1992, 1998; Goldberg et al., 2006), and the posterior intraparietal area (PIP; Shikata et al., 1996; Sakata et al., 1998; for reviews see Cavada and Goldman-Rakic, 1989a,b; Lewis and Van Essen, 2000a; Grefkes and Fink, 2005). The proposed areas have been distinguished by differences in patterns of connections, single unit properties, sometimes sensory maps, and cortical architecture. Although the terms for areas, as well as their number and boundaries, vary somewhat across investigators, it is clear that at least eight to ten separate fields exist in PPC of these monkeys, which appear to have a major involvement in guiding motor behavior (Johnson et al., 1996; Fogassi et al., 2001; Marconi et al., 2001; Grefkes and Fink, 2005). The rostral areas of PPC of macaques receive more direct somatosensory information, mainly from area 2 and areas of lateral parietal cortex, and their projections include primary motor and premotor areas (Johnson et al., 1996; Luppino et al., 1999; Lewis and Van Essen, 2000b; Tanne-Gariepy et al., 2002; Kaas, 2004). Other more caudal PPC fields receive auditory and especially visual inputs and project to an array of premotor areas (Pandya and Kuypers, 1969; Baizer et al., 1991; Matelli et al., 1998; Shipp et al., 1998; Lewis and Van Essen, 2000b; Marconi et al., 2001; Nakamura et al., 2001; de la Mothe et al., 2006; Smiley et al., 2007). In addition, PPC neurons have been shown to be active during sensory stimulation and specific motor acts (for review see Andersen, 1989). Thus, the dominant view is that PPC areas are involved in sensorimotor transformations using visual, somatosensory, and auditory signals to encode plans of action, especially arm-reaching movements, that are differentially relayed to areas of premotor cortex and then to primary motor cortex for movement execution (for review see Cohen and Andersen, 2002). The important role of PPC in the integration of sensory information for guidance of motor behavior is supported by the finding that lesions of this region cause impairments of spatial abilities in sensory guided reaching (for review see Jeannerod and Farne, 2003).
Much less is known about the functional organization of posterior parietal cortex in New World monkeys and in prosimian primates. In this study, we used long trains (~500 msec) of intracortical microstimulation in anesthetized prosimian galagos to evaluate further the potential of the approach to reveal the functional subdivisions of posterior parietal cortex. Some of these regions were also injected with anatomical tracers in order to reveal their connections (see companion paper). Experiments were on prosimian primates (galagos), because of our extended understanding of the motor system in this primate (Wu et al., 2000; Stepniewska et al., 2005a,b; Fang et al., 2005, 2006, 2008) and because a shallow intraparietal sulcus in galagos simplifies the exploration of PPC. More importantly, experiments on prosimian primates, a major branch of the primate radiation, could help to establish how PPC organization is similar or variable across primate taxa. The anesthetized preparation also offered us the possibility to have long stimulation sessions (12 hours or more) over a large region of cortex, something more difficult to complete in the awake, behaving animal. We evoked meaningful behaviors and were able to distinguish sectors of PPC for evoked defensive and aggressive postures, hand-to-mouth movements, reaching movements, and eye movements. Some of these results have been briefly described earlier (Stepniewska et al., 2004, 2005a). They demonstrate that posterior parietal cortex of galagos is involved in mediating aspects of complex adaptive behaviors in a manner similar to that described for macaque monkeys.
MATERIALS AND METHODS
Experiments were carried out on 11 adult galagos (Otolemur garnetti; Table 1). In all cases, intracortical microstimulation (ICMS) with long (500 msec) trains of electrical pulses was used to delineate functional areas in the PPC. In two of these cases, frontal cortex (motor and premotor) was also mapped. All experimental procedures followed the guidelines of the National Institutes of Health Guide for the care and use of laboratory animals and the guidelines of the Vanderbilt University Animal Care and Use Committee.
TABLE 1.
Summary of Experimental Cases
| Microstimulation |
|||
|---|---|---|---|
| PPC |
|||
| Case# | Dorsal | Ventral | M1-PM |
| 03–56L | + | ||
| 03–64L | + | + | |
| 03–74R | + | + | |
| 04–04L | + | + | |
| 04–07L | + | + | |
| 04–39L | + | + | + (short) |
| 04–47R | + | + | |
| 05–15L | + | + | + (long) |
| 05–20L | + | + | + (long) |
| 05–28L | + | + | + (short) |
| 09–09L* | + | + | |
In case 09-09L only the most anterior part of PPC was stimulated. This case was not included in our quantitative analysis.
Surgery and microstimulation
Surgical procedures were performed with animals under isoflurane anesthesia and aseptic conditions. After the skull was opened, the dura was retracted and the cortex digitally photographed. Isoflurane was replaced with ketamine hydrochloride diluted with physiological saline (1:4) delivered intravenously with an infusion pump at a rate that maintained a stable level of anesthesia (30–50 mg/kg/hour) and did not profoundly suppress cortical responsiveness. To delineate areas in PPC, the region around the intraparietal sulcus (IPS) was explored with stimulating microelectrodes (Fig. 1), and sites of microstimulation were marked on a high-resolution photograph of the exposed cortex. Stimulation was applied by a Master 8 stimulator (A.M.P.I.) with a biphasic stimulus isolator (Bak Electronics Inc.). Current was delivered with a low-impedance tungsten microelectrode inserted perpendicular to the cortical surface to a depth 1.5–1.8 mm, a level that was optimal for eliciting responses. Penetrations in the cortex within IPS were placed 1–2 mm dorsal or ventral to the sulcus and progressively advanced to depth up to 5 mm in an effort to stimulate neurons in layer 5 throughout the portions of posterior parietal cortex buried in the IPS sulcus. Stimuli consisted of trains of 0.2 msec biphasic pulses, presented at 300 Hz, and extended over a duration (500 msec) long enough to evoke complex, coordinated movements, similar to those of natural behavior. Current levels ranged from 20 μA to 400 μA, starting at lower levels and increasing over subsequent trains until a movement was evoked or the maximum stimulation level was reached (current thresholds below 100 μA evoked movements only occasionally). Consecutive stimulus trains delivered to the cortex were usually separated by 30 seconds or more. Electrode penetrations were placed in a grid with 0.5–1 mm between penetrations. To locate primary motor (M1) and premotor (PM) areas for reference, short 60-msec trains were applied in most cases, but in two cases we applied long train current pulses to both PPC and M1-PM regions (Table 1; motor cortex results will be published separately). For some sites, we also varied stimulation parameters (current amplitude; type of pulse, biphasic vs. cathodal; short vs. long stimulus trains) and found that the duration of the stimulation train is a critical factor in producing a complete, coordinated movement.
Figure 1.
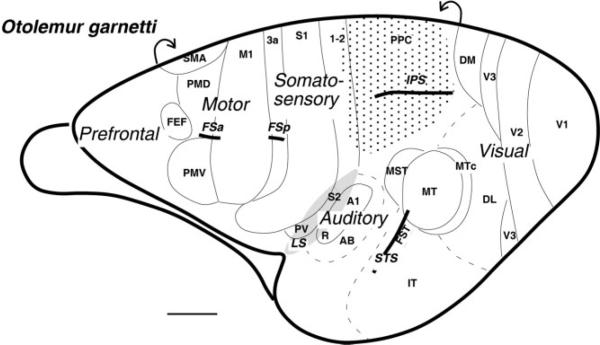
Subdivisions of the galago cortex shown on the lateral aspect of the left hemisphere. The region of cortex explored in the present study is shaded with dots. Motor areas are based on Wu et al. (2000) and Fang et al. (2005) and include the primary motor cortex (M1), the dorsal and ventral premotor areas (PMD and PMV), supplementary motor area (SMA), and frontal eye field (FEF). Somatosensory areas are from Wu and Kaas (2003) and include primary (S1) and secondary (S2) somatosensory areas, areas 1 and 2, and ventral somatosensory area (PV). Visual areas are from Lyon and Kaas (2002) and include primary visual area (V1), second (V2) and third (V3) visual areas, dorsolateral (DL) and dorsomedial (DM) visual areas, middle temporal (MT) and middle temporal crescent (MTc) areas, middle superior temporal area (MST), fundal superior temporal area (FST), and inferotemporal area (IT). Auditory areas are from Kaas and Hackett (2000) and include primary auditory cortex (A1), rostral primary auditory area (R), and auditory belt (AB). Part of auditory belt is hidden in the lateral sulcus. The partially opened lateral sulcus (LS) is shaded. The intraparietal sulcus (IPS), the superior temporal sulcus (STS), the frontal anterior (FSa), and the posterior (FSp) sulci are indicated. Scale bar = 5 mm.
At the end of the stimulation session, electrolytic lesions were placed to mark strategic stimulation sites that indicate functional borders and other sites of interest. Lesions were made by passing direct current of 10 μA during electrode withdrawal (continuous lesion) and were marked on a high-resolution photograph of the exposed cortex along with penetration sites.
Perfusion and histological processing
In the end of each experimental procedure, animals were euthanized with an overdose of sodium pentobarbital (Euthasol) and, when areflexive, were perfused through the heart with buffered physiological saline followed by 2% paraformaldehyde and then 2% paraformaldehyde with 10% sucrose. The brain was removed, and in most cases the cortex was separated from the brainstem, unfolded, manually flattened between two glass plates (see, e.g., Stepniewska et al., 2005c), and submerged in 30% sucrose at 4°C for cryoprotection. One to three days later, cortical tissue was frozen and cut parallel to the surface into two series of 40–50-μm-thick sections. In three cases, the brain was cut in the coronal plane. Typically, every other section was stained for myelinated fibers (Gallyas, 1979) or cytochrome oxidase (CO; Wong-Riley, 1979) to reveal cortical architecture. CO and myelin preparations allow sensorimotor (M1, -3a, and -3b) and visual (MT, V2, and V1) areas to be recognized histologically, as in our previous studies (Stepniewska et al., 1993; Preuss et al., 1996; Collins et al., 2001; Fang et al., 2005). In cases with tracer injections, a series of one of three or two of four sections was processed to reveal tracers (see companion paper). The locations of movement zones within PPC were reconstructed from features in the processed sections. Thus, the architectonic borders in CO- and myelin-stained sections were drawn, superimposed, and aligned by using landmarks such as blood vessels, lesions, and injection sites. Microlesions made to mark functional borders helped to correlate ICMS maps with cortical architecture. The architectonic definitions of cortical regions follows those of Preuss and Goldman-Rakic (1991a,b) and Fang et al. (2005). The processed tissue showed no signs of damage at the sites of stimulation, in contrast to sites of marking lesions.
Digital images of the brain were acquired with a Canon PowerShot S2 IS digital camera. These images were cropped and adjusted for brightness and contrast in Adobe Photoshop CS3 but were otherwise unaltered. Final figures containing images and line drawings were made in Canvas 8.0 software (Deneba, Inc., Miami, FL).
In some cases, neuronal tracers were injected into physiologically different regions of posterior parietal cortex to reveal their connections (see companion paper). In these cases, injections were guided by the results of a short stimulation session. After an appropriate time of survival, an extensive stimulation session was usually used to obtain more complete maps of PPC. The perfusion and histological tissue processing in these cases followed those described above.
Analysis of evoked movements
Movements evoked by microstimulation were observed by two or three observers and classified according to type (e.g., hand-to-mouth, reaching, defensive, aggressive). The movements were also recorded on videotape at 30 frames per second for further analysis. For each stimulation site, movement sequences (mostly of forelimb or face) were synchronized with the onset of pulse trains signaled by a light-emitting diode (LED) and captured by a digital video camera (Sony DCR-HC65) mounted directly in front of the animal. Thus, the start of the stimulation train could be determined to the nearest video frame. Videotapes were analyzed frame-by-frame to verify the type of movement evoked as well as movement delay, time course, complexity, etc. Evoked movements were distinguished from the occasional spontaneous movements that sometimes occur in ketamine-anesthetized animals by the consistency of the latency and the movement sequence of the evoked behaviors.
RESULTS
Intracortical microstimulation with long trains of electrical pulses evoked meaningful behaviors from the PPC region around the anterior, but not the posterior, portion of the intraparietal sulcus (IPS). Stimulation-evoked movements tended to cluster in cortical zones that emphasized different ethological functions. Results presented here are based on the microstimulation of the PPC in 11 galagos. Preliminary results from the first few cases have been briefly described previously (Stepniewska et al., 2005a). Here we present more detailed results and results from more cases. First, we provide a qualitative description of the main types of movements evoked by stimulation, and then we describe their distributions within the PPC, thresholds, latencies, and time courses.
Characteristics of evoked movements
Movements evoked with the long stimulus train were commonly coordinated across multiple joints. These complex movements continued through the stimulation period (about 500 msec), differing from the brisk, truncated, simple movements evoked by short (60 msec or less) train stimulation from M1-PM region or sporadically also from PPC. For both types of stimulation, movements evoked from PPC were less consistent and required much higher currents than similar movements evoked from M1-PM. The complex movements evoked from PPC are the focus of the present report.
More than 90% of movements (1,241 of 1,341 along 536 penetrations) evoked from PPC with long train stimulation were complex (Tables 2, 3). The simple movements (most often eye blink or ear movements) were observed only occasionally (see Figs. 2–6). These simple movements were evoked from sites usually located in the most anterior part of explored region (see shoulder movements in cases 03-74R or ear movements in cases 04-47R and 03-64L). Such simple movements involving a single joint or a single body part were evoked from 100 sites (Table 3).
TABLE 2.
Number of Penetrations to ICMS Located in Different Body Representation Within PPC
| No. of penetrations |
||||||||
|---|---|---|---|---|---|---|---|---|
| Case # | Total | Responsive | Unresponsive | Face | EM | Forelimb | Hindlimb/trunk | Simple |
| 03-56L | 74 | 51 | 23 | 40 | – | 1 | – | 10 |
| 03-64L* | 105 | 55 | 50 | 26/66 | – | 17/34 | 2/2 | 10/10 |
| 03-74R | 83 | 65 | 18 | 18 | 2 | 32 | – | 13 |
| 04-04L* | 99 | 66 | 33 | 27/119 | 3/7 | 26/32 | 6/6 | 4/15 |
| 04-07L* | 117 | 90 | 27 | 40/99 | – | 36/207 | 11/11 | 3/3 |
| 04-39L* | 87 | 59 | 28 | 13/61 | 8/44 | 26/150 | 10 | 2/30 |
| 04-47R* | 81 | 61 | 20 | 21/21 | – | 34/156 | 4/4 | 2/2 |
| 05-15L | 26 | 21 | 5 | 12 | – | 8 | – | 1 |
| 05-20L | 58 | 39 | 19 | 27 | – | 10 | – | 2 |
| 05-28L* | 43 | 29 | 14 | 9/9 | – | 10/42 | 6/11 | 4/14 |
| All | 773 | 536 | 237 | 233/472 | 13/53 | 200/672 | 39/44 | 51/100 |
In 6 animals (marked with asterisks) multiple sites were stimulated along some penetrations close to the IPS. In these cases the first figure shows number of penetrations and the second figure shows number of stimulated sites along those penetrations.
TABLE 3.
Number and Percentage of All Stimulation Sites (Dorsolateral PPC + the Depth of IPS) from Which Different Complex Movements Were Evoked by ICMS
| Stimulation sites |
Face |
Forelimb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case# | All | Responsive | Total | Aggressive | Defensive | EM | Total | Hand-to-mouth | Defensive | Reach | Hindlimb/trunk | Simple |
| 03-56L | 74 | 51 68.9% |
40 | 6 15% |
34 85% |
– | 1 | – | 1 100% |
– | – | 10 |
| 03-64L* | 162 | 112 69.1% |
66 | – | 66 100% |
– | 34 | 15 44% |
18 53% |
1 3% |
2 | 10 |
| 03-74R | 83 | 65 78.3% |
18 | 8 44.4% |
10 55.6% |
2 | 32 | 10 31.3% |
11 34.4% |
11 34.4% |
– | 13 |
| 04-04L* | 212 | 179 84.4% |
119 | 9 7.6% |
110 92.4% |
7 | 32 | 7 21.9% |
13 40.6% |
12 37.5% |
6 | 15 |
| 04-07L* | 347 | 320 92.2% |
99 | 25 25.3% |
74 74.7% |
– | 207 | 95 45.9% |
112 54.1% |
– | 11 | 3 |
| 04-39L* | 323 | 295 91.3% |
61 | 4 6.6% |
57 94.4% |
44 | 150 | 38 25.3% |
92 61.3% |
20 13.3% |
10 | 30 |
| 04-47R* | 203 | 183 90.1% |
21 | 18 85.7% |
3 14.3% |
– | 156 | 44 28.2% |
104 66.7% |
8 5.1% |
4 | 2 |
| 05-15L | 26 | 21 80.8% |
12 | 8 66.7% |
4 33.3% |
– | 8 | 4 50% |
3 37.5% |
1 12.5% |
– | 1 |
| 05-20L | 58 | 39 67.2% |
27 | 17 63% |
10 37% |
– | 10 | 2 20% |
4 40% |
4 40% |
– | 2 |
| 05-28L* | 90 | 76 84.4% |
9 | 9 100% |
– | – | 42 | 2 4.8% |
23 54.7% |
17 40.5% |
11 | 14 |
| All | 1578 | 1341 85% |
472 | 104 22% |
368 78% |
53 | 672 | 217 32.3% |
381 56.7% |
74 11% |
44 | 100 |
Asterisks mark cases in which the multiple sites along the depth of intraparietal sulcus were stimulated.
Figure 2.
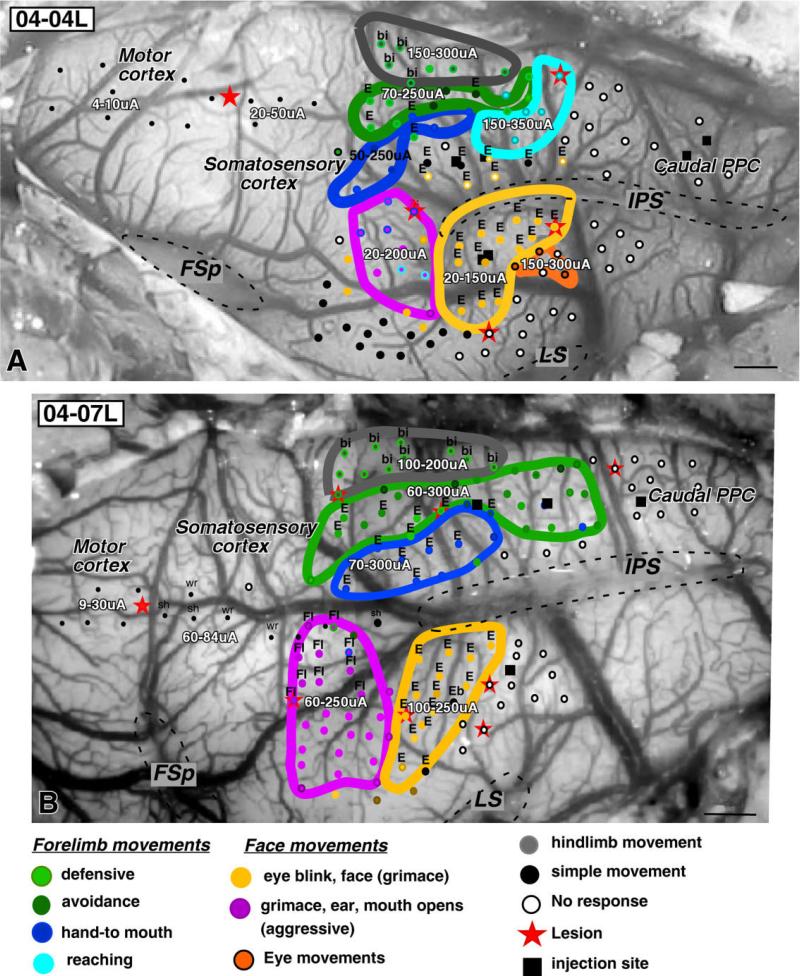
Organization of complex movement zones in PPC of galagos 04-04L (A) and 04-07L (B) shown on the exposed dorsolateral surface of the brain. Cortical sites around the intraparietal sulcus (IPS) that were stimulated with 500-msec trains are marked as different color dots according to the evoked movement. Clusters of the same dots (movements) are outlined in the corresponding color. Dorsal PPC represents complex forelimb movements (defensive, green; hand-to-mouth, dark blue; reaching, light blue) that are arranged topographically (see text). Ventral PPC has posterior and anterior zones of complex face movements, representing defensive (yellow) and aggressive (purple) movements. More caudally, few stimulation sites represent eye movements (orange). The large black dots in the anterolateral region indicate sites where an eye blink was evoked. These sites were unique to case 04-04L and were not considered in the analysis. For each movement zone, the range of current thresholds for evoked movements is shown. Sites in motor cortex (small black dots) were stimulated with short 60-msec stimulus trains to produce simple movements that allowed us to locate motor and somatosensory cortex for reference. Dashed lines mark contours of sulci. bi, Bilateral; E, ear; FL, forelimb; FSp, posterior frontal sulcus; IPS, intraparietal sulcus; sh, shoulder; wr, wrist. Modified from Stepniewska et al. (2005a). Scale bars = 1 mm.
Figure 6.
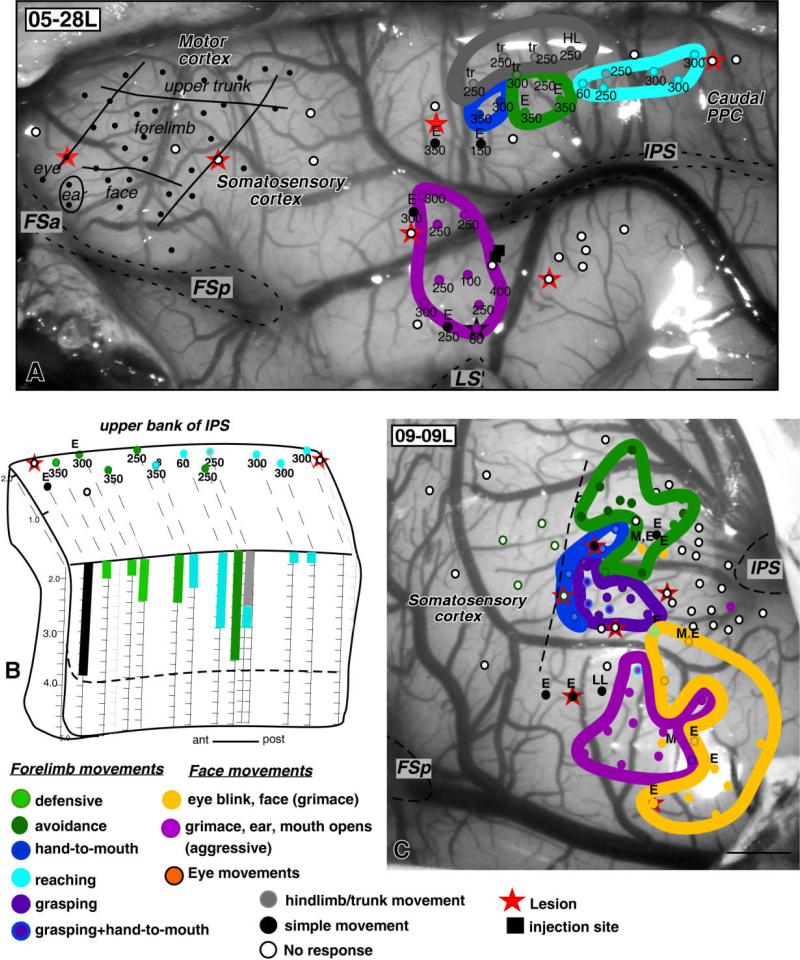
The organization of PPC in cases 05-28L (A,B) and 09-09L (C). In case 05-28L, the low numbers of stimulation sites were mostly used to determine the locations of injection sites. However, as in other cases, three complex forelimb movement zones and hindlimb zone were found in the dorsal PPC and aggressive face zone in the ventral PPC (A). In this case, defensive face movements were not evoked. B shows stimulation sites along the upper bank of IPS in case 05-28L, from which some defensive and reaching movements were evoked. One stimulation site represented hindlimb movement superficially and reaching movement deeper in the sulcus, and one stimulation site (black) represented simple ear movement. In case 09-09L (C), numerous cortical sites anterior to IPS were stimulated in search of PPC grasping zone. A small (about 1 mm in diameter) zone where stimulation with 300 μA current threshold evoked grasping movements was found in front of IPS. Stimulation sites medial to the grasping zone evoked defensive forelimb movements, and stimulation of sites lateral to the grasping zone evoked aggressive and defensive face movements. E, ear; FSa, anterior frontal sulcus; FSp, posterior frontal sulcus; IPS, intraparietal sulcus; LS, lateral sulcus; LL, lower lip; M, mouth; tr, trunk. Other conventions as in Figures 2 and 3. Scale bars = 1 mm.
Complex movements typically depended on the stimulation site and involved face, forelimb, or hindlimb/trunk musculature and less frequently eye movements. Generally, we observed five major types of movements (see Table 3). Three of them involved the forelimb, and the other two involved the face. Forelimb movements were usually coordinated across multiple joints and occurred contralateral to the side of stimulation, although some bilateral movements were also observed. Face movements were usually bilateral, with the contralateral musculature movement being stronger than ipsilateral. At no site were forelimb or face movements evoked exclusively on the side ipsilateral to stimulating electrode.
Forelimb defensive movements accounted for more than half (56.7%; Table 3) of all stimulation-induced forelimb movements. Across individuals, this class of movements was evoked at 381 cortical sites. Defensive forelimb movements involved retraction of the contralateral shoulder, causing forelimb withdrawal. When the forelimb retraction was combined with lifting the hand into the space beside the head with the palm faced outward, we considered it a protective or blocking movement (see also Graziano et al., 2002a; Graziano, 2006). The defensive movements, either avoidance or protective, were quite often associated with a backward folding of the ear, usually quite strong. Drawings illustrating these and other complex movements can be found in Stepniewska et al. (2005a).
Hand-to-mouth (body) movements accounted for 32.3% (Table 3) of all forelimb movements. These movements consisted of shaping the hand into a grasp posture, the wrist and forearm supinating to orient the hand toward the mouth (or body), and bringing the hand toward the mouth (or body). Some of the hand-to-mouth movements were linked with mouth opening. At some cortical sites, stimulation drove the hand to the body without an obvious supination (as in hand to mouth movements) or pronation (as sometimes in protective movement). These movement sites (together with lifting movements) were quite common, and they were usually scattered across the forelimb zone. Here we classified these movements as hand-to-mouth movements when they occurred without a shoulder retraction or as a defensive movement when they occurred with a shoulder retraction.
Reaching movements were less common (about 11% of all forelimb movements; Table 3). The arm straightened and hand extended outward distant from the body, either forward, side-ward, or upward. The reaching movements were often, but not always, associated with opening of the grip. On few occasions, reaching occurred together with wrist dorsi flexion and digits flexion in grasping-like movement (case 04-47R). Grasping movements in isolation, however, were evoked from only one case (09-09L, see Fig. 6C), although the grasping movements were quite often evoked by stimulation of the PPC in owl monkeys and M1-PM region of both galagos and owl monkeys (Stepniewska et al., 2006, 2007, 2008). Across individuals, bringing the hand to the mouth (or body) was evoked at 217 cortical sites, and extension of the arm in reaching posture was evoked at 74 sites (Table 3).
Two other types of movements commonly evoked from PPC involved the face. Defensive face movements accounted for 78% of all face movements (Table 3). They consisted of an eye blink and a contraction of the muscles around the eye, causing a squint, and a contraction of the muscles of the snout causing the upper lip to lift and the skin on the snout to wrinkle upward toward the eye (grimace). Electrical stimulation of the left cortex caused the right eye to close entirely, the left eye to close partially, and the face to contract into grimace. Very often, the defensive face movement involved a movement of the ear in which the pinna was flattened against the side of the head and rotated downward. The downward component of the ear movement was characteristic of defensive face behavior and was never observed concurrently with forelimb defensive behavior. Moreover, ear movements linked to face defensive movements were usually less strong than ear movements linked to defensive forelimb movements. Similar movements, with the addition of the mouth opening wide and the upper lip withdrawing to expose teeth, were classified as aggressive behavior (about 22% of all face movements), although it could be also considered a confrontational gesture. Sometimes, the face movements were associated with defensive or hand-to-mouth forelimb movements. The most complex movement that we observed in the present study was grasping followed by a hand-to-mouth movement correlated with mouth opening. This sequence was evoked from three sites in case 04-47L. Often such complex sequences of movements were observed also when stimulating motor cortex (unpublished data).
Eye movements were not consistently evoked from PPC. However, in a few cases (three of ten animals), eye movements were observed when stimulating the most lateral portion of the ventral PPC as well as the lower bank of the IPS (see Figs. 2–4). These movements were difficult to study, because the first stimulus delivered to a site that evoked eye movement was usually followed by a period of spontaneous eye movements. Most often, ICMS evoked horizontal saccades into the contralateral visual hemifield, although few downward eye movements were observed as well.
Figure 4.
The organizations of PPC in galagos 03-74R (A) and 04-47R (B,C) are shown on dorsolateral views of the cortical surface of the right hemisphere. A: In case 03-74R, complex forelimb movement zones for reaching (light blue), hand-to-mouth (dark blue), and defense (green) are marked with colored lines in dorsal PPC. Reaching movements were evoked from two spatially separated cortical zones in this case. Zones of complex face defensive (yellow) and aggressive (purple) movements as well as the small zone of eye movements (orange) are marked in the ventral PPC. Large black dots in the anterodorsal portion of the cortex represent simple shoulder movements evoked with long-train stimulation. Smaller black dots in the frontal cortex represent sites of simple shoulder movements evoked by short-train stimulation. B: At left, three zones of complex forelimb movements and small medial zone of complex hindlimb movements (gray) are marked in the dorsal PPC of case 04-47R. In the ventral PPC, stimulation of numerous cortical sites evoked aggressive face movements (purple), and stimulation of a few more caudal sites evoked defensive face movements (yellow). C: At right, stimulation sites along the dorsal bank of IPS evoked mostly defensive (green) and hand-to-mouth movements (blue). bi, Bilateral; E, ear; FL, forelimb; FSp, posterior frontal sulcus; IPS, intraparietal sulcus; LS, lateral sulcus; sh, shoulder. Conventions as in Figures 2 and 3. Scale bars = 1 mm.
We also observed complex movements involving the forelimb and the hindlimb that could be classified as climbing movements. These, most often bilateral movements consisted of hip, knee, and ankle flexion with simultaneous shoulder, elbow, and wrist extension. In a few sites, stimulation evoked similar hindlimb movements with pushing downward movements of the forelimb. Because the galago was restrained in the stereotaxic apparatus, components of these full body movements might have been missed. Nevertheless, the movements resembled those of an animal that was trying to support the body, stand up, climb, or jump. Although hindlimb movements were most often associated with forelimb movements, stimulation of a few, most medially located sites evoked hindlimb movements alone (Figs. 2–6). They consisted of hip, knee, and ankle flexion, the hip movement being the strongest. Sometimes less complex movements involving two joints (e.g., knee and ankle) or just one joint (e.g., hip) were observed. Overall, the movement patterns in PPC reflected a somatotopic pattern, with the most medial sites producing hindlimb movements and progressively more lateral sites involving forelimb, face, and eye movements.
Preliminary results for the grasping zone in PPC
In our initial set of experiments, we did not identify a grasping zone in PPC of studied galagos, although a grasping zone was recognized in the frontal motor cortex of one of the cases in this study (see Fig. 5C), as well as in other galagos with more systematic stimulation of frontal motor region (Stepniewska et al., 2007). We have also identified a grasping zone in PPC of New World owl and squirrel monkeys (owl monkey, Stepniewska et al., 2006; squirrel monkey, unpublished observations). Because the grasping movements in these monkeys were evoked from the very rostral PPC, we made an effort to explore extensively the most rostral part of PPC, just anterior to the IPS in one additional galago (case 09-09L, Fig. 6C). We located a small (about 1 mm in diameter) zone where stimulation with a current of 300-μA or less evoked grasping movements. For some of these stimulation sites, grasping movements were associated with forearm supination. Supination movements alone were evoked in the vicinity of the grasping zone. Stimulation sites medial to the grasping zone evoked defensive forelimb movements and stimulation of sites lateral to the grasping zone evoked aggressive and defensive face movements. In a couple of sites, these face movements were associated with forelimb reaching postures (see also case 08-15L in companion paper). Our electrode penetrations in case 09-09L were often 0.25 mm apart, so stimulation sites were distributed more densely in this case than in previous cases of this study, in which electrode penetrations were 0.5–1 mm apart. Although further study is needed, these results suggest that galagos, as well as monkeys, have a grasping zone in rostral PPC.
Figure 5.
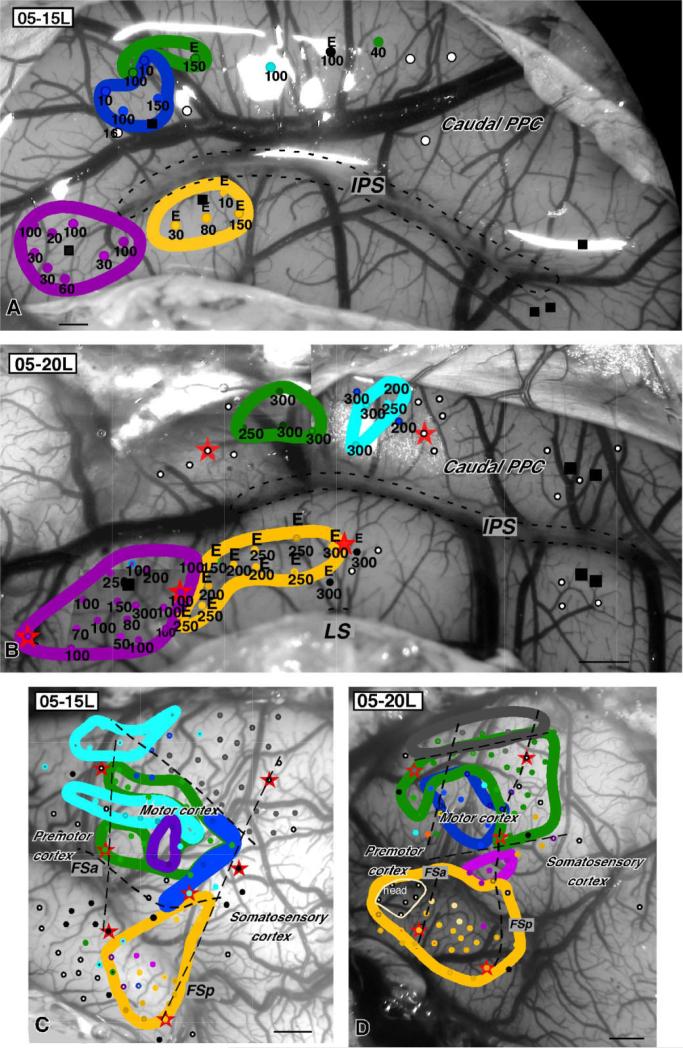
The organization of PPC in cases 05-15L (A) and 05-20L (B). Sites and zones where complex forelimb and face movements were evoked from motor cortex are marked in both cases on the dorsolateral surface of left hemisphere (C,D). In both cases, regions of primary motor cortex and premotor cortex were stimulated with long stimulus trains, evoking complex face, forelimb, and hindlimb movements similar to those evoked from PPC. Color code (see previous legends) is the same as for PPC. bi, Bilateral; E, ear; ext, extension; fl, forelimb; FSa, anterior frontal sulcus; FSp, posterior frontal sulcus; LL, lower lip; M, mouth; T, tongue; Tr, trunk. Conventions as in Figures 2 and 3. Scale bars = 1 mm.
Organization of movement zones across the posterior parietal cortex
Stimulation of sites in the region around the anterior part of the intraparietal sulcus, that extended from 1–2 mm caudal to primary somatosensory cortex (S1) to about the midpoint of the IPS, with long stimulus trains evoked complex movements in all studied galagos (Table 1). In six of those cases, a large PPC region above and below the IPS was stimulated, resulting in comprehensive movement maps (Figs. 2–4). In one additional case (03-56L, not illustrated), only the ventral region of PPC was explored. In three other cases, microstimulation was used mostly as a guide for tracer injections, resulting in somewhat less comprehensive maps (see Figs. 5, 6) that were nevertheless quite comparable to other cases. In total, 1,578 cortical sites were studied in 773 electrode penetrations in the cortex around and within the IPS (Tables 2, 3).
Movements were evoked from 1,341 sites; 1,241 (more than 90%) of those movements were complex, 100 were simple movements, and 237 sites were not responsive (Tables 2, 3). The simple movements were usually evoked from sites in the most anterior part of the region explored in front of IPS, probably in areas 1–2 (see shoulder movements in cases 03-74R, Fig. 4A; or ear movements in cases 04-47R and 03-64L, Figs. 3C, 4B), but a few were scattered throughout the extent of anterior PPC. Stimulation of the posterior PPC even with high currents did not evoke movements.
Figure 3.
The organization of PPC in galagos 04-39L and 03-64L. A,C: Dorsolateral view of exposed cortex of the left hemisphere, with dots indicating sites penetrated with stimulating electrode. The number below each dot indicates thresholds current for complex movements. Same-color dots mark sites from which the same complex movements were evoked. Functional zones of complex hindlimb, forelimb (defensive, hand-to-mouth, and reaching), and face movements are outlined with thick, colored lines. In case 03-64L (C), only one stimulated site in the dorsal PPC evoked a reaching movement, and stimulation of ventral PPC evoked only defensive and not aggressive face movements. The defensive face movements extended into the region representing the aggressive movements in other cases, making the defensive face zone much larger than in other cases. In case 04-39L (A), short-train stimulation of numerous sites in frontal cortex (small black dots) evoked simple movements of hindlimb, trunk, forelimb, and face. Borders between different body representations are marked with thin black lines. Two sites in M1 that were stimulated with long trains evoked complex forelimb movements (large green dots, defensive; large blue dots, hand-to-mouth). Thin black lines running through red stars mark rostral and caudal borders of M1. B,D: The intraparietal sulcus (IPS) has been opened up to show the faces of medial and lateral banks. The lower bank has been rotated for viewing so that the posterior edge is anterior. Dots mark electrode penetrations that involved the cortex of these banks. Stimulation occurred every 0.2 mm along the depth of each penetration (vertical lines). The penetrations are coded to indicate the types of evoked responses and the depth where these responses were evoked. Digext, digits extension; E, ear; Eb, eye blink; El, elbow; FSa, anterior frontal sulcus; FSp, posterior frontal sulcus; HL, hindlimb; LS, lateral sulcus; sh, shoulder. Other conventions as in Figure 2. Scale bars = 1 mm.
Typically, the type of evoked behavior depended on the stimulation site. Thus, different movements were evoked from dorsal PPC (medial to IPS) vs. ventral PPC (lateral to IPS) as well as anterior vs. posterior PPC. As described above these, complex movements involved face, forelimb, and hindlimb musculature and less frequently eye movements. The movements were clustered into small PPC zones devoted to different classes of action. The parcellations of the galago PPC into territories for different categories of action are shown in Figures 2–6. The movements were arranged in a similar manner across the PPC in all studied cases, although the cases were different in detail.
Overall, the organization of PPC was somatotopic, with the face movements represented more laterally (lateral to and within lower bank of IPS) and the forelimb and hindlimb movements represented more medially (medial to and within the upper IPS bank). Evoked forelimb (defensive, hand-to-mouth, reaching) and face (defensive and aggressive) behaviors were present in all cases, but all categories of complex movements, including hindlimb and eye movements, were evoked only in two cases (04-04L, Fig 2A; 04-39L, Fig. 3A). Microstimulation results from both cases indicate that evoked movements clustered into similar arrangements of seven cortical zones devoted to different movement patterns reflecting different ethological functions. Results from other cases, although less complete, conform to the same overall pattern, general to PPC organization.
Ventral PPC
Stimulation of the ventral PPC (582 cortical sites along 265 electrode penetrations) led to repeatable, short-latency, defensive movements of the face (368 sites). Most often, electrical stimulation caused the contralateral eye to close and the face to contract into a grimace. Very often, ear movement was involved in this complex behavior (304 sites). Stimulation of some sites in the cortex lateral to the IPS included an additional shoulder movement, usually retraction (defensive like forelimb movement, see cases 03-64L, Fig. 3C; 04-47R, Fig. 4B). Some hand-to-mouth movement sites were also found in ventral PPC (Fig. 4B). Less frequently, simple eye blinks or ear movements (67 sites) were observed. In one case (03-74R, Fig. 4A), a toe movement was evoked from defensive face zone. The region of defensive face postures included both cortex ventral to the IPS and cortex of the lower bank of IPS (Fig. 3; see also Figs. 3B, 4B in Stepniewska et al., 2005a). In one case (04-04L), the defensive face region also included the deep portions of upper bank of IPS (see Fig. 3A in Stepniewska et al., 2005a). In the majority of cases the most anterior defensive face sites coincided with the level of the anterior IPS tip or extended no more than 1 mm rostral to it. The most ventral face defensive sites were located about 2 mm dorsal to the tip of lateral sulcus (LS).
Similar movements with the addition of mouth opening to expose the teeth (aggressive behavior) were evoked in nine cases from 104 cortical sites (Table 3) that clustered in a small area (about 2–3 mm wide) in front of the defensive face zone. In case 03-64L, stimulation of cortical sites in this location caused a face grimace with a backward ear movement characteristic for the “defensive face zone” (Fig. 3C). As a consequence, the “defensive face zone” was twice as large in this case as in other cases. At some stimulation sites in the aggressive face zone, especially those located most dorsally, the face movements were associated with forelimb movement (35 of 104 sites), either flexion (04-04L, 04-07L, 04-39L, 04-47R) or less often extension (04-04L, 05-20L).
Stimulation of the cortex lateral to the “face defensive zone” evoked eye movements from 53 sites along 13 penetrations (Table 2). These mostly horizontal (at one penetration, downward) eye movements were not consistently evoked from ventral PPC. They were observed in just three of ten studied animals. As mentioned above, usually quite high levels of current (up to 400 μA) were necessary to produce eye movements. Most eye movements were evoked from caudal sites in ventral PPC (04-04L and 04-39L), but in case 03-74L such movements were evoked from more anterior sites located between the defensive and the aggressive face zones (Fig. 4A).
Dorsal PPC
Stimulation of dorsal PPC (759 sites along 271 electrode penetrations) most commonly evoked complex forelimb movements (672 sites) that were sometimes associated with ear movement (168 sites). Defensive movements (protective and avoidance) were evoked from 381 cortical sites (Table 3, Figs. 2–6). Medially these defensive forelimb movements were associated with hindlimb movements (20 sites in Figs. 2, 3A). Unlike movements evoked from other regions of cortex, the defensive movements evoked from medial sites were typically bilateral, involving sometimes all limbs. Bringing hand to the mouth (or body) and reaching movements were also represented in dorsal PPC, although less frequently than defensive movements. Hand-to-mouth movements were evoked from 217 sites, and reaching movements were evoked from 74 cortical sites (32.3% and 11% of all forelimb movement sites, respectively; see Table 3). In most cases, reaching movements were less numerous than other types of evoked forelimb movements, but, in case 03-74R (Fig. 4A), reaching movements were as numerous as defensive or hand-to-mouth movements (11 sites, about 35% of all forelimb movements evoked in this case; see also case 08-15L, Fig 8A, in companion paper). In contrast, only one stimulated site resulted in reaching movement in cases 03-64L and 05-15L (Figs. 3C, 5A), and in case 04-07 (Fig. 2B) no such movements were found. Although different forelimb movements could be elicited from neighboring cortical sites, reaching sites tended to be located in the posterior part of PPC (but see case 03-74R, Fig. 4A), and hand-to-mouth movements were grouped more anteriorly and close to the IPS (Figs. 2–6). The region representing complex forelimb postures extended from the dorsal PPC into the depth of the IPS (see Figs. 3, 4, 6). Typically, complex forelimb movements could be evoked from sites along the entire depth of the dorsal bank of IPS (case 04-07L; see Fig 4B in Stepniewska et al., 2005a). However, these movements were limited to the upper portion of the dorsal bank in some cases (Fig. 3, 6B). At some sites, ear and eye blink movements or simply ear movements (seven sites) were also elicited from the forelimb PPC region.
In six cases, stimulation of the most medial part of dorsal PPC evoked complex hindlimb movements (total 44 sites, Table 3). Although the hindlimb movements were usually contralateral, stimulation of four sites in case 04-39L evoked bilateral hindlimb movements (Fig. 3A). Some of the movements involving hindlimb and forelimb were also bilateral (12 sites).
Movement thresholds
Movements were elicited consistently with the lowest current (threshold) from the cortical sites located at depths of 1.8–2.0 mm. Currents up to 400 μA were used. The biphasic currents used in this study, even as high as 400 μA, did not detectably damage the brain tissue; the evoked movements remained the same over numerous stimulation trials. Also, no signs of tissue damage were seen in the histological material.
Current thresholds for evoking movements ranged from 20 μA to 400 μA and were generally lower for the sites located anteriorly around the tip of IPS (see Figs. 2–6). Values of the median and mean current threshold for each movement zone in PPC are listed in Table 4. Threshold values differed from case to case (possibly as a result of small differences in anesthesia levels), and they were also lower for somatic movements than for eye movements. Although eye movements could be evoked from some sites with currents as low as 60 μA or 100 μA (case 04-39L, Fig. 3A), most often such movements required current of 300 μA. Variations in current thresholds necessary to evoke the same movement were seen across sites throughout PPC. Thresholds could be even much different for two adjacent sites representing the same movements. For instance, in case 05-28L, the same reaching movement was evoked from two closely positioned cortical sites with 60 μA and 250 μA current, respectively (Fig. 6A).
TABLE 4.
Median/Mean Thresholds (in μA) for Zones Representing Different Body Movement in PPC (Ranges are Shown in Parentheses)
| Forelimb |
Face |
||||||
|---|---|---|---|---|---|---|---|
| Case# | Hindlimb/trunk | Head-to-mouth | Defensive | Reach | Defensive | Aggressive | EM |
| 03–64L | 275/275 (250–300) n = 2 | 300/317 (250–400) n = 3 | 250/262 (200–350) n = 13 | 250/250 (250) n = 1 | 200/208 (30–300) n = 26 | – | |
| 03–74R | – | 300/275 (200–400) n = 10 | 300/300 (300) n = 11 | 300/282 (100–300) n = 11 | 300/263 (200–300) n = 10 | 300/262 (200–300) n = 8 | 400/400 (400) n = 2 |
| 04–04L | 250/250 (150–300) n = 6 | 100/153 (50–250) n = 7 | 150/166 (80–250) n = 13 | 250/260 (150–350) n = 6 | 100/101 (20–150) n = 18 | 120/129 (20–200) n = 9 | 250/233 (150–300) n = 3 |
| 04–07L | 150/155 (100–200) n = 11 | 150/157 (70–300) n = 10 | 150/165 (60–300) n= 26 | – | 200/204 (100–250) n = 15 | 100/110 (60–250) n = 25 | – |
| 04–39L | 150/157 (80–300) n = 10 | 70/85 (20–80) n = 3 | 90/119 (50–250) n = 16 | 250/193 (30–300) n = 7 | 150/168 (60–300) n = 9 | 100/113 (50–150) n = 4 | 300/237 (60–300) n = 8 |
| 04–47R | 50/73 (40–150) n = 4 | 175/166 (50–300) n = 10 | 150/185 (60–300) n = 20 | 300/300 (300) n = 4 | 150/207 (70–400) n = 3 | 150/156 (80–250) n = 18 | – |
| 05–15L | – | 55/68 (10–150) n = 4 | 100/97 (40–150) n = 3 | 100/100 (100) n = 1 | 55/68 (10–150) n = 4 | 45/59 (20–100) n = 8 | – |
| 05–20L | – | 275/275 (250–300) n = 2 | 300/290 (250–300) n = 4 | 275/263 (200–300) n = 4 | 250/230 (150–300) n = 10 | 100/125 (50–300) n = 17 | – |
| 05–28L | 250/250 (250) n = 6 | 325/325 (300–350) n = 2 | 325/313 (25–350) n = 3 | 250/243 (60–300) n = 5 | – | 250/229 (80–300) n = 9 | – |
The lowest values for each case are bold and underlined.
Our comparison of thresholds for different somatic movements revealed that in most cases the face defensive movements, especially those with a component of aggression, required the lowest current thresholds to be evoked (Table 4). Often, the values of current thresholds necessary to evoke such behavior were 80 μA or lower. In case 05-15L (Fig. 5A), for instance, thresholds ranging from 20 μA to 100 μA (median 45 μA) were sufficient to evoke movements from all (eight) sites stimulated in the face aggressive zone. In two other cases (04-39L and 04-47R), however, face aggressive behavior required somewhat higher thresholds than other types of movements, such as hand-to-mouth or hindlimb movements (Table 4).
Thresholds for evoking forelimb movement sequences were generally a little higher than those necessary to evoke face defensive movements, although this finding was not consistent from case to case. Whereas in case 05-15L thresholds below 150 μA (median below 100 μA) evoked all types of forelimb movements, in case 05-28L the thresholds for forelimb movements were much higher, ranging up to 350 μA (median 250–325 μA).
Overall, we have not noticed a major difference in the thresholds for different types of complex forelimb movements, although in most cases hand-to-mouth movements required somewhat lower currents than defensive or reaching movements. Thresholds as low as 25 μA or 30 μA evoked defensive or reaching postures at some cortical sites. In case 04-39L, reaching movements were evoked with even lower thresholds than face aggressive movements. Also in case 04-47R, median/mean thresholds were lowest for hindlimb movements rather than face defensive movements. In both cases (04-39L and 04-47R), the sites with the lowest thresholds were in the anterior part of the stimulated region. The amount of current needed to evoke behavioral responses depended more on the locations of the stimulated sites than the type of behavior being evoked. Thus, movement thresholds were lower for the sites located anteriorly around the tip of IPS than for more posterior sites (Figs. 3–6). The anterior location of aggressive face zone (with the lowest thresholds) and quite posterior location of eye movement zone (with highest thresholds) conform to this pattern. However, the posteriorly located sites for reaching movements evoked with quite low thresholds in case 04-39L (Fig. 3A) do not conform to this pattern.
Movement initiation and termination times
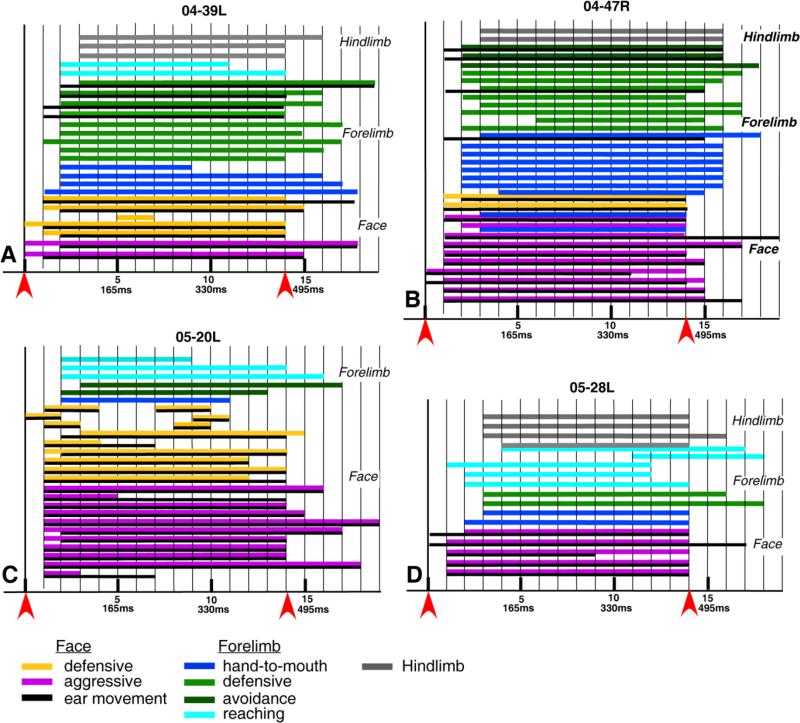
Behaviors recorded on videotape were analyzed frame-by-frame to verify the type and complexity of the evoked movement. In some animals, we also determined the reaction time and time course of the movement sequences (Fig. 7). The analysis was performed on each of the 25 trials in case 04-39L, 32 trials in case 04-47R, 26 trials in case 05-20L, and 17 trails in case 05-28L. In every case, some movements were seen in the first or the second video frame after stimulation onset. The duration of each frame was 33.3 msec, so the latencies for those movements were between zero and 67 msec. Face movements usually had the shortest latencies, starting typically in the second video frame (33–67 msec). Most face movements lasted at least to the end of a stimulus train, but on a few occasions they occurred just briefly (100–133 msec). Sometimes, we observed such brief face movements twice during the 500-msec stimulation period (Fig. 7C). When a face movement involved the ear, usually both movements appeared simultaneously with less than a 67-msec delay, but sometimes an ear movement followed the face movement with an additional 33-msec delay (see Fig. 7A–C). Face and ear movements usually stopped at the same time, although sometimes one of them lasted longer than the other. When stimulation evoked aggressive face movement, which involved grimace, and ear and jaw movements, the grimace movement usually occurred within 33 msec after the stimulus onset, followed by the ear and jaw movements one frame (33 msec) later. Forelimb movements were generally more delayed than face movements and were initiated within the first two video frames, thus within 67 msec. When the ear was involved with the complex forelimb movement, the ear movement usually occurred one frame (see Fig. 7A,B) or even two frames (see Fig. 7B) before forelimb movement. Forelimb movements were also more delayed when associated with a face defensive movement (see Fig. 7B), and the latency for an ear or grimace face movement (33–99 msec) was shorter than for forelimb movements (Fig. 7B). Hindlimb movements were more delayed than any other movements observed in this study, and the majority of them started within the third or fourth video frame after the stimulus onset (100–133 msec).
Figure 7.
A–D: Durations of face, forelimb, and hindlimb movements evoked from PPC in four cases. Face movements started the earliest, and hindlimb movements started the latest. Most of the movements start within 33–67 msec after stimulus train onset and end at the end of stimulation. The red arrowheads mark the onset (left) and offset (right) of the 500-msec stimulation train. The vertical lines separate the 33.3-msec video frames. The colored horizontal lines indicate the types of movement for each case and the duration of that movement. If any movement occurred within a video frame, the bar extends through the whole frame. A moment that was detected in the frame is attributed to the start of the frame, although it could have started later in the frame. Likewise, the movement in the last frame is extended through the complete frame, although it could have ended within the frame. Thus, the precision of the response measures is limited by the frame rate. Color code is the same as in Figures 2–6.
Generally, most movements continued during the stimulation period, with return to a normal resting posture as soon as the stimulation train ended. Sometimes movements continued past the stimulation period and stopped at the same or similar delay after stimulation offset as they began after stimulation onset.
DISCUSSION
Although the organization of PPC has been extensively studied in Old World macaque monkeys and in humans (for review see Rizolatti et al., 1998; Bremmer et al., 2001; Grefkes and Fink, 2005), very little is known about the PPC organization in prosimian primates, or even in New World monkeys. However, unlike the case in most mammals, the posterior parietal region of all primates, including prosimian galagos, is a large region that is likely to contain functionally distinct subdivisions. Recently, long trains (500 msec) of stimulating electrical pulses delivered via microelectrodes have been used effectively to reveal aspects of motor cortex and posterior parietal cortex organization in macaque monkeys (Graziano et al., 2002a,b; Cooke et al., 2003), and we have adopted this approach to study the PPC in galagos. The main conclusions that stem from our study is that the posterior half of PPC in galagos is not directly involved in evoking motor behavior, whereas the anterior half of PPC consists of as many as seven or more subareas where electrical stimulation can evoke different complex movement patterns that appear to have ethological significance. In addition, these subareas together reflect a global somatotopic pattern with the most medial part of the responsive zone participating in hindlimb movements, a middle part in forelimb movements, and the most lateral part in face and eye movements. These results are discussed further below in the context of what else is known about PPC organization in galagos. Next, the results are related to findings in macaque monkeys and other primates, as well as tree shrews (close relatives of primates), in an effort to understand what is similar and different across these taxa.
Insofar as the galagos in this study were anesthetized during the microstimulation experiments, the evoked movements were not the result of any conscious urge to move. However, the anesthesia likely had a dampening effect on the neural circuits involved in the evoked behaviors, and we would expect more vigorous and possibly more complete patterns of movements at lower stimulation thresholds from experiments on awake galagos. In addition, because movements were monitored visually, subtle patterns of muscle activity, detectable with EMG recordings, were likely missed.
Organization of posterior parietal cortex in galagos
The posterior parietal cortex of galagos can be roughly defined as the region of cortex between the narrow strip of somatosensory cortex along the caudal border of area 3b or S1 (area 1–2) and the most rostral well-defined dorsomedial visual area (DM), also known in macaque as V3a (Fig. 1). This posterior parietal region, in our estimate, extends from the medial margin of the hemisphere to perhaps 3–4 mm lateral to the intraparietal sulcus, where temporal visual and auditory areas are encountered. In contrast to the case in the anterior half of PPC, electrical stimulation throughout the posterior half of PPC failed to evoke body movements. It is possible that movements could be evoked from the posterior half of PPC in unanesthetized galagos, but the marked difference in the two halves in the present cases suggests that the anterior PPC is much more intimately involved in motor performance. This supposition is supported by the differential cortical connection patterns of the two divisions of PPC (Fang et al., 2005; see also companion paper).
At least most of the posterior half of PPC in galagos receives visual inputs from visual areas DM and V3 (Beck and Kaas, 1998a; Lyon and Kaas, 2002). Moreover, patterns of label after injections of tracers in the middle temporal visual area (MT) and narrow middle temporal crescent area (MTc) bordering MT suggest that both areas also have connections with posterior PPC (Wall et al., 1982; Krubitzer and Kaas, 1990; Kaskan and Kaas, 2007). Thus, there are probably four or more visual areas that distribute visual information to the posterior half of PPC in galagos. Similar connections exist in monkeys (for review see Krubitzer and Kaas, 1990; Beck and Kaas, 1998b). There is also evidence that in monkeys FST and MST project to posterior PPC (Boussaoud et al., 1990; Kaas and Morel, 1993). Although MST and FST connections have not yet been described in galagos, they are likely sources of additional visual inputs to posterior PPC. Thus, PPC appears to be highly devoted to the further processing of visual information that is relayed from visual areas that are considered to be parts of the dorsal stream of visual processing (Ungerleider and Desimone, 1982).
The anterior half of PPC in galagos receives major inputs from the posterior half of PPC, as well as inputs from subdivisions of somatosensory cortex (Wu and Kaas, 2003; Fang et al., 2005), including those from the second somatosensory area (S2) and the parietal ventral area (PV) as well as the narrow somatosensory belt along the posterior border of S1 (area 3b) that is in the position of area 1 of anthropoid primates (areas 1 + 2 of some authors; e.g., Wu and Kaas, 2003; see also Preuss and Goldman-Rakic, 1991b). In monkeys, some of the inputs to anterior PPC are from cortex dorsal and medial to primary auditory cortex that could include second-order or higher order auditory areas, so auditory inputs are possible as well (see Pandya and Kuypers, 1969; de la Mothe et al., 2006). Neurons in anterior PPC project to motor and premotor areas of galagos, including M1, SMA, PMD, and PMV (Fang et al., 2005). Only the frontal eye field appears to have inputs from cortex of the intraparietal sulcus in the posterior half of PPC (Fang et al., 2005). Thus, our present analysis implicates posterior PPC largely in visual functions that are mediated via connections with anterior PPC and then frontal motor and premotor areas. Some visual inputs are also relayed directly to anterior PPC and then to the frontal motor areas (see companion paper). Neurons throughout PPC, especially in the posterior half, also project to granular frontal cortex of galagos (Preuss and Goldman-Rakic, 1991a), providing another pathway to premotor cortex (Fang et al., 2005). In addition, there are widespread connections within anterior PPC, providing a connectional framework for different sectors to interact. Our analysis of PPC connection patterns is more extensive in our companion paper on the ipsilateral connections of anterior PPC (Stepniewska et al., 2009).
Comparison with results from other primates
PPC has not been extensively explored with microstimulation in other primates, although the results of early studies with brain surface stimulation in macaques suggest that movements might be evoked by microstimulation from much of the region (Fritsch and Hitzig, 1870). The traditional large divisions of PPC of Brodman (1909), areas 5 and 7, have been partially retained and partially subdivided by subsequent investigators so that the intraparietal region of macaques includes a number of variously named areas, identified by connection patterns, architecture, and neuron response properties (for reviews see Lewis and Van Essen, 2000a,b; Gattas et al., 2005). The ventral intraparietal area (VIP) in the depth of intraparietal sulcus is the only part of PPC of macaques that has been explored with 500-msec trains of electrical pulses used by Graziano and coworkers for stimulating frontal motor cortex (Cooke et al., 2003). VIP was first defined by Maunsell and Van Essen (1983) as the projection zone of visual area MT in macaques. Neurons in VIP also have somatosensory inputs and respond to tactile stimuli, while projecting to frontal motor cortex (for review see Graziano and Cooke, 2006). Stimulation of VIP in macaques resulted in a sequence of movements that Cooke et al. (2003) described as defensive and avoidance movements (eye closure, facial grimacing, and hand movements to the site of the face). Another more lateral area (on flattened cortex), the lateral intraparietal area (LIP), originally defined by connections with the frontal eye field, has been implicated in the control of eye movements (for review see Andersen et al., 1992; Andersen and Buneo, 2002). More anterolaterally in PPC, the anterior intraparietal area (AIP) is considered to be involved in the visual guidance of grasping (see, e.g., Sakata et al., 1995; Fogassi et al., 2001), whereas a more posterior and medial region, the parietal reach region (PRR), occupied largely by the previously defined medial intraparietal area (MIP), is thought to be involved in the spatial guidance of reaching (see, e.g., Andersen et al., 1998; Batista et al., 1999). Thus, there are suggestions of an overall similarity in the functional organization of PPC in galagos and macaques. Unfortunately, comparable microstimulation data have been gathered for only part of PPC in macaques (VIP), and neuron response properties have been studied only in macaques. Thus, a detailed comparison between PPC of macaques and galagos is not possible. In addition, there are differences in organization that indicate that caution is needed in proposing an area-by-area correspondence. In galagos, the anterior half of PPC is the portion responsive to long-train microstimulation. In macaques, this portion would correspond to cortex along the caudal border of area 2, area 5a medially, and area 7b laterally, including AIP and part of VIP, but little or none of LIP and MIP. Thus, the positions of some of the macaque areas that might be included in the parietal motor strip are not comparable in location to those of the anterior motor strip of galagos. As another difference, VIP, LIP, and MIP (parietal reach region) are all parts of the parietal cortex that receive dense visual inputs in macaques, whereas the anterior PPC motor strip is largely free of such direct visual inputs in galagos. Insofar as the research emphasis in macaque PPC has been on understanding the functions of the intraparietal cortex, less is known about cortex medial and lateral to the IPS, so there is little evidence for a general somatotopic organization from medial to lateral in anterior PPC of macaques. Perhaps some of the apparent differences will seem less prominent as more results are obtained from PPC of both species. However, it seems possible that the division of PPC into anterior and posterior strips with anterior multisensory and motor guidance functions and posterior visual functions represents a pattern that emerged in early primates and was most modified in Old World anthropoids. Further comparative studies, including studies on New World anthropoids, could be very useful.
Ethological interpretation of the movement patterns
After the descriptions of Graziano and coworkers (Graziano et al., 2002a; Cooke et al., 2003; Graziano, 2006) of movement patterns evoked from frontal motor cortex and the ventral intraparietal area of macaque monkeys, we have been impressed with the tendency of movement patterns evoked from PPC in galagos to conform to sequences that appear to have adaptive behavior consequences under natural conditions (e.g., defensive movements, reaching, retrieving, climbing, change of gaze). This classification has some face validity, in that such behavior patterns are commonly observed in a range of primate taxa as well as in other mammals. To the extent that these categories are valid, they suggest that there are specific networks for a number of classes of adaptive behaviors in primate brains and that these networks include different subregions of PPC together with different subregions of frontal motor cortex. As details of the motor pattern evoked from the functional subregions of anterior PPC vary for sites within each subregion, we assume that variations in the evoked motor patterns within and across the major functional subregions are based on variations in the spatial distributions of the extrinsic connections of the stimulated neurons. Thus, not only are neurons over the extents of small regions of PPC involved in specific classes of ethologically relevant behaviors but the variations in the behavior result from involvements of different populations of neurons within each functional region. Furthermore, it seems likely that the borders of functional regions are not sharp, insofar as classes of evoked behavior appear to be most similar when nearby neurons across a border region are successively stimulated. A more precise understanding of how spatial distributions of cortical neurons relate to complex patterns of movement may emerge when natural behavior patterns are more fully studied in galagos and when motor areas are stimulated in awake, freely moving galagos.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Burish, Dr. Omar Gharbawie, and Dr. Nicole Young for assistance with the microstimulation mapping and helpful comments on the article, Dr. Lisa de la Mothe for help with the figures, Mary Feurtado for surgical assistance, and Laura Trice and Mary Varghese for technical assistance.
Grant sponsor: National Institutes of Health; Grant number: NS 16446 (to J.H.K.); Grant number: NS 055843 (to I.S.).
LITERATURE CITED
- Andersen RA. Visual and eye movement functions of the posterior parietal cortex. Annu Rev Neurosci. 1989;12:377–403. doi: 10.1146/annurev.ne.12.030189.002113. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;2:840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Batista AP, Buneo CA, Cohen YE. Posterior parietal areas specialized for eye movements (LIP) and reach (PRR) using a common coordinate frame. Novartis Found Symp. 1998;218:109–122. 171–175. doi: 10.1002/9780470515563.ch7. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Arnold AP. Noxious effects of excessive currents used for intracortical microstimulation. Brain Res. 1975;96:103–107. doi: 10.1016/0006-8993(75)90579-x. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol. 1991a;66:1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991b;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in prosimian primates. J Comp Neurol. 1998a;398:162–178. [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in new world owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus). J Comp Neurol. 1998b;400:18–34. doi: 10.1002/(sici)1096-9861(19981012)400:1<18::aid-cne2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Brodman K. Barth; Leipzig: 1909. Vergleichende Lokalizationslehre der Grosshirnhinde in Ihren Prinzipien Dargestellt auf Grund des Zellenbaues. [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in a prosimian primate (Galago garnetti). J Comp Neurol. 2001;431:155–167. [PubMed] [Google Scholar]
- Cooke DF, Taylor CSR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci. 1999;2:88–93. doi: 10.1038/4594. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti). Neuroscience. 2006;143:987–1020. doi: 10.1016/j.neuroscience.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Corpus callosum connections of subdivisions of motor and premotor cortex, and frontal eye field in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2008;508:565–578. doi: 10.1002/cne.21706. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fritsch GT, Hitzig E. On the electrical excitability of the cerebrum. In: Von Bonin G, editor. Some papers on the cerebral cortex. Charles C. Thomas; Springfield IL: 1870. 1960. [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gattas R, Nascimento-Silva S, Soares JG, Lima B, Jansen AK, Diogo AC, Farias MF, Botelho MM, Mariani OS, Azzi J, Fiorani M. Cortical visual areas in monkeys: location, topography, connections, columns, plasticity and cortical dynamics. Philos Trans R Soc Lond B Biol Sci. 2005;360:709–731. doi: 10.1098/rstb.2005.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: the role of the lateral intraparietal area in visual behavior. Prog Brain Res. 2006;155:157–175. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:2621–2635. doi: 10.1016/j.neuropsychologia.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002a;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T, Cooke DF. The cortical control of movement revisited. Neuron. 2002b;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink G. The functional organization of the intraparietal sulcus in human and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Farne A. The visuomotor functions of posterior parietal areas. Adv Neurol. 2003;93:205–217. [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti Cortical network for visual reaching; physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec. 2004;281A:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J Neurosci. 1993;13:534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan PM, Kaas JH. Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti). Anat Rec. 2007;290:349–66. doi: 10.1002/ar.20440. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 2000a;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lewis J, Van Essen D. Corticocortical connections of visual sensorimotor, multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000b;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 2002;59:453–461. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol. 1998;402:327–352. [PubMed] [Google Scholar]
- Maunsell JHR, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Microstimulation of the lateral wall of the intraparietal sulcus compared with the frontal eye field during oculomotor tasks. J Neurophysiol. 1999;81:1443–1448. doi: 10.1152/jn.1999.81.3.1443. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kuroda T, Wakita M, Kusunoki M, Kato A, Mikami A, Sakata H, Itoh K. From three-dimensional space vision to prehensile hand movements: the lateral intraparietal area links the area V3A and the anterior intraparietal area in macaques. J Neurosci. 2001;21:8174–8187. doi: 10.1523/JNEUROSCI.21-20-08174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991a;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared with the anthropoid primate Macaca. J Comp Neurol. 1991b;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui K. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Philos Trans R Soc Lond B Biol Sci. 1998;353:1363–1373. doi: 10.1098/rstb.1998.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata E, Tanaka Y, Nakamura H, Taira M, Sakata H. Selectivity of the parietal visual neurons in 3D orientation of surface of stereoscopic stimuli. Neuroreport. 1996;7:2389–2394. doi: 10.1097/00001756-199610020-00022. [DOI] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur J Neurosci. 1998;10:3171–3193. doi: 10.1046/j.1460-9568.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 2007;502:894–923. doi: 10.1002/cne.21325. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PY, Dengler-Crish CM, Kaas JH. 2004 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2004. Cortical areas in the posterior parietal cortex of prosimian galagos identified by microstimulation. Program No. 191.4. Online. [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005a;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang P-C, Kaas JH. 2005 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2005b. Cortical connections of physiologically identified subdivisions of posterior parietal cortex in pros-imian galagos. Program No. 617.7. Online. [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex. 2005c;15:809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Burish MJ, Kaas JH. 2006 Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. Intracortical microstimulation uncovers map of complex movements in the posterior parietal cortex of New World owl monkeys. Program No. 549.7. Online. [Google Scholar]
- Stepniewska I, Burish MJ, Kaas JH. Society for Neuroscience; San Diego, CA: 2007. Complex movements evoked by intracortical microstimulation from the frontal motor region of prosimian galagos. Program No. 828.7. 2007 Neuroscience Meeting Planner. Online. [Google Scholar]
- Stepniewska I, Burish MJ, Gharbawie OA, Kaas JH. 2008 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. Mapping the frontal motor region in New World monkeys with long train intracortical microstimulation. Program No. 78.6. Online. [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang P-C, Kaas JH. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal vs. ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RLW, editors. Analysis of visual behavior. MIT Press; Cambridge, MA: 1982. pp. 544–586. [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Wall JT, Symonds LL, Kaas JH. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J Comp Neurol. 1982;211:193–214. doi: 10.1002/cne.902110208. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]