Abstract
Rationale
Chronic nicotine administration decreases the functioning of the cystine-glutamate antiporter system xc_ which is hypothesized to promote nicotine-taking and -seeking behaviors. N-acetylcysteine (NAC), a cystine pro-drug, increases the activity of the cystine-glutamate antiporter system xc_. Thus, NAC could potentially reverse nicotine-induced alterations in glutamatergic transmission and decrease nicotine taking and seeking.
Objectives and Methods
To test this hypothesis in the present study, the effects of acute NAC treatment (30, 60, 90 mg/kg i.p.) on nicotine (fixed- and progressive-ratio schedules) and food (fixed-ratio schedule) self-administration were assessed in rats. In addition, the effects of acute NAC treatment on cue-induced reinstatement of nicotine- and food-seeking behaviors were investigated. Finally, the effects of repeated daily NAC administration (60 mg/kg, i.p., 14 days) on nicotine and food self-administration were assessed.
Results
Acute NAC administration decreased nicotine self-administration but not food responding under a fixed-ratio schedule of reinforcement. In addition, acute NAC administration showed a non-significant trend in attenuating nicotine self-administration under a progressive-ratio schedule that was similar to the dose-response function under the fixed-ratio schedule. Furthermore, repeated NAC administration decreased nicotine self-administration from day 6 to 14 compared with vehicle treatment, with no indication of tolerance development. By contrast, repeated NAC administration decreased food responding from day 6 to 8 compared with vehicle treatment, and showed rapid development of tolerance. Finally, NAC administration attenuated cue-induced reinstatement of nicotine and food seeking.
Conclusions
Altogether, these findings suggest that NAC may be useful in promoting smoking cessation in humans.
Keywords: motivation, extinction, glutamate, food responding, drug abuse, dependence
Tobacco smoking is a chronic addictive disorder characterized by high rates of relapse (Shiffman et al. 2008). Considering that tobacco smoking is a significant preventable cause of morbidity and mortality, there is an urgent need to develop effective and inexpensive treatments to promote smoking cessation and prevent relapse to smoking. The reinforcing effects of nicotine, a major psychoactive component of tobacco smoke, are widely accepted to be responsible for the initiation and maintenance of the tobacco smoking habit (Stolerman and Jarvis 1995).
Glutamatergic transmission plays an important role in the development of nicotine dependence (Kalivas 2009; Liechti and Markou 2008). Repeated exposure to nicotine leads to neuroadaptations in glutamatergic substrates, resulting in decreased glutamate signaling, and thus the development of a hypothesized hypoglutamatergic state (for reviews, see Kenny and Markou 2004; Markou 2008). For example, during early withdrawal, chronic nicotine self-administration results in downregulation of predominantly presynaptic mGlu2/3 receptor function in the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex, amygdala, hippocampus, and hypothalamus, indicating decreased synaptic glutamate levels (Liechti et al. 2007). Furthermore, during early withdrawal, chronic nicotine self-administration experience resulted in increased expression of postsynaptic N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor subunits in the VTA, again suggesting a decrease in synaptic glutamate levels (Kenny et al, 2009). This hypoglutamatergic state may play an important role in maintaining nicotine seeking and the reinstatement of nicotine-seeking behavior upon cessation of nicotine administration (Markou 2008). Thus, restoring the nicotine-induced alterations in glutamatergic transmission may help promote smoking cessation and prevent the reinstatement of nicotine seeking (Liechti and Markou 2008).
The cystine-glutamate exchanger, a heterodimer that consists of a catalytic subunit (xCT) and a 4F2hc subunit, plays an important role in the regulation of extrasynaptic glutamate levels. The xCT subunit is responsible for both the binding and exchange of intracellular glutamate for extracellular cystine across the plasma membrane of glial cells (Lo et al. 2008). Our previous work showed that chronic nicotine self-administration in rats reduced the expression of xCT in mesolimbic regions that mediate the reinforcing effects of nicotine, such as the NAc and VTA (Knackstedt et al. 2009).
Previous work showed that administration of N-acetylcysteine (NAC), a cystine pro-drug that activates the cystine-glutamate exchanger (Griffith, 1999; Meister 1985), restored cocaine-induced decreases in glutamate levels (Baker et al. 2003) and xCT expression in the NAc (Knackstedt et al. 2010). In addition, NAC blocked cue-, cocaine-, and heroin-induced reinstatement of drug seeking in rats (Baker et al. 2003; Zhou and Kalivas 2008). Based on these earlier studies involving drugs of abuse, such as cocaine and heroin, we hypothesize here that NAC administration will also influence the effects of nicotine.
In the present study, the effects of acute NAC administration on nicotine and food self-administration were assessed using the fixed-ratio schedule of reinforcement in rats. In addition, the effects of acute NAC administration on the motivational effects of nicotine were assessed using the progressive-ratio schedule of reinforcement. Because medications for smoking cessation need to be administered chronically in humans, the effects of repeated NAC administration on nicotine and food self-administration were also assessed. Finally, the effects of NAC on cue-induced reinstatement of nicotine- and food-seeking behavior were investigated.
METHODS AND MATERIALS
Animals
Male Wistar rats (Charles River, Raleigh, NC), weighing 300-350 g at the start of the experiments, were housed two per cage on a reverse 12/12 h light/dark cycle in a temperature- and humidity-controlled vivarium. All behavioral testing occurred during the dark phase of the light/dark cycle. The animals had unrestricted access to water except during testing, and were food-restricted to 20 g of rat chow per day. Animal procedures were conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the University of California, San Diego Animal Care and Use Committee.
Drugs
(-)Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in saline; the pH was adjusted to 7.0 ± 0.5 with sodium hydroxide. The solution was then filtered through a 0.22 μm syringe filter (Fisher Scientific, Pittsburgh, PA). Nicotine doses are reported as base concentrations. NAC (Sigma-Aldrich, St. Louis, MO) was dissolved in saline, and administered intraperitoneally (i.p.) 2.5 h prior to testing. A 2.5 h pretreatment time was chosen based on demonstrations that glutamate levels were elevated in rat brain approximately 2.5 h after NAC administration (Baker et al. 2003). NAC was administered according to a within-subjects Latin-square design. The NAC doses used (0, 30, 60, 90 mg/kg) were selected based on previously published studies (Baker et al. 2003; Kau et al. 2008). In every experiment, the animals were habituated to i.p. injections by administering two i.p. saline injections one week prior to the initiation of the Latin-square involving NAC/vehicle treatment. The antibiotic ticarcillin-clavulanate (100 mg/ml, GlaxoSmithKline, Research Triangle Park, NC) dissolved in heparinized (33.3 U/ml, Abraxis, Schaumburg, IL) saline (Hospira, Lake Forest, IL) was administered intravenously immediately after surgery and daily after every nicotine self-administration session.
Apparatus: Testing Chambers
Standard operant conditioning chambers (24 × 30 × 28 cm; Med Associates, St. Albans, VT) were used in all of the experiments as described previously (Liechti et al. 2007).
Food training and food-maintained responding
Naive rats were gradually trained to lever press for food (45 mg Noyes food pellets) on a fixed-ratio 5 timeout 20 s (FR5 TO20 s) schedule over a period of 4-5 days as previously described (Vlachou et al. 2011). Rats that were used to assess the effects of NAC on food-maintained responding were trained for an additional 2-3 weeks (5 days per week) to match the training of their nicotine self-administering counterparts. NAC treatment was administered only when rats showed less than 20% variability in responding over three consecutive days.
Nicotine self-administration training under the fixed-ratio schedule of reinforcement
After food training, the animals used in the nicotine self-administration studies were surgically prepared with an intravenous catheter inserted into the right jugular vein under isoflurane (1-3% in oxygen) anesthesia as described previously (Liechti and Markou 2007). After surgery, the animals were allowed to recover for at least 7 days before nicotine self-administration training was initiated. During nicotine self-administration training, animals self-administered nicotine (0.03 mg/kg/infusion) during 1 h sessions for 4-5 weeks (5 days per week) as described previously (Liechti et al. 2007), and then drug manipulations were implemented.
Nicotine self-administration training under the progressive-ratio schedule of reinforcement
After recovery from intravenous catheterization surgery, naive rats were initially trained to self-administer nicotine (0.03 mg/kg/infusion) under the FR5 TO20 s schedule for two weeks. Subsequently, the rats self-administered nicotine under the progressive-ratio schedule of reinforcement, as described previously (Paterson et al. 2004). The breakpoint, defined as the highest ratio successfully completed, was used to determine the effects of NAC on the motivation to self-administer nicotine. The rats were trained for 7 days on the progressive-ratio schedule of reinforcement to allow them to establish stable nicotine self-administration under this schedule. Performance was considered stable when there was less than 20% variability and an average of four or more infusions over three consecutive daily sessions.
Extinction and cue-induced reinstatement of nicotine- and food-seeking behavior
Extinction and cue-induced reinstatement sessions were described in detail previously (Vlachou et al. 2011). Briefly, animals with chronic nicotine/food self-administration experience underwent extinction training for 10 days. The rats used in the cue-induced reinstatement of nicotine seeking procedure were animals used earlier to examine the effects of repeated NAC administration on nicotine self-administration under a fixed-ratio schedule of reinforcement. Only animals that reached the predetermined criterion of extinction (<30% responding on the active lever for three consecutive days compared with active lever presses during the last three nicotine self-administration days prior to the initiation of extinction training) by the end of the 10th extinction session were included in the study.
The rats used to test for cue-induced reinstatement of food-seeking behavior were naive rats trained to self-administer food, as described above. After the acquisition of food self-administration training (1 week), the animals had an additional 3 weeks of food self-administration training sessions.
The first reinstatement session was conducted immediately after the 10-day extinction phase. Reinstatement sessions were initiated by the presentation of a single noncontingent cue light (20 s). After the cue light was switched off, both the active and inactive levers were extended into the operant conditioning chamber, and subsequent cue light (20 s) presentations required five responses on the active lever. The nicotine group received the delivery of a saline infusion (0.1 ml over 1 s), in addition to the presentation of the cue light.
During the reinstatement sessions cue-induced responding was measured as the number of responses on the active lever throughout the test session, including during saline infusions and timeout periods. Reinstatement was defined as having a minimum of a 50% increase in active lever responses compared with the mean number of active lever responses during the three preceding extinction sessions.
Experimental Designs
Experiment 1: Effects of acute NAC administration on nicotine self-administration and food-maintained responding under a fixed-ratio schedule of reinforcement
The effects of NAC (0, 30, 60, and 90 mg/kg, i.p.; 2.5 hr pretreatment) were assessed in naive animals that showed stable nicotine (n = 20) or food (n = 10) self-administration behavior using a within-subjects Latin-square design. At least 7 days elapsed between drug administrations, and the rats were required to exhibit stable baseline performance prior to each NAC administration.
Experiment 2: Effects of acute NAC administration on the motivation to self-administer nicotine under a progressive-ratio schedule of reinforcement
The effects of acute NAC (0, 30, 60, and 90 mg/kg, i.p.; 2.5 hr pretreatment) administration on nicotine self-administration were assessed under a progressive-ratio schedule of reinforcement in rats (n =14) using a within-subjects Latin-square design. At least 7 days were allowed to elapse between drug administrations, and the rats were required to exhibit stable baseline performance prior to each NAC administration. NAC was expected to remain active for the entire duration of the experiment, because the half-life of NAC has been measured to be 6.25 hours in humans (Olsson et al. 1988). However it must be acknowledged that the half-life of NAC in rodents may be less than 6.25 hours, because rodents have faster metabolism than humans. The effects of acute NAC on food responding were not assessed under the progressive-ratio schedule of reinforcement because no statistically significant effect of acute NAC administration on food responding was observed in Experiment 1 (see Results below).
Experiment 3: Effects of repeated NAC administration on nicotine self-administration and food-maintained responding under a fixed-ratio schedule of reinforcement
The effects of 14-day NAC/vehicle administration were assessed using a between-subjects factorial design with four groups of naive rats: (i) NAC (60 mg/kg/day, i.p.) administration in nicotine self-administering animals (n = 14); (ii) saline administration in nicotine self-administering animals (n = 13); (iii) NAC (60 mg/kg/day, i.p.) administration in food-self-administering animals (n = 8); and (iv) saline administration in food-self-administering animals (n = 9). NAC/vehicle was administered 2.5 hrs prior to nicotine/food self-administration session on each day. The groups were balanced for weight and nicotine/food intake before the initiation of treatments. Rats were allowed to self-administer nicotine or food for six additional days after the termination of the NAC/saline treatment.
Experiment 4: Effects of NAC on cue-induced reinstatement of nicotine- and food-seeking behavior
After the completion of Experiment 3, the rats that were self-administering nicotine (n = 27) were tested under extinction conditions for 10 daily sessions. All of the animals from Experiment 3 reached the predetermined criterion of extinction (see above) by the end of the 10th extinction session. The animals then underwent six reinstatement sessions. The first reinstatement session was conducted after vehicle administration (V1; 2.5 h pretreatment, i.p.) to ensure that the subjects showed reliable reinstatement. Only rats that exhibited nicotine-seeking behavior (n=21), defined in the Methods above, during this first reinstatement session were included in the remainder of the experiment (six rats were excluded because they did not meet the reinstatement criterion). After the first reinstatement session, NAC (30, 60, and 90 mg/kg, i.p.) or saline (V2) was administered 2.5 hrs prior to each of four reinstatement test sessions according to a within-subjects Latin-square design. After the completion of the Latin-square design, one final reinstatement session was conducted after vehicle administration (V3; 2.5 h pretreatment, i.p.). Each of the reinstatement sessions was preceded by three daily extinction sessions to re-extinguish responding. The three reinstatement sessions that were conducted after vehicle administration were used to assess the stability of cue-induced reinstatement of nicotine-seeking behavior over time.
Four independent naive groups of rats (n = 11-13 per group) that responded for food were tested with different doses of NAC (0, 30, 60, and 90 mg/kg, i.p.). The groups were balanced for weight and food responding before treatment. A single reinstatement test session after the extinction phase was done on food trained rats because previous work in our laboratory indicated that repeated reinstatement sessions in food-trained rats are not feasible because food-seeking behavior exhibits rapid extinction with repeated reinstatement testing (Bespalov et al, 2005).
Data Analyses
The effects of acute NAC on nicotine and food self-administration (Experiment 1) and nicotine breakpoints (Experiment 2) were analyzed by two and one-way repeated-measures analyses of variance (ANOVAs), with Dose as the within-subjects factor, and Reinforcer as the between-subject factor. The effects of repeated NAC on nicotine and food self-administration (Experiment 3) were analyzed by two-way repeated-measures ANOVAs, with Time as the repeated measure and Treatment as the between-subjects factor. The effect of treatment history on extinction and cue-induced reinstatement was analyzed by a two-way repeated-measures ANOVA, with Cue condition as the repeated measure and Treatment history as the between-subjects factor. Reinstatement data (Experiment 4) in nicotine- and food-trained rats were analyzed with three-way and follow-up two-way repeated-measures ANOVAs, with Cue condition and Dose as the repeated measures, and Reinforcer as the between-subjects factor. Additionally, a two-way repeated-measures ANOVA, with Cue condition and Vehicle session as the repeated measures, was performed on the three different vehicle reinstatement sessions (i.e., one immediately after the 10-day extinction phase, one as part of the Latin-square design, and one after the completion of the Latin-square design). Post hoc comparisons were made with Bonferroni post hoc tests after statistically significant effects in the ANOVAs. All data were analyzed using Prism 4.0 software (GraphPad, San Diego, CA).
RESULTS
Experiment 1: Effects of acute NAC administration on nicotine self-administration and food-maintained responding under a fixed-ratio schedule of reinforcement
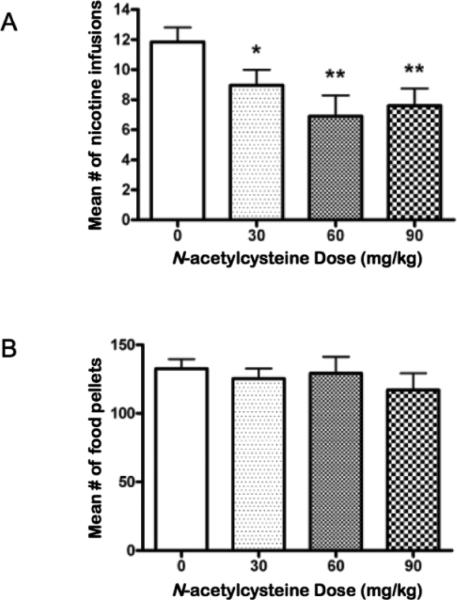
The baseline mean ± SEM (mean of the last 3 days) number of nicotine infusions earned just before vehicle administration in nicotine and food self-administering rats was 11.85 ± 0.89 and 141.9 ± 4.43, respectively. A two-way repeated measures ANOVA (reinforcer × dose) indicated a main effect of reinforcer (F1,28 = 1126, p < 0.0001), but no main effect of dose (F3,84 = 1.26, n.s.) and no reinforcer × dose interaction effect (F3,84 = 0.63, n.s.). A follow-up one way ANOVA that directly addressed the primary experimental question posed indicated a significant decrease in nicotine self-administration after NAC administration (F3,19 = 2.529, p < 0.01; Fig. 1a). Post hoc tests indicated that NAC significantly reduced nicotine self-administration at the 30 (p < 0.05), 60 (p < 0.01), and 90 (p < 0.01) mg/kg doses compared with self-administration after vehicle administration. Another follow-up one-way ANOVA on food responding indicated no statistically significant effect of NAC on food-maintained responding (Fig. 1b). No effects of dose order on nicotine or food self-administration were revealed by the ANOVA (data not shown).
Figure 1.
The effects of acute NAC administration on (a) nicotine self-administration (n = 20) and (b) food-maintained responding (n = 10) under a FR5 schedule of reinforcement. Asterisks (*p < 0.05, **p < 0.01) indicate difference from the 0 mg/kg NAC condition.
Experiment 2: Effects of acute NAC administration on the motivation to self-administer nicotine under a progressive-ratio schedule of reinforcement
The baseline mean ± SEM (mean of the last 3 days) number of nicotine infusions earned before vehicle administration was 8.29 ± 0.63. Acute NAC administration had no significant effect on break points for nicotine, although upon visual inspection, a similar dose-response curve to that of nicotine self-administration under a fixed-ratio schedule of reinforcement was observed (Fig. 2). No effects of dose order on nicotine self-administration were revealed by ANOVA analyses (data not shown).
Figure 2.
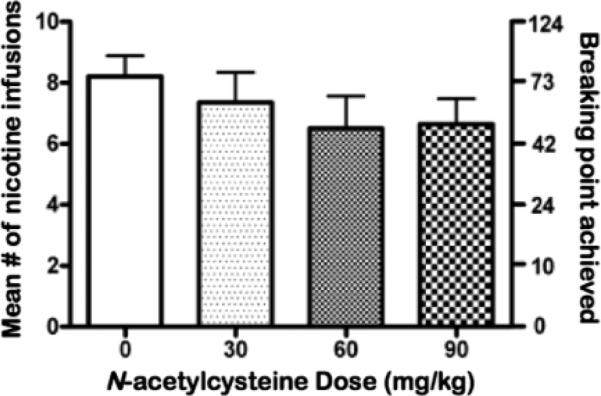
The effects of acute NAC administration on the number (mean ± S.E.M) of nicotine infusions earned (left y-axis) and corresponding nicotine break points (mean ± S.E.M) achieved (right y-axis; n = 14) under a progressive-ratio schedule of reinforcement.
Experiment 3: Effects of repeated NAC administration on nicotine self-administration and food-maintained responding under a fixed-ratio schedule of reinforcement
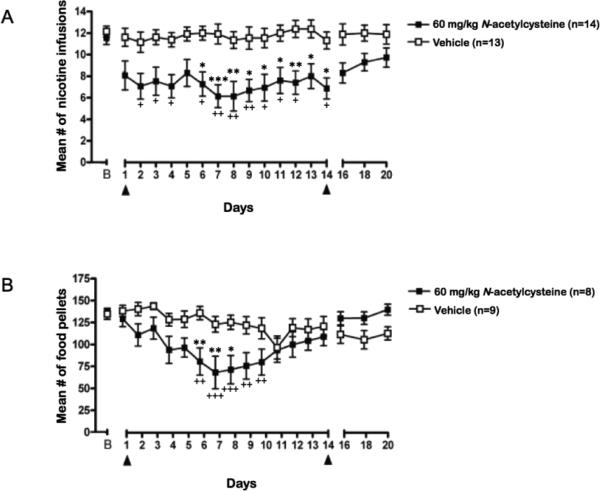
Baseline mean ± SEM nicotine infusions earned during the 3 days of nicotine self-administration before the initiation of repeated NAC or vehicle treatment for the NAC and vehicle treatment groups were 11.6 ± 0.7 and 12.1 ± 0.5, respectively, and are shown as data point B in Fig. 3a. Repeated NAC administration decreased nicotine self-administration across days, whereas vehicle did not, reflected by a significant Time × Treatment interaction (F22, 572 = 2.25, p < 0.001), and significant main effects of Treatment (F1, 572 = 14.59, p < 0.0007) and Time (F22, 572 = 2.88, p < 0.0001). Post hoc tests indicated that NAC significantly reduced nicotine self-administration on days 6-14 compared with nicotine self-administration of the vehicle-treated group on the same days (p < 0.05; Fig. 3a).
Figure 3.
The effects of repeated NAC/vehicle administration on (a) nicotine self-administration (n = 13-14/group) and (b) food responding (n = 8-9/group); In both graphs B= Baseline responding; Arrows indicate the start and end of NAC/vehicle treatment. Asterisk signs (*p < 0.05, **p < 0.01, ***p < 0.001) indicate difference compared with vehicle treated group; Plus signs (+p < 0.05, ++p < 0.01, +++p < 0.001) indicate difference in compared with baseline responding.
The mean ± SEM number of food pellets earned during the 3 days of food-maintained responding before repeated NAC or vehicle treatment was initiated was 136.7 ± 4.5 and 134.7 ± 6.2, respectively, and are shown as data point B in Fig. 3b. Repeated NAC, but not vehicle, treatment decreased food self-administration across days (Fig. 3b), reflected by a significant Time × Treatment interaction (F22, 330 = 6.89, p < 0.0001), and a significant main effect of Time (F22,330 = 9.44, p < 0.0001). No significant main effect of Treatment was found. Post hoc tests indicated that NAC significantly reduced food self-administration on days 6-8 compared with food self-administration of the vehicle-treated group on the same days (p < 0.05; Fig. 3b).
Experiment 4: Effects of NAC on cue-induced reinstatement of nicotine- and food-seeking behavior
The ANOVA indicated that regardless of treatment history (i.e., chronic NAC or chronic saline in Experiment 3), the animals exhibited similar behavior during the extinction and reinstatement sessions. Specifically, a two-way repeated-measures ANOVA revealed a significant main effect of Cue Condition (F1,19 = 55.14, p < 0.0001), but no significant Cue condition × Treatment history interaction and no main effect of Treatment history. Post hoc tests showed significant (p < 0.001) cue-induced reinstatement after vehicle treatment in animals with history of NAC or saline treatment. Therefore, the data from the two groups of animals (i.e., NAC and saline treatment history) were pooled for the analysis of cue-induced reinstatement of nicotine-seeking behavior using a Latin-square design. Furthermore, no effects of dose order on nicotine or food seeking were revealed by the ANOVA (data not shown).The mean number of active lever presses over the last 3 days of nicotine self-administration prior to the extinction phase was 58.0 ± 0.7 (mean ± SEM). The mean number of active lever presses over the last 3 days of food-maintained responding prior to the extinction phase was 1039.0 ± 49.6 (mean ± SEM).
A three-way ANOVA (reinforcer × cue condition × dose), with dose as a repeated measure, indicated statistically significant effects of reinforcer (F1,60 = 78.86, p < 0.0001), cue condition (F1,60 = 53.48, p < 0.0001), and dose (F3,180 = 5.60, p < 0.001), reinforcer × cue Condition (F1,60 = 27.668, p < 0.0001) and cue condition × dose (F3,180 = 5.36, p < 0.01) interactions, but no reinforcer × dose (F3,180 = 1.06, n.s.) or reinforcer × cue condition × dose (F3,180 = 1.48, n.s.) interaction. The three-way ANOVA was followed up with separate two-way repeated-measures ANOVA's for each reinforcer.
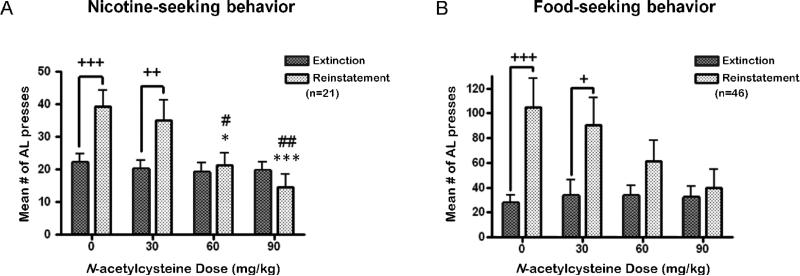
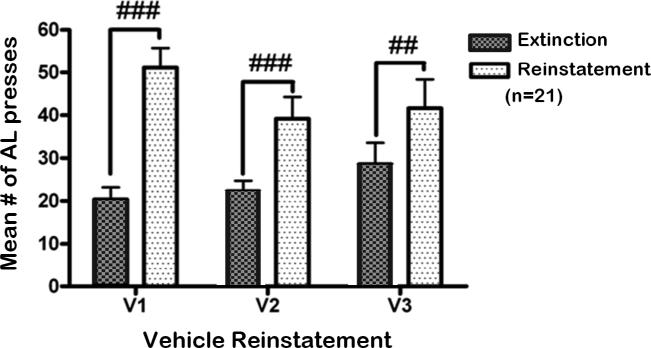
For the nicotine group, a two-way repeated-measures ANOVA indicated a significant cue condition × dose interaction (F3,80 = 5.85, p < 0.01), and significant main effects of cue condition (F1,80 = 10.51, p < 0.01) and dose (F3,80 = 3.85, p < 0.05). Post hoc tests indicated that on the days that the animals were pretreated with vehicle or 30 mg/kg NAC, a significant increase in active lever responding was observed upon presentation of the nicotine-associated cues compared with responding during the preceding extinction sessions (p < 0.01; Fig. 4a). Importantly, compared with responding after vehicle administration, NAC administration significantly attenuated cue-induced reinstatement of nicotine seeking at the 60 (p < 0.05) and 90 (p < 0.001) mg/kg doses. Furthermore, no difference in the reinstatement of nicotine-seeking behavior after vehicle administration was observed over time (Fig. 5). A significant main effect of cue condition (F1,60 = 78.52, p < 0.001), and a significant time × cue Condition interaction (F2,60 = 5.55, p < 0.0001) were observed. Post hoc tests indicated that all three reinstatement sessions that were preceded by vehicle administration were characterized by significantly higher response rates compared with the mean number of responses during the three extinction sessions that preceded each reinstatement session (p < 0.01). Because NAC was administered only prior to the reinstatement sessions and not prior to the extinction sessions, post hoc tests indicated no difference in responses across the various extinction sessions (Fig. 5).
Figure 4.
The effects of acute NAC administration on cue-induced reinstatement of (a) nicotine-(n = 21) and (b) food-seeking (n = 11-13) behavior in rats. AL= active lever presses. Plus signs (+p < 0.05, ++p < 0.01, +++p < 0.001) indicate difference from the preceding extinction condition. Asterisk signs (*p < 0.05, ***p < 0.001) indicate difference from the 0 mg/kg NAC (vehicle) condition; Pound signs (#p < 0.05, ##p < 0.01) indicate difference from the 30 mg/kg NAC condition.
Figure 5.
The effects of vehicle administration on cue-induced reinstatement of nicotine seeking over time and with repeated cue-induced reinstatement testing (n = 21/dose). AL=active lever presses. V1= first reinstatement session conducted immediately after the 10 day extinction phase; V2 = reinstatement session after vehicle administration conducted as part of a within-subjects Latin-square design; V3 = reinstatement session after vehicle administration conducted after completing the Latin-square design. Pound signs (##p < 0.01, ###p < 0.001) indicate difference from extinction.
For the food group, a significant cue condition × dose interaction (F3,42 = 2.86, p < 0.05), and a significant main effect of cue condition (F1,42 = 20.55, p < 0.0001) were found. No significant main effect of dose was observed. Post hoc tests indicated that animals pretreated with vehicle or 30 mg/kg NAC exhibited a significant increase in active lever responding in response to the presentation of food-associated cues compared with their respective extinction sessions (p < 0.05; Fig. 4b). In contrast, cue-induced reinstatement of food-seeking behavior was attenuated in animals pretreated with NAC at the 60 and 90 mg/kg doses.
DISCUSSION
Acute NAC administration decreased nicotine self-administration, but not food-maintained responding, under a fixed-ratio (FR5) schedule of reinforcement. Acute NAC administration showed a non-significant trend in attenuating nicotine self-administration under a progressive-ratio schedule, exhibiting a similar dose-response function as under the fixed-ratio schedule. Repeated administration of NAC significantly decreased both nicotine and food self-administration under the fixed-ratio schedule of reinforcement. This effect of repeated NAC administration showed no tolerance development for nicotine-maintained responding. In contrast, the development of tolerance to NAC-induced decreases in food-maintained responding was evident. Finally, acute NAC administration attenuated cue-induced reinstatement of both nicotine and food seeking. These results indicate that pharmacological activation of the cystine-glutamate exchanger after acute and repeated NAC administration attenuated the reinforcing effects of nicotine in rats. Furthermore, the data suggest that activation of the cystine-glutamate exchanger with NAC attenuated the motivational impact of stimuli previously associated with nicotine or food.
Glutamatergic neurotransmission plays an important role in mediating the reinforcing effects of nicotine. Inhibition of glutamatergic neurotransmission either via blockade of postsynaptic metabotropic glutamate 5 (Glu5) receptors or NMDA receptors or activation of predominantly presynaptic mGlu2/3 receptors decreased nicotine self-administration (Kenny et al. 2009; Paterson et al. 2003; Liechti et al. 2007). Nevertheless, NAC that increases extrasynaptic glutamate levels by exchanging extracellular cystine for intracellular glutamate also decreases nicotine self-administration. One possible mechanism by which extrasynaptic glutamate increase can reduce synaptic glutamate transmission is by activating extrasynaptic inhibitory presynaptic mGlu2/3 receptors (Moussawi and Kalivas 2010; Kupchik et al. 2011; Moussawi et al. 2011). Thus, the effects of acute NAC administration on nicotine self-administration are possibly mediated via activation of inhibitory presynaptic mGlu2/3 receptors.
As described above, acute NAC administration selectively attenuated nicotine self-administration compared with food self-administration under an FR-5 schedule. This finding is consistent with findings from previous studies using compounds that blocked glutamatergic neurotransmission either via blockade of postsynaptic metabotropic glutamate 5 (Glu5) receptors (Paterson et al. 2003) or activation of predominantly presynaptic mGlu2/3 receptors (Liechti et al. 2007). As a caveat to this finding, it must be mentioned that rates of responding for each reinforcer (nicotine vs. food) were different under an FR-5 schedule; this difference in responding could have potentially influenced the effects of NAC on nicotine vs. food self-administration. Nevertheless, it should be noted that high rates of responding, such as those supported by food as the reinforcer, are more likely to be affected by drug manipulations than low response rates such as those supported by nicotine (Sanger & Blackman,1976). In addition, even though both nicotine- and food- self-administering rats were similarly food-restricted in this study, it is possible that food-restriction could have differentially affected the effects of NAC on nicotine vs. food self-administration.
Repeated NAC administration significantly decreased nicotine self-administration on days 6-14 and food responding on days 6-8 compared with responding of the vehicle-treated group on the same days during the 14-day treatment. Thus, repeated NAC administration reduced both food and nicotine self-administration compared with vehicle administration by the sixth day of treatment. However, rapid tolerance developed to the effects of NAC on food-maintained responding, whereas the effects on nicotine self-administration persisted throughout the duration of treatment. Consistent with the present findings, a small double-blind study in humans showed that repeated NAC administration reduced the number of cigarettes smoked daily over a period of 4 weeks when alcohol consumption was taken into consideration (Knackstedt et al. 2009). The present results suggest that NAC continues to be effective in attenuating the reinforcing effects of nicotine after repeated administration, while only having transitory effects on the reinforcing effects of natural reinforcers, such as food.
Acute NAC administration in rats blocked cue-induced reinstatement of both nicotine and food seeking. The blockade of cue-induced reinstatement of nicotine seeking is also consistent with results from numerous studies reporting blockade of cue/drug-induced reinstatement of cocaine and heroin seeking in rats after NAC administration (Amen et al. 2011; Baker et al. 2003; Reichel et al. 2011; Zhou and Kalivas 2008). Previous work showed that acute NAC administration at the doses tested in this study had no effect on locomotor activity (Madayag et al. 2007; Murray et al. 2011); thus, the present findings cannot be attributed to locomotor suppressant effects of NAC. However, it should be noted that animals in the nicotine self-administration group were initially food trained prior to their nicotine self-administration training. Thus, when animals were learning to self-administer nicotine they were also simultaneously undergoing extinction training for food reward. Thus, the extinction training after the nicotine self-administration phase was the second extinction training for these animals, and this may have influenced extinction and reinstatement of nicotine-seeking behavior. Nevertheless taken together, the decrease in responding to nicotine- and food-associated stimuli after NAC administration suggests disruption of conditioned behavioral responding.
Drug-associated cues enhance glutamatergic neurotransmission (Bell et al. 2000; Hotsenpiller et al. 2001) that is hypothesized to result in reinstatement of drug seeking. Consistent with this hypothesis, decreased glutamate neurotransmission either by agonist actions at presynaptic mGlu2/3 receptors (Liechti et al. 2007) or antagonist actions at postsynaptic mGlu5 receptors (Bespalov et al. 2005) blocked reinstatement of nicotine seeking. As described above, a possible mechanism by which NAC can decrease glutamatergic neurotransmission is via activation of extrasynaptic mGlu2/3 receptors. mGlu2/3 receptor activation attenuated both conditioned behavioral and neurochemical responses to cues/contexts-associated with nicotine and natural rewards, such as food and sucrose in rats (Bossert et al. 2006; D'Souza et al. 2011; Liechti et al. 2007). Importantly, attenuation of cocaine seeking by NAC was reversed after administration of an mGlu2/3 receptor antagonist, demonstrating that reinstatement prevention by NAC is mGlu2/3 receptor-dependent (Kupchik et al. 2011; Moussawi et al. 2011). Finally, in vivo and in vitro experiments showed that NAC administered to cocaine-withdrawing rats reversed decreases in extracellular glutamate levels, re-established tone onto mGlu2/3 receptors, and returned synaptic glutamate release to the level of control animals (Kupchik et al. 2011; Moussawi et al. 2011). Altogether, the data suggest that NAC attenuates nicotine and food seeking behavior possibly via activation of mGlu2/3 receptors.
NAC-induced attenuation of cue-induced reinstatement of food seeking suggests that in addition to blocking nicotine cravings in humans after a period of abstinence, NAC may also interfere with cravings for food. Abstinent smokers often complain about increased food cravings and consumption, resulting in weight gain (Friedman and Siegelaub 1980). Thus, in humans, this pattern of results could potentially be seen as a positive effect.
In humans, NAC administration decreased the urge to gamble in pathological gamblers (Grant et al. 2007). Importantly, NAC administration decreased the number of cigarettes smoked daily by smokers (Knackstedt et al. 2009), and the subjective rewarding effect of smoking a cigarette after a brief period of abstinence (Schmaal et al. 2011). Finally, NAC has few to no adverse side effects (LaRowe et al. 2006) and is available as an over-the-counter nutritional supplement. Altogether, NAC potentially meets the demands of effectiveness, accessibility, and low cost for the treatment of nicotine addiction.
In conclusion, the results of the present study showed that acute NAC administration attenuated the reinforcing effects of nicotine without affecting food-maintained responding. Notably, this attenuation of the reinforcing effects of nicotine was maintained after repeated NAC administration, indicating the lack of tolerance to its effects. Finally, NAC attenuated cue-induced reinstatement of both nicotine- and food-seeking behavior in rats. These data support the prospect that NAC administration will promote smoking cessation and block cue-induced cravings for cigarettes and food in abstinent smokers. Taken in the context of preliminary clinical findings in humans (see above), the present findings suggest that NAC could be an effective therapeutic option in promoting smoking cessation and preventing relapse in abstinent smokers.
Acknowledgements
This work was supported by National Institutes of Health grants DA11946 to AM. AR-N was supported by National Science Foundation Training Grant HRD-0331537 to California State University, and MSD was supported by Tobacco-Related Disease Research Program (TRDRP) of the State of California Individual Postdoctoral Fellowship 19FT-0045. The authors thank Mr. Michael Arends for editorial assistance.
Footnotes
Conflict of interests: Athina Markou has received contract research support from Bristol-Myers Squibb Co., F. Hoffman-La Roche, Pfizer, and Astra-Zeneca, and honoraria/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years. Dr. Markou has a patent on the use of metabotropic glutamate compounds for the treatment of nicotine dependence. The remaining authors report no financial conflicts of interests.
REFERENCES
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Liechti ME, Ramirez-Niño AM, Kuczenski R, Markou A. The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology. 2011;36:2111–2124. doi: 10.1038/npp.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Siegelaub AB. Changes after quitting cigarette smoking. Circulation. 1980;61:716–723. doi: 10.1161/01.cir.61.4.716. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-Acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.10.024. doi: 10.1016/j.biopsych.2011.10.024 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence: implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. The xc- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Methods for the selective modification of glutathione metabolism and study of glutathione transport. Methods Enzymol. 1985;113:571–585. doi: 10.1016/s0076-6879(85)13077-6. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00330.x. doi: 10.1111/j.1369-1600.2011.00330.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABA(B) receptor agonists baclofen and CGP44532 decreased nicotine self- administration in the rat. Psychopharmacology. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction following cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337:487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: A review of the literature. Pharmacol Biochem Behav. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addict Res. 2011;17:211–216. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14-20. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Guery S, Froestl W, Banerjee D, Benedict J, Finn MG, Markou A. Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2011;215:117–128. doi: 10.1007/s00213-010-2119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]