Abstract
Blood transfusions have become indispensable to treat the anemia associated with a variety of medical conditions ranging from genetic disorders and cancer to extensive surgical procedures. In developed countries, the blood supply is generally adequate. However, the projected decline in blood donor availability due to population ageing and the difficulty in finding rare blood types for alloimmunized patients indicate a need for alternative red blood cell (RBC) transfusion products. Increasing knowledge of processes that govern erythropoiesis has been translated into efficient procedures to produce RBC ex vivo using primary hematopoietic stem cells, embryonic stem cells, or induced pluripotent stem cells. Although in vitro-generated RBCs have recently entered clinical evaluation, several issues related to ex vivo RBC production are still under intense scrutiny: among those are the identification of stem cell sources more suitable for ex vivo RBC generation, the translation of RBC culture methods into clinical grade production processes, and the development of protocols to achieve maximal RBC quality, quantity, and maturation. Data on size, hemoglobin, and blood group antigen expression and phosphoproteomic profiling obtained on erythroid cells expanded ex vivo from a limited number of donors are presented as examples of the type of measurements that should be performed as part of the quality control to assess the suitability of these cells for transfusion. New technologies for ex vivo erythroid cell generation will hopefully provide alternative transfusion products to meet present and future clinical requirements.
Keywords: Anemia, Adult hematopoietic stem cells, Cord blood, Embryonic stem cells, Induced pluripotent stem cells, Erythropoiesis
Introduction
The history of transfusion outlines the pathway for successful implementation of an innovative therapy. The transfer of blood from healthy donors to patients with insufficient levels of red blood cells (RBCs) is a cell therapy conceived in the 17th century when William Harvey provided definitive experimental evidence for blood circulation. In 1665, Richard Lower reported the first successful dog-to-dog transfusion. The first successful transfusion in humans is attributed to James Blundel who, in 1818, performed a life saving husband-to-wife transfusion for postpartum hemorrhage. The discovery of the heterogeneity of major (A, B, O, and Rhesus [Rh]) blood types by Karl Landsteiner, recognized by the Nobel committee in 1930, and the establishment of blood banks in the 1940–1950s, eventually made transfusion therapy safe and widely available [1].
Blood transfusion is an essential part of modern patient care. Although 92 million donations are made yearly worldwide (www.who.int/worldblooddonorday/en/), blood is a scarce human resource. In western countries, the blood supply is usually sufficient or even in excess [2], whereas in developing countries, the supply rarely meets existing needs. However, the increasing population >60 years of age, together with the growth in blood transfusions to support advanced surgical procedures and medical treatments in older individuals, has led to projections that even in industrialized countries the blood supply will no longer be adequate by 2050 [3]. Providing blood for chronically transfused patients and patients with rare blood types is an additional challenge because of the risks of alloimmunization, a complex immune reaction resulting in development of antibodies against antigens present on RBC. Since transfusions are routinely matched for ABO and Rh-D blood types, patients may develop antibodies against other Rh and minor blood group antigens [4]. In addition, RBC antibodies may develop during pregnancy and following transplantation [4]. These antibodies pose serious consequences (hemolysis, organ failure, and even death) if the patient is transfused with RBC expressing the cognate antigen. Incompatible transfusions may occur when antibodies become undetectable. Because of this low but consistent risk, alloimmunized patients are transfused with blood from matched donors identified through targeted recruitment programs. Despite these efforts, blood for alloimmunized patients is often unavailable. These considerations underscore the importance of developing alternative transfusion products.
Although the use of RBC generated in vitro for transfusion has been suggested for several years, this concept was considered unrealistic due to the complexity of the skills involved in its realization. This concept has gained new momentum due to recent scientific and technical discoveries in the field. This review summarizes recent advances in ex vivo RBC production for transfusion purposes and discusses scientific and logistic barriers in this rapidly developing field.
Formulation of the Concept: Establishment of Massive In Vitro Expansion Methods For Human Erythroid Cells
Hematopoietic stem cells (HSCs) give rise to mature erythroid cells through a series of intermediate differentiation stages including hematopoietic progenitor cell populations (HPC) capable to form colonies in semisolid cultures (the burst-forming unit-erythroid and colony-forming unit-erythroid) and morphologically recognizable erythroid precursor cells (Fig. 1) [5]. In the final step of erythroid differentiation, orthochromatic erythroblasts extrude their nucleus and egress from the bone marrow into the blood stream as circulating reticulocytes. This process is positively regulated by erythropoietin (EPO). EPO exerts its action by binding to a specific receptor (EPO-R) expressed on both erythroid progenitor and precursor cells. At the progenitor cell levels, EPO-EPO-R binding activates proliferation while at the precursor level, it promotes maturation [5]. These effects are mediated by a finely tuned positive regulation of the expression and activity of the major erythroid transcription factor GATA1 [6]. By contrast, members of the tumor necrosis factor (TNF) family (CD95L and TRAIL) induce erythroblast death or differentiation arrest by activating caspase-mediated cleavage of GATA1 [7]. Although caspases are also activated during terminal erythroid differentiation, in this context HSP70 protects GATA1 from cleavage allowing cells to undergo final maturation [8].
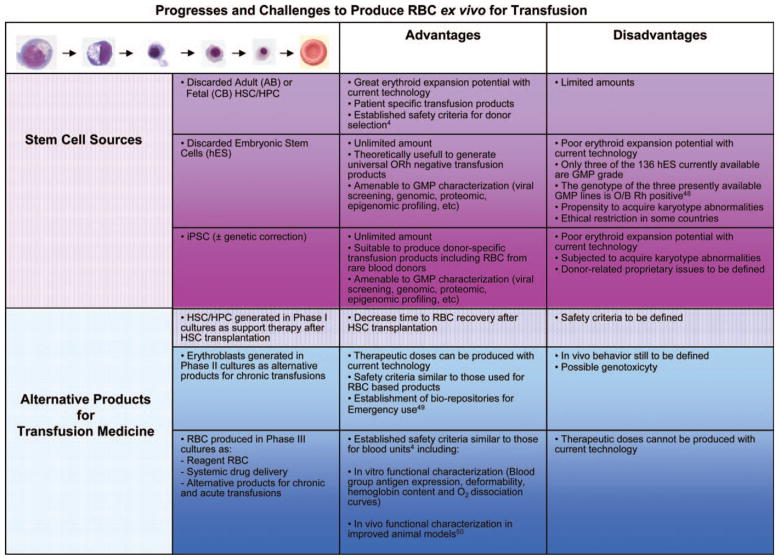
Figure 1.
Outline of progressively more mature human erythroid cells generated ex vivo under conditions of massive expansion and of the stem cell sources, alternative transfusion products, and relative safety criteria currently under development. Abbreviations: CB, cord blood; GMP, good manufacturing practice; hES, human embryonic stem; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; iPSC, induced pluripotent stem cell.
Methods for cultivating small amounts of lineage-committed blood progenitor cells have been known for almost two decades [9]. However, massive production of RBC ex vivo was made possible by the discovery that dexamethasone and estradiol, the ligands for the glucocorticoid and estrogen receptors, respectively, greatly increase the cell yield of erythroid cultures [10–13]. Currently, the cultures to generate large numbers of human RBC in vitro are structured in three phases devised to optimize progenitor (phase 1), erythroblast (phase 2) expansion, and terminal maturation (phase 3), respectively (Fig. 1 and [14]). Phase 1 cultures are stimulated with growth factors that promote proliferation of HSCs and of their immediate progeny (stem cell factor [SCF], FLT-3L, and thrombopoietin). Phase 2 cultures are stimulated with growth factors (SCF, interleukin-3, and EPO) plus dexamethasone and estradiol to promote expansion of erythroid progenitor cells (EPCs) by blocking maturation and activating telomerase activity (thus preventing chromosome senescence). Phase 3 cultures contain EPO and insulin-like growth factor 1 to induce maturation and either murine MS-5 cells or human mesenchymal cells to promote enucleation. The stromal cells probably exert in vitro the same functions (physical support and optimization of iron metabolism) performed by macrophages in the erythroblast island in vivo [15].
The major obstacle to the use of cultured RBC for transfusion is the high costs of their production. A unit of blood contains numbers of RBC equivalent to one-tenth of those present in the circulation (2.5 × 1012 RBC). Since under normal circumstances, humans make 2 × 1011 RBC per day, the production of 2.5 × 1012 RBC in vivo requires at least 12–13 days and occurs in compartmentalized areas of the marrow, the erythroid island, that, by minimizing cell interactions, allows production of great cell numbers in relatively small volumes. In vitro, progression from HSC/HPC to RBC also occurs within 12–13 days, but the cells mature in a disorganized environment that is permissive for cell interaction. To reduce the inhibitory effects exerted by negative regulatory factors (including TRAIL) released by erythroblasts as they mature [16, 17], cell interactions are minimized in vitro by maintaining the cells at densities <106 cells per milliliter. Therefore, production of 2.5 × 1012 RBC requires >2,500 l of culture media and growth factors in milligram quantities [14]. A conservative estimate indicates that the cost to produce a unit of blood ex vivo is between $8,000–15,000 versus the current cost of $200–230 for a unit of donated blood and $700–1,200 for a unit of phenotypically matched blood.
Proof-of-Principle In Animal Models and First-in-Human Transfusion of Cultured RBC
The functionality of cultured erythrocytes once injected in the bloodstream was first proved by Nakamura and coworkers, who showed that RBC obtained from an immortalized embryonic stem cell (ESC) line can protect mice from lethal anemia in a model of transfusion [18]. Recently, Douay and coworkers have provided the first proof-of-principle in man for the use of ex vivo-expanded RBC as transfusion product [19]. In this study, 10 million RBCs (the equivalent of 2 ml of blood) were generated ex vivo under good manufacturing practice (GMP) conditions from CD34pos cells obtained by apheresis from an adult volunteer. The survival in vivo of these expanded autologous RBC labeled with 51Cr (the only method accepted by the U.S. Food and Drug Administration) was comparable to that of native RBC.
In addition to RBC, it is conceivable that cells generated at the end of earlier phases of the production process may be considered as transfusion products (Fig. 1). The Douay laboratory had previously established that ex vivo produced human erythroblasts fully mature into RBC when injected into immunocompromised animal models [20]. This study not only demonstrated that nucleated erythroid cells may undergo terminal differentiation in vivo but also provided a proof-of-principle that erythroblasts may represent an alternative transfusion product (Fig. 1). Preliminary support for this possibility is also provided by the clinical observation that 40–80 ml of matched cord blood (CB), which contains 4–8 × 1010 RBCs plus ~4–8 × 107 erythroblasts, is successfully used for occasional transfusions in developing countries [21]. Transfusion of nucleated erythroblasts, which may proliferate and generate at least 4–64 additional cells before differentiation into enucleated RBC in vivo could not treat acute blood losses but could be beneficial for chronically anemic patients (such as patients with hemoglobinopathies) because the frequency of transfusion would be reduced (these RBC would be younger than RBC in a unit of blood and survive longer in vivo) and reduce, rather than increase, iron overload. In addition, the transfusion would be effective with lower numbers of cells (1/64 of 2.5 × 1012 i.e., ~4 × 1010) reducing the costs and technical challenges of production (GMP facilities to produce 4 × 1010 RBC already exists). However, since transfusion of nucleated cells poses potential leukemogenic risks, these products should be extensively evaluated for genotoxicity.
Resolution of Scientific and Logistic Barriers
The identification of the order in which the scientific and logistic barriers should be overcome will play an important role in determining the success with which this innovative idea will be translated into clinical practice. These priorities are summarized below.
Choosing the Best Stem Cell Source to Derive Cultured RBC
One of the most important aspects of RBC production ex vivo is the source of stem cells. The ideal stem cell source should be available as discarded material, have unlimited availability, be suitable to attain full erythroid maturation, and not be immunogenic. CB is a source of HSC/HPC which is currently largely discarded. A small percent of CB is collected for HSC transplantation [22]. CB units below the volume threshold required for transplantation (>90 ml) are currently discarded. These units are candidates for ex vivo expansion of RBC because each unit may theoretically generate 10–75 RBC products [23]. Therefore, in principle, CB-generated RBC could supplement blood with rare phenotypes used for hemoglobinopathic patients which represent ~1% of transfused units (130,000 transfusions per year in the U.S.). Unfortunately, these low volume CBs are presently not stored and their blood group phenotype unknown.
The RBCs used for first-in-human administration were derived from autologous CD34pos cells mobilized by granulocyte-colony stimulating factor (G-CSF). Although G-CSF mobilization is routinely performed to mobilize HSC/HPC for transplantation and is currently used by some blood centers to increase the numbers of granulocytes harvested for transfusion to neutropenic patients with serious infections, it may pose unacceptable donor risks for routine RBC production. The leukoreduction byproducts (buffy coats) of adult blood (AB) donations are additional sources of HSC/HPC currently discarded. When cultured in humanized media, that is, media formulated with clinical grade culture components, buffy coats generate as many RBC as the HSC/HPC contained in low volume CB [24]. This observation suggests that buffy coats discarded during blood processing may be used to generate additional transfusion products from donors with rare blood phenotypes, reducing the overall number of donations from these generous individuals.
ESCs and, more recently, induced pluripotent stem cells (iPSCs) have been investigated as potential sources for the generation of universal ORh negative RBC (Fig. 1). ESCs were shown to be capable of producing mature functional erythrocytes on a large scale [25]. Lately, iPSC obtained from human fetal and adult fibroblasts [26] and from neonatal fibroblasts [27] were used to produce RBC in vitro. Despite their advantage of unrestrained replication, both ESC- and iPSC-derived erythroid progenitors had low proliferation rates compared with CB-derived cells and suboptimal levels of erythroid enucleation. It has been calculated that HSCs derived from human ESC (hESC) and iPSC generate 500 erythroblasts each versus 104–105 erythroblasts generated by one CB/AB-derived CD34pos cell. Thus, production of a transplantation product from hESC and iPSC requires enormous amplification of the hESC and iPSC themselves. Regardless of these limitations, iPSC may be generated from any donor and represent a promising stem cell source to produce RBC for transfusion of alloimmunized patients. In fact, it has been calculated that iPSC from only three rare blood donors would be sufficient to match more than 99% of alloimmunized patients in need of RBC transfusions in France, suggesting that iPSC banking could represent an unlimited source for RBC production [23]. Notably, both ESC- and iPSC-derived erythroid cells expressed mostly embryonic and fetal globins [28–31]. This observation suggests that RBC derived from genetically uncorrected iPSC may represent an alternative autologous transfusion product for patients with hemoglobinopathies.
In view of these promising future applications, the procedures used to derive RBC from iPSC urgently need to be optimized in order to reach clinical standards. However, even with optimized experimental procedures, the large-scale generation of RBC directly from iPSC/ESC will be a costly and time-consuming process. One possible solution to this problem is to generate EPC lines from ESC or iPSC by epigenetic/genetic treatment, which are able to massively differentiate into mature RBC. These cell lines would be equivalent to the megakaryocytic cell lines recently derived from human ESC capable to generate massive numbers of platelets ex vivo [32]. Another interesting possibility allowed by advanced reprogramming technology is to reprogram any somatic cell directly into erythroblasts by-passing the pluripotent state by over-expression of suitable genes. A successful example of this approach is represented by the recent demonstration that over-expression of p45NF-E2/Maf turns human fibroblasts into megakaryocytes [33].
Unexpected sources for ex vivo RBC production may alternatively come from differentiated erythroid cells endowed with high proliferative ability. Recently, a population of self-renewing erythroblasts was discovered in early mouse embryos, and it was shown to possess self-renewal abilities and extensive proliferative capacity ex vivo [34]. In principle, a human homolog of this population may represent an unlimited cell source to produce transfusable RBCs ex vivo. The identification of these cells, however, would involve studies on human embryos that are subjected to ethical limitations. Since the mechanisms of erythroid cell development are well conserved between mice and men, it is conceivable that studies on embryonic murine erythroblasts may lead to the identification of genes that maintain these cells in a self-renewal state and that over-expression of these genes may confer self-renewal ability also to human erythroblasts expanded ex vivo from currently discarded primary stem cell sources (AB and CB).
A caveat of these technologies is the tendency of immortalized cells to acquire karyotypic abnormalities. In principle, this should not represent a problem as mature RBCs do not contain genetic material. In addition, the same procedures (leukoreduction plus radiation) developed to prevent alloimmunization to human leucocyte antigen (HLA) present on leukocytes and graft versus host disease after transfusion may assure that ex vivo-generated RBCs are completely devoid of nucleated elements capable of proliferation [19] (although irradiation reduces RBC shelf life). However, eventual karyotypic abnormalities acquired by the stem cell source may be reflected in their progeny as alterations of the blood group antigen profile, making genotoxicity testing of the stem cell sources an important safety control for ex vivo-generated transfusion products. In addition to alterations in blood group antigen profile, ex vivo-generated RBC may acquire neoantigens if proteins present in the culture media adhere to their surface.
Quantitative and Qualitative Issues in RBC Production
Generating sufficient amounts of RBC is probably the first technical obstacle to overcome to turn transfusion with ex vivo-expanded RBC into a reality. This barrier has several facets. The growth factors used to stimulate the culture such as SCF (which has been recently shown to induce massive erythroblast expansion through Notch activation [35]) and glucocorticoids play an interrelated role in regulating erythroblast expansion and the concentration/time of administration with which they should be used in culture to obtain optimal expansion is probably still to be determined. In addition, it has been recently appreciated that the gene encoding the human glucocorticoid receptor is highly polymorphic and may encode up to 260 isoforms of the receptor each one with a different transcriptional activity [36]. More studies are required to understand how expression of these different isoforms affects erythroblast expansion and whether the polymorphismic state of this gene may predict the potency of a donor to generate erythroblasts in vitro [14].
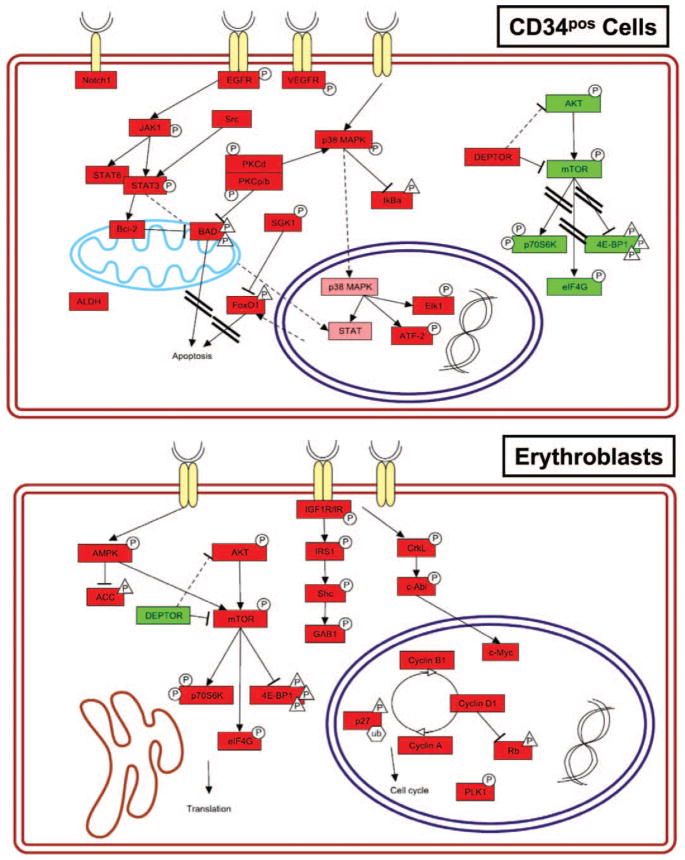
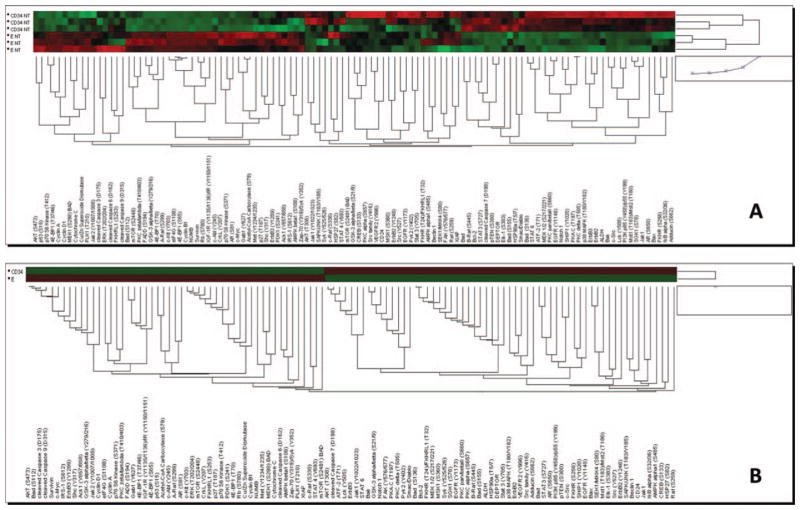
A molecular characterization of the signaling pathways activated during erythroid differentiation may indicate novel approaches to promote erythroblast expansion. Reverse-phase proteomic analysis of CB-derived erythroblasts compared with CD34pos progenitors is presented in Figures 2 and 3. These analyses reveal that the pathways prevalently active in CD34pos cells are those involved in inhibition of apoptosis, transcription, and proliferation while those more active in erythroblasts are those involved in cell cycle and transcription/translation. In addition, the low levels of mitochondrial antiapoptotic mediators expressed that characterize the erythroblasts may make these cells more prone to apoptotic stimuli. The unique picture of positive- and negative-feedback interactions between these pathways, which characterize the erythroblasts, are likely to determine the specificity of the response of these cells to external growth stimuli. It is foreseen that pharmacological agents capable to specifically activate erythroid-specific proliferation pathways and/or inhibit apoptosis may be successfully used in future implementation of large-scale erythroid cultures.
Figure 2.
Signaling pathways activated in cord blood-derived CD34pos hematopoietic progenitor cells (upper panel) and in erythroblasts at day 6 of differentiation (erythroblasts, lower panel). Selected statistically significant proteins are shown. Phosphorylated and total proteins were distinguished as hyperphosphorylated/-expressed (red) and hypophosphorylated/-expressed (green). The shape of the phosphorylations symbols differs for activating (circle) and inhibitory (triangle) phosphorylations. Signaling pathway representation was made with PathVisio v2.0. Nonparametric statistical analysis (Wilcoxon rank sum test) was performed with Jump v5.1 (SAS Institute, Cary, NC) using endpoints relative intensity values. For Wilcoxon analysis, all significant levels were set at p < .05 (for technical details see [37]).
Figure 3.
Unsupervised hierarchical clustering of cord blood-derived CD34pos hematopoietic progenitor cells versus erythroblasts at day 6 of differentiation of all the endpoints examined in reverse-phase proteomic analysis (A—separate triplicates and B—triplicate averages). Unsupervised hierarchical clustering was performed with Jump v5.1 using endpoints relative to intensity values (for technical details see [38]).
It is also possible that each phase of the RBC production process may require for optimal growth a specifically formulated media, as exemplified by the recent studies on the effects of iron on erythropoiesis. It has been recently demonstrated that the type 2 transferrin receptor (which regulates hepcidin production by liver cells) is a component of the EPO-R complex and is required for efficient erythropoiesis [39]. This evidence indicates that iron-regulatory proteins contribute more directly than previously thought to the regulation of erythroid cell production and that different iron concentrations may promote erythroblast expansion and maturation [40]. In vivo, the macrophage adjusts the iron supply for the stage of erythroblast maturation. Whether optimal expansion in vitro is dependent on modulation of the media iron concentration as the cells mature and by further addition of key regulators of iron homeostasis, such as hepcidin, is still to be determined.
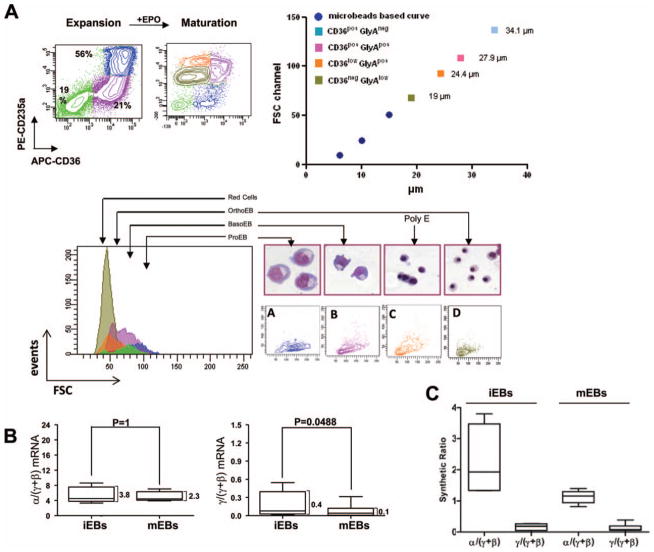
To be considered suitable for transfusion, the quality of in vitro-generated RBC should be defined in terms of antigenic profile, hemoglobin content, and physical properties. Many of these issues have been already outlined (http://www.clinicaltrials.gov/ct2/show/NCT00929266) and preliminary data on RBC expanded from two donors of Caucasian descendence are available [19]. Extensive characterization of the physical properties and hemoglobin content of erythroid cells expanded from 19 Caucasian donors have also been published [14, 41]. Although ex vivo-generated erythroblasts are large (they range in size from 30 to 50 μm), they significantly reduce their diameter upon exposure to EPO, going from 40.1 6 ± 1.4 to 11.6 6 0.× μm after 96 hours of culture in EPO (Fig. 4A). The size of ex vivo-generated RBC is similar to that of in vivo-generated cells although they remain slightly macrocytic. These ex vivo-generated erythroid cells express great levels of alpha hemoglobin stabilizing protein (AHSP), BCL11A, and globin genes with high donor-to-donor heterogeneity [42]. Although α-globin was preferentially expressed in immature erythroblasts, the α/(γ+β) globin ratio nearly normalized during maturation (Fig. 4B). The presence of AHSP likely compensates for the preferential accumulation of α-globin. Despite a modest decrease in the γ/(γ+β) mRNA and synthetic ratio with maturation (Fig. 4C), ex vivo-generated immature and mature erythroblasts contain γ-globin in slightly greater amounts than RBC generated in vivo (0.12–0.20 pg per cell with respect to a total protein content of 18.7–23.7 per cell) [42].
Figure 4.
In vitro maturation of human erythroid cells. (A): Upon exposure to EPO for 4 days, human erythroblasts generated from peripheral blood mononuclear cells undergo a synchronous maturation characterized by increased expression of Glycophorin A (CD235a), loss of CD36 (the thrombospondin receptor) expression, decrease in cell size, and morphologic changes including nuclear condensation. (B): At the mRNA level, iEBs and mEBs generated in cultures express excessive α-globin mRNA (α/[γ+β] mRNA ratio >2) and high levels of γ/(γ+β) ratio. With maturation, the α/(γ+β) ratio remains unbalanced while the γ/(γ+β) ratio is significantly reduced. (C): At the protein level, with maturation, the α/(γ+β) globin chain synthetic ratio becomes approximately balanced and the γ/(γ+β) ratio is reduced. Modified and reproduced by permission from [14] and [42]. Abbreviations: EPO, erythropoietin; FSC, forward scatter; iEB, immature erythroblast; mEB, mature erythroblast.
Extensive changes in the structure and properties of the plasma membrane occur during erythroblast maturation and during the transition from reticulocyte to erythrocyte [43]. Understanding membrane biogenesis during erythropoiesis is crucial to develop biomarkers to monitor the production process. A set of surface antigens that identifies distinct erythroid cell populations in mice have been defined [44]. Although these markers do not identify the corresponding human cells, it is conceivable that comparable markers for human cells exist and that their identification may lead to the development of quality control biomarkers for RBC production ex vivo.
The transmembrane complexes embedded in the lipid bilayer are essential to RBC functions and deformability. Numerous clinically relevant blood group antigens are present on these complexes [43]. Therefore, studies of blood group antigen expression on ex vivo-expanded RBC provide insights both on membrane structure and on potential immunogenicity. Anstee and his colleagues have been studying the genetic and biochemical basis of blood group antigen expression in erythroid cells generated ex vivo for many years. More recently, this group has reported that appropriate levels of expression of band 3 and Rh-associated glycoprotein on the RBC surface is assured by a physical interaction established at the beginning of the maturation process [45].
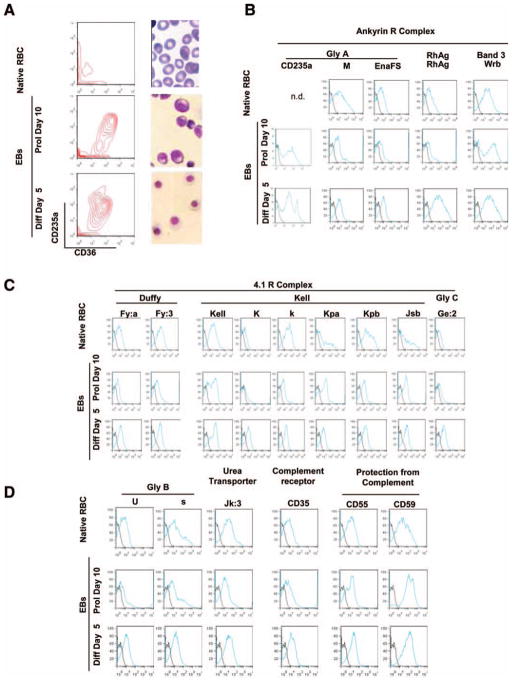
Studies on RBC antigen profiles of erythroblasts-expanded ex vivo are often performed on cells expanded from Caucasian donors. However, in the U.S., phenotypically matched donors for sickle cell anemia patients are most likely to be African Americans. Blood centers are starting to pay attention to the hypothesis that the ethnicity of the donor may affect the yields of neutrophil and progenitor cells collected by apheresis [46]. However, little attention is paid on a possible effect of ethnicity on the properties of RBC generated ex vivo. We have characterized the ability of mononuclear cells (MNC) from one donor of African American descent to generate erythroid cells in culture. Over three separate cultures, these MNC generated great numbers of erythroid cells (the fold increase by day 14 was 30 vs. fold increases of 1.7–30 from 26 different donors of Caucasian descent) [47]. The blood group antigen profile of RBC generated ex vivo in three separate cultures from this donor was reproducible and consistent with the profile predicted by DNA-based genotyping. The expression of these antigens on in vivo- and ex vivo-generated erythroid cells is compared in Figure 5. Ex vivo-expanded cells expressed normal levels of the antigens present on both the Ankyrin R (GPA, M/N, EnaFS, RhAG, and band 3) and 4.1R (Duffy, Kell, and GPC) complexes as well as antigens located on Glycophorin B, the Urea transporter, the complement receptor, and receptors that protect RBC from complement-mediated lysis. Although encouraging, additional studies on a larger number of donors of multiple ethnicities are necessary to assess whether the genetic program of the stem cell source (primary or epigenetically/genetically reprogrammed stem cells) and/or the culture components alter the blood group antigen expression of the ex vivo-expanded RBC leading to unpredictable immunogenicity.
Figure 5.
Blood group antigen expression of RBCs from the blood of an African-American donor (native RBCs) and of erythroid cells generated ex vivo from the same donor. (A): CD36/CD235a profiling and May-Grunwald staining of the native RBCs and of the erythroblasts generated in 10-day of culture (Prol day 10) and induced to mature with erythropoietin for 5 days (Diff day 5). (B): Flow cytometry analyses of the various cell types using antibodies provided by The New York Blood Center recognizing antigens present on proteins on (B) the Ankyrin R (glycophorin A [GPA, CD235a, M, and EnaFS], RhAG, and band 3 [Wrb]) and (C) the 4.1R (Duffy [Fya and Fy3], Kell [Kell prot, K/k, Kpa/Kpb, and Jsb], and glycophorin C [GPC and Ge2]) complexes, and (D) on other important membrane proteins (glycophorin B [GPB, s, and U], urea transporter [Kidd and Jk3], the complement receptor [CD35], and inhibitors of complement-mediated lysis [CD55 and CD59]) (see also [41]). Abbreviations: EB, erythroblast; RBC, red blood cell.
Achieving Complete RBC Maturation Ex Vivo
The last stages of erythroid maturation proceed through erythroblast enucleation with subsequent formation of a reticulocyte, followed by a step of extensive membrane remodeling and autophagic elimination of organelles to yield the mature RBC [48]. Reticulocytes are motile cells with a relatively rigid membrane; through final maturation, they lose volume and surface area and acquire the flexibility that allows their passage through the narrowest vessels (Fig. 1). Multiple pathways including chromatin condensation, actomyosin motors, and vesicle trafficking act in concert to ensure RBC terminal maturation, and a correct accomplishment of such processes is instrumental to obtain functional RBC [49].
In the process of RBC generation ex vivo, achieving high levels of erythroblast enucleation is considered an important requirement. However, enucleation remains one of the rate-limiting steps of ex vivo RBC production. While 60% enucleation has been achieved in ESC-derived erythroid cultures [25], in iPSC-derived erythroid cultures, the percentage of enucleation was only 4%–10% [26]. Therefore, it seems necessary to elucidate the mechanisms that underlie the process of erythroblast enucleation in order to devise pharmacological strategies for increasing the efficiency of this process in vitro. Recent insights into the events that drive terminal erythroid maturation have shown that histone deacetylases (and specifically the HDAC2 isoform) are required for chromatin condensation and enucleation in mouse fetal erythroblasts [50]. The fact that glucocorticoids are strong inhibitors of HDAC2 [51] may explain the poor enucleation efficiency of human erythroblasts generated in phase 2 cultures and suggests that nuclear condensation and enucleation of erythroid cells generated in the presence of glucocorticoids may be improved by adding HDAC activators in phase 3 cultures.
The use of stromal cells in phase 3 cultures introduces a poorly defined source of potentially immunogenic and/or infectious agents posing an additional challenge to the development of GMP RBC production processes. Hence, there is an active search to identify biochemical substitutes for these cell lines. The first compounds to be described to sustain efficient erythroblast enucleation in the absence of feeder layers were mifepristone and plasmanate [52]. More recently, factors that promote the functionality of proteins involved in vesicle trafficking, such as vacuolin-1, have been shown to increase the percentage of enucleated cells [53].
The Ultimate Challenge: Identification of Clinical Applications Achievable with Current Technologies
Presently, the greatest challenge in Transfusion Medicine is to identify adequate financial support to develop programs to increase blood donations in low-income countries and to establish and maintain blood processing/storage facilities. Production of RBC ex vivo for transfusion is presently an unrealistic and expensive proposition even in industrialized countries. To maintain momentum in the field of ex vivo RBC expansion, it is important to identify intermediate clinical goals for these cells achievable with current technologies.
Examples of applications currently achievable are reagent RBCs for antibody identification in alloimmunized patients and drug delivery for personalized therapy [14]. Reagent RBC are RBC obtained from highly selected donors (often paid) with rare blood group phenotypes/genotypes. These RBCs are incorporated in diagnostic kits/devices used to identify alloimmunized individuals (both patients and blood donors) and to determine the specificity of the antibodies detected [4]. Although these assays can be performed with RBC from several milliliters of blood, periodic shortages of reagents RBC occur because donors may be unavailable or are temporarily/permanently deferred from donation. Since only ~2 × 108 RBCs will be sufficient to perform 100 of these assays and 109–1010 RBC can be expanded ex vivo with current technology from the buffy coat of as little as 25 ml of blood, RBC-expanded ex vivo from the buffy coat of an entire rare blood donation (500 ml) would provide sufficient amounts of reagent red cells for alloantibody identification in the U.S. for 1 year.
The potential to use RBC as a vehicle for targeted drug delivery has been considered for some time. The recent improvements in retroviral technology have opened the possibility to use RBC derived from genetically modified HSC/HPC as a vehicle for therapeutic recombinant proteins. Proof-of-principle for the use of RBCs for systemic drug delivery was obtained in mouse models for Hemophilia, an X-linked recessive congenital disorder of coagulation due to factor VIII or IX deficiency [54]. It is conceivable that transfusion of RBCs expanded in vitro from autologous CD34pos cells might be a feasible option to achieve therapeutic levels of factor IX also in Hemophilia patients. It is foreseen that progress in these intermediate clinic applications will assure that the ultimate goal of ex vivo-expanded RBC, transfusion, will be achieved.
The amount of RBC required for phase 1 safety studies (the equivalent of 2–5 ml of blood) is achievable with current technologies. The first-in-human administration in an autologous setting was registered with the NIH (http://www.clinicaltrials.gov/ct2/show/NCT00929266) [19]. Additional small-scale clinical trials in low-risk situations are required to submit the investigational new drug (IND) application that would pave the way for more extensive clinical studies. The next step toward the clinical application of ex vivo-generated RBC for transfusion is therefore a first-in-human administration in an allogenic setting in normal volunteers and eventually in selected low-risk patient populations. These trials may require only local Institutional Review Board approval but must be registered on clinicaltrials.gov. Studies with small numbers of transfusion recipients using 2–5 ml of blood will provide preliminary information on safety but cannot address efficacy. These studies will determine in vivo cell survival and address issues of immunogenicity. Alloimmunized individuals currently donating blood as a source of antiserum for blood group antigen typing may represent a motivated normal population to volunteer for the first allogenic transfusion study because they may be occasionally challenged (where permitted) with incompatible blood to boost antibody production. The identification of the low-risk patient population for the first safety study requires additional considerations. To be eligible for the study, the patient should be anemic and scheduled for an elective transfusion. The study may be designed as comparison of in vivo survival of 5 ml of ex vivo-expanded RBC transfused concurrently with a unit of blood from the same donor. Techniques to label the two cell types with different tracers are available. Although alloimmunized sickle cell anemia patients and patients with other hemoglobinopathies are those most likely to volunteer for these clinical studies because they are more aware of the potential importance of the information to be gained, they may not be included in these initial studies because of their susceptibility to hyperhemolysis syndrome [55]. Adult patients undergoing transfusion for other reasons (chronic anemia unresponsive to EPO) may represent better candidates for initial studies. Once the IND is submitted and approved, it will be possible to conduct the first clinical trials to assess efficacy. The most important criterium in the selection of the patient population for this study is body weight. Although infants are considered to be a vulnerable population, they may benefit from transfusion of 5–10 ml/kg of blood. Safety and feasibility study to assess the efficacy of CB for transfusion in small children with cerebral palsy or undergoing arterial switch operations in the first hours of life have been performed [56, 57]. Therefore, a trial in a carefully chosen clinical condition requiring chronic transfusions in pediatric patients may be able to assess the efficacy of transfusion with the small volumes of blood that can be produced with current technology. At this point, critical for the advancement of the field will be the identification of the appropriate conditions suited for the clinical trial.
Acknowledgments
We thank the assistance of Drs. B. Ghinassi for flow cytometry determination, G. Hashmi for blood group antigen genotyping with the HEA-Bead Chip Immunocor, and G. Halverston for providing blood group antigen-specific antibodies. This study was supported by a grant from the Italian “Centro Nazionale Sangue” and by institutional funds from the Istituto Superiore Sanità.
Footnotes
Author contributions: A.Z., F.M., S.V., G.F., C.W., and A.R.M.: wrote and reviewed the manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
References
- 1.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–2626. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Report of the US Department of Health and Human Services. The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: Services, T.U.D.o.H.a.H; 2010. [Google Scholar]
- 3.Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion. 2010;50:584–588. doi: 10.1111/j.1537-2995.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 4.Zimring JC, Welniak L, Semple JW, et al. Current problems and future directions of transfusion-induced alloimmunization: Summary of an NHLBI working group. Transfusion. 2011;51:435–441. doi: 10.1111/j.1537-2995.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papayannopoulou Th, Abkowitz J, D’Andrea A, et al. Biology of erythropoiesis, erythroid differentiation and maturation. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, Heslop H, editors. Hematology: Basic Principles and Practice. 5. Philadelphia, PA: Elsevier; 2009. pp. 276–299. [Google Scholar]
- 6.Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol. 2005;16:137–147. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.De Maria R, Zeuner A, Eramo A, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- 8.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- 9.Peschle C, Testa U, Valtieri M, et al. Stringently purified human hematopoietic progenitors/stem cells: Analysis of cellular/molecular mechanisms underlying early hematopoiesis. Stem Cells. 1993;11:356–370. doi: 10.1002/stem.5530110503. [DOI] [PubMed] [Google Scholar]
- 10.Fibach E, Manor D, Oppenheim A, et al. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989;73:100–103. [PubMed] [Google Scholar]
- 11.Panzenbock B, Bartunek P, Mapara MY, et al. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658–3668. [PubMed] [Google Scholar]
- 12.von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- 13.Migliaccio G, Di Pietro R, di Giacomo V, et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio AR, Whitsett C, Migliaccio G. Erythroid cells in vitro: From developmental biology to blood transfusion products. Curr Opin Hematol. 2009;16:259–268. doi: 10.1097/MOH.0b013e32832bcaa2. [DOI] [PubMed] [Google Scholar]
- 15.Chasis JA, Mohandas N. Erythroblastic islands: Niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 17.Migliaccio G, Masiello F, Tirelli V, et al. Under HEMA conditions, self-replication of human erythroblasts is limited by autophagic death. Blood Cells Mol Dis. 2011;47:182–197. doi: 10.1016/j.bcmd.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Hiroyama T, Miharada K, Sudo K, et al. Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS One. 2008;3:e1544. doi: 10.1371/journal.pone.0001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giarratana MC, Rouard H, Dumont A, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neildez-Nguyen TM, Wajcman H, Marden MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 21.Ende M, Ende N. Hematopoietic transplantation by means of fetal (cord) blood. A new method. Va Med Mon (1918) 1972;99:276–280. [PubMed] [Google Scholar]
- 22.Broxmeyer HE. Umbilical cord transplantation: Epilogue. Semin Hematol. 2010;47:97–103. doi: 10.1053/j.seminhematol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyrard T, Bardiaux L, Krause C, et al. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: Potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011;25:206–216. doi: 10.1016/j.tmrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio G, Sanchez M, Masiello F, et al. Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 2010;19:453–469. doi: 10.3727/096368909X485049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiroyama T, Miharada K, Kurita R, et al. Plasticity of cells and ex vivo production of red blood cells. Stem Cells Int. 2011;2011:195780. doi: 10.4061/2011/195780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KH, Nelson AM, Fields PA, et al. Diverse hematopoietic potentials of five human embryonic stem cell lines. Exp Cell Res. 2008;314:2930–2940. doi: 10.1016/j.yexcr.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang KH, Huang A, Hirata RK, et al. Globin phenotype of erythroid cells derived from human induced pluripotent stem cells. Blood. 2010;115:2553–2554. doi: 10.1182/blood-2009-11-252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CJ, Mitra K, Koya M, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS One. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papapetrou EP, Lee G, Malani N, et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29:73–78. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura S, Takayama N, Hiromitsu N, et al. Platelet production system using an immortalized magakaryoctic cell line derived from human pluripotent stem cells. Blood. 2011;118:3a. [Google Scholar]
- 33.Wang Y, Ono Y, Ikeda Y, et al. Induction of megakaryocytes from fibroblasts by p45NF-E2/Maf. Blood. 2011;11:908a. [Google Scholar]
- 34.England SJ, McGrath KE, Frame JM, et al. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeuner A, Francescangeli F, Signore M, et al. The Notch2-Jagged1 interaction mediates stem cell factor signaling in erythropoiesis. Cell Death Differ. 2011;18:371–380. doi: 10.1038/cdd.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Cidlowski JA. The human glucocorticoid receptor: One gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.VanMeter A, Signore M, Pierobon M, et al. Reverse-phase protein microarrays: Application to biomarker discovery and translational medicine. Expert Rev Mol Diagn. 2007;7:625–633. doi: 10.1586/14737159.7.5.625. [DOI] [PubMed] [Google Scholar]
- 38.Van Iersel MP, Kelder T, Pico AR, et al. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forejtnikova H, Vieillevoye M, Zermati Y, et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010;116:5357–5367. doi: 10.1182/blood-2010-04-281360. [DOI] [PubMed] [Google Scholar]
- 40.Keel SB, Doty RT, Yang Z, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 41.Ghinassi B, Themeli M, Chang K-H, et al. Comparative blood group profiling of human erythroid cells (EBs) generated from adult blood (AB), Cord Blood (CB), human embryonic stem cells (hESC) and induced pluripotent stem cells (iPS) Blood. 2011;118:475. (travel award) [Google Scholar]
- 42.Varricchio L, Fabucci ME, Alfani E, et al. Compensated variability in the expression of globin-related genes in erythroblasts generated ex vivo from different donors. Transfusion. 2010;50:672–684. doi: 10.1111/j.1537-2995.2009.02483.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Mohandas N, An X. Membrane assembly during erythropoiesis. Curr Opin Hematol. 2011;18:133–138. doi: 10.1097/MOH.0b013e32834521f3. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Liu J, Heck S, et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satchwell TJ, Bell AJ, Pellegrin S, et al. Critical band 3 multiprotein complex interactions establish early during human erythropoiesis. Blood. 2011;118:182–191. doi: 10.1182/blood-2010-10-314187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carilli AR, Sugrue MW, Rosenau EH, et al. African American adult apheresis donors respond to granulocyte-colony-stimulating factor with neutrophil and progenitor cell yields comparable to those of Caucasian and Hispanic donors. Transfusion. 2012;52:166–172. doi: 10.1111/j.1537-2995.2011.03253.x. [DOI] [PubMed] [Google Scholar]
- 47.Tirelli V, Masiello F, Sanchez M, et al. Effects of ontogeny, ethnicity, gender and loss of companion cells on ex-vivo expansion of erythroid cells for transfusion. Blood. 2011;118:563. [Google Scholar]
- 48.Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keerthivasan G, Wickrema A, Crispino JD. Erythroblast enucleation. Stem Cells Int. 2011;2011:139851. doi: 10.4061/2011/139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji P, Yeh V, Ramirez T, et al. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95:2013–2021. doi: 10.3324/haematol.2010.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li LB, Leung DY, Martin RJ, et al. Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma. Am J Respir Crit Care Med. 2010;182:877–883. doi: 10.1164/rccm.201001-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miharada K, Hiroyama T, Sudo K, et al. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 53.Keerthivasan G, Small S, Liu H, et al. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116:3331–3340. doi: 10.1182/blood-2010-03-277426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol. 2006;24:1017–1021. doi: 10.1038/nbt1227. [DOI] [PubMed] [Google Scholar]
- 55.Win N, New H, Lee E, et al. Hyperhemolysis syndrome in sickle cell disease: Case report (recurrent episode) and literature review. Transfusion. 2008;48:1231–1238. doi: 10.1111/j.1537-2995.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 56.Chasovskyi K, Fedevych O, Vorobiova G, et al. Arterial switch operation in the first hours of life using autologous umbilical cord blood. Ann Thorac Surg. 2012 doi: 10.1016/j.athoracsur.2012.01.104. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Papadopoulos KI, Low SS, Aw TC, et al. Safety and feasibility of autologous umbilical cord blood transfusion in 2 toddlers with cerebral palsy and the role of low dose granulocyte-colony stimulating factor injections. Restor Neurol Neurosci. 2011;29:17–22. doi: 10.3233/RNN-2011-0572. [DOI] [PubMed] [Google Scholar]