Abstract
Context
A triple-marker approach for chronic kidney disease (CKD) evaluation has not been well studied.
Objective
To evaluate whether combining creatinine, cystatin C, and urine albumin-to-creatinine ratio (ACR) would improve identification of risks associated with CKD compared with creatinine alone.
Design, Setting, and Participants
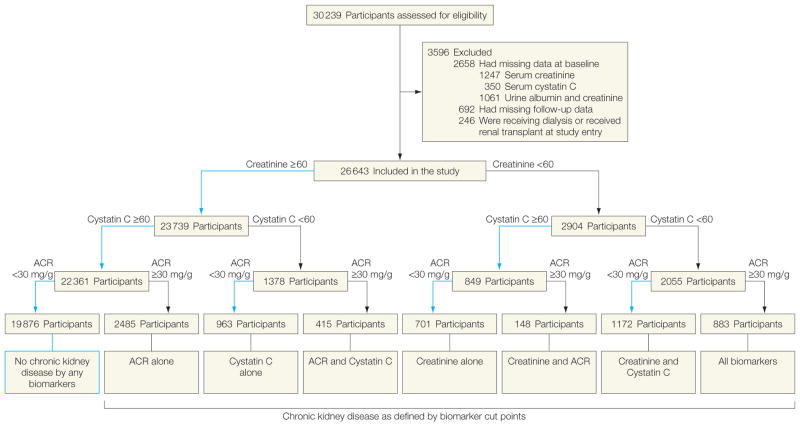
Prospective cohort study involving 26 643 US adults enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study from January 2003 to June 2010. Participants were categorized into 8 groups defined by estimated glomerular filtration rate (GFR) determined by creatinine and by cystatin C of either <60 or ≥60 mL/min/1.73 m2 and ACR of either <30 or ≥30 mg/g.
Main Outcome Measures
All-cause mortality and incident end-stage renal disease with median follow-up of 4.6 years.
Results
Participants had a mean age of 65 years, 40% were black, and 54% were women. Of 26 643 participants, 1940 died and 177 developed end-stage renal disease. Among participants without CKD defined by creatinine, 24% did not have CKD by either ACR or cystatin C. Compared with those with CKD defined by creatinine alone, the hazard ratio for death in multivariable-adjusted models was 3.3 (95% confidence interval [CI], 2.0–5.6) for participants with CKD defined by creatinine and ACR; 3.2 (95% CI, 2.2–4.7) for those with CKD defined by creatinine and cystatin C, and 5.6 (95% CI, 3.9–8.2) for those with CKD defined by all biomarkers. Among participants without CKD defined by creatinine, 3863 (16%) had CKD detected by ACR or cystatin C. Compared with participants who did not have CKD by any measure, the HRs for mortality were 1.7 (95% CI, 1.4–1.9) for participants with CKD defined by ACR alone, 2.2 (95% CI, 1.9–2.7) for participants with CKD defined by cystatin C alone, and 3.0 (95% CI, 2.4–3.7) for participants with CKD defined by both measures. Risk of incident end-stage renal disease was higher among those with CKD defined by all markers (34.1 per 1000 person-years; 95% CI, 28.7–40.5 vs 0.33 per 1000 person-years; 95% CI, 0.05–2.3) for those with CKD defined by creatinine alone. The second highest end-stage renal disease rate was among persons missed by the creatinine measure but detected by both ACR and cystatin C (rate per 1000 person-years, 6.4; 95% CI, 3.6–11.3). Net reclassification improvement for death was 13.3% (P<.001) and for end-stage renal disease was 6.4% (P<.001) after adding estimated GFR cystatin C in fully adjusted models with estimated GFR creatinine and ACR.
Conclusion
Adding cystatin C to the combination of creatinine and ACR measures improved the predictive accuracy for all-cause mortality and end-stage renal disease.
Chronic kidney disease (CKD) is currently defined as a creatinine-based estimated glomerular filtration rate (GFR) of less than 60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio (ACR) of 30 mg/g or higher.1 Clinical laboratories are routinely reporting estimated GFR, and electronic medical records often alert clinicians to the presence of CKD on this basis alone.2 Because routine assessment of the ACR is only recommended for persons with diabetes,3 initial CKD detection in routine practice is primarily limited to serum creatinine testing.
Chronic kidney disease is associated with increased risk of adverse outcomes, including death, cardiovascular events, and the development of end-stage renal disease.4,5 Serum creatinine levels are affected by muscle mass, age, and race,6 and estimated GFRs are less reliable for assessing renal function when GFR is more than 60 mL/min2.7 Therefore, current practice and staging systems based primarily on serum creatinine may mis-classify individuals when assessing these risks.8 Alternative methods have been suggested to improve detection and classification of CKD, including improved estimated GFR equations9 and the combination of categories of ACR and creatinine-based estimated GFR.10,11 These approaches have not yet been adopted in international guidelines.12 Another available tool to detect kidney disease is serum cystatin C, an alternative biomarker of kidney function that is a better predictor of death and cardiovascular events than creatinine and is also less affected by age, race, or muscle mass.13,14 Although testing for cystatin C is available in the United States, it is not routinely used in clinical practice.
Despite these advances in kidney disease evaluation, a triple-marker approach for the detection and classification of CKD using creatinine, cystatin C, and ACR has not been well evaluated. This is an important and timely research question because guidelines for the evaluation and staging of CKD are currently being revised with the explicit objective of developing staging systems that accurately reflect prognosis for CKD complications.15–18 We designed this study to evaluate the yield of adding CKD definitions based on ACR and cystatin C to forecast risk compared with a CKD definition using creatinine-based estimates alone. We hypothesized that cystatin C and albuminuria would add complementary risk information among persons with and without CKD, as defined by creatinine-based estimated GFR (GFRcreatinine).
METHODS
Subjects
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a large, population-based cohort study originally designed to identify factors that contribute to the excess stroke mortality in the stroke belt of the United States and to the excess stroke risk of black Americans.19 REGARDS recruited black and white participants who were 45 years or older, beginning January 2003. Participants were randomly sampled and were recruited by mail and then by telephone, followed by an in-home visit. Participants who were free of cancer and, at the time of the initial telephone call were able to answer the questions and were not living in an assisted living home, were included in the study. By design, approximately 50% of the sample was recruited in North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas (the stroke belt states). The other 50% were recruited from the remaining 40 continental states.
Kidney function was assessed in 30 239 participants.20 For these analyses, we excluded 1247 participants who were missing baseline data for serum creatinine, 350 for serum cystatin C, and 1061 for urine albumin and creatinine. We also excluded 692 participants without follow-up data and 246 who were receiving dialysis or had received a renal transplant at study entry, for a total sample size of 26 643. Consent was obtained verbally by a trained interviewer and later in writing. All appropriate institutional review boards approved this study.
Primary Predictors
Our predictors of interest were estimates of GFR by creatinine and cystatin C (GFRcystatin C) and albuminuria expressed as ACR. Blood was collected from participants during an in-home examination after a 12-hour fast. Serum creatinine was measured and calibrated to isotope dilution mass spectrometry-traceable methods.21 Cystatin C was measured by particle-enhanced immunonephelometry (N Latex Cystatin C on the BNII, Formerly, Dade Behring, Now Siemens AG, Munich, Germany).13 Urine albumin was measured by nephelometry using the BNII ProSpec nephelometer (Now Siemens AG), and urine creatinine by the Jaffe method using the Modular-P chemistry analyzer (Roche/ Hitachi, Basel, Switzerland).
We defined stage 3 or higher CKD as an estimated GFR of less than 60 mL/min/1.73 m2 using the CKD Epidemiology Collaboration (CKD-Epi) equation for creatinine22:
and the CKD-Epi cystatin C equation:
The cystatin C formula was developed from the pooling of several cohorts with GFR measured by iothalamate.23
We defined albuminuria as a spot urine ACR of 30 mg/g or higher.1
Ascertainment of Outcomes
The outcomes of interest were all-cause mortality and incident end-stage renal disease. All participants engaged in a telephone follow-up interview at 6-month intervals. Participants’ proxies reported deaths via telephone or mail. Death reports were confirmed by review of death certificates or by linkage to the social security death index to verify the date of death.24 Deaths reported until June 30, 2010, were included. Incident end-stage renal disease was ascertained by linkage to the US Renal Data System (http://www.usrds.org), which obtains data on persons who initiate dialysis or receive a kidney transplant. End-stage renal disease cases were identified through August 31, 2009.
Covariates of Interest
Baseline participant information was first collected via a telephone interview. A trained technician then conducted an in-home examination for the anthropometric and clinical examination, electrocardiogram, specimen collection, and inventory of medications. Age, race, sex, smoking history, income, and educational attainment were determined by self-report during the telephone interview. Prevalent cardiovascular disease was defined by any one of the following: electrocardiographic evidence of a myocardial infarction, self-report of a cardiac procedure (coronary artery bypass graft surgery or angioplasty), self-reported myocardial infarction, or self-reported stroke. Hypertension was defined by self-reported use of antihypertensive medications or an average of the second and third seated blood pressure measures: systolic 140 mm Hg or higher or diastolic 90 mm Hg or higher. Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting blood glucose level of 126 mg/dL or higher, or a nonfasting blood glucose concentration of 200 mg/dL or higher. Blood and urine were collected, processed locally, and sent to a central laboratory for measurements. (To convert glucose from mg/dL to mmol/L, multiply by 0.0555.)
Statistical Analyses
The purpose of this study was to evaluate a simple and clinically applicable tool to detect and risk stratify CKD in practice with 3 available markers. In a first step, we calculated the frequency of CKD defined by all possible combinations of estimated GFR using serum creatinine, cystatin C, and ACR into 8 mutually exclusive groups. We evaluated characteristics of participants by CKD group at baseline. Participants were categorized into these 8 groups by estimated GFRcreatinine less than 60 and 60 mL/min/ 1.73m2 or higher, estimated GFRcystatin C less than 60 and 60 mL/min/1.73m2 or higher, and ACR less than 30 and 30 mg/g or higher (Figure 1). We specifically chose these cut points because they reflect the current clinical definition of CKD and because they have been identified as important risk thresholds for CKD complications.25 The use of mutually exclusive categories of creatinine, cystatin C, and ACR also removes the possibility of colinearity because the filtration marker and ACR concentrations do not enter the statistical models. We then evaluated associations of these CKD categories with risks of death and end-stage renal disease. Because CKD is clinically defined by estimated GFRcreatinine of less than 60 mL/min/1.73 m2 in most general clinical settings, we stratified analyses first by presence or absence of CKD by creatinine. To evaluate the utility of cystatin C and ACR in confirming a CKD diagnosis, we categorized persons with CKD defined by creatinine into the following 4 groups: (1) CKD defined by creatinine alone: estimated GFRcystatin C of 60 mL/min/1.73 m2 or higher and ACR less than 30 mg/g; (2) CKD defined by creatinine plus ACR: estimated GFRcystatin C of 60 mL/min/1.73 m2 or higher and ACR of 30 mg/g or higher; (3) CKD defined by creatinine plus cystatin C: estimated GFRcystatin C of less than 60 mL/min/1.73 m2 and ACR less than 30 mg/g; and (4) CKD defined by all biomarkers: estimated GFRcystatin C of less than 60 mL/min/1.73 m2 and ACR of 30 mg/g or higher. Second, to determine the ability of cystatin C or ACR to detect CKD missed by creatinine, we repeated this categorization among persons with estimated GFRcreatinine of 60 mL/min/1.73 m2 or higher at baseline: (1) no CKD defined by any biomarker: estimated GFRcystatin C of 60 mL/min/1.73 m2 or higher and ACR of less than 30 mg/g, (2) CKD defined by ACR alone: estimated GFRcystatin C of 60 mL/min/1.73 m2 or higher and ACR of 30 mg/g or higher, (3) CKD defined by cystatin C alone: estimated GFRcystatin C less than 60 mL/min/1.73 m2 and ACR less than 30 mg/g, and (4) CKD defined by ACR plus cystatin C: estimated GFRcystatin C less than 60 mL/min/1.73 m2 and ACR of 30 mg/g or higher. Across each of these 8 groups, we calculated all-cause mortality and end-stage renal disease rates per 1000 person-years and 95% confidence intervals (CIs). We then compared adjusted risks using multivariate Cox proportional hazard models, separately for persons with and without CKD based on creatinine.
Figure 1. Chronic Kidney Disease Definitions Using a Triple-Marker Approach of Creatinine, Cystatin C, and Albumin-to-Creatinine Ratio.
The blue lines indicate normal results. Creatinine and cystatin C-based data refer to creatinine-based and cystatin C–based estimated glomerular filtration rate, mL/min/1.73 m2, respectively. ACR indicates albumin-to-creatinine ratio.
In a second set of analyses, we evaluated the effect on prognosis of diagnosing CKD stage 3 or higher by each biomarker. Specifically, we compared the risks of death and end-stage renal disease associated with defining CKD stage 3 by estimated GFRcreatinine or estimated GFRcystatin C concentrations. We determined mortality and end-stage renal disease rates per 1000 person-years for each of the above 4 groups and constructed multivariable adjusted models, including adjustment for ACR level.
For all analyses, the follow-up interval was defined for each participant as the elapsed time between the phlebotomy date at the in-home visit to the date of the last confirmed follow-up telephone call, the confirmed date of death, or date of end-stage renal disease ascertainment.
Finally, we evaluated whether adding estimated GFRcystatin C to models with estimated GFRcreatinine and ACR would improve risk classification. We used 2 different methods to evaluate the improvement in the prediction of end-stage renal disease or death (separately) when adding estimated GFRcystatin C to the models: the net reclassification improvement and relative integrated discrimination improvement.26,27 The net reclassification improvement without categories quantifies the accuracy of risk prediction for persons moving into higher- or lower-risk groups based on the addition of estimated GFRcystatin C. First, we compared CKD definitions using 4 groups vs 8 groups. We determined the proportions of the cohort who were classified as higher or lower risk with the addition of cystatin C and determined the event rates for each group. In addition to the net reclassification improvement model, we used relative integrated discrimination improvement to evaluate whether the added predictive value of cystatin C would remain significant when estimated GFRcreatinine, estimated GFRcystatin C, and log ACR were included as linear predictors. We conducted these analyses because we were specifically interested in whether adding estimated GFRcystatin C to the models resulted in improved prediction, even when the markers are modeled as continuous variables. The relative integrated discrimination improvement specifically calculates the relative improvements in sensitivity and specificity for multivariable models with and without the addition of estimated GFRcystatin C. For both of the discrimination indices, empirical 95% CIs and P values were calculated using a bootstrap approach with 1000 iterations. All analyses were conducted using SAS 9.1 (SAS Institute Inc, Cary, North Carolina). A P value of <.05 was considered statistically significant.
RESULTS
Cohort Characteristics
The 26 643 study participants had a mean (SD) age of 65 (9) years. Overall, 40% self-identified as black, 54% were women, 21% had diabetes, and 59% had hypertension (eTable 1 available at http://www.jama.com). The 3596 excluded individuals had similar baseline characteristics for estimated GFR, age, prevalence of diabetes, lipid levels, body mass index, and fasting glucose levels; however, those excluded were more likely to be black (53% vs 40%) and to have an educational attainment of less than high school (17% vs 12%). Characteristics of included participants by all 8 CKD groups are presented in Table 1. Participants with no CKD by all markers were the youngest and had the lowest prevalence of diabetes, hypertension, and cardiovascular disease.
Table 1.
Baseline Characteristics of Participants by Chronic Kidney Disease Group Using Creatinine, Cystatin C, and Albumin-to-Creatinine Ratio
| No. (%) of Participants
|
||||||||
|---|---|---|---|---|---|---|---|---|
| No Chronic Kidney Disease (n = 19 876) | Chronic Kidney Disease Defined by Biomarker Measure
|
|||||||
| ACR Alone (n = 2485) | Cystatin C Alone (n = 963) | ACR + Cystatin C (n = 415) | Creatinine Alone (n = 701) | Creatinine + ACR (n = 148) | Creatinine + Cystatin C (n = 1172) | All Measures (n = 883) | ||
| Estimated GFR, normal: ≥60 ml/min/1.73m2 | ||||||||
| Creatinine | Normal | Normal | Normal | Normal | Abnormal | Abnormal | Abnormal | Abnormal |
|
| ||||||||
| Cystatin C | Normal | Normal | Abnormal | Abnormal | Normal | Normal | Abnormal | Abnormal |
|
| ||||||||
| ACR, normal: <30 mg/g | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal |
|
| ||||||||
| Age, mean (SD), y | 63 (9) | 65 (9) | 70 (9) | 69 (10) | 71 (8) | 73 (10) | 74 (9) | 71 (9) |
|
| ||||||||
| Women | 10 935 (55) | 1286 (52) | 478 (50) | 177 (43) | 433 (62) | 79 (53) | 666 (57) | 404 (46) |
|
| ||||||||
| Black | 7629 (38) | 1296 (52) | 325 (34) | 189 (46) | 272 (39) | 82 (55) | 401 (34) | 438 (50) |
|
| ||||||||
| Educational attainment < high school | 1996 (10) | 416 (17) | 174 (19) | 78 (19) | 93 (13) | 33 (22) | 220 (19) | 188 (21) |
|
| ||||||||
| Income <20 000, US $ | 2966 (15) | 587 (24) | 236 (25) | 116 (28) | 128 (18) | 36 (24) | 288 (25) | 251 (28) |
|
| ||||||||
| Diabetes | 3112 (16) | 953 (38) | 249 (26) | 204 (49) | 137 (20) | 47 (32) | 357 (31) | 445 (50) |
|
| ||||||||
| Hypertension | 10 442 (52) | 1815 (73) | 700 (73) | 339 (82) | 496 (71) | 121 (82) | 967 (83) | 766 (87) |
|
| ||||||||
| Prevalent CVD | 3384 (17) | 668 (27) | 323 (33) | 181 (44) | 188 (27) | 48 (32) | 482 (41) | 409 (47) |
|
| ||||||||
| Cholesterol, mean (SD), mg/dL | ||||||||
| Total | 193 (39) | 193 (41) | 185 (41) | 181 (44) | 192 (44) | 189 (44) | 183 (39) | 183 (44) |
|
| ||||||||
| LDL | 115 (34) | 114 (36) | 109 (35) | 104 (34) | 112 (38) | 109 (36) | 105 (33) | 106 (36) |
|
| ||||||||
| HDL | 53 (17) | 51 (17) | 46 (14) | 45 (14) | 54 (16) | 52 (18) | 48 (16) | 46 (16) |
|
| ||||||||
| Fasting glucose, mean (SD), mg/dL | 99 (27) | 117 (51) | 101 (28) | 117 (50) | 101 (28) | 105 (32) | 102 (29) | 114 (53) |
|
| ||||||||
| BMI, mean (SD) | 29 (6) | 30 (7) | 31 (7) | 32 (8) | 28 (6) | 29 (6) | 30 (7) | 30 (7) |
| Estimated GFR, median (IQR), mL/min/1.73 m2 | ||||||||
| Cystatin C | 92 (27) | 87 (29) | 55 (7) | 54 (9) | 70 (14) | 67.7 (9) | 48 (14) | 41 (18) |
|
| ||||||||
| Creatinine | 91 (20) | 91 (23) | 71 (15) | 71 (16) | 55 (7) | 55 (7) | 49 (14) | 43 (19) |
Abbreviations: ACR, albumin-to-creatinine ratio; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factors: To convert total cholesterol, LDL, and HDL from mg/dL to mmol/L, multiply by 0.0259; glucose from mg/dL to mmol/L, multiply by 0.0555.
Over median follow-up periods of 4.6 years (maximum, 7.28 years) for death and 4.6 years (maximum, 6.57 years) for end-stage renal disease, 1940 participants died and 177 developed incident end-stage renal disease.
Risks of Death and End-Stage Renal Disease
Overall, 2904 participants (11%) were classified as having CKD based on estimated GFRcreatinine. Among them, 701 participants (24%) had CKD defined by estimated GFRcreatinine alone and 148 participants (5%) had CKD defined by estimated GFRcreatinine and ACR, whereas CKD was defined by creatinine and cystatin C for 1172 participants (40%) and by all biomarkers for 883 participants (30%). Among 23 739 participants with no CKD defined by creatinine, 3863 (16%) had CKD detected by ACR, cystatin C, or both (Figure 1).
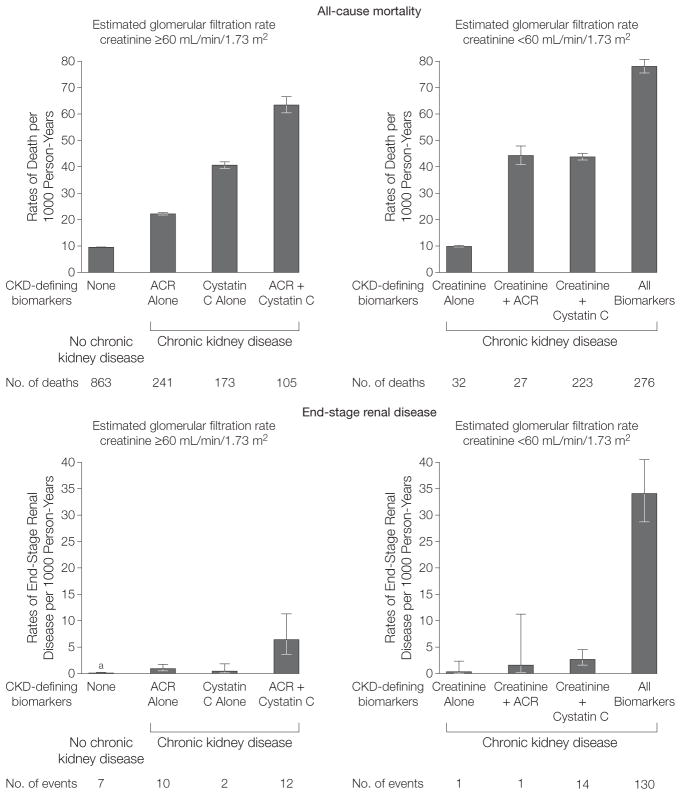
Among individuals classified as having CKD defined by creatinine, both cystatin C and ACR identified individuals at higher risk of death and end-stage renal disease (Figure 2). The mortality rates of participants with CKD defined by creatinine and ACR and CKD defined by creatinine and cystatin C were 4.4 times higher and those with CKD defined by all biomarkers were 7.8 times higher than the mortality rates of those with CKD defined by creatinine alone. Furthermore, participants with CKD defined by creatinine alone had mortality risks similar to those who did not have CKD by any of the 3 markers (rates per 1000 person-years were 9.9 and 9.6, respectively). Participants with CKD defined by all biomarkers had rates of end-stage renal disease that were 103-fold higher than those with CKD defined by creatinine alone (Figure 2). Among persons with CKD by all 3 markers, the risk of death is twice that for end-stage renal disease. In contrast, for all other groups, the risk of death is more than 10-fold higher than that of end-stage renal disease.
Figure 2. Association of Chronic Kidney Disease Definitions With All-Cause Mortality and End-Stage Renal Disease.
Error bars indicate 95% confidence intervals; ACR, albumin-to-creatinine ratio. aNo chronic kidney disease (CKD) from all biomarker measures: 0.08 (95% CI 0.04–0.17) per 1000 person-years.
Estimated GFRcystatin C and albuminuria were also independently associated with higher risk of death and end-stage renal disease among persons with no CKD defined by creatinine at baseline than those whose CKD was. Among participants with no CKD creatinine at baseline, those with no CKD by all 3 biomarkers had the lowest rate of death and end-stage renal disease of all the groups. Compared with participants with no CKD, those with CKD defined by ACR alone had a 2.3-fold higher rate of death, those with CKD defined by cystatin C had a 4.2-fold higher rate of death, and those with CKD defined by ACR plus cystatin C had a 6.6-fold higher rate of death. A similar pattern was observed for end-stage renal disease. The second highest risk group for end-stage renal disease were participants with CKD defined by ACR and cystatin C but for whom CKD was otherwise not detected by creatinine (Figure 2).
In multivariate analyses, having either CKD defined by ACR or cystatin C was associated with increased risk of death among persons with or without a CKD determination at baseline (Table 2). Compared with persons with CKD defined by creatinine alone, the adjusted hazard ratios (HRs) were 3.3 (95% confidence interval [CI], 2.0–5.6) for those with CKD defined by creatinine plus ACR, 3.2 (95% CI, 2.2–4.7) for those with CKD defined by creatinine plus cystatin C, and 5.6 (95% CI, 3.9–8.2) for those with CKD defined by all biomarkers. Among persons initially classified as not having CKD defined by creatinine, those with CKD defined by ACR plus cystatin C had the largest multivariable adjusted HR of death, followed by those with CKD defined by cystatin C alone and by ACR alone (Table 2).
Table 2.
Mortality Associated With Cystatin C, Estimated Glomerular Filtration Rate, and Albuminuria
| No. of Patients | Total No. of Deaths | HR (95% CI)
|
||
|---|---|---|---|---|
| Adjusted Model 1a | Adjusted Model 2b | |||
| Estimated GFR Creatinine ≥60 mL/min/1.73 m2 | ||||
| No CKD all | 19 876 | 863 | 1 [Reference] | 1 [Reference] |
|
| ||||
| CKD defined by biomarker measuresc | ||||
| ACR alone | 2485 | 241 | 1.9 (1.6–2.2) | 1.7 (1.4–1.9) |
|
| ||||
| Cystatin C alone | 963 | 173 | 2.5 (2.1–3.0) | 2.2 (1.9–2.7) |
|
| ||||
| ACR + Cystatin C | 415 | 105 | 3.9 (3.1–4.7) | 3.0 (2.4–3.7) |
|
| ||||
| Estimated GFR Creatinine <60 mL/min/1.73 m2 | ||||
| CKD defined by biomarker measuresc | ||||
| Creatinine alone | 701 | 32 | 1 [Reference] | 1 [Reference] |
|
| ||||
| Creatine + ACR | 148 | 27 | 3.7 (2.2–6.2) | 3.3 (2.0–5.6) |
|
| ||||
| Creatinine + Cystatin C | 1172 | 223 | 3.5 (2.4–5.1) | 3.2 (2.2–4.7) |
|
| ||||
| All biomarkers | 883 | 276 | 6.6 (4.6–9.6) | 5.6 (3.9–8.2) |
Abbreviation: ACR, albumin-to-creatinine ratio; CI, confidence; CKD, chronic kidney disease; GFR, glomerular filtration rate; HR, hazard ratio.
Model 1 adjusts for age, race, sex, income, and educational attainment.
Model 2 adjusts for the above plus hypertension, diabetes, prevalent cardiovascular disease, smoking status, and body mass index.
See “Methods” section for definitions of biomarker measures.
CKD Stage 3 Comparisons
Eight percent of participants had an estimated GFR of less than 60 mL/min/ 1.73 m2 by both markers: 5% by cystatin C alone, and 3% by creatinine alone. The prevalence of albuminuria was 17% for those with CKD defined by estimated GFRcreatinine alone, 30% for those with CKD defined by estimated GFRcystatin C alone, and 43% with CKD defined by both markers. Participants with estimated GFR less than 60 mL/ min/1.73 m2 by creatinine alone had no significant increase in mortality or end-stage renal disease risk in adjusted models, whereas those with an estimated GFR less than 60 mL/min/1.73 m2 by cystatin C had higher mortality and end-stage renal disease risk despite their estimated GFRcreatinine being less than 60 mL/min/1.73 m2 (Table 3).
Table 3.
Risk of Death and End-Stage Renal Disease Associated With Chronic Kidney Disease Stage 3 by Estimated Glomerular Filtration Rate Using Creatinine and Cystatin Ca
| Biomarker Measures, Estimated GFR mL/min/1.73 m2 | No. of Participants | No of Events | Rates per 1000 Person-Years | HR (95% CI)
|
|
|---|---|---|---|---|---|
| Adjusted Model 1b | Adjusted Model 2c | ||||
| All-Cause Mortality Over 4.6 y | |||||
| Creatinine + Cystatin C, ≥60 | 22 361 | 1104 | 10.9 (10.9–11.0) | 1 [Reference] | 1 [Reference] |
|
| |||||
| Creatinine alone, <60 | 849 | 59 | 15.4 (14.9–15.9) | 1.0 (0.7,1.2) | 0.9 (0.7–1.1) |
|
| |||||
| Cystatin C alone, <60 | 1378 | 278 | 47.0 (45.8–48.2) | 2.6 (2.2–2.9) | 2.1 (1.9–2.5) |
|
| |||||
| Creatinine + Cystatin C, <60 | 2055 | 799 | 57.8 (56.6–59.1) | 2.8 (2.5–3.1) | 2.1 (1.9–2.4) |
|
| |||||
| End-Stage Renal Disease Over 4.6 y | |||||
| Creatinine + Cystatin C, ≥60 | 22 361 | 17 | 0.2 (0.1–0.3) | 1 [Reference] | 1 [Reference] |
|
| |||||
| Creatinine alone, <60 | 849 | 2 | 0.5 (0.1–2.2) | 3.9 (0.9–16.9) | 2.5 (0.6–10.9) |
|
| |||||
| Cystatin C, <60 | 1378 | 14 | 2.2 (1.3–3.8) | 12.6 (6.2–25.9) | 5.8 (2.8–12.1) |
|
| |||||
| Creatinine + Cystatin C, <60 | 2055 | 144 | 15.8 (13.5–18.6) | 90.5 (53.2–153.9) | 26.1 (14.9–45.7) |
Abbreviation: CI, confidence; HR, hazard ratio.
Stage 3 chronic kidney disease is defined as estimated glomerular filtration rate of less than 60 mL/min/1.73 m2.
Mortality model adjusts for age, race, sex, income, educational attainment, hypertension, and diabetes. End-stage renal disease model adjusts for age, race, sex, hypertension, and diabetes.
Mortality model adjusts for the above plus hypertension, diabetes, prevalent cardiovascular disease, smoking status, body mass index, waist circumference, and log albumin-to-creatinine ratio. End-stage renal disease model adjusts for the above plus log albumin-to-creatinine ratio.
Discrimination and Reclassification Improvement
Adding estimated GFRcystatin C as a measure for CKD resulted in 5.2% of the cohort being reclassified to a higher risk group, whereas 3.2% were reclassified to a lower risk group. Participants who were reclassified to a higher risk group by adding estimated GFRcystatin C to the model had a 3-fold higher mortality risk (10% during follow-up) than those who were reclassified to a lower risk group (3% mortality risk). Similarly, the risk of end-stage renal disease was almost 4-fold higher (0.62%) for those reclassified upward than those whose risk was lowered by adding the estimated GFRcystatin C measure (0.15%). The net reclassification improvement for death was 13.3% (95% CI, 12.3%–13.7%; P<.001) and the net reclassification improvement for end-stage renal disease was 6.4% (95% CI, 5.5%–6.7%, P<.001). Adding estimated GFRcystatin C as a continuous variable to the fully adjusted model resulted in a significant relative integrated discrimination improvement for death (9.5%; 95% CI, 2.7%–20.6%) but not for end-stage renal disease (0.04%; −21.7% to 27.1%).
COMMENT
We evaluated a triple-marker approach for the assessment of kidney disease in a large cohort of black and white adults across the United States. Using serum creatinine, cystatin C, and albuminuria resulted in an increased ability to discriminate risk of death and end-stage renal disease. Cystatin C and albuminuria were both strongly and independently associated with all-cause mortality among persons with or without CKD defined by creatinine-based estimated GFR. The risk of future end-stage renal disease was concentrated within the subset of participants who had CKD defined by all 3 markers. The second highest risk group for end-stage renal disease was missed by creatinine but was detected by cystatin C and ACR.
Implications of Findings for CKD Confirmation
Our findings confirm prior reports that albuminuria quantification and cystatin C can improve risk stratification among those with CKD detected by creatinine.8,11,24,28 The current results extend prior findings to highlight that measurements of both cystatin C and albuminuria are complementary to identify individuals with CKD who have an increased risk of mortality and incident end-stage renal disease. Several groups are currently advocating new international guidelines that more accurately reflect prognosis of CKD15–18 and have proposed adding ACR to staging of CKD.28 Our results suggest that a triple-marker approach using both ACR and cystatin C to confirm CKD more accurately discriminates prognosis for death and progression to end-stage renal disease than creatinine and ACR alone. These findings have important clinical relevance. According to data from the third and fourth National Health and Nutrition Surveys (NHANES), more than 9.9 million persons in the United States have CKD stage 3 with an estimated GFR between 45 and 60 mL/min/1.73 m2. Among those, 2.4 million would be identified as being at higher risk with ACR, and cystatin C would identify another 2.9 million at elevated risk.29 These data, taken together, support the observation that the clinical presentation of low GFR in the absence of albuminuria is common.30,31 Moreover, 25% of participants in the REGARDS study were labeled as having CKD defined by creatinine but who had no CKD defined by ACR or cystatin C were not at an increased risk of death or end-stage renal disease. The NHANES data suggest that among the 9.9 million persons with CKD and estimated GFR between 45 and 60 mL/min/1.73 m2, 2.6 to 4.6 million would have no CKD by ACR or cystatin C, and thus are likely at low risk. The use of a triple-marker renal panel that improves prognostic ability could both reduce unwarranted referrals and unnecessary work-ups for low-risk individuals and would prioritize specialty care and interventions to individuals at highest risk. Using cystatin C to confirm CKD, particularly among persons with estimated GFRs between 45 and 60 mL/ min/1.73 m2, would optimize risk stratification of CKD.
Implications of Findings for CKD Detection
In addition, cystatin C and albuminuria can detect CKD in persons who are missed by estimated GFRcreatinine but have elevated risk of death and end-stage renal disease. In our sample, 1 in 6 persons had CKD undetected by creatinine. In the REGARDS study, more CKD was detected by cystatin C and ACR than by creatinine (14% vs 11%).
Our findings illustrate the potential implications of universal screening for CKD using a triple-marker approach. It remains unclear whether early detection of CKD would be cost-effective.32 Current guidelines suggest regular screening with quantification with ACR alone among individuals with diabetes. The role of cystatin C in screening is not yet known. In our cohort, 4% of persons were detected as having CKD by cystatin C alone. These persons were at significantly higher risk of death compared with persons with no CKD. Because universal screening may not be practical, future studies should identify risk factors associated with occult CKD in order to develop targeted approaches that would maximize the yield of novel CKD screening strategies.
Our study is limited by the lack of direct GFR measurements. However, recent studies have suggested that even iothalamate measures can have daily variations of up to 8%,33 and these are cumbersome, costly, and rarely available in large epidemiological studies. No general population study has evaluated prognosis using a gold standard GFR estimate with an exogenous filtration marker. However, our findings with end-stage renal disease end points suggest that a triple-marker approach can also detect persons at highest risk of CKD progression. In addition, we relied on a 1-time measure of biomarkers including albuminuria, which is known to be variable, particularly at lower ranges. Moreover, because end-stage renal disease is an outcome that develops over decades, our event rate for end-stage renal disease limits our statistical power.
In conclusion, our findings suggest that adding cystatin C to creatinine and albuminuria for risk prediction can more accurately reclassify persons and candistinguish important prognostic differences, namely a 3-fold risk of death and 4-fold risk of end-stage renal disease. Future studies are needed using the triple-marker approach to evaluate clinicals trategies that may reduce these risks.
Acknowledgments
Funding/Support: This research project is supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Additional funding was provided by an investigator-initiated grant-in-aid from Am-gen Corp. Dr Peralta is funded by the National Institutes of Diabetes and Digestive and Kidney Diseases grant 1K23SK082793-01 and a Robert Wood Johnson Harold Amos award. Dr Shlipak is funded by grants R01AG034853 (PI), R01DK066488 (PI), R01HL085757 (Co-Investigator), R01DK078124 (Co-Investigator), and R21HL091217 (Co-Investigator) and by an American Heart Association Established Investigator Award (0640012N; PI)
Role of the Sponsor: Amgen had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript.
Footnotes
Additional Contributions: We thank the staff members at the USRDS Coordinating Center for their assistance with the linkage to the USRDS under a data-use agreement between the USRDS and the University of Alabama at Birmingham. The manuscript was sent to Amgen for review prior to submission for publication. The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Online-Only Material: eTable 1 is available at http://www.jama.com.
Author Contributions: Drs Peralta, Shlipak, Muntner, and Judd had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Peralta, Shlipak, Cushman, Muntner, Warnock.
Acquisition of data: Zhang.
Analysis and interpretation of data: Peralta, Shlipak, Judd, Cushman, McClellan, Zakai, Safford, Muntner, Warnock.
Drafting of the manuscript: Peralta.
Critical revision of the manuscript for important intellectual content: Peralta, Shlipak, Judd, Cushman, McClellan, Zakai, Safford, Zhang, Muntner, Warnock.
Statistical analysis: Judd.
Obtained funding: Peralta, Safford, Warnock.
Study supervision: Shlipak, Cushman, Muntner.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Warnock reported that he is a consultant for Amgen Corp and has received research support from Amgen. Dr Cushman reported receiving research support from Amgen. Dr McClellen reported receiving research support from Amgen. Otherwise, no other conflicts of interest were reported.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 2.den Hartog JR, Reese PP, Cizman B, Feldman HI. The costs and benefits of automatic estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol. 2009;4(2):419–427. doi: 10.2215/CJN.04080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molitch ME, DeFronzo RA, Franz MJ, et al. American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27(suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. 2008;73(11):1310–1315. doi: 10.1038/ki.2008.67. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354 (23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 8.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22(1):147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, de Jong PE, Coresh J, et al. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report [published online ahead of print December 8] Kidney Int. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Sarnak M, Katz R, et al. Cystatin-C and risk for mortality and cardiovascular disease in elderly adults. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19(5):844–846. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 16.Couser WG. Chronic kidney disease the promise and the perils. J Am Soc Nephrol. 2007;18(11):2803–2805. doi: 10.1681/ASN.2007080964. [DOI] [PubMed] [Google Scholar]
- 17.de Jong PE, Gansevoort RT. Fact or fiction of the epidemic of chronic kidney disease—let us not squabble about estimated GFR only, but also focus on albuminuria. Nephrol Dial Transplant. 2008;23 (4):1092–1095. doi: 10.1093/ndt/gfn028. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009;53(3 suppl 3):S4–S16. doi: 10.1053/j.ajkd.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 20.McClellan W, Speckman R, McClure L, et al. Prevalence and characteristics of a family history of end-stage renal disease among adults in the United States population: Reasons for Geographic and Racial Differences in Stroke (REGARDS) renal cohort study. J Am Soc Nephrol. 2007;18(4):1344–1352. doi: 10.1681/ASN.2006090952. [DOI] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51 (3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warnock DG, Muntner P, McCullough PA, et al. REGARDS Investigators. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56(5):861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonelli M, Muntner P, Lloyd A, et al. Using pro-teinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154(1):12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 29.Foley RN, Wang C, Snyder JJ, Collins AJ, Cystatin C. Levels in U.S. Adults, 1988–1994 Versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009;4(5):965–972. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375(9721):1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 31.Wright JT, Jr, Bakris G, Greene T, et al. African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 32.Jaar BG, Khatib R, Plantinga L, Boulware LE, Powe NR. Principles of screening for chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(2):601–609. doi: 10.2215/CJN.02540607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2011;56(1):39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]