Abstract
Objective
Delirium duration is predictive of long-term cognitive impairment (LTCI) in Intensive Care Unit (ICU) survivors. Hypothesizing that a neuroanatomical basis may exist for the relationship between delirium and LTCI, we conducted this exploratory investigation of the associations between delirium duration, brain volumes and LTCI.
Design, Setting, and Patients
A prospective cohort of medical and surgical ICU survivors with respiratory failure or shock.
Measurements
Quantitative high resolution 3-Tesla brain magnetic resonance imaging was used to calculate brain volumes at discharge and three-month follow-up. Delirium was evaluated using the Confusion Assessment Method for the ICU; cognitive outcomes were tested at three- and twelve-month follow-up. Linear regression was used to examine associations between delirium duration and brain volumes, and between brain volumes and cognitive outcomes.
Results
A total of 47 patients completed the MRI protocol. Patients with longer duration of delirium displayed greater brain atrophy as measured by a larger ventricle-to-brain ratio (VBR) at hospital discharge [0.76, 95% confidence intervals (CI) (0.10, 1.41); p=0.03] and at 3-month follow-up [0.62 (0.02, 1.21), p=0.05]. Longer duration of delirium was associated with smaller superior frontal lobe [−2.11 cm3 (−3.89, −0.32); p=0.03] and hippocampal volumes at discharge [−0.58 cm3 (−0.85, −0.31), p<0.001] – regions responsible for executive functioning and memory, respectively. Greater brain atrophy (higher VBR) at three months was associated with worse cognitive performances at twelve months [lower RBANS battery score −11.17 (−21.12, −1.22), p=0.04]. Smaller superior frontal lobes, thalamus, and cerebellar volumes at three months were associated with worse executive functioning and visual attention at twelve months.
Conclusions
These preliminary data show that longer duration of delirium is associated with smaller brain volumes up to three months after discharge, and that smaller brain volumes are associated with LTCI up to 12 months. We cannot, however, rule out that smaller preexisting brain volumes explain these findings.
Keywords: MRI, delirium, cognitive impairment, ICU, sepsis, neuroimaging, brain, frontal lobe, critical care, intensive care, mechanical ventilation, aging, geriatrics, encephalopathy
Individuals are more likely to survive critical illness today than 20 years ago (1). Research has, however, demonstrated that intensive care unit (ICU) survivors often experience new or exacerbated cognitive impairment months to years following intensive care treatment (2–8), and this long-term cognitive impairment (LTCI) is similar phenotypically to an acquired brain injury with profound deficits in executive functioning, memory and attention (3, 7–10).
Reports of neuroanatomical effects of disease or injury, such as brain atrophy and ventricular enlargement following traumatic brain injury (TBI) (11) and anoxic injury (12–15), have profoundly influenced the understanding, treatment and research of such disorders. Indeed, the important understanding that global brain atrophy and ventricular enlargement in patients with both TBI and anoxic injury is associated with enduring cognitive impairments strongly influenced our methodological design of this investigation (15–17). Similar information is lacking for other critically ill populations without overt neurological injury (such as trauma or overt anoxia) but who nevertheless frequently have evidence of significant LTCI (3, 7–10). While there are many potential predictors of LTCI in general medical and general surgical ICU patients, one potentially modifiable risk factor is delirium duration (6, 10).
The purposes of our study were two-fold: 1) to explore the hypothesis that increased duration of delirium in medical and surgical ICU survivors was associated with smaller brain volumes, both globally and in areas that play an important role in executive functioning (e.g., frontal lobes) and memory (e.g., hippocampus); and 2) to examine if brain volumes were associated with LTCI globally and in brain areas associated with executive functioning (e.g., frontal lobes), memory (e.g., hippocampus), and attention (e.g., parietal cortex, cerebellum).
Methods
Study Design and Population
The VISIONS (VISualizing Icu SurvivOrs Neuroradiological Sequelae) study was a prospective, convenience sample neuroimaging study that was nested within an ongoing prospective cohort study evaluating the role of delirium in long term cognitive impairment in patients with respiratory failure or shock. Patients eligible for the VISIONS study were >18 years of age, surviving the ICU admission in the medical, surgical or cardiac ICUs at Vanderbilt University Medical Center (Nashville, TN) or Saint Thomas Hospital (Nashville, TN) between June 2006 and December 2009. Exclusion criteria were presence of neurological disease with known brain lesions, TBI, history of severe dementia or anoxic brain injury that would confound delirium and LTCI diagnoses, blindness, deafness, non-English speaking, cardiopulmonary bypass within 3 months of ICU admission (to avoid potential bypass-related cognitive impairment), and presence of delirium at hospital discharge. Specific exclusion criteria due to the use of magnetic resonance imaging (MRI) were patient weight >300 pounds, claustrophobia, and MRI contraindications (e.g., pacemakers or other implanted metal incompatible with MRI).
The institutional review boards at Vanderbilt and Saint Thomas approved the study. Patients were enrolled in the parent study within 72 hours of ICU admission with respiratory failure or shock. Written informed consent was obtained from surrogates for collection of demographic and in-hospital data, including delirium evaluation; patients were then self-consented prior to MRI imaging once off mechanical ventilation and free of delirium.
Baseline and Demographic Characteristics
Baseline clinical and demographic data were collected by study staff at enrollment in the parent study. Pre-hospitalization baseline cognitive abilities were assessed through surrogate interview using the validated Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF) (18). The IQCODE-SF has good reliability, high sensitivity (75–100%) and specificity (68–86%) as a screening test for dementia. Patients scoring 4 or higher (18) were further evaluated with the Clinical Dementia Rating Scale (CDR) (19, 20). Patients with a CDR score of 3, indicating severe preexisting dementia, were excluded. Severity of illness was measured using the Acute Physiology and Chronic Health Evaluation II score (APACHE II) (21) and the Sequential Organ Failure Assessment (SOFA) score (22).
Measures of Delirium (Exposure), Covariates and Cognitive Outcomes
Delirium was assessed twice daily up to 30 days after study enrollment by trained research nurses using the Confusion Assessment Method for the ICU (CAM-ICU) (23, 24). Sedation level was measured via the Richmond Agitation-Sedation Scale (RASS) (25, 26). Delirium duration was defined as the total number of days a patient experienced delirium during hospitalization (up to 30 days). Sepsis on a daily basis throughout the ICU stay was defined by the presence of two Systemic Inflammatory Response Criteria (SIRS), known or suspected infection, and further adjudication by clinical personnel.(27)
Cognitive outcomes were tested at three and twelve months using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (28).The RBANS is a “stand alone” tool for the evaluation of cognitive functioning widely employed in the assessment of elderly and medically ill patients (29, 30).
The RBANS generates “Index Scores” based on subtest raw scores, which are interpreted based on the following classification system: 69 and below – Extremely Low, 70 to 79 – Borderline, 80 to 89 – Low Average, 90 to 109 – Average, 110 to 119 – High Average, 120 to 129 – Superior, 130 and above – Very Superior. The RBANS assesses immediate and delayed memory, attention and concentration, visual spatial construction, and language. Executive functioning and visual attention were assessed using the Trail Making Test Parts B and Part A, respectively (31, 32).The tests and scoring system are described in Appendix 1. Cognitive testing was performed by investigators trained in administering neuropsychological tests, who were blind to clinical data and duration of delirium.
MRI Parameters including Ventricle-to-Brain Ratio and Brain Structures
Patients were scanned in a Philips Achieva 3T MRI scanner (Philips Medical Systems, Inc., Best, The Netherlands) at hospital discharge and 3-month follow-up. Scanning included T1-weighted 3D turbo field echo image covering the whole brain, 170 slices, TR=8.0ms, TE=3.7ms, SENSE factor=2, voxel size=1mm isotropic, FOV=256 × 256 × 170.
The following brain volumes were chosen a priori and investigated as the key neuroanatomic findings of interest in this study: ventricle-to-brain ratio (VBR), total brain volumes (i.e., sum of total gray and white matter volumes), hippocampus, superior frontal lobes, total frontal lobes (including superior frontal regions), thalamus, precuneus (postero-medial portion of the parietal lobe), rostral anterior cingulate (rACC) gyrus, and cerebellum volumes. Volumes for the hippocampus, superior frontal, total frontal lobes, precuneus, rACC, and cerebellum include right and left hemisphere volumes combined. The VBR is a widely used measure of brain atrophy and is considered a better measurement of atrophy than total brain volume, since it corrects for brain size and has stronger associations with cognitive performance (33). The VBR is a measure of generalized, diffuse brain volume loss and an index of white matter integrity (34). VBR was calculated by dividing total ventricular volume (lateral, III and IV ventricle volumes) by total brain parenchymal volume × 100.
Superior frontal lobes were analyzed separately from total frontal lobes because we hypothesized superior frontal lobes may be differentially impacted by delirium. ICU survivors have been shown to have executive functioning deficits (4, 5, 35), and evidence suggests that the superior frontal lobes are more precisely linked to executive functioning (36, 37) than the remaining frontal lobe regions, which (in addition to executive functioning) also subserve language, motor, and working memory. The hippocampus is important for declarative memory and is hypothesized to play a role in both delirium and cognitive impairment (38–40). Investigators who assessed brain volumes were blind to patient identity and all medical information.
MRI image processing
Volumetric segmentation was performed using FreeSurfer image analysis suite v4.0.2 (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA; https://surfer.nmr.mgh.harvard.edu). FreeSurfer is an automated program. Freesurfer morphometric procedures have good test-retest reliability across scanner manufacturers and across field strength (41, 42). One investigator (E.K.) did the image data set up and checking. The volumetric segmentation procedures are described elsewhere in detail (41, 43–50). Briefly, image analysis included motion correction, removal of non-brain tissue (43), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter into structural volumes (45, 46), intensity normalization (51), tessellation of the gray and white matter boundaries, automated topology correction (43, 47), and surface deformation (48–50). Brain volumes were automatically calculated by summing the volumes of brain regions/units for each structural volume of interest. Subcortical region segmentations were derived automatically in FreeSurfer, as each voxel in the normalized brain volume is assigned a label that corresponds to its neuroanatomical substrate (e.g., hippocampus). The data were visually inspected post-processing and corrected where necessary to ensure data accuracy (e.g., removal of skull and dura matter and accurate delineation of gray/white matter and pial surfaces).
Statistical Analyses
Descriptive statistics are presented using medians and interquartile ranges for continuous variables and proportions for categorical variables. To examine associations between duration of delirium and each brain volume measure, we used separate linear regression models for each brain region, adjusting for 3 covariates:(a) age at study enrollment, (b) highest SOFA score during the ICU stay (22), and (c) presence of sepsis at any time during the ICU stay. Each regression coefficient estimates the difference in volume of the region of interest between patients with delirium duration at the 75th versus the 25th percentile of our population. To avoid overfitting (i.e., finding significant results due solely to noise), we limited our choice of covariates to these three confounders we judged a priori as potentially the most important (age, sepsis and highest SOFA). Separate models were used for discharge and three-month outcomes.
To strengthen our understanding of these relationships, we also conducted a sensitivity analysis, which adjusted for potential preexisting cognitive impairment, using the IQCODE-SF (18, 52). We used the IQCODE-SF as a continuous variable because it allows us to use all the information we have, without using artbitrary cut offs, and provides the most statistical power to detect any association with the outcome, or to adjust for any confounding effect it may have on the relationship between brain volumes and delirium or long-term cognitive performance. This sensitivity analysis was important because we were unable to obtain pre-ICU imaging due to the emergent nature of our patient recruitment criteria (acute respiratory failure/shock) and among available surrogate markers, the IQCODE-SF has been shown to be moderately correlated with brain atrophy (52). Nonetheless, due to the number of covariates in our main model and the possibility of overfitting, we could not adjust for this in our main analyses.
The associations between brain volumes and cognitive outcomes (i.e., global RBANS score, memory, executive functioning, attention and concentration, visual spatial construction, language) were also evaluated using separate linear regression models for three- and twelve-month cognitive follow-up, adjusting both models for age at study enrollment and presence of sepsis at any time during ICU stay.
Given the hypothesis-generating nature of this pilot study, we did not adjust for multiple comparisons, preferring to examine all possible associations in order to inform future research.
R version 2.13 was used for all analyses (53). In reporting results of linear regression for continuous variables, we compared the 75th percentile value of the independent variable of interest of our population to the 25th percentile, to provide a more clinically meaningful comparison.
Results
Baseline Demographics
A total of 335 patients were screened between June of 2007 and December of 2009; 10 refused consent, 142 met MRI exclusions, 20 died before being discharged, 60 were delirious at hospital discharge, and 41 were discharged before being consented. The remaining 62 were all enrolled in this cohort study. Of those enrolled 12 experienced either physical or psychological discomfort in the scanner and were unable to complete scanning procedures, and 3 had poor quality MRI imaging. Thus MRI imaging was available in 47 patients at hospital discharge. At three-month follow-up, 3 patients died, 1 moved out of area, 3 were not scanned due to chronic health conditions, 1 was claustrophobic, and 3 had poor quality imaging, resulting in 36 patients with MRI imaging at 3 months.
Patients were severely ill at ICU admission, with a median APACHE II score of 24 [interquartile range (IQR), (18, 29)] and a median SOFA score of 9 (7, 12). The patients’ median age was 58 (48, 65) years (see Table 1). Only two patients had preexisting cognitive impairment as defined by a score of >4 on the IQCODE-SF. These 2 patients were then further assessed for cognitive impairment using the CDR; both had mild cognitive impairment (CDR 0.5) (20). Delirium occurred in 70% of patients during their critical illness, with more than 25% of patients experiencing three or more days
Table 1.
Baseline Demographics and Clinical Characteristicsa
| Characteristic | Cohort, n=47 |
|---|---|
| Age at enrollment | 58 (48, 65) |
| Female, % (n) | 38% (18) |
| Education, years | 12 (12, 14) |
| IQCODE-SF | 3 (3, 3.06) |
| Baseline cognitive impairment, % (n/total) | 4% (2) |
| APACHE II at enrollment | 24 (18, 29) |
| SOFA score at enrollment | 9 (7, 12) |
| Mechanical Ventilation, % (n) | 44 (94%) |
| Sepsis during ICU stay, % (n) | 57% (27) |
| Admission diagnoses, % (n) | |
| Sepsis/acute respiratory distress syndrome | 30% (14) |
| Hepatobiliary/Pancreatic Surgery | 21% (10) |
| CHF/MI/Cardiogenic shock | 13% (6) |
| ARDS w/o infection | 9% (4) |
| COPD/Asthma | 6% (3) |
| Vascular Surgery | 4% (2) |
| Other Diagnoses | 17% (8) |
| ICU length of stay | 4 (2, 6) |
| Hospital length of stay | 9 (6, 13) |
| Delirium prevalence, % (n/total) | 70% (33) |
| Delirium duration, days | 1 (0, 3) |
| Coma duration, days | 1 (0, 2) |
All results expressed as median (interquartile range), or % (n/total).
Abbreviations:
IQCODE-SF, Short Informant Questionnaire of Cognitive Decline in the Elderly; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, Intensive Care Unit; COPD, Chronic Obstructive Pulmonary Disease; ARDS, Acute Respiratory Distress Syndrome; MI, myocardial infarction; CHF, Congestive Heart Failure; SOFA, Sequential Organ Failure Assessment; MI, Myocardial Infarction; Other diagnoses include colonic surgery, ENT surgery, gastric surgery, vascular surgery neurological disease, pulmonary other, renal failure, seizures/status epilepticus and transplants (excluding liver).
Delirium and Brain Volumes
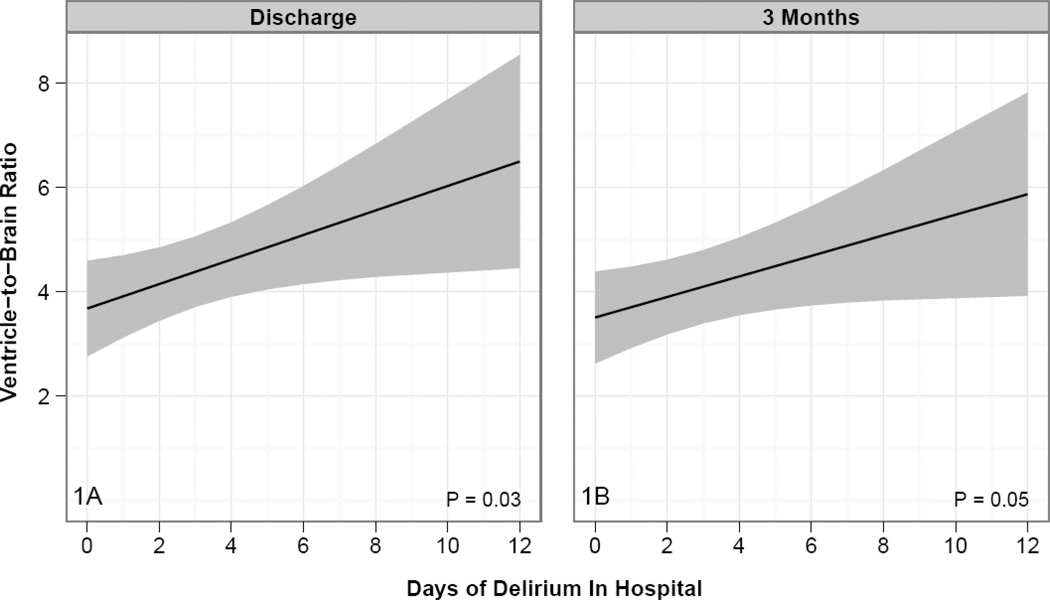
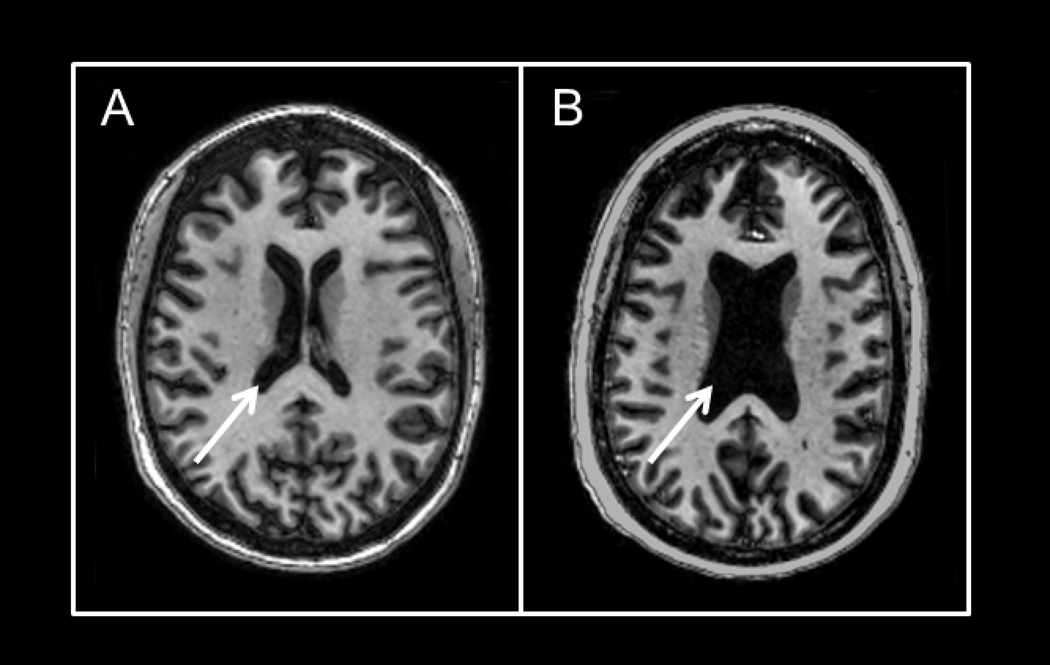
The results of the multivariable models for duration of delirium and brain volumes are shown in Table 2 and Appendix 2. After adjusting for age, highest SOFA score and sepsis during the ICU stay, longer duration of delirium was independently associated with larger ventricle-to-brain ratios (VBR) (i.e., more global brain atrophy) such that compared to a patient who experienced 0 days of delirium (25th percentile of delirium duration), a patient who experienced 3 days of delirium (75th percentile) had, on average, a VBR that was 0.76 greater at hospital discharge [95% confidence interval, CI (0.10, 1.41); p = 0.03] and 0.62 greater at three-month follow-up [95% CI (0.02, 1.21); p = 0.05] (Figure 1). Mean values of VBRs for our cohort as a whole at discharge and at three months were 4.0±1.9 and 3.8±1.7, respectively. The VBR in our patients is much larger (i.e., represents greater brain atrophy) than mean VBR of 2.08, for normal adults ages 56–65 years (54). We report an example of significant brain atrophy in a young adult patient who experienced delirium for 12 days versus a matched age patient without delirium (Figure 3). This clinical example illustrates how atrophy might develop even in a young delirious patient who had no evidence of pre-existing cognitive impairment.
Table 2.
Relationship between Brain Volumes and Duration of Deliriuma
| Anatomical Region | Hospital Discharge Differences in brain volumes (95% CI) associated with 3-day increase in delirium duration |
P-value | Three-month Follow-up Differences in brain volumes (95% CI) associated with 3-day increase in delirium duration |
P-Value |
|---|---|---|---|---|
| VBR | 0.76 (0.10, 1.41) | 0.03 | 0.62 (0.02, 1.21) | 0.05 |
| Gray Matter | −13.91 (−30.42, 2.59) | 0.11 | −17.34 (−37.06, 2.39) | 0.10 |
| White Matter | −3.71 (−30.04, 22.61) | 0.78 | −12.91 (−43.21, 17.40) | 0.41 |
| Hippocampus | −0.58 (−0.85, −0.31) | <0.001 | −0.28 (−0.66, 0.11) | 0.17 |
| Superior Frontal Lobe | −2.11 (−3.89, −0.32) | 0.03 | −2.36 (−4.30, −0.41) | 0.02 |
| Frontal Lobe | −6.63 (−13.14, − 0.38) | 0.04 | −5.68 (−16.46, 5.1) | 0.31 |
| Thalamus | −0.11 (−0.76, 0.55) | 0.75 | −0.48 (−1.09, 0.12) | 0.13 |
| Precuneus | −0.76 (−1.56, 0.04) | 0.07 | −0.65 (−1.64, 0.33) | 0.21 |
| rACC | −0.11 (−0.32, 0.11) | 0.35 | −0.09 (−0.33, 0.15) | 0.46 |
| Cerebellum | 1.19 (−4.02, 6.4) | 0.66 | −0.94 (−7.29, 5.41) | 0.77 |
[NOTE: representing two of our three leading hypotheses, the bolded rows are used as interpretive examples below] The point estimates and 95% confidence intervals represent differences in brain volumes in relationship to the duration of delirium. As in these examples, all point estimates shown in this table are for adjusted differences in brain volumes between patients who had 3 days of delirium (75th percentile of the duration of delirium) vs. those who had 0 days of delirium (25th percentile).
EXAMPLE 1: Using the 1st line of the table (since VBR represented our overarching hypothesis about global atrophy), patients who experienced 3 days of delirium had, on average, a ventricle-to-brain ratio that was 0.62 higher at 3-month follow-up MRI scanthan a patient who had 0 days of delirium. The VBR is higher when the volume of the patients’ ventricles is larger relative to the volume of global brain structures, indicating more brain atrophy.
EXAMPLE 2: Reading from the 5th line of the table (since the superior frontal lobes represented one of our two leading hypotheses about a specific brain region, due to the predominant neuropsychological domain deficit of executive function in cohorts to date), patients who experienced 3 days of delirium had, on average, a 2.36 cm3 smaller total frontal lobe volume at 3-month follow-up MRI scan than a patient who had 0 days of delirium Volume units per each area of the brain were measured in cm3; VBRs were calculated by dividing total ventricular volume (lateral, III and IV ventricle volumes) by total brain parenchymal volume × 100. All results were adjusted for age, highest ICU SOFA score, and presence of sepsis.
Abbreviations: CI, confidence interval; VBR, ventricle-to-brain ratio; rACC, rostral anterior cingulate cortex
Figure 1. Delirium Duration and Ventricle-to-Brain Ratio in ICU Patients.
Increased duration of delirium was independently associated with smaller overall brain volumes (defined by ventricle-to-brain ratio [VBR]) both at hospital discharge and at three-month follow-up, after adjusting for age, highest SOFA score in the ICU, and presence of sepsis. Increased VBR indicates increase in cerebral spinal fluid due to loss of brain volume. VBR is shown on the y-axis of figures 1a and 1b. The x-axis for all figures represents the duration of delirium measured in days. The solid black line indicates adjusted brain volume for a given value of delirium duration, adjusted for confounders; the 'ribbon' indicates the 95% confidence bounds.
Figure 3. Representative example of lateral ventricle size in 46-year-old female and 42-year-old female ICU survivors with no preexisting cognitive impairment.
Axial T1-weighted brain images in 2 ICU survivors. Figure 3a depicts relatively normal ventricular volume (see arrow) in a 46-year-old female who did not experience delirium in the ICU. Patient had a history of respiratory and heart failure. She was admitted to a medical ICU due to acute respiratory distress syndrome (ARDS) and was subsequently intubated and managed through the ICU without ever developing delirium. Figure 3b depicts enlarged ventricles (see arrow) in a 42-year-old female who did develop delirium in the ICU. Patient was admitted to the hospital after reporting fever and dyspnea with a chest X-ray and other laboratory data confirming community acquired pneumonia and ARDS. The patient was admitted to the ICU and mechanically ventilated, experiencing 12 days of delirium and then resolution. There was no preexisting history of neurological impairment, and surrogate questioning for preexisting cognitive impairment was also negative
Duration of delirium was independently associated with volume loss in the superior frontal lobes – indicative of superior frontal lobe atrophy – at both discharge [point estimate for 3 versus 0 days of delirium = −2.11 (−3.89, −0.32) cm3; p = 0.03] and at the three-month follow-up scan [−2.36 (−4.3, −0.41) cm3; p = 0.02](Figure 2). The mean superior frontal lobe volumes were 32.5 ± 4.8 at discharge and 33 ± 5 at three months. Increased duration of delirium was also associated with smaller hippocampal volumes at discharge [point estimate for 3 versus 0 days of delirium = −0.58(−0.85, −0.31) cm3; p < 0.001], but this relationship was not significant at the three-month follow-up scan (Figure 2). The mean hippocampal volume at discharge was 6.19 ± 0.97 and at 3 months was 6.2 ± 1.1.
Figure 2. Delirium Duration, Hippocampal and Superior Frontal Lobe Volumes.
Longer duration of delirium was independently associated with smaller volumes in the hippocampus at discharge (2A at discharge and 2B at three-month follow-up) and the superior portion of the frontal lobe at both scans (2C at discharge and 2D at three-month follow-up). Brain volumes are shown in cm3 on the y-axis. The x-axis is duration of delirium measured in days. The solid black line indicates adjusted brain volume for a given value of delirium duration, adjusted for confounders; the 'ribbon' indicates the 95% confidence bounds.
Neither sepsis nor severity of illness was associated with any brain volume changes in our analyses. Older age was associated with greater VBR at discharge [0.98 (0.35, 1.6); p= 0.004] and three months [0.97 (0.37, 1.58); p= 0.004], smaller gray matter volume at discharge [−17.17 (−33.14, −1.19); p= 0.04], smaller hippocampal volumes at discharge [0.33 (0.01, 0.64); p=0.04], and smaller thalamic volumes at three months [−0.84 (−1.46, −0.23); p=0.01]. Age was not associated with volumes in other brain regions of interest. In our sensitivity analysis, adjusting for preexisting cognitive function (measured by the IQCODE-SF) did not significantly alter the relationship between delirium duration and the aforementioned brain volumes.
Brain volumes and neuropsychological outcomes
Greater VBR at three months, indicative of more brain atrophy, was associated with worse cognitive performance at 12 months (as defined via the global RBANS score) (Table 3). A patient who had a VBR of 5.3 (75th percentile of VBR at three-month imaging), compared to a patient with a VBR of 2.18 (25th percentile), had an average RBANS score 11 points lower at twelve-month testing [−11.17 (−21.12, −1.22); p=0.04]. Given that the mean 12-month RBANS score in our population was 79±14, a change of 11 points is clinically significant and reflects a level of cognitive performance that is “borderline” to “extremely low”. Borderline scores are defined via the RBANS as being between 70 and 79, with extremely low scores falling below 69. Similarly, smaller gray matter volumes, indicative of brain atrophy, measured at three months were also associated with worse cognitive performance (as defined via the global RBANS score) at both three- and twelve-month testing.
Table 3.
Relationship between Brain Volumes at Three months versus Long-term cognitive impairments at Twelve-month follow-up
| Neurocognitive domains and brain anatomical regions |
Neurocognitive scores differences (95% CI) associated with brain volumes |
P-value |
|---|---|---|
| Global cognitive performance (RBANS) | ||
| - VBR | −11.17 (−21.12, −1.22) | 0.04 |
| - Gray Matter, cm3 | 6.68 (0.27, 13.09) | 0.05 |
| - White Matter, cm3 | 3.71 (−2.84, 10.25) | 0.28 |
| Executive functioning | ||
| - Superior frontal lobe, cm3 | 4.78 (−0.32, 9.89) | 0.08 |
| - Thalamus, cm3 | 8.28 (1.59, 14.97) | 0.02 |
| - rACC, cm3 | 3.77 (−1.1, 8.63) | 0.14 |
| - Cerebellum, cm3 | 6.48 (0.63, 12.33) | 0.04 |
| Immediate Memory | ||
| - Hippocampus, cm3 | 3.35 (−1.56, 8.26) | 0.19 |
| Visual attention | ||
| - Superior frontal lobe, cm3 | 6.67 (2.78, 10.56) | 0.003 |
| - Thalamus, cm3 | 8.3 (2.74, 13.86) | 0.008 |
| - rACC, cm3 | 4.60 (0.58, 8.62) | 0.03 |
| - Cerebellum, cm3 | 7.96 (3.42, 12.49) | 0.002 |
The point estimate (95% confidence interval) represents differences in neurocognitive scores at twelve-month follow-up for patients with brain volumes at the 75th vs. the 25th percentile of our population. Tests reflect several different domains of cognitive function, as described in the Appendix 1
EXAMPLE: When considering the relationship of VBR at 3 months versus cognitive impairment at 12 months, a patient with a VBR of 5 (75th percentile of VBR of our population) had an RBANS global score which was, on average, 11 points lower than a patient with a VBR of 2.18 (25th percentile), indicating that more atrophy (i.e., greater ventricle-to-brain ratio) at three months was associated with worse global cognitive performance at 12 months. This neuropsychological test battery index score drop of 11 points could potentially represent a change in category (e.g., low average to borderline) according to the classification scheme shown for the RBANS in.Appendix 1
| Index Score | Classification |
|---|---|
| 130 and above | Very superior |
| 120–129 | Superior |
| 110–119 | High average |
| 90–109 | Average |
| 80–89 | Low average |
| 70–79 | Borderline |
| 69 and below | Extremely low |
All results adjusted for age and presence of sepsis in the ICU.
Abbreviations: CI, confidence interval; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; VBR, ventricle-to-brain ratio; rACC, rostral anterior cingulate cortex
Associations were also detected between worse executive functioning, as measured by the Trail Making Test Part B, and both smaller thalamic and smaller cerebellar volumes. Compared to a patient with a thalamic volume of 12.7 cm3 at three months, a patient with a thalamic volume of 10.7 cm3 would have, on average, a twelve-month Trails B score that is lower by 8.28 (1.59, 14.97) points [p = 0.02]. Similarly, a patient with a cerebellar volume of 95.6 cm3 at three months would have, on average, a twelve-month Trails B score 6.5 (0.63, 12.33) points lower than a patient with a volume of 112.5 cm3 [p = 0.04] (Table 3). For a patient with an executive functioning score of 40±13 (reflecting the mean of our population at 12 months), a 6- to 8- point decrease (as seen in the regression models for the thalamus and the cerebellum) would result in a change in classification from normal to mild/moderate impairment (scores between 40 to 60 are considered normal) (Appendix 1). There were trends towards an association between smaller superior frontal lobe volumes at three months and worse executive functioning (Table 3).
Smaller superior frontal lobe volume at three months was associated with worse visual attention scores at 12 months, as measured with the Trail Making Test Part A (Appendix 1). A patient with a superior frontal lobe volume of 30 cm3 (25th percentile) had a visual-attention score 6.7 points lower [95%CI (2.78, 10.56); p= 0.003] than a patient with a volume of 35 cm3 (75th percentile of superior frontal lobe volume at three-month scans). For a patient with a visual-attention score of 38 ± 11 (reflecting the mean of our population at 12 months), a 6 point decrease would result in a change in classification from normal to mild to moderate impairment (scores between 40 to 60 are considered normal). Similarly, smaller thalamus, rostral anterior cingulate, and cerebellum volumes at three months were associated with worse visual attention scores at twelve months.
No association was found between hippocampal volumes and scores on tests of immediate memory. Age was not associated with any cognitive outcomes at three- or twelve-month follow-up in our analysis. Sepsis during the ICU stay was associated with worse executive functioning at three months [−5.42 (−10.76, −0.07); p=0.05].
Discussion
To our knowledge, this hypothesis-generating study is the first prospective neuroimaging investigation to incorporate data on delirium duration as well as neuroanatomical and cognitive outcomes in a cohort of survivors of general medical and general surgical critical illness. Increased duration of delirium was associated with smaller total brain volumes (e.g. larger VBR) at hospital discharge and three-month follow-up in these ICU survivors. In addition, duration of delirium was associated with smaller superior frontal lobe and hippocampal volumes, which were stated a priori as regions of interest given their association with executive functioning and declarative memory, respectively –cognitive domains known to be commonly impaired in ICU survivors (3, 7, 9, 10). The second finding of this study was that smaller total brain volumes at three-month follow-up were associated with worse cognitive performance at twelve months. Associations were also detected between smaller cerebellum, thalamic, and rostral anterior cingulate volumes at three-month follow-up and worse executive functioning at 12 months. Finally, smaller superior frontal lobes, cerebellum, and thalamic volumes at three-month follow-up were associated with visual attention impairment at 12 months.
The findings of our investigation are preliminary and do not prove directionality. Nonetheless, they are informative and may be useful to generate important hypotheses regarding mechanisms subtending the development of cognitive impairment in patients who experience delirium during an ICU stay. Delirium duration was recently shown to be associated with worse cognitive performance in both ICU and non-ICU patients (6, 55). Combining these data with our findings, a direction for future research could be to examine the following hypotheses: A) delirium duration will be associated with brain atrophy; B) brain atrophy will be associated with cognitive impairment; C) delirium duration will be associated with cognitive impairment. Investigating these neuroanatomical and behavior hypotheses has the potential to provide insights into the pathogenesis of delirium and LTCI and brain behavior relationships in ICU survivors.
Recent studies have associated delirium with decreased cognitive function,(4–6, 55) yet the mechanism linking delirium to long-term cognitive impairment remains poorly understood (56). Nonspecific brain damage, such as occurs in critical illness, may result in general brain volume reduction manifested by reduced gyral volumes, increased sulcal space, ventricular enlargement, and an increase in cerebral spinal fluid (57).The ventricle-to-brain ratio or VBR (total brain volume divided by ventricular volume × 100) is indeed an excellent indicator of generalized brain atrophy (58).Our observations that delirium is associated with global brain atrophy (e.g., larger VBRs) and smaller superior frontal lobe and hippocampal volumes, areas associated with executive functioning and declarative memory, may suggest a neuroanatomical basis for understanding the link between delirium and LTCI.
The temporal relationship between the onset of delirium and the development of atrophy cannot be addressed by this study because of the inability to perform pre-ICU scans. It is thus possible that the brain atrophy in our patients preceded the ICU admission. We, however, undertook a number of steps to try to minimize the potential confounding due to lower baseline brain volumes. First, we excluded patients with severe dementia or those with significant neurological disease. Second, we used the IQCODE as a continuous variable, thus eliminating artificial cutoffs, and allowing us to better study its role as a confounder in its entire spectrum. Our low median IQCODE score of 3 (IQR 3,3.06) attests to the fact that our patient population did not have significant prior cognitive impairment, potentially reducing the likelihood of significant prior atrophy in these patients and thus the related confounding effect.
Our patient population was relatively young and with minimal preexisting cognitive impairment or neurological injury. Based on these baseline demographics and study exclusion criteria, one would not expect significant atrophy prior to ICU admission. Another potential cause of delirium, brain atrophy and LTCI observed in our patients is cerebral hypoperfusion. Several small studies (59, 60) have reported dramatic reduction in regional cerebral blood flow (rCBF) during acute delirium in the frontal and parietal lobes, thalamus, right temporal and occipital lobes and the pons. Reduced cerebral blood flow, if maintained for a sufficient length of time, would potentially result in cell death and neuronal loss, leading to atrophy. Additionally, systemic inflammation as in sepsis –a frequent diagnosis in critically ill patients– can lead to neuronal death and brain atrophy (61, 62).
Brain volume loss in our patients was present at hospital discharge and 3 months. It is important to note that studies have reported brain atrophy may occur over a period of days rather than months, (63–66) raising the possibility that atrophic processes for these patients may have occurred during hospitalization. Animal studies have also shown significant hippocampal cell loss within hours of sepsis induction using a lipopolysaccharide model (67), although in our investigation, no such association was identified. Similarly, hippocampal atrophy has been reported in pigs within 12 hours after an induction of acute respiratory distress syndrome–another common syndrome in critically ill patients (68).
The association between brain atrophy at three months and neuropsychological outcomes at twelve months parallels findings in patients who experienced TBI and anoxic brain injury (15–17). In fact, previous studies showed global atrophy (as expressed by VBR) and hippocampal atrophy were correlated with cognitive performance and memory impairments in patients who experienced TBI and anoxic brain injury (15–17).In our population, larger VBRs were associated with worse global cognitive performance. Supportive of the same finding is that larger frontal lobes volumes –here indicative of less atrophy– were associated with better visual attention test scores. A recent longitudinal non-ICU cohort (69) also found an association between frontal lobe atrophy and early and prolonged executive dysfunction, linking MRI findings with cognitive impairment. In our patients, associations between volumes of specific areas of the brain (i.e., cerebellum, thalamus, and rostral anterior cingulate) and executive functioning were also detected. We did not find a relationship between hippocampal volumes and memory as reported in patients with TBI and anoxic brain injury (16), though this could be a type II error. These data further emphasize the cognitive impairments observed in our ICU survivors involving executive functioning, memory and attention are common following critical illness, similar to reports from previous studies (3, 7, 9, 10).
Our study has several strengths and limitations that warrant discussion. Important strengths of this study include the prospective cohort design, uniform protocol of MRI scanning at 2 time points, and detailed in-hospital data collection, including the delirium assessments performed twice daily by trained research personnel using a valid and reliable delirium assessment tool (the CAM-ICU) (23, 24) and sedation scale (the RASS) (25, 26). Additionally, brain volumes were obtained using an automated image analysis program; investigators who assessed brain volumes were blind to patient identity and all medical information. While significant atrophy is obvious on brain imaging, using an automated volumetric analysis reduces potential investigator bias.
Important limitations include the population included in this study, which represented a convenience sample of patients admitted to the ICUs during the study period. Another important limitation was the sample size, which limited our ability to reliably adjust for a multitude of potential confounders. At the same time, this study is one of the largest prospective neuroimaging investigations in survivors of critical illness, and we prospectively designed it to allow for the adjustment of key variables (age, sepsis, and severity of illness, with preexisting cognitive impairment in a sensitivity analysis) while at the same time avoiding overfitting. Nonetheless, we were not able to include other important confounding factors such as drug and alcohol abuse, use of psychotropic medications, and history of psychiatric disorders. As in most studies of emergently critically ill patients, we were unable to study our patients prior to their acute ICU admission, and as such pre-illness brain volumes could not be obtained. As stated previously, it is possible that brain atrophy was present in some patients prior to critical illness. Therefore we cannot with certainty determine the temporality of the association between delirium and brain atrophy. The many variables of brain volume and cognitive outcomes recorded over twelve months may have increased the chance of type 1 error. We addressed this by limiting the outcomes to a global measure of atrophy (VBR) and specific regions of interest chosen a priori, and by avoiding voxel-wise analyses; however, there is still the possibility of finding significant relationships due solely to chance. Finally, patients with delirium at discharge were not included in this study because these patients are unable to follow commands to perform the required tasks in the MRI scanner. Nonetheless, future larger studies should evaluate these delirious patients for alterations in brain perfusions, though ability to perform imaging might be limited by hyperactive behavior.
Conclusions
As more people survive their ICU stays due to advancement of technology, many find themselves unable to return to their previous state of functioning, employment and cognitive abilities. Neuroimaging offers a rarely tapped, quantitative method by which occult patterns of brain injury may be identified and measured in ICU survivors. The results of our study offer preliminary information for the development of a testable hypothesis, which may connect delirium duration with brain structural integrity and cognitive impairment in ICU survivors. Future studies are now needed, using both anatomical and functional neuroimaging, to evaluate further these preliminary findings and to elucidate mechanisms of any potentially modifiable exposures for this apparent ICU-accelerated or ICU-acquired brain injury.
Acknowledgement
Funding: National Institutes of Health (AG027472, AG034257, RR024975, EB001628), Saint Thomas Foundation (Nashville, TN) and Veterans Affairs Tennessee Valley Geriatric Research, Education, and Clinical Center (GRECC)
Appendix: Neurocognitive Assessments
| Test | Description | Area Measured | Scoring |
|---|---|---|---|
| Global RBANS (35)a | This score is a composite score of the five domains included in the RBANS: immediate memory, delayed memory, attention, language, visuospatial/constructional | Global neuropsychological functioning | Age and education adjusted index scores which rely on the following classification scheme:
Index Score Classification 130 and above Very superior 120–129 Superior 110–119 High average 90–109 Average 80–89 Low average 70–79 Borderline 69 and below Extremely low |
| Digit Span (35) | A test requiring a subject to listen and immediately repeat a progressively longer sequence of digits forward and backward. | Attention/concentration | Scores from the Digit Span subtest contribute to the Attention Index Score. |
| Digit symbol coding (35) | A timed test requiring a subject to populate a series of empty boxes using an answer key. | Information/processing speed | Scores from the Digit Symbol Coding subtest contribute to the Attention Index Score. |
| List Learning (35) | A test requiring a subject to repeat as many words as possible (from a list of 10) over a period of 4 trials. | Immediate memory | Scores from the List Learning subtest contribute to the Immediate Memory Index Score. |
| Story memory (35) | A test requiring a subject to repeat a short story from memory over a total of 2 trials. | Immediate memory | Scores from the Story Memory subtest contribute to the Immediate Memory Index Score. |
| Figure copy (35) | A test requiring a subject to copy a complex geometric design. | Visuospatial/Constructional | Scores from the Figure Copy subtest contribute to the Visuospatial/Constructional Index Score. |
| Line orientation (35) | A test requiring a subject to match 2 lines on a page in front of them with 2 lines from a picture containing a series of lines. | Visuospatial/Constructional | Scores from the Line Orientation subtest contribute to the Visuospatial/Constructional Index Score. |
| Picture naming (35) | A test requiring a subject to identify and accurately provide the names of objects presented to them via a series of pictures. | Language | Scores from the Picture Naming subtest contribute to the Language Index Score. |
| Semantic fluency (35) | A test requiring a subject to name as many examples from a given semantic category such as fruits and vegetables or animals. | Language | Scores from the Semantic Fluency subtest contribute to the Language Index Score. |
| Trailmaking Test A (38) | A timed test requiring subjects to connect a series of consecutive numbers on a page. | Visual attention | T-scores adjusted by age, education, gender, and ethnicity. T-scores of between 40–60 are considered normal Lower scores reflect worse performance. (39) |
| Trailmaking Test B (38) | A timed test requiring subjects to connect a series of alternating numbers and letters on a page in a specified sequence. | Executive functioning | T-scores adjusted to age, education, gender, and ethnicity using the Heaton manual. T-scores between 40–60 are considered normal. Lower scores reflect worse performance. (39) |
Abbreviations: RBANS, repeatable battery for the assessment of neuropsychological status
The Global RBANS score is a composite index score derived from scores the five index scores - immediate memory, delayed memory, attention, language, and visuospatial/constructional. Lower scores on the RBANS, as reflected in the classification scheme, reflect poorer performance. The Global RBANS score as well as the five index scores have a mean of 100 and a SD of 15 and can be interpreted accordingly – e.g. scores of 85 and 115 and 1 SD below the mean and 1 SD above the mean, respectively. Index scores are generated from raw subtest scores, as reflected in the Table.
Appendix 2
Brain volumes at hospital discharge and at three-month follow-up a
| Anatomical Region | Hospital Discharge Brain volumes for the entire cohort (N=47) |
Three-month Follow-up Brain volumes for the entire cohort (N=47) |
Hospital Discharge Brain volumes for patients scanned at discharge and at three-month follow-up (N=36) |
Three-month Follow-up Brain volumes for patients scanned at discharge and at three-month follow-up (N=36) |
|---|---|---|---|---|
| VBR | 3.5 (2.4, 5.3); 4±1.9 | 3.8 (2.2, 5.0); 3.8±1.7 | 3.4 (2.2, 5.3); 3.9±1.8 | 3.8 (2.2, 5.0); 3.8±1.7 |
| Gray Matter, cm3 | 366 (346, 394); 373±45 | 348 (375, 403); 381±50 | 365 (347, 394); 374±46 | 375 (348, 403); 381±50 |
| White Matter, cm3 | 496 (457, 521); 496±66 | 483 (−443, 524); 489±70 | 488 (455, 521); 494±70 | 483 (443, 524); 484±70 |
| Hippocampus, cm3 | 6.4 (6.04, 6.68); 6.2±0.97 | 6.4 (5.9, 6.8); 6.2±1.1 | 6.5 (6.0, 6.7); 6.1±1.1 | 6.4 (5.9, 6.8); 6.2±1.1 |
| Superior Frontal Lobe, cm3 | 31.8 (29.7, 34.5); 32.5±4.8 | 33 (30, 35); 33±5 | 31.9 (29.8, 34.4); 32.4±4.8 | 33 (30, 35); 33±5 |
| Frontal Lobe, cm3 | 133 (123, 139); 134±18 | 133 (121, 144); 133±27 | 134 (120, 139); 134±19 | 133 (121, 144); 133±27 |
| Thalamus, cm3 | 11.5 (10.7, 12.4); 11.7±1.7 | 11.6 (10.7, 12.7); 11.7±1.7 | 11.5 (10.6, 12.4); 11.6±1.5 | 11.6 (10.7, 12.7); 11.7±1.7 |
| Precuneus, cm3 | 15.2 (14.2, 16.3); 15.4±2.4 | 15.8 (14.4, 17.0); 15.9±2.4 | 15.4 (14.2, 16.3); 15.6±2.1 | 15.8 (14.4, 17.0); 15.9±2.4 |
| rACC, cm3 | 3.2 (2.89, 3.54); 3.3±0.6 | 3.16 (2.89, 3.42); 3.2±0.6 | 3.2 (2.91, 3.54); 3.3±0.5 | 3.2 (2.89, 3.42); 3.2±0.6 |
| Cerebellum, cm3 | 104 (96, 113); 105±13 | 104 (96, 113); 106±15 | 105 (94, 114); 105±14 | 104 (96, 113); 106±15 |
All results expressed as median (interquartile range) and mean ± SD.
Abbreviations: CI, confidence interval; VBR, ventricle-to-brain ratio; rACC, rostral anterior cingulate cortex
Abbreviations: CI, confidence interval; VBR, ventricle-to-brain ratio; rACC, rostral anterior cingulate cortex
Neurocognitive scores at three and at twelve-month follow-upa
| Cognitive domains | Neurocognitive scores at three-month follow-upb |
Neurocognitive scores at twelve- month follow-upb |
|---|---|---|
| Global cognitive performance (RBANS) | 83 (76, 89); 82 ±10 | 79 (69, 92); 79±14 |
| - Extremely low, N (%) | 14% (6) | 26% (8) |
| - Borderline, N (%) | 28% (12) | 26% (8) |
| - Low average, N (%) | 33% (14) | 16% (5) |
| - Average, N (%) | 26% (11) | 32% (10) |
| - High average, N (%) | 0% (0) | 0% (0) |
| - Superior, N (%) | 0% (0) | 0% (0) |
| - Very superior, N (%) | 0% (0) | 0% (0) |
| Delayed Memory (RBANS) | 88 (82, 97); 88.4±9.7 | 76 (65, 95); 78±18 |
| - Extremely low, N (%) | 2% (1) | 29% (10) |
| - Borderline, N (%) | 11% (5) | 24% (8) |
| - Low average, N (%) | 43% (19) | 12% (4) |
| - Average, N (%) | 43% (19) | 35% (12) |
| - High average, N (%) | 0% (0) | 0% (0) |
| - Superior, N (%) | 0% (0) | 0% (0) |
| - Very superior, N (%) | 0% (0) | 0% (0) |
| Immediate Memory (RBANS) | 87 (80, 103); 90±14 | 83 (76, 94); 84±17 |
| - Extremely low, N (%) | 11% (5) | 21% (7) |
| - Borderline, N (%) | 14% (6) | 18% (6) |
| - Low average, N (%) | 27% (12) | 26% (9) |
| - Average, N (%) | 43% (19) | 32% (11) |
| - High average, N (%) | 5% (2) | 0% (0) |
| - Superior, N (%) | 0% (0) | 3% (1) |
| - Very superior, N (%) | 0% (0) | 0% (0) |
| Attention (RBANS) | 88 (72, 100); 86±16 | 85 (80, 104); 91±20 |
| - Extremely low, N (%) | 18% (8) | 13% (4) |
| - Borderline, N (%) | 18% (8) | 13% (4) |
| - Low average, N (%) | 16% (7) | 32% (10) |
| - Average, N (%) | 43% (19) | 23% (7) |
| - High average, N (%) | 5% (2) | 13% (4) |
| - Superior, N (%) | 0% (0) | 3% (1) |
| - Very superior, N (%) | 0% (0) | 3% (1) |
| Visuospatial construction (RBANS) | 72 (64, 84); 75±14 | 78 (64, 84); 74±13 |
| - Extremely low, N (%) | 43% (19) | 39% (13) |
| - Borderline, N (%) | 16% (7) | 24% (8) |
| - Low average, N (%) | 30% (13) | 30% (10) |
| - Average, N (%) | 9% (4) | 6% (2) |
| - High average, N (%) | 2% (1) | 0% (0) |
| - Superior, N (%) | 0% (0) | 0% (0) |
| - Very superior, N (%) | 0% (0) | 0% (0) |
| Language (RBANS) | 91 (86, 98); 91±10 | 90 (87, 96); 92±10 |
| - Extremely low, N (%) | 2% (1) | 3% (1) |
| - Borderline, N (%) | 7% (3) | 3% (1) |
| - Low average, N (%) | 26% (11) | 39% (13) |
| - Average, N (%) | 65% (28) | 48% (16) |
| - High average, N (%) | 0% (0) | 3% (1) |
| - Superior, N (%) | 0% (0) | 3% (1) |
| - Very superior, N (%) | 0% (0) | 0% (0) |
| Visual attention | ||
| (Trailmaking Test Part A) Executive functioning | 40 (31.8, 45.2); 39.3±9.4 | 40 (32, 46); 38±11 |
| (Trailmaking Test Part B) | 40 (35, 47); 42±9.1 | 38 (31, 50); 40±13 |
All results expressed as median (interquartile range) and mean (SD), unless otherwise noted.
| Index Score | Classification |
|---|---|
| 130 and above | Very superior |
| 120–129 | Superior |
| 110–119 | High average |
| 90–109 | Average |
| 80–89 | Low average |
| 70–79 | Borderline |
| 69 and below | Extremely low |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest
Reference List
- 1.Williams TA, Dobb GJ, Finn JC, et al. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36:1523–1530. doi: 10.1097/CCM.0b013e318170a405. [DOI] [PubMed] [Google Scholar]
- 2.Gunther ML, Jackson JC, Ely EW. The Cognitive Consequences of Critical Illness: Practical Recommendations for Screening and Assessment. Critical Care Clinics. 2007;23:491–506. doi: 10.1016/j.ccc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 4.Jones C, Griffiths RD, Slater T, et al. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32:923–926. doi: 10.1007/s00134-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 5.Sukantarat KT, Burgess PW, Williamson RC, et al. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60:847–853. doi: 10.1111/j.1365-2044.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 6.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson JC, Girard TD, Gordon SM, et al. Long-Term Cognitive and Psychological Outcomes in the Awakening and Breathing Controlled Trial. Am J Respir Crit Care Med. 2010;182:183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlenbach WJ, Hough CL, Crane PK, et al. Association Between Acute Care and Critical Illness Hospitalization and Cognitive Function in Older Adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins RO, Jackson JC. Short- and long-term cognitive outcomes in intensive care unit survivors. Clin Chest Med. 2009;30:143–153. doi: 10.1016/j.ccm.2008.11.001. ix. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Collingridge D, et al. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 11.Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. J Head Trauma Rehabil. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bigler ED, Alfano M. Anoxic encephalopathy: neuroradiological and neuropsychological findings. Archives of Clinical Neuropsychology. 1988;3:383–396. [PubMed] [Google Scholar]
- 13.Greer DM. MRI in anoxic brain injury. Neurocrit Care. 2004;1:213–215. doi: 10.1385/NCC:1:2:213. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins RO, Bigler ED. Hypoxic and anoxic conditions of the CNS. In: Morgan JE, Ricker JH, editors. Textbook of Clinical Neuropsychology. Swets & Zeitlinger; 2005. [Google Scholar]
- 15.Hopkins RO, Gale SD, Johnson SC, et al. Severe anoxia with and without concomitant brain atrophy and neuropsychological impairments. Journal of the International Neuropsychological Society. 1995;1:501–509. doi: 10.1017/s135561770000059x. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins RO, Tate DF, Bigler ED. Anoxia versus traumatic brain injury: amount of tissue loss not etiology, alters cognitive and emotional function. Neuropsychology. 2005;19:233–242. doi: 10.1037/0894-4105.19.2.233. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SC, Bigler ED, Burr RB, et al. White matter atrophy, ventricular dilatation, and intellectual functioning following traumatic brain injury. Neuropsychology. 1994;8:307–315. [Google Scholar]
- 18.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 20.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 27.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 28.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Manual. San Antonio: Psychological Corporation; 1998. [Google Scholar]
- 29.Benitez A, Horner MD, Bachman D. Intact cognition in depressed elderly veterans providing adequate effort. Arch Clin Neuropsychol. 2011;26:184–193. doi: 10.1093/arclin/acr001. [DOI] [PubMed] [Google Scholar]
- 30.Landgraff NC, Whitney SL, Rubinstein EN, et al. Cognitive and physical performance in patients with asymptomatic carotid artery disease. J Neurol. 2010;257:982–991. doi: 10.1007/s00415-009-5449-z. [DOI] [PubMed] [Google Scholar]
- 31.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 32.Reitan RM, Wolfson D. The Halstead Reitan Neuropsychological Test Battery. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 33.Bigler ED, Neeley ES, Miller MJ, et al. Cerebral volume loss, cognitive deficit and neuropsychological performance: comparative measures of brain atrophy: I. Dementia. J Int Neuropsychol Soc. 2004;10:442–452. doi: 10.1017/S1355617704103111. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20:263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- 35.Christie JD, Biester RC, Taichman DB, et al. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care. 2006;21:125–132. doi: 10.1016/j.jcrc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Mesulam MM. Priciples of Behavioral and Cognitive Neurology. New York: Oxford University Press; 2000. [Google Scholar]
- 37.Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.08.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 39.Janz DR, Abel TW, Jackson JC, et al. Brain autopsy findings in intensive care unit patients previously suffering from delirium: a pilot study. J Crit Care. 2010;25:538.e7–538.e12. doi: 10.1016/j.jcrc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunther ML, Jackson JC, Ely EW. Loss of IQ in the ICU brain injury without the insult. Med Hypotheses. 2007 doi: 10.1016/j.mehy.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 41.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 42.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 43.Segonne F, Pacheco J, Fischl B. Geometrically Accurate Topology-Correction of Cortical Surfaces Using Nonseparating Loops. Medical Imaging, IEEE Transactions on. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Salat DH, van der Kouwe AJW, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Salat DH, Busa E, et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. Medical Imaging, IEEE Transactions on. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dale AM, Sereno MI. Improved Localization of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 50.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 51.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Medical Imaging, IEEE Transactions on. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 52.Henon H, Pasquier F, Durieu I, et al. Medial temporal lobe atrophy in stroke patients: relation to pre-existing dementia. J Neurol Neurosurg Psychiatry. 1998;65:641–647. doi: 10.1136/jnnp.65.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 54.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning five decades of life. American Journal of Neuroradiology. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 55.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maclullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 57.Graham DI, Gennarelli TA, McIntosh TK. Trauma. In: Graham DI, Lantos PI, editors. Greenfield's Neuropathology. Seventh Edition. London, Arnold: Hodder Headline Group; 2002. [Google Scholar]
- 58.Bigler ED. Neuroimaging correlates of functional outcome. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury and Medicine: Principles and Practice. New York, Demos: 2007. pp. 201–224. [Google Scholar]
- 59.Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 60.Fong TG, Bogardus ST, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. Journal of Gerontology: Medical Sciences. 2007;61A:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 61.Semmler A, Okulla T, Sastre M, et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Sharshar T, Annane D, de la Grandmaison GL, et al. The neuropathology of septic shock. Brain Pathol. 2004;14:21–33. doi: 10.1111/j.1750-3639.2004.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 64.Gordon N. Apparent cerebral atrophy in patients on treatment with steroids. Dev Med Child Neurol. 1980;22:502–506. doi: 10.1111/j.1469-8749.1980.tb04355.x. [DOI] [PubMed] [Google Scholar]
- 65.Suchyta MR, Jephson A, Hopkins RO. Neurologic Changes during Critical Illness: Brain Imaging Findings and Neurobehavioral Outcomes. Brain Imaging and Behavior. 2010;4:22–34. doi: 10.1007/s11682-009-9082-3. [DOI] [PubMed] [Google Scholar]
- 66.Lagenstein I, Kühne D, Sternowsky HJ, et al. Computerized Cranial Transverse Axial Tomography (CTAT) in 145 Patients with Primary and Secondary Generalized Epilepsies GÇô West Syndrome, Myoclonic-Astatic Petit Mal, Absence Epilepsy. Neurop+ñdiatrie. 1979;10 doi: 10.1055/s-0028-1085310. 15,28. [DOI] [PubMed] [Google Scholar]
- 67.Semmler A, Frisch C, Debeir T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Fries M, Bickenbach J, Henzler D, et al. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology. 2005;102:761–767. doi: 10.1097/00000542-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Cardenas VA, Chao LL, Studholme C, et al. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]