Abstract
We studied cortical connections of functionally distinct movement zones of the posterior parietal cortex (PPC) in galagos identified by intracortical microstimulation with long stimulus trains (~500 msec). All these zones were in the anterior half of PPC, and each of them had a different pattern of connections with premotor (PM) and motor (M1) areas of the frontal lobe and with other areas of parietal and occipital cortex. The most rostral PPC zone has major connections with motor and visuomotor areas of frontal cortex as well as with somatosensory areas 3a and 1-2 and higher order somatosensory areas in the lateral sulcus. The dorsal part of anterior PPC region representing hand-to-mouth movements is connected mostly to the forelimb representation in PM, M1, 3a, 1-2, and somatosensory areas in the lateral sulcus and on the medial wall. The more posterior defensive and reaching zones have additional connections with nonprimary visual areas (V2, V3, DL, DM, MST). Ventral aggressive and defensive face zones have reciprocal connections with each other as well as connections with mostly face, but also forelimb representations of premotor areas and M1 as well as prefrontal cortex, FEF, and somatosensory areas in the lateral sulcus and areas on the medial surface of the hemisphere. Whereas the defensive face zone is additionally connected to nonprimary visual cortical areas, the aggressive face zone is not. These differences in connections are consistent with our functional parcellation of PPC based on intracortical long-train microstimulation, and they identify parts of cortical networks that mediate different motor behaviors.
Keywords: intraparietal cortex, motor areas, somatosensory cortex, visual cortex, movement, behavior
The posterior parietal cortex (PPC) of primates is known to be involved in sensorimotor functions, as connections with visual and somatosensory areas as well as frontal motor and premotor areas suggest (Cavada and Goldman-Rakic, 1989a,b; for review see Cavada and Goldman-Rakic, 1993; Lewis and Van Essen, 2000a; Cavada, 2001; Matelli and Luppino, 2001; Grefkes and Fink, 2005). There have been many efforts to subdivide PPC into subregions or cortical areas of functional significance, including those based on cortical architecture, patterns of connections, microelectrode recordings, fMRI in humans, and even microstimulation (Pandya and Seltzer, 1982; Cavada and Goldman-Rakic, 1989a,b, 1993; Lewis and Van Essen, 2000a,b; Geyer et al., 2005; Grefkes and Fink, 2005). Recently, Graziano and coworkers (2002; for review see Graziano and Aflalo, 2007) introduced an additional way to parcellate motor and premotor cortex in macaques based on behavior evoked by the stimulation with long trains of electrical pulses (see companion paper), and they extended this approach to the intraparietal cortex (Cooke et al., 2003; Graziano and Cooke, 2006). These authors evoked defensive movements of the hand and head from a region of PPC, which they identified as the ventral intraparietal area (VIP). Our own studies based on the long stimulus train approach were directed toward prosimian galagos, in part because we are very interested in determining differences and similarities in the brain organization across primate taxa and in part because the intraparietal sulcus is short and shallow in galagos so that proportionally more of the PPC is accessible on the dorsolateral cortical surface. Somewhat to our surprise, we discovered that complex movements could be evoked from stimulation sites throughout the anterior half of PPC in anesthetized galagos, which simplified the task of using extensive microelectrode stimulation series to explore the functional organization of the region. The results allowed us to divide anterior PPC into separate regions where retrieving, reaching, defensive, and aggressive behaviors could be evoked, while conforming to an overall somatotopy with hindlimb and forelimb “climbing” movements from the most medial sites, followed by forelimb and the face movements more laterally (Stepniewska et al., 2005a, 2009). As the locations of these movement zones were in the same relative locations across individuals, we reasoned that their connections could be studied and that the different types of movements evoked from each region likely reflected different patterns of connections with motor areas of the frontal lobe. In addition, we postulated that each type of behavior would be guided by a different pattern of sensory inputs, so that inputs from visual areas, areas of the posterior half of PPC with visual inputs, and somatosensory areas would also differ. Thus, we used long-train microstimulation procedures to identify zones of cortex involved in different types of complex movements, injected tracers into the functionally defined zones, and then plotted the ipsilateral cortical connections of these zones. Specifically, we studied the connections of hand-to-mouth, defensive, and reaching regions of the forelimb movement zone and defensive and aggressive regions of the face movement zone. An abstract of some of the present findings has been presented elsewhere (Stepniewska et al., 2005b).
MATERIALS AND METHODS
Experiments were carried out in nine galagos (Otolemur garnetti). In seven of these galagos, intracortical microstimulation (ICMS) with long (500 msec) trains of electrical pulses was used to delineate functional zones in the PPC, and injections of different anatomical tracers were placed in functionally defined zones to reveal their connections. In two other galagos, PPC injections were made without physiological control. Altogether, 16 tracer injections were made in zones of cortex around the intraparietal sulcus devoted to different classes of complex movements. In the same galagos, an additional 11 injections were made in the posterior visually related part of PPC that was unresponsive to ICMS, and these results will be published separately. See Table 1 for a summary of all injection cases. All experimental procedures followed the guidelines of the National Institutes of Health Guide for the care and use of laboratory animals and were approved by the Vanderbilt University Animal Care and Use Committee.
TABLE 1.
Summary of Experimental Cases
| Case# | Plane of section | Section thickness μm | ICMS | Anterior PPC |
Posterior PPC |
||
|---|---|---|---|---|---|---|---|
| Dorsal Forelimb zone | Ventral Face zone | Dorsal | Ventral | ||||
| 04–04L | flat | 50 | yes | FR(hm) | BDA(d) | FE | |
| 04–07L | flat | 50 | yes | FR(hm),FE(d) | BDA(d) | CTB | |
| 04–26L | flat | 50 | no | BDA | FR | FB | |
| 04–39L | flat | 50 | yes | FR(d) | FE(d) | BDA | FB |
| 04–47L | flat | 50 | no | FE | FR | BDA | |
| 05–15L* | coronal | 50/40 | yes | FB(hm) | WGA-HRP(a) | FR | BDA |
| 05–20L | coronal | 50 | yes | WGA-HRP(a) | FR | BDA | |
| 05–28L | coronal | 50 | yes | FR(a) | |||
| 08–15L | flat | 50 | yes | CTB(r),FB(5?) | |||
in case 05–15 the brain was cut into two blocks anterior and posterior that were cut into 50 and 40um sections, respectively. Movements: d, defensive; hm, hand to mouth; r, reach; a, aggressive.
Surgery, microstimulation, and tracer injections
Surgical operations were performed under aseptic conditions as described in details in our companion article (Stepniewska et al., 2009). After we exposed the left hemisphere of the brain, the region around the intraparietal sulcus was explored with stimulating microelectrodes to identify areas in the PPC engaged in different complex movements. After a short-lasting microstimulation session that was used to locate the sites for injections, we placed different tracers [wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP) 2% in distilled water (Sigma, St. Louis, MO); biotinylated dextran amine (BDA) 10% in 10 mM phosphate buffer (Molecular Probes, Eugene, OR); fluororuby (FR), fluoroemerald (FE), 10% in distilled water (Sigma); fast blue (FB) 3% in distilled water (Sigma)] into three or four physiologically defined zones in the PPC. Tracers were pressure injected with a 1–5-μl Hamilton syringe. A total of approximately 0.05 μl of WGA-HRP and 1 μl of other tracers was injected at two depths, 1.5 mm and 1 mm below the pial surface. After the injections, cortex was covered with gelfilm, the skull was closed by cementing a cap made of dental cement, and the skin was sutured. Animals were allowed to recover from anesthesia and were carefully monitored during recovery, receiving antibiotics and analgesics as needed. After 5–7 days, galagos were anesthetized, and the skull was reopened for a more extensive session of microstimulation of additional points in the injected hemisphere. In addition to stimulating the posterior parietal region to complete the ICMS map, we stimulated parts of the frontal motor region (see Stepniewska et al., 2009) to identify functional regions where transported label from the tracer injections was expected. At the end of the stimulation session, small electrolytic lesions were placed to mark the borders between functional zones and other sites of interest. Animals were then given a lethal dose of barbiturate and perfused through the heart, first with phosphate-buffered saline (PBS; 0.9% NaCl) and then with fixative (2–4% paraformaldehyde), followed by fixative with 10% sucrose. The brain was removed, and in most cases cortex was separated from the thalamus, manually flattened (see Stepniewska et al., 2003), and stored overnight in 30% sucrose at 4°C.
Tissue processing and data analysis
Tissue sections were cut on a freezing microtome either parallel to the surface of flattened cortex or in the coronal plane at 50 μm thickness (see Table 1). Alternating sets of sections were processed to reveal transported tracers or architectonic features of the cortex. The sections processed for tracers were divided into serial sets, with one set mounted on glass unstained for examination of neurons with retrogradely transported fluorescent dyes. Another set of sections with WGA-HRP, BDA, or CTB as labels were processed according to the existing protocols (Gibson et al., 1984; Bruce and Grofova, 1992; Veeman et al., 1992). When injections of two or three of these tracers were placed in the same case, procedures were used to reveal each of them on the same sections. Sections treated for HRP were reacted with tetramethylbenzidine (TMB) as chromogen and ammonium molybdate (Olucha et al., 1985) and stabilized in diamidinobenzidine (DAB). To reveal the BDA, sections were incubated in a 1:500 dilution of avidin-biotin-peroxidase complex (Vector ABC Elite kit; Vector Laboratories, Burlingame, CA) according to the protocol of Veeman et al. (1992). Finally, the immunocytochemical reaction was performed to reveal CTB (Bruce and Grofova, 1992). The sections were rinsed and blocked in 3% normal rabbit serum and incubated in a 1:5,000 dilution of goat anticholera toxin B subunit (List Biological, Campbell, CA) at 4°C, rinsed again, and placed in a 1:200 dilution of biotinylated rabbit anti-goat IgG (Vector Laboratories). CTB antibody was raised against purified cholera toxin B subunit, and reacted tissue from uninjected animals did not produce any staining. The CTB reaction product was visualized by using the avidin-biotin-immunoperoxidase method with a Vectastain ABC kit (Vector Laboratories) and Vector VIP substrate kit (Vector Laboratories SK 4600).
For cortical architecture, a set of flattened cortex sections was stained for myelinated fibers (Gallyas, 1979) and another set for cytochrome oxidase (CO; Wong-Riley, 1979). For brains cut coronally, an additional set of sections was stained for Nissl substance. Labeled neurons were plotted at high resolution with Leitz microscope coupled to an x,y encoder and a Macintosh G3 computer running Igor Pro Software. Patterns of label were superimposed and aligned with architectonic borders drawn from adjacent CO-, myelin-, or Nissl-stained sections by using landmarks such as blood vessels, lesions, and injection sites. For each case, we prepared a composite drawing showing the relationship of injection sites and transported label to the electrophysiological and architectonic maps of posterior parietal, motor, and premotor areas. The architectonic definitions of cortical regions follows those of Preuss and Goldman-Rakic (1991a) and Fang et al. (2005). All final reconstructions were composed in Canvas 8.0 software (Deneba, Inc., Miami, FL).
RESULTS
Our microstimulation (ICMS) study has shown that long-train stimulation in galagos evokes different meaningful behaviors in different parts of anterior PPC (Stepniewska et al., 2005a, 2009). Several functionally different zones were identified within the anterior PPC region. Here we describe the ipsilateral cortical connections of these zones. The patterns of ipsilateral connections were determined for 16 tracer injections in nine galagos (Table 1). The locations of these injections in a standardized view of the functional zones of PPC are indicated in Figure 1. Seven injections were placed dorsal to the intraparietal sulcus (IPS), eight just below the IPS, and one just anterior to the IPS. Dorsal PPC injections were in forelimb complex movement zones, and ventral PPC injections involved face movement zones. The exact locations of injection sites with respect to the physiological zones identified in each case are shown in the companion paper (Stepniewska et al., 2009). To reveal the overall pattern of connections, as well as their laminar distribution, the distributions of label are presented on flattened views of the left cortex (six cases) or in a series of coronal sections through the left hemisphere (three cases).
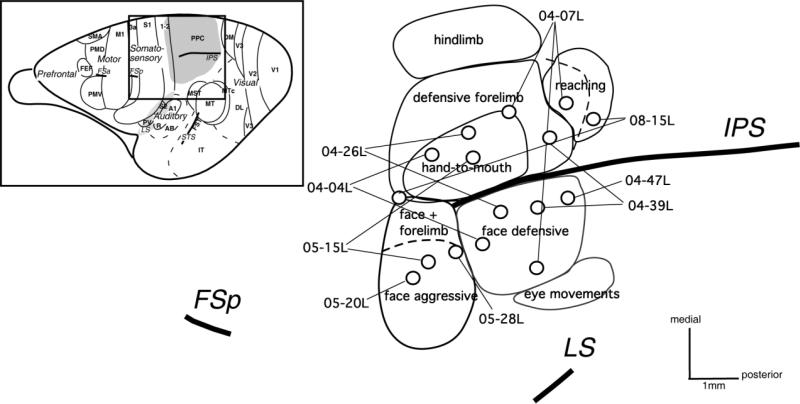
Figure 1.
The location of injection sites (circles) in the behavioral PPC zones (outlined by solid lines) in all studied galagos. In total, 16 injections were made in the responsive-to-microstimulation part of PPC. Seven injections are located in the dorsal forelimb PPC region, with three injections in hand-to-mouth zone, two injections in defensive forelimb zone, one at the border of these two zones, and one in the reaching zone. Injection in case 04-07L, although in the defensive face zone, is located as caudally as the reaching zone in other studied galagos (dashed line shows the caudal limit of defensive forelimb zone in this case, which expands much more caudally than in other cases). Eight injections were in the ventral PPC face region, three in face aggressive zone and five in face defensive zone. The most rostral injection in case 08-15L (probably in area 5) was located in the region devoted to forearm extension combined with face defensive movement. Left upper corner: Dorsolateral view of the galago brain with areas and sulci indicated and the stimulated PPC region marked with a gray pattern. Scale bar = 1 mm.
Definition of cortical areas
Cortical areas and functional zones within the posterior parietal and motor regions were identified by physiological and architectonic criteria. We used microstimulation to identify and distinguish motor and PPC areas by their patterns of evoked movements and the current levels needed to evoke these movements. Generally, both posterior parietal and motor regions were characterized by systematic representations of body movements from hindlimb to face in a mediolateral sequence across the cortex, but current levels required to evoke movements were significantly lower for M1 than for other regions. On the other hand, PPC required the highest levels of current (~300 μA) and long-train stimuli to evoke movements, whereas the short-train stimulation that reliably evoked movements from primary motor cortex (M1) and dorsal and ventral premotor areas (PMD and PMV) was not effective for PPC. The physiological borders between areas, somatotopic representations, and behavioral zones in M1, PM, and PPC were variously marked with microlesions, so that physiological results could be related to cortical architecture (see Figs. 2–14).
Figure 2.
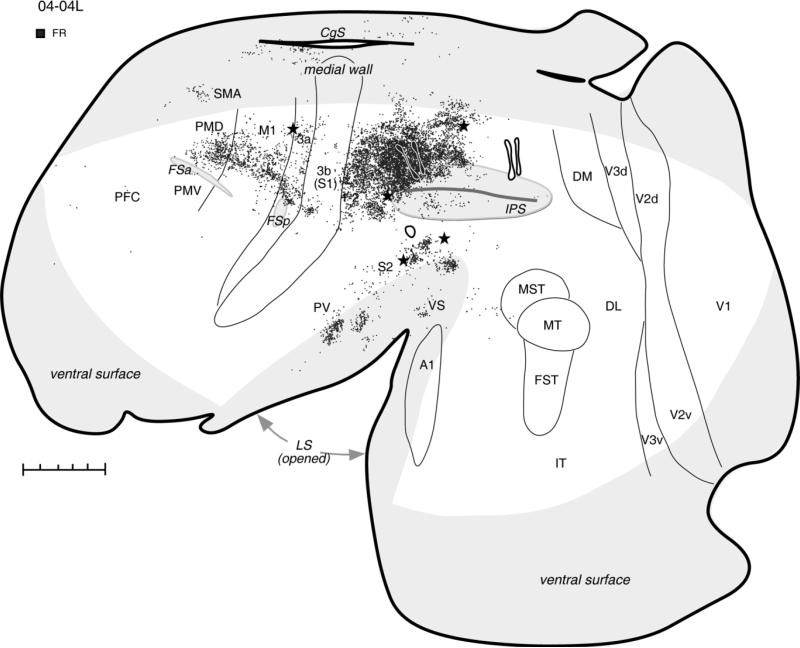
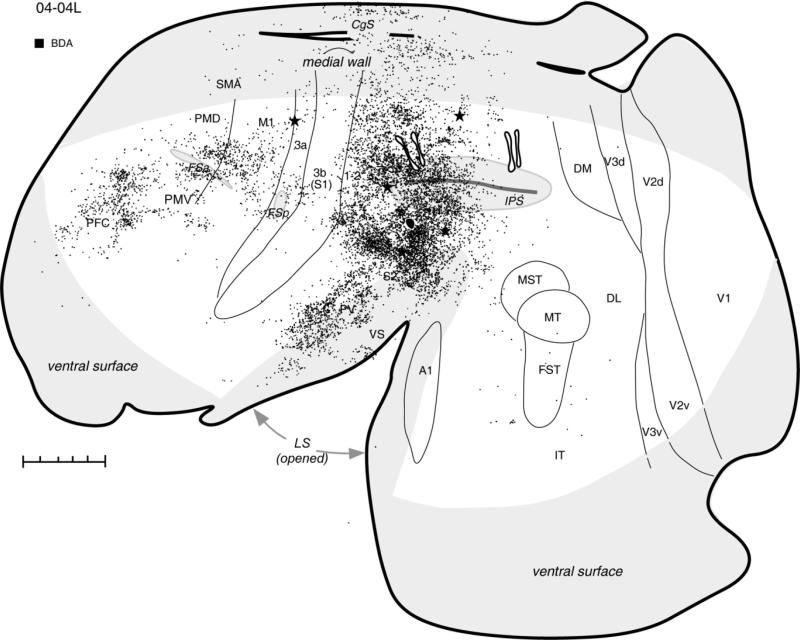
Distribution of labeled neurons after FR injections (two black spots outlined with white) in the hand-to-mouth zone, shown in a schematic of flattened cortex in case 04-04L. Dots demarcate the location of labeled cells. Injections of two other tracers, BDA in the ventral PPC and FE in the posterior PPC, are also marked for reference. Thin lines indicate the approximate borders of areas defined by physiological mapping and patterns of myelination. Thick lines outline the section or indicate breaks in the section due to sulci. Stars mark electrolytic lesions. For the detailed microstimulation map of this case see Figure 2A in our companion paper. The cortical areas on the medial wall and hidden within the lateral sulcus are shown in light gray. For abbreviations see list. Scale bar = 5 mm.
Areal borders were also determined by cortical architecture. Flattened cortex sections allowed us to visualize directly not only the surface-view patterns of labeled cells but also the architectural features of the cortex. In flattened sections stained for CO or myelinated fibers, sensory cortical areas were easier distinguished than motor areas. Primary somatosensory area 3b stood out as a densely myelinated and CO-dark band extending mediolaterally and curving rostrally where it represents face and mouth (see Krubitzer and Kaas, 1990; Wu and Kaas, 2003; Fang et al., 2005). Anteriorly adjacent area 3a is usually less myelinated and lighter in CO than area 3b. Also cortex caudal to area 3b (area 1-2 in Fig. 1 or Sp of Preuss and Goldman-Rakic, 1991a) is myelin and CO lighter than area 3b, but a little darker than the PPC around the intraparietal sulcus. PPC was quite homogenous in the flattened preparation, and we were not able to distinguish differences in myelination or CO density between dorsal and ventral PPC zones or between rostral and caudal zones of the dorsal PPC. Only the small zone located below the IPS from which we evoked face movements was different in appearance from the rest of PPC. This ventral PPC part was distinguished by somewhat denser myelination and higher CO concentration.
Frontal motor cortex, including area 3a, is less myelinated than area 3b of somatosensory cortex, with the primary motor area, M1, a little darker than premotor areas. Galagos do not have a central sulcus separating the motor region from the parietal somatosensory region. Instead, small FSa and FSp sulci mark the anterior and posterior M1 borders. The location of FSa and FSp sulci, as well as of IPS, were apparent in the flattened preparations as depressions that produced lighter myelin-stained regions or actual holes in sections, and they were used as the important landmarks. Among other areas, primary auditory (A1) and middle temporal visual (MT) areas were easily recognized by their dense myelination and CO-dark appearance (Krubitzer and Kaas, 1990). Visual areas V1 and V2 were also easily recognized by their characteristic CO and myelin patterns, demonstrating blobs in V1 and in most favorable sections stripes in V2 (Lyon and Kaas, 2002; Kaskan and Kaas, 2007). In favorable sections, area DM has been distinguished from PPC as more densely myelinated.
Because the coronal plane reveals the laminar pattern of cortical PPC connections, the brain was cut coronally in three cases. Cortical architecture in these cases was examined as well. As described previously (Preuss and Goldman-Rakic, 1991a; Wu et al., 2000; Fang et al., 2005), M1 is characterized by the presence of large pyramidal cells in layer 5 and lack of an obvious layer 4. Areas PMD and PMV have few large pyramidal cells in layer 5, and layer 4 is thin or absent in PMD, but notable in PMV. Area 3b can be distinguished by densely packed cells in layers 4 and 3. Such cells are less densely packed in area 3a, which also has an apparent layer 4. Area 3b is more myelinated than areas M1 or 3a. The cortex between area 3b and the IPS [area 1-2 or the posterior somatosensory area (Sp) of Preuss and Goldman-Rakic, 1991a] is quite homotypical, with isolated, large pyramidal cells in layer 5 and less myelination than area 3b, but more myelinated than posteriorly adjacent PPC. The dorsal part of PPC (above the IPS) is quite homogenous, although laterally it is a little bit more myelinated with thicker layer 4 than medially. The ventral PPC can be divided into at least two regions, anterior and posterior, with the anterior region more myelinated and containing larger pyramidal cells in layer 5 than posterior PPC region.
In all cases, architectonically identified areas as well as sulci and other landmarks were used to locate the relative positions of cortical areas for which the architecture was not distinctive, especially in flattened preparations. The architectonic boundaries were always compared with the physiological borders based on ICMS data, when available.
The forelimb movement zone
Microstimulation of the dorsal PPC, corresponding to area 7d of Preuss and Goldman-Rakic (1991a), evoked at least three main categories of complex forelimb movements in different portions of PPC: hand-to-mouth (or hand-to-body), defensive (either protective or avoidance), and reaching (Stepniewska et al., 2005a, 2009). Tracers were injected into each of the functional zones.
The hand-to-mouth zone
The zone representing hand-to-mouth movements was injected in three cases (see Table 1). In case 04-04L, two small injections of FR were placed close to each other above the IPS, where they invaded the dorsal bank of the sulcus (Fig. 2). These injections labeled neurons in somatosensory and motor areas, leaving visual areas free of label. The dense distribution of labeled neurons extended rostrocaudally from injection site through adjoining parts of the anterior PPC and area 1-2. Anterior to the IPS, area 3b contained only a small focus of labeled cells. The caudal limit of this expanse of labeled neurons was coincident with the lesion that marked the border between anterior part of PPC that was responsive to microstimulation and the posterior PPC that was not (see Fig. 2A in companion paper). This lesion also marked the approximate border between forelimb and dorsally adjacent hindlimb region of anterior PPC. Thus, the major focus of labeled connections covered the forelimb region of PPC, as well as parietal area 1-2. Small patches of labeled neurons were also found laterally in the lower bank of IPS and below the IPS. Other foci of labeled neurons were in the forelimb representation of somatosensory area 3a (above the posterior frontal sulcus; FSp), and in the somatosensory areas (PV, S2, VS) of the upper bank and the depth of the lateral sulcus (LS). Numerous cells were labeled in the forelimb representation of M1 and PMD. In PMD, neurons were labeled mostly in its caudal part bordering area M1, where shoulder and trunk movements are represented (Wu et al., 2000). Fewer labeled neurons were in the motor areas on the medial surface, including the regions of the supplementary motor area (SMA) and the rostral cingulate cortex of the dorsal bank of cingulate sulcus (CgS). Occasional cells were labeled in the lower bank of CgS, the caudal half of PPC, the prefrontal cortex (PFC), and the cortex between the tip of LS and areas MST-MT.
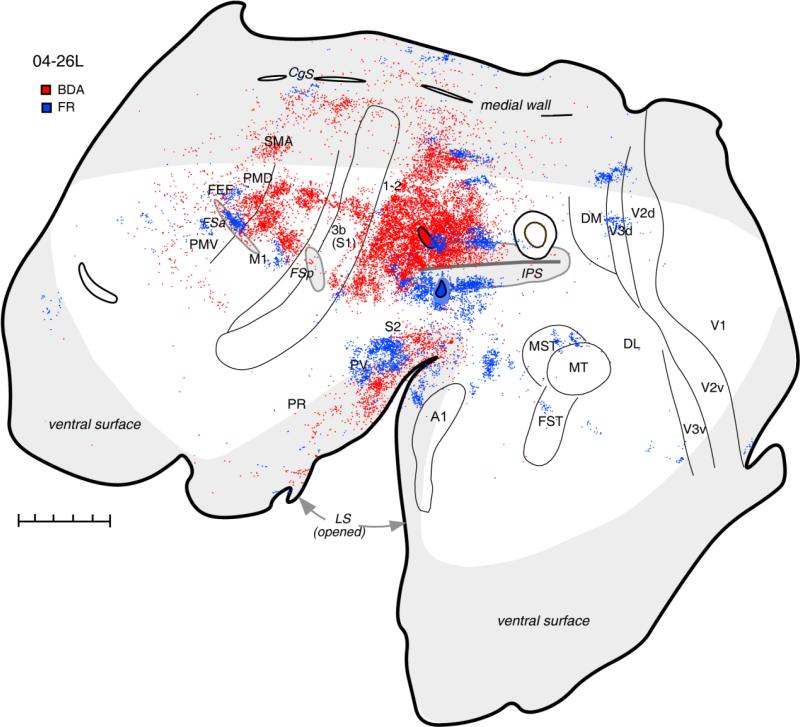
A very similar pattern of connections was observed in case 04-26L (Fig. 3), with a BDA injection made without physiological control. The injection was close to the rostral tip of IPS just above the sulcus in the region from which complex hand-to-mouth movements were evoked by ICMS in other cases (see Fig. 1). Most labeled neurons were in the surround of the injection site covering the anterior half of PPC cortex above the IPS and extending on the dorsal bank of the sulcus. This focus of dense label spread rostrally toward area 3b, covering partially the anterior PPC and probably area 1-2. A thin strip of the cortex adjacent to area 3b was less strongly labeled. An additional focus of labeled cells was found in the medial PPC (area 7m of Preuss and Goldman-Rakic, 1991a), and this labeled region was much more extensive than in case 04-04L. Many neurons were also labeled in the portions of somatosensory areas 3a and 3b as well as in the region of areas S2, PV, and VS in the lateral sulcus. As in case 04-04L, motor areas M1, caudal PMD, and SMA were very strongly labeled. Large, distinct patches of labeled neurons found in M1 may relate to the existence of functional subdivisions within this region of galagos (Wu et al., 2000), similar to those described for monkeys (Stepniewska et al., 1993; Gould et al., 1986; Preuss et al., 1997; Rathelot and Strick, 2009). Some neurons were labeled in the rostral and caudal cingulate cortex. A few labeled neurons were in areas PMV and PFC. Except for the occasional labeled cell, the ventral part of rostral PPC and caudal half of PPC as well as visual cortex were unlabeled. Thus, rostrodorsal PPC has major links with motor cortex and higher order of somatosensory fields.
Figure 3.
Distribution of neurons labeled after injections in the hand-to-mouth (BDA) and face defensive (FR) zones of the anterior PPC shown on the flattened cortex in galago 04-26L. Another injection in the posterior PPC is shown for reference. Injection of FR in the face defensive zone (below and in the ventral bank of IPS) labeled patches of neurons in somatosensory and motor areas as well as in auditory belt and in the extrastriate visual areas, especially V3 and MST. Injection of BDA in the hand-to-mouth zone produced results similar to the hand-to-mouth zone injections in case 04-04L. Conventions are the same as in Figure 2. For abbreviations see list. Scale bar = 5 mm.
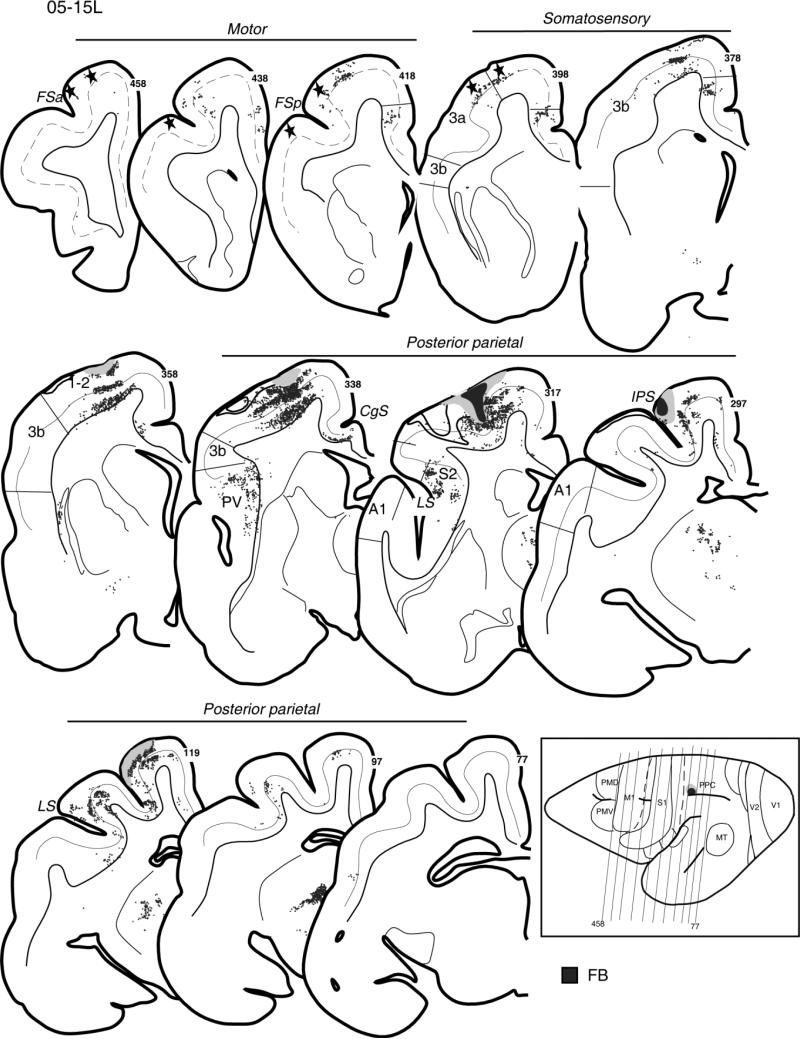
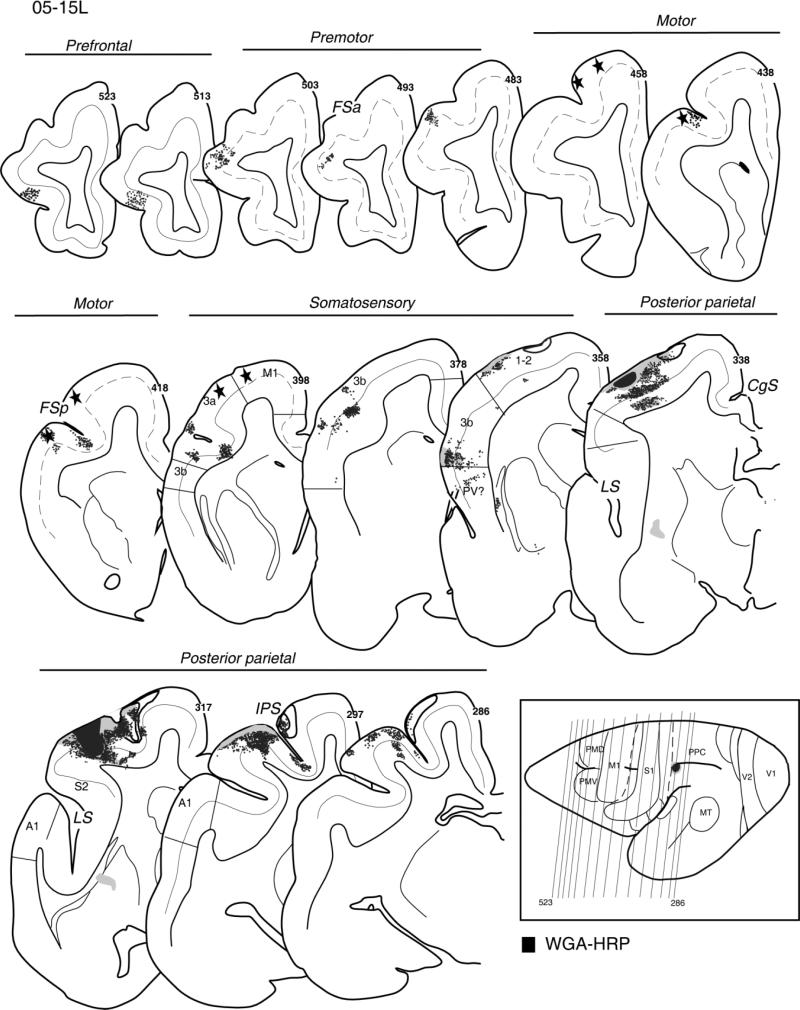
In case 05-15L, a fast blue (FB) injection into the hand-to-mouth zone of PPC partially involved the upper bank of the sulcus (Fig. 4). This injection was somewhat more rostral than the two other dorsal injections described above. The injection densely labeled adjoining parts of the rostral half of PPC and a broad extent of cortex along the upper bank of IPS. Parietal somatosensory areas 3a, 3b, and 1-2 were also labeled in the lateral portions devoted to the forelimb, as was the physiologically identified forelimb portion of M1 that was marked by lesions (Fig. 4, sections 458–398). Other foci of labeled neurons were located in the somatosensory areas within the territory of PV and S2 of the lateral sulcus (Fig. 4, sections 338–317) and on the medial surface in the cingulate cortex. For this injection, there was no evidence of inputs from premotor cortex, insofar as the labeled neurons were not found in front of most rostral lesions marking the PM/M1 border (Fig. 4, section 458). Labeled neurons were distributed mostly in the supragranular layer 3 and the infragranular layer 5 of the cortical areas; a few labeled neurons were found in other layers, mainly layers 2 and 6. The results suggest that the rostral portion of PPC representing hand-to-mouth movements has major connections with somatosensory and motor fields but is isolated from direct visual and auditory inputs.
Figure 4.
Distribution of labeled neurons after injection of FB in the hand-to-mouth zone of anterior PPC, shown on the series of coronal sections arranged from rostrally (458) to caudally (77) in galago 05-15L. The injection core is marked as black and the diffusion zone as gray. Stars indicate electrolytic marking lesions. The location of layer 4 is indicated by the solid line. In M1, which lacks layer 4, the dashed line marks the border between supra- and infragranular layers. Cortical motor, somatosensory, and posterior parietal areas with retrogradely labeled neurons (small dots) in the supra- and infragranular cortical layers are indicated. In the lower right corner, the anteroposterior arrangement of coronal sections is marked with thin lines on the dorsolateral view of the left hemisphere. For the detailed microstimulation map of this case see Figure 5A in our companion paper. Conventions as in Figure 2.
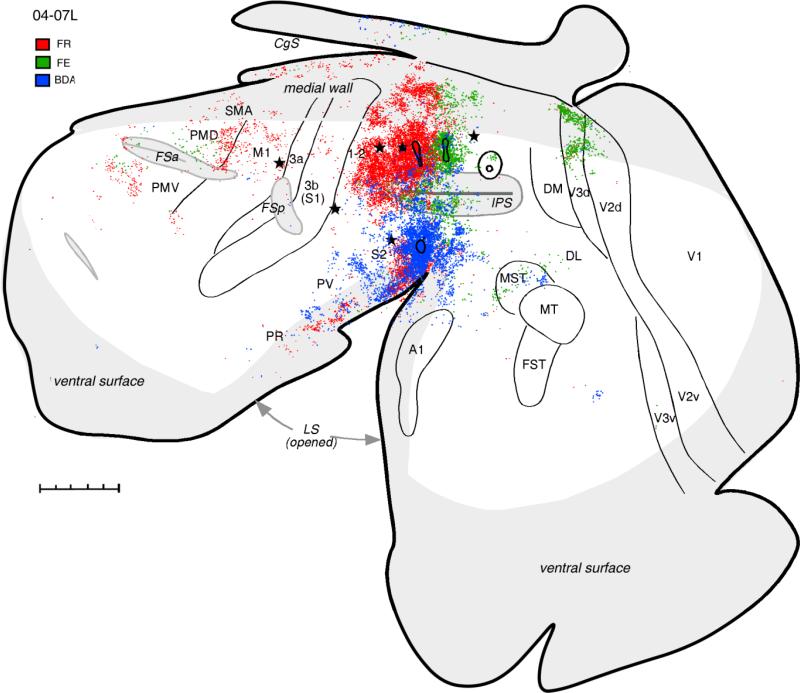
Injections of two tracers, FR and FE, in case 04-07L were also located in the dorsal PPC, but a little more caudally than in cases described above. The injection of FR covered mostly the hand-to-mouth movements zone (Fig. 1), although some forelimb defensive movements were evoked from this region as well (see Fig. 2B in companion paper). Again, the anterodorsal PPC and the medial PPC and somatosensory areas in the lateral sulcus (especially S2) devoted to the forelimb were strongly labeled by this injection (Fig. 5). Fewer FR-labeled neurons were found in the forelimb zones of somatosensory areas 3a, 3b, and 1-2 and motor areas M1 and SMA compared with cases 04-04L and 04-26L. Among the motor areas, caudal PMD contained the largest number of labeled neurons, confirming the strong connections between this region and anterodorsal PPC. Additional labeled neurons were found in the region rostral to PMV, although some of these cells might belong to PMV and some to FEF. Although no labeled cells were found in the caudal visually related part of PPC, some patches of labeled neurons coincided with the CO-dense stripes of area V3d, indicating some visual input to the injected part of dorsal PPC.
Figure 5.
Distributions of labeled neurons after injections of tracers FR (red dots), FE (green dots), and BDA (blue dots) in different locations in anterior PPC in case 04-07L. The pattern of connections after the FR injection that partially covered hand-to-mouth and defensive forelimb zones is similar to other cases with hand-to-mouth zone injections, although less label was obvious in M1 in this case. Note also the presence of label in the extrastriate visual area V3d. FE injection in the forelimb defensive zone labeled the most cells in adjacent parts of PPC and in extrastriate visual areas. Fewer cells were labeled in the premotor and cingulate cortex, and only few in the somatosensory areas. Injection in the face defensive zone labeled the most neurons in the somatosensory areas of the lateral sulcus and in the auditory belt and visual area MST. Injection in posterior PPC is shown for reference. For the detailed microstimulation map of this case see Figure 2B in our companion paper. Conventions as in Figure 2. Scale bar = 5 mm.
In summary, the hand-to-mouth zone of anterior PPC has major connections with hand representation in somatosensory and motor fields but is isolated from direct auditory and visual inputs. The most rostral portion of the hand-to-mouth zone maintained these connections, although projections from premotor cortex were not found. An injection at the caudal border of the hand-to-mouth zone, possibly also involving the defensive movement zone, labeled fewer neurons in areas 3a, 3b, and 1-2 and a few neurons in visual area dorsal V3, representing the lower visual hemifield. Neurons projecting to the hand-to-mouth zone were located in both supragranular and infragranular layers, suggesting that projection areas had both feedforward and feedback roles.
The defensive forelimb zone
The second injection (FE) made in case 04-07L was placed caudal to FR injection and overlapped the PPC region representing defensive forelimb movements (see Fig. 1). In other cases, some reaching movements were also evoked from this region, although reaching movements were not evoked in case 04-07L. The pattern of connections revealed by FE injection (Fig. 5) was quite different from the patterns described above. Although similar areas of PPC on the dorsolateral and medial surface were heavily labeled, additional dense patches of labeled neurons were found dorsomedially in dorsal visual area V3 and the rostral half of V2d. Some neurons labeled in visual cortex were rostral, medial, and caudal to MT (MST and DL). Connections with the premotor cortex were sparser and involved mostly anterior PMD. A few labeled neurons were also in the region of FEF. Large regions of cortex, including M1, SMA, anterior cingulate motor areas, and somatosensory areas, were devoid of label, and only a few cells were labeled in area 1-2 and the cortex of lateral sulcus.
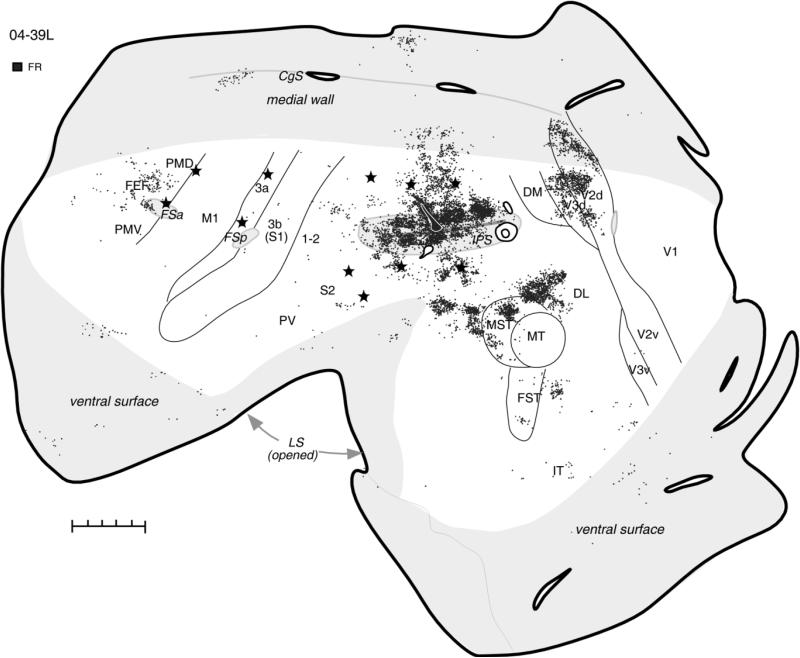
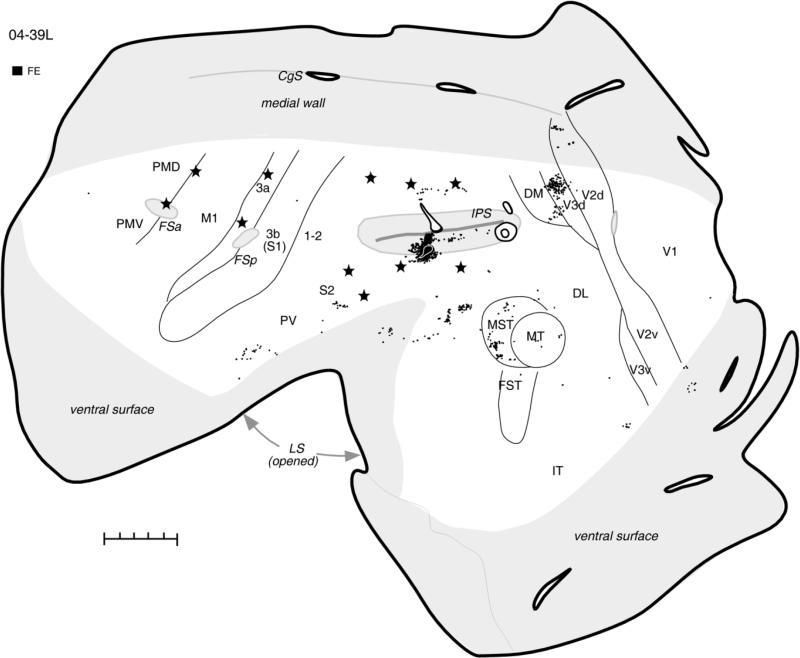
The pattern of defensive forelimb zone connections was further established in case 04-39L, in which an FR injection site involving mostly the upper bank of IPS resulted in very extensive and strong label (Fig. 6). Although defensive movements predominated in this region, some reaching movements were also evoked by stimulation of the dorsal bank of IPS (Fig. 3A in companion paper). Patches of labeled neurons were distributed along the entire length of IPS, covering the dorsal bank and deeper parts of ventral bank of the sulcus, including the posterior half of PPC, where movements are not evoked. Foci of strong label were found in the visual areas DL and MST and in dorsal V3 (V3d) and V2 (V2d), where labeled neurons formed stripes. Single neurons were also labeled in the ventral V2 and V3 parts. Dense patches of labeled cells anterior to MST were possibly in higher-order auditory areas (Kaas and Hackett, 2000). Inferotemporal cortex (IT) and parietal cortex between the rostral tip of IPS and S1 (areas 1-2 and 5) had some small patches of label. Anterior parts of PMD and PMV, FEF, and rostral cingulate cortex contained some labeled neurons as well, but in other motor areas (M1, SMA) and somatosensory areas (3a, 3b, 1-2, S2, PV, VS) only the occasional single neuron was labeled.
Figure 6.
The pattern of cortical connections in case 04-39L after injection of FR into forelimb defensive PPC zone is comparable to the pattern found after similar injection site in case 04-07L. In this case, however, the injection site involved mostly the dorsal bank of IPS, which caused very dense label in most extrastriate areas and sparse label in the premotor and cingulate cortex. For the detailed microstimulation map of this case see Figure 3A,B in our companion paper. Conventions as in Figure 2. Scale bar = 5 mm.
In summary, the defensive forelimb zone is characterized by inputs from visual areas and possibly higher order auditory areas, but there are a few somatosensory or motor connections as well. Connections intrinsic to PPC are broadly distributed along the length of the IPS. However, it is important to recognize that connection patterns were determined for the posterior part of defensive forelimb zone, and the connections of the anterior part (Fig. 1) were not determined.
The reaching zone
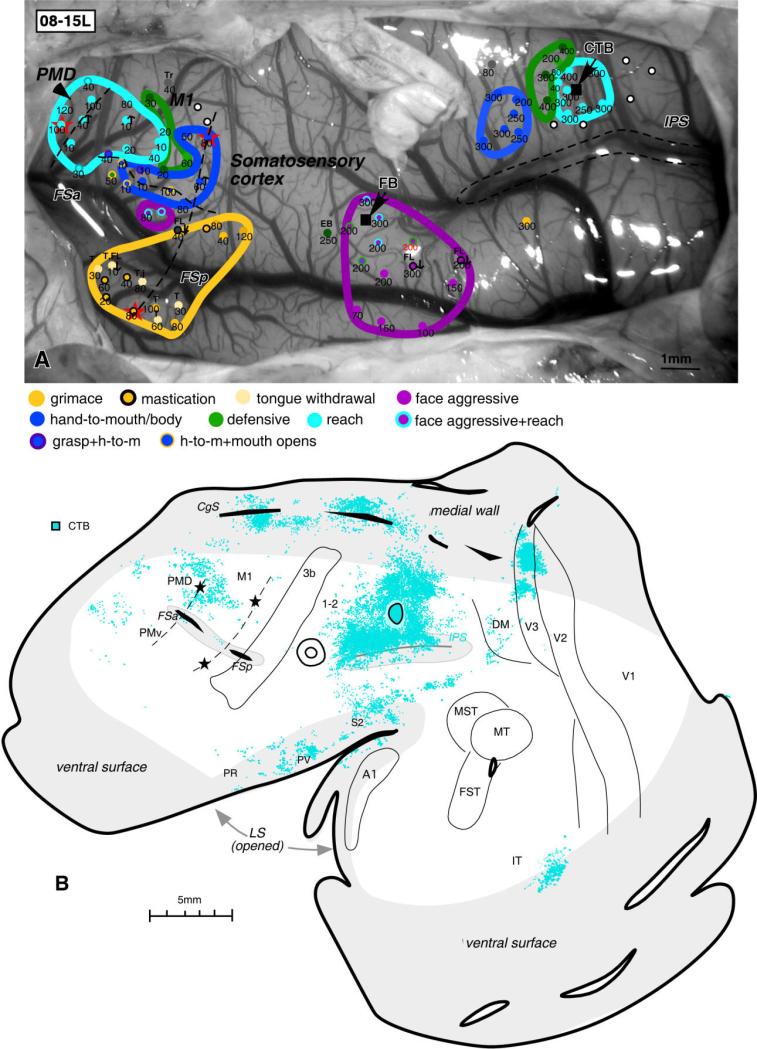
In case 08-15L, injection of CTB was centered in the reaching PPC zone (Fig. 7A). Although in this case we concentrated on mapping the reaching zone, some defensive and hand-to-mouth movements were also evoked from more anterior parts of PPC. After the injection of CTB into the reaching zone, labeled neurons were densely distributed around the injection site, including the whole dorsal PPC region representing forelimb movements, and some of the caudal PPC region unresponsive to ICMS (Fig. 7B). This main zone of labeled neurons involved the cortex on the medial wall and the dorsal bank of IPS, where numerous labeled cells were found. In contrast, the ventral bank of IPS (where the face is mostly represented) contained much sparser label. As in cases 04-07L (Fig. 5) and 04-39L (Fig. 6), large patches of neurons were labeled in dorsal part of visual area V3 representing paracentral and peripheral vision. Labeled neurons were also seen in the anterior V2. A few labeled cells were in area DM and in the cortex between IPS and MT/MST. The cortex in the cingulate sulcus contained two main foci of label, which coincided with the rostral and caudal parts of the cingulate cortex. In the frontal region, quite dense label was found in the region of the caudal PMD and the rostral M1. These foci of label coincided well with the region of PMD and M1 from which reaching movements were evoked (see Fig. 7A; region around the most rostral lesion). In this case, more label was found in the somatosensory cortex of dorsal bank of lateral sulcus than in case 04-07L. A patch of label was also found in the IT. In summary, the middle portion of dorsal PPC representing reaching forelimb movements interconnects rostral and caudal regions of the PPC and relays visual information (mostly from area V3) to dorsal premotor, and motor fields.
Figure 7.
Pattern of label resulting from injection of tracers into PPC in case 08-15L. A: ICMS map and the location of injections (black squares) shown on the dorsolateral view of the left brain hemisphere. Penetration sites and type of evoked motor response are shown as colored circles. Numbers below indicate the current thresholds. Electrolytic lesions in frontal cortex are shown as red stars. B: Distribution of neurons labeled by CTB injection into the reaching PPC zone. Note the dense label in rostral M1 and caudal PMD (around the marking lesion), from which reaching movements were evoked by ICMS. Other conventions as in Figure 2. CTB, cholera toxin subunit B; E, ear; EB, eye blink; FB, fast blue; FL, forelimb; h-to-m, hand-to-mouth; T, tongue; Tr, trunk. Scale bar = 5 mm.
Evidence for a rostral reach zone
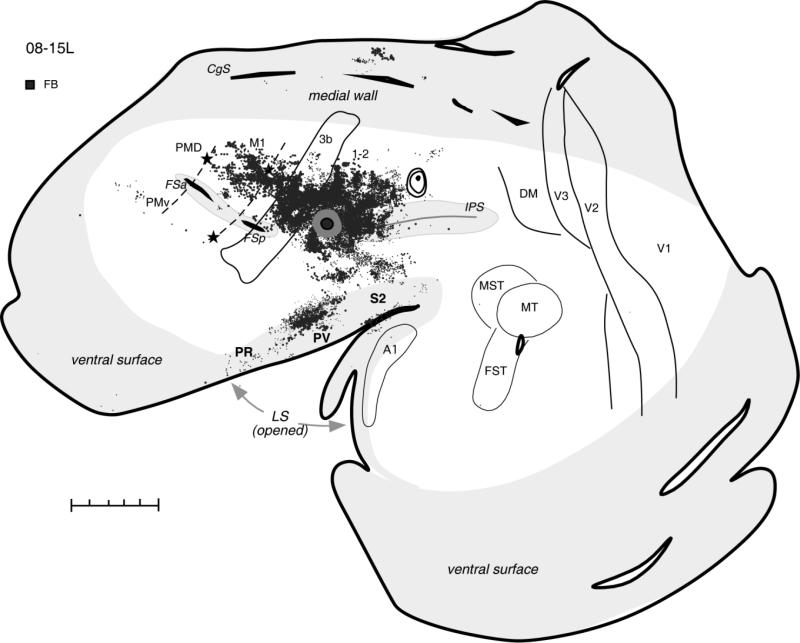
In the same case (08-15L), an injection of another tracer (FB) was made between the rostral tip of IPS and the somatosensory cortex (Fig. 8). The core of this injection was located in the region that might be considered area 5 (Preuss and Goldman-Rakic, 1989a), but the tracer diffusion zone involved part of the caudal area 1-2. This region was responsive to ICMS, which evoked strong forelimb extension and a weaker face grimace movement (Fig. 7A). This injection densely labeled adjoining parts of rostral PPC spreading anteriorly across the forelimb representations in somatosensory areas 3b and 3a. Other foci of labeled neurons were in the somatosensory areas of the upper lip of the lateral sulcus. Also, primary motor cortex contained many labeled neurons in the region devoted mostly to the forelimb. Smaller foci of label were in cingulate cortex and anterior PMV, but PMD was free of label. Except for the occasional labeled neurons, the caudal visual half of the PPC and other visual areas as well as auditory areas remained unlabeled as well. The results indicate that most rostral forelimb portion of PPC has major connections with somatosensory and motor fields, while not having direct inputs from visual or auditory areas, including even those visually related zones in caudal PPC.
Figure 8.
Distributions of labeled neurons after injection of FB in the most rostral part of PPC responsive to ICMS in case 08-15L. In this case, most labeled cells were found in the somatosensory areas of anterior parietal cortex and in the lateral sulcus, as well as in the motor cortex. Premotor and cingulate cortex had fewer labeled cells, and visual areas were free of label. For the detailed microstimulation map of this case see Figure 8A. Conventions as in Figure 2. Scale bar = 5 mm.
Face movement zone
Stimulation of the rostral half of ventral PPC evoked two types of face movements, defensive and aggressive (see Stepniewska et al., 2005a). Another zone produced eye movements, but no injections were placed in this zone. Defensive face movements were sometimes associated with the forelimb movements, and they were evoked from the cortical region below and within the lower bank of IPS. This location corresponds to area 7b and 7s according to architectonic description of galago cortex by Preuss and Goldman-Rakic (1991a). Aggressive face movements were evoked from the cortex rostrally adjacent to defensive face zone, and this cortical region may involve parts of areas 7b and 5 of Preuss and Goldman-Rakic (1991a).
The defensive face zone
Anatomical tracers were injected into the defensive face zone of ventral PPC in five cases (Table 1). In two cases (04-04L and 04-07L), small tracer injections were placed laterally between the rostral tip of IPS and the tip of lateral sulcus (Fig. 1). In three other cases, injections were either limited to the lower bank of IPS (04-39L) or partially (04-47L) or substantially (04-26L) involved the cortex of IPS lower bank (Fig. 1).
The BDA injection in case 04-04L was located in the center of the defensive face zone, which was marked by three lesions (Fig. 9). The injection labeled neurons in a wide mediolateral band of adjoining cortex extending from the upper bank of the lateral sulcus (LS) to the medial wall. Thus, the lateral portion of PPC was widely connected to other parts of PPC, except for the caudal half of PPC, which contained only few labeled neurons. This main zone of labeled neurons included the anteriorly adjacent somatosensory area 1-2 and face and forelimb representations of areas S2 and PV within the lateral sulcus. Somatosensory areas 3a and 3b were almost free of labeled neurons. Some neurons were labeled in the cingulate cortex of the medial wall. The parts of motor (M1) and premotor (PMD, PMV) areas representing face and forelimb were also labeled, as was the prefrontal cortex on the dorsolateral surface. Except for the occasionally labeled neurons in the cortex lateral and rostral to MT, visual areas were free of label.
Figure 9.
Distribution of labeled neurons in the flattened cortex after injection of BDA into face defensive PPC zone in the case 04-04L. This injection placed in the very rostral part of the defensive zone labeled neurons in rostral half of PPC, motor, premotor and cingulate areas as well as somatosensory areas, especially area 1-2 and higher order somatosensory areas in the lateral sulcus. Only a few neurons were found in extrastriate areas. For the detailed microstimulation map of this case see Figure 2A in our companion paper. Conventions as in Figure 2. Scale bar = 5 mm.
Another BDA injection (case 04-07L) placed close to the lateral border of defensive face zone (Fig. 1), in a location that corresponds to the areas 7a/7b junction (Preuss and Goldman-Rakic, 1991a), labeled neurons mostly in the adjacent PPC region and surrounding areas (Fig. 5). Thus, cortex within the anterior half of IPS and dorsal PPC, as well as above and within the upper bank of lateral sulcus, had a significant distribution of labeled neurons. Some neurons were also labeled in the ventral bank of LS, possibly in area VS, and the auditory belt. including a few neurons in primary auditory cortex (A1) and cortex ventral to the cingulate sulcus (CgS). Except for occasional neurons in M1 and PMD for this injection, there was no evidence of inputs from frontal cortex and somatosensory areas 3a and 3b, although some labeled neurons were detected in area 1-2. Again, the caudal half of PPC was mostly devoid of label. Only few small foci of label were observed in area MST, IT, and the cortex between MT and LS. Only a few neurons were labeled in areas V3d and the anterior part of V2d. Thus, cortex on the ventral margin of the face defensive zone does not connect significantly with visual cortex, anterior parietal cortex, or motor cortex.
A somewhat different pattern of connections was revealed by tracer injections made in the lower lip and lower bank of IPS representing defensive face movements. This part of PPC connects quite densely with somatosensory and motor areas as well as with nonprimary visual areas. In case 04-26L, FR injection was placed just below and near the rostral tip of IPS, including cortex of the lower bank of the sulcus (Fig. 3). The densest distribution of labeled neurons was in ventral PPC adjoining the injection site. The patches of labeled cells were also found in the dorsal and medial PPC. The labeled zone extended ventrally on the lower LS bank, including the regions of secondary areas of the somatosensory cortex (S2, PV, VS) near the tip of the sulcus, as well as the auditory belt. The other major concentration of labeled neurons was in motor cortex, including PMD, PMV, and M1, with the densest foci of labeled neurons just above and within the anterior frontal sulcus (FSa), the architectonic area 6ds of Preuss and Goldman-Rakic (1991a). Some labeled neurons were in the region of the frontal eye field (FEF), and others were in cingu-late cortex. Moreover, this small injection labeled the patches of neurons in visual cortex (V3d, V2d, MST, FST), demonstrating visual inputs to this PPC location.
In case 04-39 (Fig. 10), a very small FE injection was almost entirely in the lower bank of IPS, a location from which the face defensive movements as well as eye movements were elicited (see Fig. 3A in companion paper). Although this injection resulted in only a limited number of labeled neurons, it confirmed the existence of visual inputs to the face zone of PPC within the lower bank of IPS. Labeled neurons were generally distributed in the same visual cortical regions as in case 04-26L (Fig. 3). Thus, many labeled neurons were in V3 and V2, mostly in their dorsal portions representing lower visual quadrant. Visual cortex medial and rostral to MT, including MST, and cortex in the location of DM were also labeled. In contrast to the findings in case 04-26L, higher-order somatosensory areas S2, PV, and VS were labeled very sparsely, and large regions of cortex, including anterior parietal somatosensory areas, as well as premotor, motor, and cingulate areas were devoid of label. This could be due to the more posterior location of the injection site in the face defensive zone.
Figure 10.
Distribution of labeled neurons after small injection of FE in the lower bank of IPS from which face defensive movements were evoked in case 04-39L. In spite of poor transport of injected tracer, numerous labeled neurons were found in extrastriate visual areas, and their location was comparable to the location in case 04-26L with the similar injection site. For the detailed microstimulation map of this case see Figure 3A,B in our companion paper. Conventions as in Figure 2. Scale bar = 5 mm.
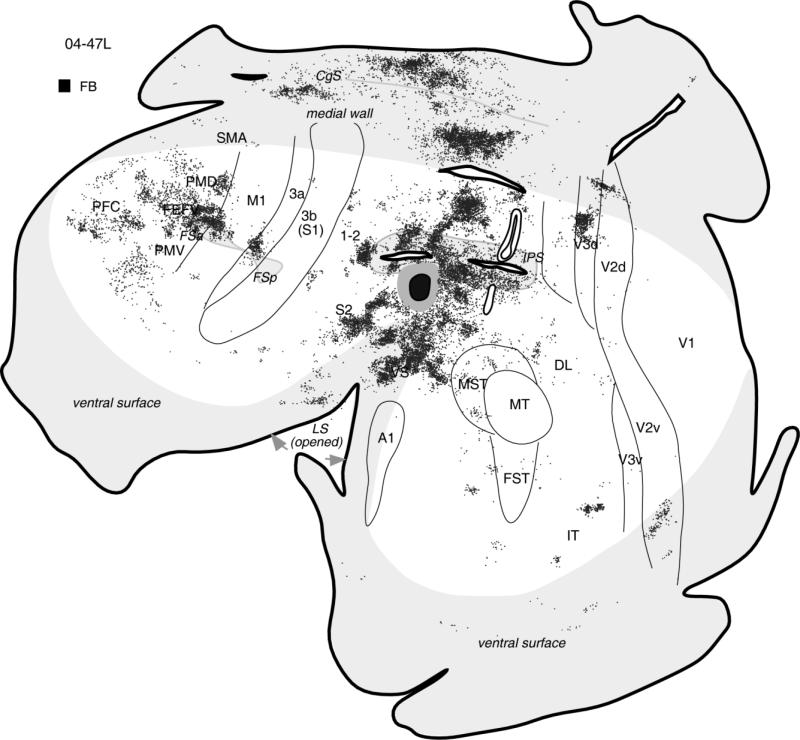
The overall pattern of ventral PPC connections is illustrated further in case 04-47L with a large FB injection (Fig. 11). The injection likely involved parts of the face defensive and eye movement zones (physiological data were not obtained) on the lateral surface and within the lower IPS bank. Labeled neurons were arranged in large clusters, with most of them in an adjoining PPC cortex and both banks of IPS. The lateral and medial PPC cortex was widely connected to the injection site as well. Ventrally dense patches of label were found in the upper and lower banks of lateral sulcus, comprising the secondary areas of the somatosensory cortex, especially S2, where two patches of dense label were found. These patches probably corresponded to face and forelimb representations of S2. Other label foci were found in somatosensory area 3a, just above FSp, and in cingulate cortex of the medial wall. The other major concentrations of labeled neurons were in motor cortex, including PMD, PMV, and M1, with the densest foci of labeled neurons in PMD, above and within FSa. Some patches of neurons were in PFC, FEF, and visual cortex, demonstrating some visual inputs to this PPC location. Among visual areas, V3d was labeled the most (as in other cases with injection in defensive face zone), but some patches of label were also found in dorsal and ventral V2 as well as MST, DL, and IT.
Figure 11.
The general pattern of corticocortical connections after large injection of FB in the ventral PPC in the location of face defensive zone in case 04-47L. The core of injection is shown in black and the diffusion zone in gray. Microstimulation data are not available for this case. Note that, as in other cases with injection in the face defensive zone, most label was in the motor and somatosensory cortical regions, but some neurons were also labeled in extrastriate visual areas. Conventions as in Figure 2.
In summary, the face defensive zone has widespread connections with the anterior half of PPC and connections with somatosensory areas of the lateral sulcus and with motor and premotor areas. The ventral parts of the zone have motor cortex connections, whereas more dorsal portions involving the lateral bank of IPS have visual connections.
The aggressive face zone
Cortex rostrally adjacent to the defensive face zone and involving the junction of area 7b and area 2-5 of Preuss and Goldman-Rakic (1991a) was the target for tracer injections in three galagos (see Table 1, Fig. 1). All three brains (05-15L, 05-20L, and 05-28L) were cut in coronal plane, and the patterns of cortical connections are presented on a series of coronal sections from the most representative case 05-15L (Fig. 12) with a large WGA-HRP injection site centered in the aggressive face zone (Fig. 1). Most labeled neurons were in the caudally adjacent ventral PPC zone representing face defensive movements and in the face representations of parietal somatosensory areas 3a, 3b, and 1-2 (Fig. 12). Some of the labeled neurons were also in the rostral PPC region above the IPS, where forelimb complex movements were represented, and in the region of area PV within the lateral sulcus. Among the motor areas, some patches of label were below the FSa-FSp sulcus in the PMV and M1 face representations. These latter connections seem to originate mostly in caudal M1 adjacent to area 3a. Ventral prefrontal cortex (PFC) contained some foci of labeled neurons. A quite similar pattern of connections with adjacent posterior parietal areas, and somatosensory and motor cortex, was revealed in case 05-28L (not illustrated), with an FR injection situated at the border between face defensive and aggressive zones. In this case, PFC also contained foci of label, although they were distributed a little more dorsally than in case 05-15L. An additional small patch of label that was not observed in case 05-15L was also found in the ventral and caudal cingulate cortex. The WGA-HRP injected in case 05-20L (not illustrated) was transported only to the cortical locations surrounding the injection site, partially confirming the connections of the aggressive face zone with parietal somatosensory areas 3b and 1-2 and anterior PPC (area 5), but leaving more distant cortical locations in the prefrontal and motor regions unlabeled. Connections with visual cortex or even with the visually related caudal PPC were not found after any of the injections in the face aggressive zone. In all cases, labeled neurons were distributed mostly in supragranular layer 3 and to a lesser degree in the infragranular layer 5.
Figure 12.
Series of coronal sections (rostral, 523, to caudal, 286) through the left hemisphere in case 05-15L with the distribution of neurons labeled following injections of WGA-HRP in the face aggressive zone of ventral PPC. The pattern of label is similar to that in cases with defensive face injections (labeled neurons in the somatosensory and motor areas of frontal cortex), but note the numerous cells in the ventral prefrontal cortex and the lack of label in the visual areas. Labeled cells are mostly in supragranular layer 3 (above thin line marking layer 4) and in infragranular layer 5 (below thin line marking layer 4). Another injection made in the dorsal forelimb zone (outlined with black) is shown for reference. Stars mark electrolytic lesions. For the detailed microstimulation map of this case see Figure 6A,B in our companion paper. Other conventions are the same as in Figure 4.
In summary, the two PPC face zones representing defensive and aggressive behaviors are interconnected and receive common inputs from motor, premotor, and parietal somatosensory areas 3a, 3b, and 1-2 as well as higher order somatosensory areas in the lateral sulcus. The aggressive face zone receives stronger connections from the parietal somatosensory areas and prefrontal cortex than face defensive zone, which receives stronger inputs from higher-order somatosensory areas (S2 and PV). Visual inputs from nonprimary visual areas seem to reach directly only the face defensive zone.
DISCUSSION
In the present study, we traced the ipsilateral cortical connections of functionally defined zones within posterior parietal cortex of galagos. We used intracortical microstimulation (ICMS) with long train duration to evoke different types of complex movements from the cortical areas around the anterior half of intraparietal sulcus (IPS), which were consequently injected with anatomical tracers. Below, we summarize and discuss the PPC connection patterns obtained, relate them to previous results from prosimian primates, and compare the present results with those from monkeys.
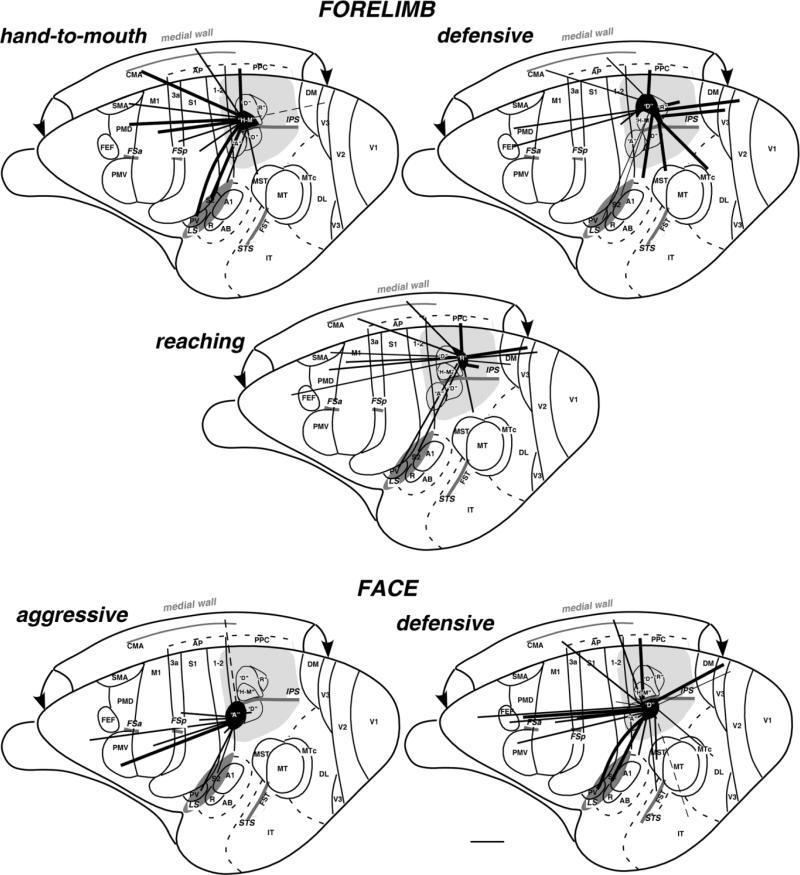
Figure 13 illustrates the overall patterns of connections of the identified movement zones in the anterior PPC region responsive to ICMS. In general, these PPC areas have dense connections with adjacent PPC regions, major connections with motor areas of frontal cortex and somatosensory areas in the anterior parietal lobe as well as in the lateral sulcus, and variable connections with the extrastriate visual cortex. Forelimb movement zones in PPC are connected to forelimb representations of somatosensory and motor regions, and face movement zones have connections with the face representations in these cortical regions. The visual connections are with parts of areas representing mostly peripheral and paracentral vision of the lower visual quadrant. PPC zones representing different movements, either of the forelimb or of the face, have distinct patterns of connections. The hand-to-mouth zone of anterior PPC has major connections with hand representation in somatosensory (areas 3a and 1-2 and less with 3b) and motor (PMD, M1, SMA, and CMA) fields but is isolated from direct visual and auditory inputs. Only the most caudal injection made in the hand-to-mouth zone, which partially involved the defensive forelimb zone, has few connections with dorsal part of visual area V3, representing the lower visual hemifield. The defensive forelimb zone is characterized by dense inputs from visual areas and possibly higher order auditory areas and fewer somatosensory or motor connections. Its motor connections are mostly with PMD. The middle portion of dorsal PPC representing reaching forelimb movements connects with more rostral and caudal regions of the PPC as well as dorsal motor and nonstriate visual fields, thus relaying visual information (mostly from area V3) to dorsal premotor, and to a lesser extent, motor cortex.
Figure 13.
Summary of corticocortical connections of the specific complex movement zones of PPC in galago brain. Functionally distinct movement zones (filled with black) and their connections (indicated by black lines) are marked on the exposed left cerebral hemisphere. Thick lines represent strong connections, and thin lines represent weak connections. Note that the hand-to-mouth zone has major connections with somatosensory and motor fields, while being isolated from direct auditory and visual inputs, whereas reaching and especially defensive forelimb zones get fewer somatosensory or motor inputs but more information from nonprimary visual areas. Face defensive and aggressive zones below the IPS receive major inputs from motor, premotor, and somatosensory areas (3a, 3b, 1-2, S2, PV). The defensive face zone receives additional input from nonprimary visual areas (e.g., V3). Sulci are marked with dark gray, and the entire region explored by ICMS is marked with light gray. A, aggressive; D, defensive forelimb or face; H-M, hand-to-mouth; R, reach. Scale bar = 5 mm.
The ventrally located face zones representing defensive and aggressive behaviors are interconnected and have widespread connections with somatosensory areas of the lateral sulcus (S2, PV) as well as parietal somatosensory areas 3a, 3b, and 1-2 and with motor and premotor areas. The aggressive face zone receives stronger connections from the parietal somatosensory areas and prefrontal cortex than the face defensive zone, which receives stronger inputs from higher order somatosensory areas (S2 and PV). Only the face defensive zone, and not the aggressive zone, receives visual inputs from nonprimary visual areas (especially area V3).
The overall patterns of connections of PPC functional zones provide some suggestions for how specific behaviors are guided by sensory information and are mediated by motor and premotor areas. The two zones of complex hand-to-mouth and reaching movements involve extensive sensory guidance by higher order somatosensory inputs, while mediating those movements by directly involving forelimb portions of M1 and PMD as well as parts of SMA and cingulate motor areas. All three hand movement fields have some direct visual input from V3, but the visual inputs are most abundant for the forelimb defensive zone, suggesting that an analysis of the visual scene is most important for defense and least important for bringing the object to the mouth. The forelimb defensive zone may also have inputs from higher order auditory or multisensory fields, suggesting that threatening sounds may also contribute to the defensive movements. Two zones for complex face movements had connections with face portions of PMV and M1, while depending on somatosensory inputs for guidance. Whereas the aggressive face zone lacked the direct visual inputs, the defensive face zone had both direct visual inputs from a number of visual areas and higher order auditory inputs. Thus, both the face and the forelimb defensive zones are similar in having visual and auditory inputs. The connections of the face defensive zone with the ventral portions of PMD, where digit, ear, and nose movements are represented (Wu et al., 2000), may function to recruit the ear movements that are part of the defensive complex or coordinate hand and face defensive movements.
PPC areal partitioning in galagos
Our microstimulation mapping of the PPC in galagos has demonstrated the existence of five subdivisions or zones representing the different motor behavior, all located in the anterior half of PPC. The three forelimb movement zones for hand-to-mouth, defensive, and reaching postures were within the dorsal PPC, and the two face movement zones representing aggressive and defensive behaviors were within the ventral PPC. The functional zones did not correspond to architectonically distinct regions, insofar as PPC was rather uniform in myelin and CO preparations, especially from flattened cortex. Previously, Preuss and Goldman-Rakic (1991a), who studied the cyto- and myeloarchitecture of PPC in galagos, also concluded that areal differences were not visibly marked.
All our movement zones in PPC of galagos are located within area 7 of Preuss and Goldman-Rakic (1991a), with the exception of the most rostral face aggressive zone, which could involve parts of their area Sp (our 1-2). Their architectonic parcellation of posterior parietal region in galagos does not reflect, however, the physiological and connectional differences between the particular movement zones or even anterior (responsive to ICMS) and posterior (unresponsive to ICMS) halves of PPC evident in this study. New architectonic studies based on differences in histochemical and immunochemical features of PPC may reveal subdivisions that correlate with functional PPC zones.
PPC connections with somatosensory areas
Recent anatomical studies in galagos (Wu and Kaas, 2003) have demonstrated the existence of most of the somatosensory areas described in monkeys. Three somatosensory areas have been identified in anterior parietal cortex of galagos: area 3b or S1 proper, a mediolateral strip of cortex along the rostral border of 3b identified as area 3a, and a mediolateral strip of cortex along the caudal border of 3b, the identity of which is less certain. In this paper, we refer to the region as area 1-2 to reflect our uncertainty in identifing this cortex as either area 1 or area 2 of monkeys. Other authors also concerned about classification of this region consider the whole region between S1 and intraparietal sulcus as “somatosensory posterior” (Sp) area (Preuss and Goldman-Rakic, 1991a) or Spost (Fogassi et al., 1994). With regard to the cortex of the lateral sulcus of galagos, Wu and Kaas (2003) also provided evidence for areas S2 and PV as well as the ventral somatosensory area (VS).
Our injections into the different movement zones of PPC revealed connections with most of the somatosensory areas. We found labeled neurons in the areas of anterior parietal cortex (3a, 3b, and 1-2) as well as in the higher order somatosensory areas of the lateral sulcus (S2, PV, and VS). Labeled neurons in the cortex immediately caudal to S2 could be the homologue of retroinsular area (Ri) of monkeys (Robinson and Burton, 1980a,b; Friedman et al., 1986; Krubitzer et al., 1995). The strongest connections with areas 3a, 3b, and 1-2 were found for the anterior hand-to-mouth zone, whereas other more posterior zones designated as reaching and defensive forelimb and face movements had sparser connections with this region. S2 and PV areas of lateral sulcus were also most densely connected to the anterior parts of PPC. Thus, injections into the cortex in front of the anterior tip of IPS (Fig. 8) as well as in the hand-to-mouth zone labeled numerous cells in the S2-PV region. S2-PV also sends inputs to the other PPC zones representing complex movements. Dense connections between anterior PPC and somatosensory areas of anterior parietal and lateral sulcus cortex in galagos have been described previously (Wu and Kaas, 2003; Fang et al., 2005).
The denser somatosensory inputs to anterior PPC areas vs. posterior PPC areas have also been described in monkeys (for review see Cavada and Goldman-Rakic, 1989a,b; Rozzi et al., 2006). In macaques, most cells in anterior parts of areas 5 and 7 (7b) respond to somatosensory stimulation (Mountcastle et al., 1975; Robinson and Burton, 1980b; Hyvarinen, 1981; Chapman et al., 1984; Andersen et al., 1987; Taoka et al., 1998; Murata et al. 2000). Cells in both regions respond to reaching and hand manipulation (Mountcastle et al., 1975; Andersen et al., 1985; Gardner et al., 2007). As expected, areas 7b and 5 are interconnected and have connections primarily with other areas involved in somatosensory processing, such as insular cortex, as well as areas S2 and PV (Andersen et al., 1987, 1990; Disbrow et al., 2003). Area 5 also has connections with areas 1, 2, and 3a (especially area 2), but connections to 3b are rare (Jones et al., 1978; Pearson and Powell, 1985; Pons and Kaas, 1986; Burton and Fabri, 1995). Caudal parts of area 5 (MIP) and area 7 (7a), although dominated by visual inputs, also have connections with the anterior parts of areas 5 (5d) and 7 (7b), respectively (Pandya and Seltzer, 1982; Sakata et al., 1973, 1995; Lewis and Van Essen, 2000b; Nakamura et al., 2001; Breveglieri et al., 2006). The majority of cells in ventral intraparietal area (VIP), hidden in the IPS, have tactile receptive fields mostly on the face and head, insofar as fewer cells represent upper body parts such as arms and hands (Colby and Duhamel, 1991; Duhamel et al., 1998; Colby and Goldberg, 1999; Avillac et al., 2005; Ishida et al., 2009). Area VIP receives projections from somatosensory areas 2, 1, and possibly 3a, although the strongest and the most consistent connections are with polysensory areas (Maunsell and Van Essen, 1983; Lewis and Van Essen, 2000b; Gamberini et al., 2009). Areas 5, 7b, and S2 probably send face-related inputs to VIP.
PPC connections with visual areas
The visual inputs to PPC come from visual areas well defined in galagos, including areas MT (Kaskan and Kaas, 2007), MST, V3, DM (Beck and Kaas, 1998), V2 (Collins et al., 2001), and other less well-understood and more associative areas. Based on our previous (Fang et al., 2005) and unpublished observations, the caudal half of PPC that is unresponsive to ICMS receives most of the inputs from visual cortex and relays it to the anterior excitable PPC. Here we demonstrated that some parts of the anterior PPC involved in motor activity receive visual inputs directly from nonprimary visual areas. Our injections in the most posterior part of the responsive-to-ICMS half of the PPC (“reaching zone” and “defensive forelimb and face zones”) labeled many cells in areas V3, rostral V2, DM, and MST, which are considered parts of the dorsal stream of visual processing, involved in visuomotor guidance of motion processing (Ungerleider and Mishkin, 1982; Felleman and Van Essen, 1991; Goodale and Milner, 1992). A few labeled neurons were inconsistently found in areas DL and IT, regions involved in the ventral visual stream, processing information for object identification (Ungerleider and Mishkin, 1982; Ungerleider and Haxby, 1994; Vaina, 1994). Inputs to PPC from visuotopically organized areas more strongly involved the lower visual field representations than the upper field representations. None of our movement zones received information from parts of visual areas representing central vision, but rather received information from their parts representing peripheral and paracentral (for regions within the upper bank of the IPS) vision. Among them, dorsal V3 seemed to have the densest connections with caudal motor-related PPC areas. No labeled cells were found in visual areas after injections placed in the most anterior hand-to-mouth and aggressive face zones.
As in galagos, in macaques the visual inputs have been shown to reach mostly caudal areas of posterior parietal cortex (Cavada and Goldman-Rakic, 1993; Kalaska, 1996; Shipp et al., 1998; for review see Caminiti et al., 1996; Cavada, 2001). In the intraparietal sulcus, the parietal reach region (Cohen and Andersen, 2002; Boussaoud et al., 1990; Marconi et al., 2001), the lateral intraparietal area (LIP; Shipp et al., 1998; Blatt et al., 1990), and the ventral intraparietal area (VIP; Maun-sell and Van Essen, 1983; Lewis and Van Essen, 2000b) receive inputs from dorsal stream areas. A clear segregation of visual and somatosensory responses was found in the inferior parietal lobule of macaques, with areas 7a, LIP, and DP being visual and visual-motor and area 7b being primarily somatosensory (Andersen et al., 1990). A similar segregation was found anatomically. Visual inputs to caudal dorsomedial (7m, MDP) and ventral (area 7a) areas as well as areas within the intraparietal sulcus (areas VIP, LIP and MIP) tend to arise either from areas that have been implicated in spatial or motion analysis or from peripheral field representations in the prestriate cortex (Baizer et al., 1991). As in galagos, cells projecting to the caudal parietal cortex in macaques were found in visual areas V2, V3, and V4 (or DL) but mainly in the far peripheral representations of both the upper and the lower visual field, as well as in the dorsal parietooccipital cortex, including the parietooccipital (PO or M) area, V3A (or DM), and the dorsal prelunate area (DP). Cortex of the superior temporal sulcus receives visual information from several motion-sensitive areas, including the middle temporal area (MT), the medial superior temporal area (MST), the fundal area of the superior temporal sulcus (FST), and the superior temporal polysensory area (STP; Seltzer and Pandya, 1994; Cusick et al., 1995; Padberg et al., 2003; for review see Andersen, 1989).
Visual inputs have also been shown to reach anterior areas of PPC (e.g., area 7b, area 5) that traditionally have been considered to be involved mostly in somatosensory processing (Sakata et al., 1973; Mountcastle et al., 1975; Kalaska, 1996; Gardner et al., 1999; Murata et al., 2000; Squatrito et al., 2001). For example, the anterior intraparietal area (AIP/7b) receives indirect visual inputs from LIP (Nakamura et al., 2001). A minority of cells (10%) in area 7b respond to visual or visual and somatosensory stimuli (Hyvarinen and Shelepin, 1979; Robinson and Burton 1980a,b; Hyvarinen, 1981). Area 7b has few direct connections with visual cortical areas (Lewis and Van Essen, 2000b), and these connections likely account for the small but consistent number of visually responsive cells that are found in this region (Mountcastle et al., 1975).
PPC connections with the motor areas of frontal cortex
There is evidence that the major subdivisions of motor cortex of anthropoid primates (M1, PMD, PMV, SMA, CMA, and FEF) also exist in prosimian galagos (Wu et al., 2000; Fang et al., 2005). Previously, we have shown that all these regions connect to the posterior parietal region around the intraparietal sulcus (Fang et al., 2005). The present results confirmed those data and revealed the specific connections of physiological zones of PPC designated to specific motor behaviors. We have shown that all studied movement zones have major connections with premotor areas, whereas connections with cingulate areas, and especially with SMA, were generally less dense. Dorsal areas of PPC (above IPS) involved in forelimb motor behavior connect to dorsal premotor cortex and forelimb representation of motor cortex, whereas ventral PPC parts (below IPS) with face movement zones are more strongly connected to lateral premotor and motor fields, representing face. Each physiological zone, however, has specific pattern of connections with the frontal motor areas. Thus, the rostral PPC zone representing hand-to-mouth movements has more connections with M1 and caudal parts of PMD, whereas more caudal PPC zones representing defensive and especially reaching movements are densely connected with rostral PMD, and connections with M1 are very weak or absent. A similar rostrocaudal pattern of connections between PPC and PM/M1 was reported by Fang et al. (2005).
Strong topographically organized connections between PPC and frontal motor areas (especially motor and premotor) have been also reported in macaque monkeys, and the general pattern of PPC connections with the motor areas is roughly similar to the patterns we decribed for galagos (Kurata, 1991; Matelli et al., 1998; Caminiti et al., 1999; Luppino et al., 1999; Marconi et al., 2001; Tanne-Gariepy et al., 2002; for review see Wise et al., 1997; Matelli and Luppino, 2001). Physiological studies in macaques have shown that connected parts of parietal and frontal motor areas have similar neuronal characteristics (Johnson et al., 1996; Luppino et al., 1999; for review see Rizzolatti et al., 1998; Matelli and Luppino, 2001). For instance, LIP, which has sensorimotor functions involving saccadic eye movements, is connected to FEF with similar functions (Petrides and Pandya, 1984; Bruce et al., 1985; Huerta et al., 1987; Selemon and Goldman-Rakic, 1988; Blatt et al., 1990; Andersen et al., 1990, 1992; Schall et al., 1995; Stanton et al., 1995; Maioli et al., 1998; Thier and Andersen, 1998; Mushiake et al., 1999), and the anterior intraparietal area is connected with the ventral premotor area, both involved in visual guidance of grasping (see, e.g., Sakata et al., 1995; Rizzolatti and Fadiga, 1998; Luppino et al., 1999; Lewis and Van Essen, 2000b; Fogassi et al., 2001). In addition, the ventral intraparietal area within the IPS and the dorsal premotor area with neurons of similar characteristic are interconnected (Colby et al., 1993; Duhamel et al., 1998; for review see Graziano and Cooke, 2006). Most recently, microstimulation with long trains of electrical pulses evoked similar defensive behaviors from both of these regions (Cooke et al., 2003, Cooke and Graziano, 2004; for review see Graziano and Cooke, 2006). Thus, the regions of PPC and frontal cortex form anatomical circuits that are involved in specific motor actions (for review see Rizzolatti et al., 1998; Matelli and Luppino, 2001).
Our microstimulation studies (Stepniewska et al., 2005a, 2009) have shown that similar patterns of complex movements such as reaching, and hand-to-mouth, or defensive movements can be also evoked from parts of motor (M1), premotor (dorsal PMD and ventral PMV), and posterior parietal (PPC) cortices of galagos. We assume that frontal and posterior parietal areas representing the same behavior are strongly interconnected. Although in the present study maps of complex movements in M1/PM were obtained for only a few galagos, we have demonstrated that regions representing the same behavior are strongly interconnected. The best evidence for dense anatomical relations between areas representing the same behavior comes from case 08-15L with injection of CTB into the reaching zone of PPC. This injection labeled numerous neurons around the most rostral lesion marking the PMD/M1 border, where our ICMS mapping revealed a zone for reaching movements. In case 05-15L, injection into the hand-to-mouth zone in PPC labeled neurons in the most posterior M1 (section 418), where most hand-to-mouth movements were found in this case (see Fig. 5A in companion paper). Another injection made in this case in the aggressive face zone of PPC labeled neurons in ventral M1 just below the level of FSa and FSp (sections 438, 418), where aggressive behaviors were also evoked (see Fig. 5A in companion paper).
Our PPC injections also revealed connections with prefrontal cortex, including the region of the frontal eye field (FEF), especially in cases with defensive forelimb and face zones. Connections of PPC with prefrontal cortex in galagos were described previously by Preuss and Goldman-Rakic (1991b). Their injections in the posterior portion of granular frontal cortex revealed strong connections with the cortex around and within the intraparietal sulcus. Injections that involved anterior PMV revealed strong connections with the ventral part of anterior PPC, in accordance with the present results.
Similar connections of functionally distinct areas of PPC (VIP, LIP, areas 7a and 7m) with individual sectors of prefrontal cortex and the FEF have been described for macaques (Cavada and Goldman-Rakic, 1989b; Lewis and Van Essen 2000b; Bullier et al., 1996). Posterior parietal cortex, FEF with surrounding cortex, and cingulate cortex are proposed parts of a parietofrontal network that is involved in coordination and control of spatial attention in human and nonhuman primates (Rizolatti et al., 1987; Morecraft et al., 1993; for review see Goldman-Rakic, 1987).
Does the organization of PPC in galagos reflect a basic pattern found in all primates?
We have subdivided the PPC of galagos into a more rostral sensorimotor region, with functionally relevant subdivisions, and a more caudal, visually dominated region. PPC in macaque monkeys has been divided based on other approaches, resulting in subdivisions reflecting the results of early architectonic studies and those from experimental studies. Evidence for some of the areas proposed for macaque monkeys has been obtained for humans (for review see Grefkes and Fink, 2005). Surprisingly, there is little evidence for how PPC is subdivided in New World monkeys (Kaas et al., 1977; Padberg et al., 2005, 2007). Thus, comparisons across these taxa are limited by a general lack of comparable information. However, we expect major similarities in the organization of PPC across primates, because primate brains share many features not necessarily characteristic of the brains of other mammals. We are encouraged by our recent microstimulation studies in New World owl (Stepniewska et al., 2006, 2008) and squirrel monkeys (unpublished data), which indicate that grasping, defensive, and reaching zones exist in PPC of these primates, and also the evidence from Cooke et al. (2003) indicating that a defensive movement zone exists in the region of VIP of macaque monkeys. Evidence for grasping and grasping regions in macaques has not yet been obtained in microstimulation studies, although this may be revealed in the future. However, the posterior parietal reach region (PPR) is involved in reaching behavior (Cohen and Andersen, 2002; Andersen et al., 1998), and the AIP region is involved in grasping behavior (Sakata et al., 1995, 1998; Murata et al., 1996, 2000). Although microstimulation studies in macaques indicate that LIP is involved in saccadic eye movements (Andersen et al., 1992), a comparable zone has not yet been identified in prosimians or New World monkeys, although we have evoked eye movements from PPC lateral to the intraparietal cortex in some of the microstimulation experiments in galagos. We have not yet fully described a grasping zone in PPC of galagos, but such a zone was recently found in the most rostral part of PPC (Stepniewska et al., 2009) in a location similar to the grasping zone of New World monkeys and AIP of macaques. Grasping movements have also been evoked from motor and premotor cortex of galagos (Stepniewska et al., 2007). Thus, we are beginning to see the outlines of a PPC organization that likely applies to all primates, one that emerged early in the course of primate evolution, and one that differs considerably from those that evolved in other mammals, including the organization found in tree shrews (Remple et al., 2006), representing a branch of mammals that diverged from a common ancestor of primates over 80 millions years ago (Murphy et al., 2004).
ACKNOWLEDGMENTS
The authors thank Dr. Omar Gharbawie and Dr Nicole Young for assistance with the microstimulation mapping and helpful comments on the article, Dr Lisa de la Mothe for help with the figures, Mary Feurtado for surgical assistance and Laura Trice and Mary Varghese for technical assistance.
Grant sponsor: National Institutes of Health; Grant number: NS 16446 (to J.H.K.); Grant number: NS 055843 (to I.S.).
Abbreviations
- A1
primary auditory area
- AB
auditory belt
- AIP
anterior intraparietal area
- AP
anterior parietal cortex
- CgS
cingulate sulcus
- CMA
cingulate motor area
- DL
dorsolateral visual area
- DM
dorsomedial visual area
- FEF
frontal eye field
- FSa
frontal sulcus, anterior
- FSp
frontal sulcus, posterior
- FST
fundal superior temporal visual area
- IT
inferotemporal area
- IPS
intraparietal sulcus
- LS
lateral sulcus
- M1
primary motor cortex
- MST
middle superior temporal visual area
- MT
middle temporal visual area
- MTc
middle temporal area, crescent
- PFC
prefrontal cortex
- PM
premotor cortex
- PMD
dorsal premotor area
- PMV
ventral premotor area
- PPC
posterior parietal cortex
- PR
rostral parietal area
- PV
ventral parietal area
- SMA
supplementary motor area
- STS
superior temporal sulcus
- S1 (or 3b)
primary somatosensory area
- S2
secondary somatosensory area
- V1
primary visual area
- VS
ventral somatosensory area
- V2
second visual area
- V2d
second visual area, dorsal
- V2v
second visual area, ventral
- V3
third visual area
- V3d
third visual area, dorsal
- V3v
third visual area, ventral
- 1-2
somatosensory areas 1 and 2
- 3a
somatosensory area 3a
LITERATURE CITED
- Andersen RA. Visual and eye movement function of the posterior parietal cortex. Annu Rev Neurosci. 1989;12:377–403. doi: 10.1146/annurev.ne.12.030189.002113. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Siegel RM, Essick GK, Asanuma C. Subdivision of the inferior parietal lobule and dorsal prelunate gyrus of macaque by connectional and functional criteria [abstract]. Invest Ophthalmol Vis Sci. 1985;23:266. [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Neurons of area 7 activated by both visual stimuli and oculomotor behavior. Exp Brain Res. 1987;67:316–322. doi: 10.1007/BF00248552. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;2:840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Batista AP, Buneo CA, Cohen YE. Posterior parietal areas specialized for eye movements (LIP) and reach (PRR) using a common coordinate frame. Novartis Found Symp. 1998;218:109–122. 171–175. doi: 10.1002/9780470515563.ch7. [DOI] [PubMed] [Google Scholar]
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8:941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in prosimian primates. J Comp Neurol. 1998;398:162–178. [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Gamberini M, Passarelli L, Fattori P. Somatosensory cells in area PEc of macaque posterior parietal cortex. J Neurosci. 2006;26:3679–3684. doi: 10.1523/JNEUROSCI.4637-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Bruce K, Grofova I. Notes on a light and electron microscopic double-labeling method combining anterograde tracing with Phaseolus vulgaris leucoagglutinin and retrograde tracing with cholera toxin subunit B. J Neurosci Methods. 1992;45:23–33. doi: 10.1016/0165-0270(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Bullier J, Schall JD, Morel A. Functional streams in occipito-frontal connections in the monkey. Behav Brain Res. 1996;76:89–97. doi: 10.1016/0166-4328(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol. 1995;355:508–538. doi: 10.1002/cne.903550404. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex. 1996;6:319–328. doi: 10.1093/cercor/6.3.319. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P, Ferraina S, Mitsuda T, Giannetti S, Squatrito S, Maioli MG, Molinari M. Early coding of reaching: frontal and parietal association connections of parieto-occipital cortex. Eur J Neurosci. 1999;11:3339–3345. doi: 10.1046/j.1460-9568.1999.00801.x. [DOI] [PubMed] [Google Scholar]
- Cavada C. The visual parietal areas in the macaque monkey: current structural knowledge and ignorance. Neuroimage. 2001;14:S21–S26. doi: 10.1006/nimg.2001.0818. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Multiple visual areas in the posterior parietal cortex of primates. Prog Brain Res. 1993;95:123–137. doi: 10.1016/s0079-6123(08)60363-5. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Spidalieri G, Lamarre Y. Discharge properties of area 5 neurones during arm movements triggered by sensory stimuli in the monkey. Brain Res. 1984;309:63–77. doi: 10.1016/0006-8993(84)91011-4. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29:517–537. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–49. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: Anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in a prosimian primate (Galago garnetti). J Comp Neurol. 2001;431:155–167. [PubMed] [Google Scholar]
- Cooke DF, Graziano MSA. Sensorimotor integration in the precentral gyrus: polysensory neurons and defensive movements. J Neurophysiol. 2004;91:1648–1660. doi: 10.1152/jn.00955.2003. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CS, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG, Seltzer B, Cola M, Griggs E. Chemoarchitectonics and corticocortical terminations within the superior temporal sulcus of the rhesus monkey: evidence for subdivisions of superior temporal polysensory cortex. J Comp Neurol. 1995;360:513–535. doi: 10.1002/cne.903600312. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Gentilucci M, Luppino G, Matelli M, Rizzolatti G. The fronto-parietal cortex of the prosimian galago: patterns of cytochrome oxidase activity and motor maps. Behav Brain Res. 1994;60:91–113. doi: 10.1016/0166-4328(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gamberini M, Passarelli L, Fattori P, Zucchelli M, Bakola S, Luppino G, Galletti C. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J Comp Neurol. 2009;513:622–642. doi: 10.1002/cne.21980. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Ro JY, Debowy D, Ghosh S. Facilitation of neuronal activity in somatosensory and posterior parietal cortex during prehension. Exp Brain Res. 1999;127:329–354. doi: 10.1007/s002210050803. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Babu KS, Reitzen SD, Ghosh S, Brown AS, Chen J, Hall AL, Herzlinger MD, Kohlenstein JB, Ro JY. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol. 2007;97:387–406. doi: 10.1152/jn.00558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Luppino G, Ekamp H, Zilles K. The macaque inferior parietal lobule: cytoarchitecture and distribution pattern of serotonin 5-HT1A binding sites. Anat Embryol. 2005;210:353–362. doi: 10.1007/s00429-005-0026-4. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Hansma DI, Houk JC, Robinson FR. A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res. 1984;298:235–241. doi: 10.1016/0006-8993(84)91423-9. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountacstle VB, Plum F, Geiger SR, editors. Handbook of physiology 5, part 1, chapter 9. American Physiological Society; Washington, DC: 1987. pp. 373–417. [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:239–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J. Regional distribution of functions in parietal association area 7 of the monkey. Brain Res. 1981;206:287–303. doi: 10.1016/0006-8993(81)90533-3. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Shelepin Y. Distribution of visual and somatic functions in the parietal associative area 7 of the monkey. Brain Res. 1979;169:561–564. doi: 10.1016/0006-8993(79)90404-9. [DOI] [PubMed] [Google Scholar]
- Ishida H, Nakajima K, Inase M, Murata A. Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21185. Jan 13 E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol. 1978;181:291–347. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Lin CS, Wagor E. Cortical projections of posterior parietal cortex in owl monkeys. J Comp Neurol. 1977;72:387–408. doi: 10.1002/cne.901710306. [DOI] [PubMed] [Google Scholar]
- Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol. 1996;74:483–498. [PubMed] [Google Scholar]
- Kaskan PM, Kaas JH. Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti). Anat Rec. 2007;290:349–366. doi: 10.1002/ar.20440. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Disbrow E. The evolution of parietal areas involved in hand use in primates. Cambridge University Press; New York: 2006. [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci Res. 1991;12:263–280. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 2000a;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lewis J, Van Essen D. Corticocortical connections of visual sensorimotor, multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000b;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 2002;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Maioli MG, Squatrito S, Samolsky-Dekel BG, Sanseverino ER. Corticocortical connections between frontal periarcuate regions and visual areas of the superior temporal sulcus and the adjoining inferior parietal lobule in the macaque monkey. Brain Res. 1998;789:118–125. doi: 10.1016/s0006-8993(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14:S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol. 1998;402:327–352. [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch Neurol. 1993;50:279–284. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuña C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Kaseda M, Sakata H. Parietal neurons related to memory-guided hand manipulation. J Neurophysiol. 1996;75:2180–2186. doi: 10.1152/jn.1996.75.5.2180. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Pevzner PA, O'Brien SJ. Mammalian phylogenomics comes of age. Trends Genet. 2004;20:631–639. doi: 10.1016/j.tig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Microstimulation of the lateral wall of the intraparietal sulcus compared with the frontal eye field during oculomotor tasks. J Neurophysiol. 1999;81:1443–1448. doi: 10.1152/jn.1999.81.3.1443. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kuroda T, Wakita M, Kusunoki M, Kato A, Mikami A, Sakata H, Itoh K. From three-dimensional space vision to prehensile hand movements: the lateral intraparietal area links the area V3A and the anterior intraparietal area in macaques. J Neurosci. 2001;21:8174–8187. doi: 10.1523/JNEUROSCI.21-20-08174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olucha F, Martinez-Garcia F, Lopez-Garcia C. A new stabilizing agent for the tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP). J Neurosci Methods. 1985;13:131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Padberg J, Seltzer B, Cusick CG. Architectonics and cortical connections of the upper bank of the superior temporal sulcus in the rhesus monkey: an analysis in the tangential plane. J Comp Neurol. 2003;467:418–434. doi: 10.1002/cne.10932. [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JG, Rosa MG, Fiorani M, Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Pearson RCA, Powell TPS. The projection of the primary somatic sensory cortex upon area 5 in the monkey. Brain Res Rev. 1985;9:89–107. doi: 10.1016/0165-0173(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J Comp Neurol. 1986;248:313–335. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared with the anthropoid primate Macaca. J Comp Neurol. 1991a;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate Galago, with comparative comments on anthropoid primates. J Comp Neurol. 1991b;310:507–549. doi: 10.1002/cne.903100404. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Jain N, Kaas JH. Multiple divisions of macaque precentral motor cortex identified with neurofilament antibody SMI-32. Brain Res. 1997;767:148–153. doi: 10.1016/s0006-8993(97)00704-x. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2006;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5). Novartis Found Symp. 1998;218:81–95. doi: 10.1002/9780470515563.ch6. discussion 95–103. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol. 1980a;192:69–92. doi: 10.1002/cne.901920105. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol. 1980b;192:93–108. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 2006;16:1389–417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Sakata H, Takaoka Y, Kawarasaki A, Shibutani H. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res. 1973;64:85–102. doi: 10.1016/0006-8993(73)90172-8. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui K. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Philos Trans R Soc Lond B Biol Sci. 1998;353:1363–1373. doi: 10.1098/rstb.1998.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bulier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur J Neurosci. 1998;10:3171–3193. doi: 10.1046/j.1460-9568.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Raffi M, Maioli MG, Battaglia-Mayer A. Visual motion responses of neurons in the caudal area PE of macaque monkeys. J Neurosci. 2001;21:RC130. doi: 10.1523/JNEUROSCI.21-04-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Sakai ST, Qi H-X, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for parkinsonian tremor. J Comp Neurol. 2003;455:378–395. doi: 10.1002/cne.10499. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005a;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang P-C, Kaas JH. 2005 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2005b. Cortical connections of physiologically identified subdivisions of posterior parietal cortex in prosimian galagos. Program No. 617.7. Online. [Google Scholar]
- Stepniewska I, Collins CE, Burish MJ, Kaas JH. 2006 Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. Intracortical microstimulation uncovers map of complex movements in the posterior parietal cortex of New World owl monkeys. Program No. 549.7. Online. [Google Scholar]
- Stepniewska I, Burish MJ, Kaas JH. 2007 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. Complex movements evoked by intracortical microstimulation from the frontal motor region of prosimian galagos. Program No. 828.7. Online. [Google Scholar]
- Stepniewska I, Burish MJ, Gharbawie OA, Kaas JH. 2008 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. Mapping the frontal motor region in New World monkeys with long train intracortical microstimulation. Program No. 78.6. Online. [Google Scholar]
- Stepniewska I, Fang P-C, Kaas JH. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal vs. ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Taoka M, Toda T, Iwamura Y. Representation of the midline trunk, bilateral arms, and shoulders in the monkey postcentral somatosensory cortex. Exp Brain Res. 1998;123:315–322. doi: 10.1007/s002210050574. [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. “What” and “where” in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingje DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- Vaina LM. Functional segregation of color and motion processing in the human visual cortex: clinical evidence. Cereb Cortex. 1994;4:555–572. doi: 10.1093/cercor/4.5.555. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Ann Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured as enucleated cats demonstrable with cytochrome-oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]