Abstract
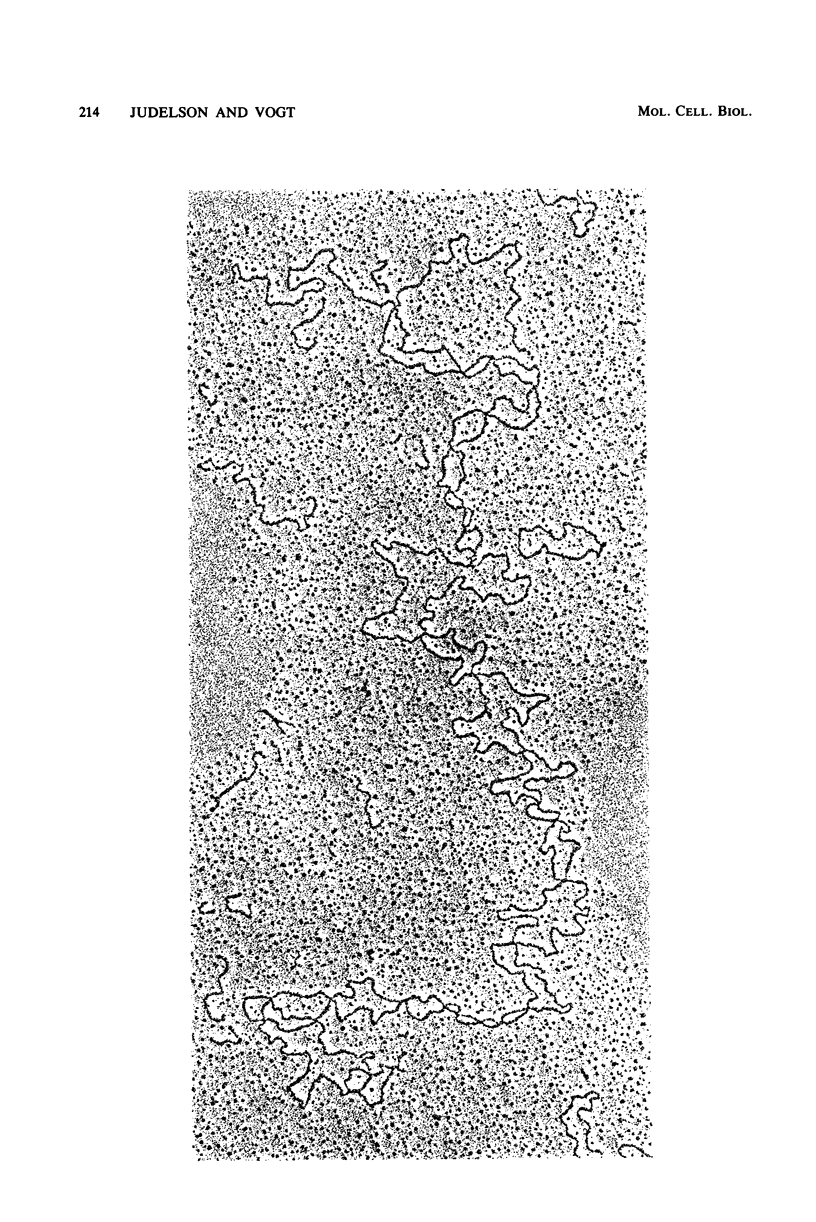
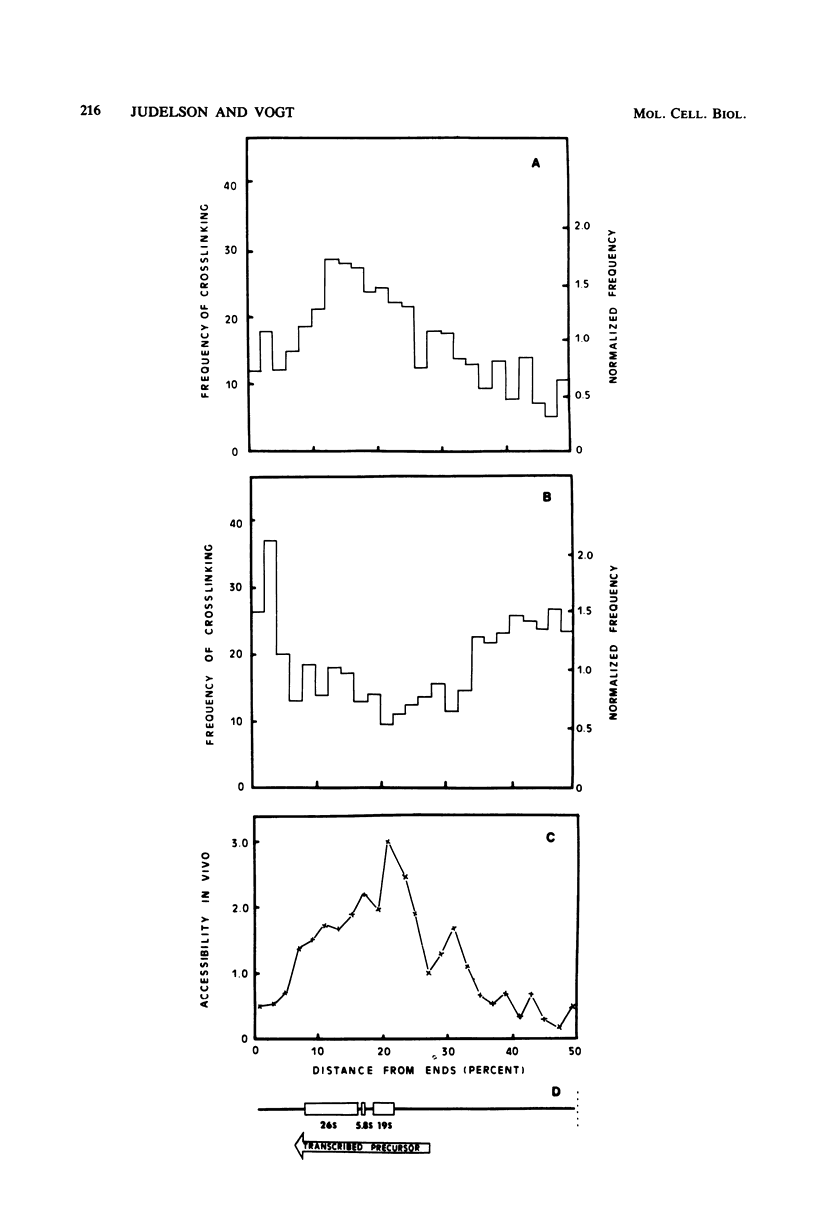
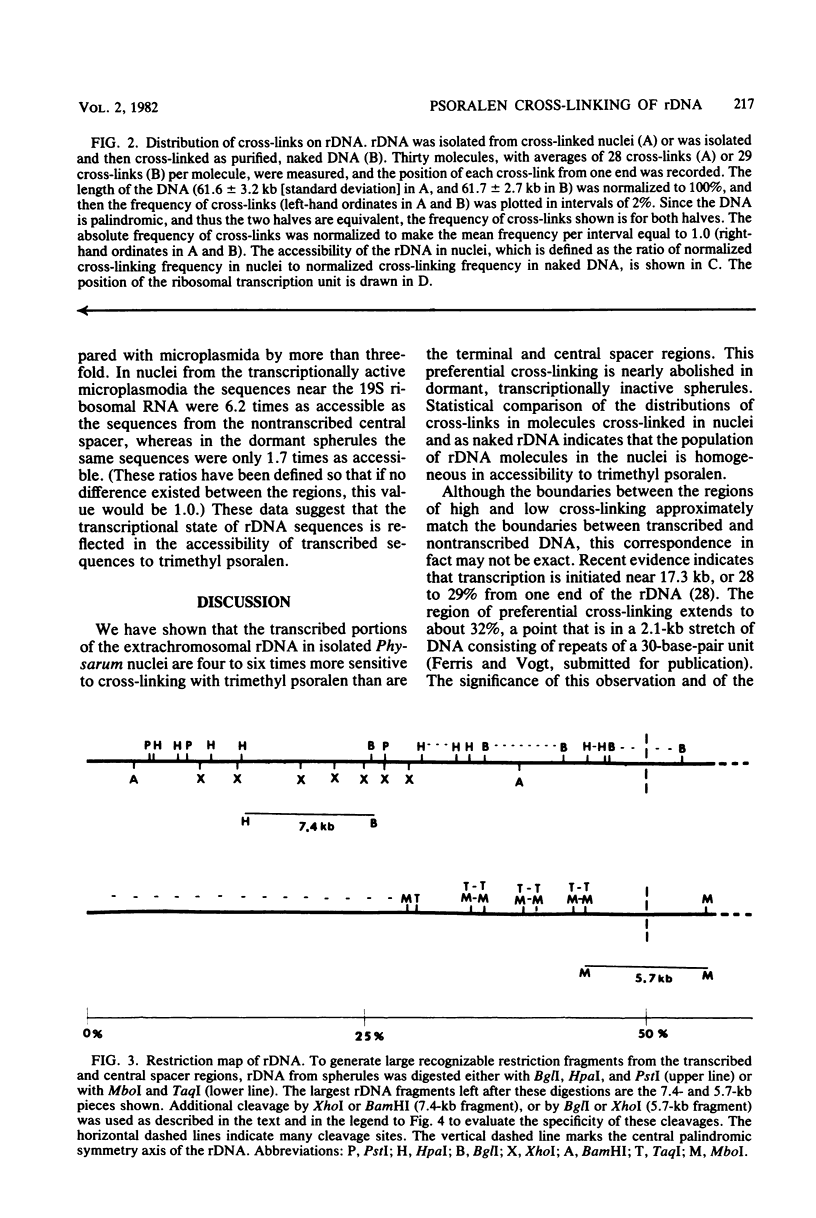
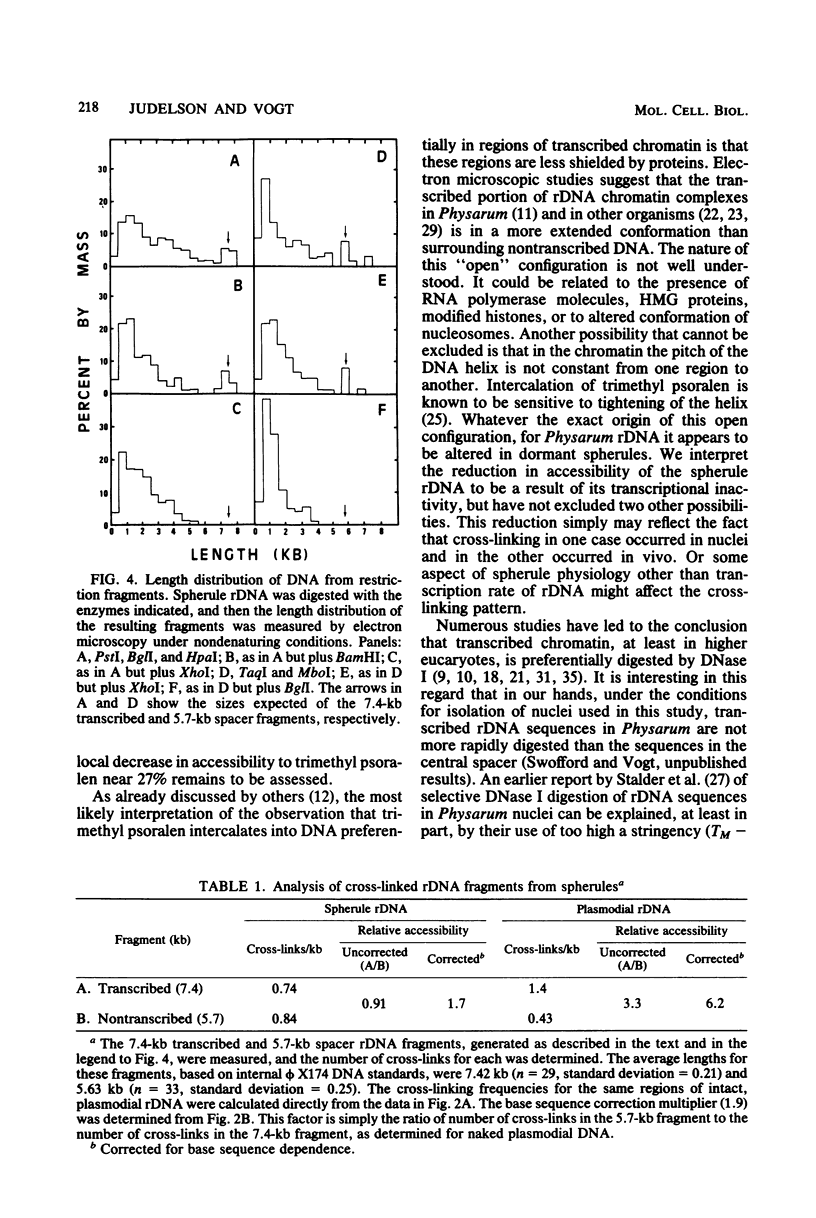
We have probed the accessibility of the genes for rRNA in Physarum polycephalum by using the photoreactive DNA cross-linking agent 4,5',8-trimethyl psoralen. Nuclei isolated from actively growing Physarum were treated with trimethyl psoralen and irradiated with 360-nm light in order to form cross-links. The palindromic, extrachromosomal rDNA then was isolated, and the positions of cross-links were determined by electron microscopy of the DNA under totally denaturing conditions. The results indicate that the frequency of cross-linking, after correction for base sequence bias of the reaction, is up to sixfold higher in the transcribed regions than in the central or the terminal spacer regions. There is no detectable heterogeneity among the different rDNA molecules or between the halves of a single molecule. Cross-linked molecules invariably occur in a linear as opposed to a cruciform structure. The preferential cross-linking of the transcribed region is nearly eliminated in spherules, a dormant transcriptionally inactive form in the Physarum life cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell G. R., Littau V. C., Melera P. W., Allfrey V. G., Johnson E. M. Unique sequence arrangement of ribosomal genes in the palindromic rDNA molecule of Physarum polycephalum. Nucleic Acids Res. 1979 Apr;6(4):1433–1447. doi: 10.1093/nar/6.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Karrer K. M. Chromatin structure of the ribosomal RNA genes of Tetrahymena thermophila as analyzed by trimethylpsoralen crosslinking in vivo. J Mol Biol. 1980 Feb 5;136(4):395–416. doi: 10.1016/0022-2836(80)90397-6. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Cech T., Potter D., Pardue M. L. Electron microscopy of DNA cross-linked with trimethylpsoralen: a probe for chromatin structure. Biochemistry. 1977 Nov 29;16(24):5313–5321. doi: 10.1021/bi00643a024. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Allfrey V. G., Bradbury E. M., Matthews H. R. Altered nucleosome structure containing DNA sequences complementary to 19S and 26S ribosomal RNA in Physarum polycephalum. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1116–1120. doi: 10.1073/pnas.75.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Campbell G. R., Allfrey V. G. Different nucleosome structures on transcribing and nontranscribing ribosomal gene sequences. Science. 1979 Dec 7;206(4423):1192–1194. doi: 10.1126/science.505006. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Matthews H. R., Littau V. C., Lothstein L., Bradbury E. M., Allfrey V. G. The structure of chromatin containing DNA complementary to 19 S and 26 S ribosomal RNA in active and inactive stages of Physarum polycephalum. Arch Biochem Biophys. 1978 Dec;191(2):537–560. doi: 10.1016/0003-9861(78)90392-2. [DOI] [PubMed] [Google Scholar]
- Klug A., Rhodes D., Smith J., Finch J. T., Thomas J. O. A low resolution structure for the histone core of the nucleosome. Nature. 1980 Oct 9;287(5782):509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- Lohr D., Hereford L. Yeast chromatin is uniformly digested by DNase-I. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4285–4288. doi: 10.1073/pnas.76.9.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Panet A., Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977 Aug;11(4):933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Pruitt S. C., Grainger R. M. A mosaicism in the higher order structure of Xenopus oocyte nucleolar chromatin prior to and during ribosomal gene transcription. Cell. 1981 Mar;23(3):711–720. doi: 10.1016/0092-8674(81)90434-7. [DOI] [PubMed] [Google Scholar]
- Scheer U. Changes of nucleosome frequency in nucleolar and non-nucleolar chromatin as a function of transcription: an electron microscopic study. Cell. 1978 Mar;13(3):535–549. doi: 10.1016/0092-8674(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Stalder J., Braun R. Isolation of a minichromosome containing the ribosomal genes from Physarum polycephalum. Biochemistry. 1979 Feb 6;18(3):484–490. doi: 10.1021/bi00570a017. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Degradation of the ribosomal genes by DNAse I in Physarum polycephalum. Eur J Biochem. 1978 Oct;90(2):391–395. doi: 10.1111/j.1432-1033.1978.tb12616.x. [DOI] [PubMed] [Google Scholar]
- Sun I. Y., Johnson E. M., Allfrey V. G. Initiation of transcription of ribosomal deoxyribonucleic acid sequences in isolated nuclei of Physarum polycephalum: studies using nucleoside 5'-[gamma-S]triphosphates and labeled precursors. Biochemistry. 1979 Oct 16;18(21):4572–4580. doi: 10.1021/bi00588a018. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F., Gurdon J. B. Transcription of cloned Xenopus ribosomal genes visualised after injection into oocyte nuclei. Nature. 1978 Nov 16;276(5685):292–294. doi: 10.1038/276292a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Structure of ribosomal DNA in Physarum polycephalum. J Mol Biol. 1976 Sep 25;106(3):567–587. doi: 10.1016/0022-2836(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]




