Abstract
Background
One-year mortality outcomes in the PARTNER trial showed that transcatheter aortic valve implantation (TAVI) was noninferior to surgical aortic valve replacement (sAVR) in patients who were eligible for sAVR (cohort A), and superior to standard treatment in patients who were ineligible for sAVR (cohort B).
Objective
To update a previous report on the safety, effectiveness, and cost-effectiveness of TAVI, published in 2012.
Data Sources
A literature search was performed on September 11, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2011, until September 11, 2012.
Review Methods
Randomized controlled trials investigating TAVI in comparison to sAVR or standard treatment were included for analysis. Results were summarized descriptively.
Results
At 2-year follow-up, mortality in cohort A was similar between the TAVI and sAVR groups. Rates of stroke/transient ischemic attack, major vascular complications, and moderate/severe paravalvular aortic regurgitation were significantly higher in the TAVI group, but rate of major bleeding was significantly higher in the sAVR group. Mortality in cohort B was significantly lower with transfemoral (TF) TAVI than with standard treatment, but rate of stroke was significantly higher with TF TAVI.
TF TAVI resulted in a more rapid improvement in quality of life scores than sAVR, but this difference was not sustained at 6 and 12 months. Patients who underwent transapical TAVI did not have a greater early improvement in quality of life compared to sAVR patients. Compared to standard treatment, TF TAVI resulted in a greater improvement in quality of life scores at all time points.
Incremental cost-effectiveness ratios were in favour of TAVI for inoperable patients in the base-case analysis, but varied widely for operable patients.
Conclusions
The findings of the 2-year follow-up with respect to mortality and adverse events were consistent with those of the 1-year follow-up. TAVI was also associated with improvement in quality of life, although results varied by cohort. Consistent with the 2012 report, TAVI may be cost-effective for patients who are not candidates for surgery.
Plain Language Summary
Narrowing of 1 of the heart valves (called aortic valve stenosis) makes it difficult for the heart to work properly. Often, patients have surgery to replace the narrowed valve, but surgery is too risky for some. In 2012, Health Quality Ontario published a report on a less invasive treatment option called transcatheter aortic valve implantation (TAVI). This report reviews information published since the 2012 report: the results of a 2-year follow-up of TAVI patients, and studies exploring patients’ quality of life.
Background
Objective of Analysis
The objective of this analysis is to update a previous report on the safety, effectiveness, and cost-effectiveness of transcatheter aortic valve implantation (TAVI), published by Evidence Development and Standards, Health Quality Ontario, in 2012. (1)
Clinical Need and Target Population
Aortic valve stenosis (AVS) is the narrowing of the aortic valve. AVS can result from the progressive build-up of calcium and the formation of scar tissue on a normal valve or on one damaged by an episode of rheumatic fever. The disease spectrum ranges from minor focal leaflet thickening with normal valve function to severe calcification and stiffness of the leaflets. Left untreated, the obstruction gradually results in pressure overload and left ventricular hypertrophy. (2) Symptoms of AVS include shortness of breath during physical activity, chest pain, dizziness, and syncope. Severe AVS represents the end stage of the disease spectrum. (3)
Studies suggest that degenerative AVS is an active disease process associated with underlying risk factors, rather than an inevitable consequence of aging. (4) Factors that lead to AVS include low-density lipoprotein cholesterol, smoking, male sex, diabetes, hypertension, and renal failure. (3;5;6)
It is important to diagnose and treat AVS, as it can eventually result in heart failure. Symptomatic patients who are managed medically have a poor prognosis. (7) Balloon valvuloplasty may result in temporary relief of symptoms, but it is associated with a high rate of restenosis and long-term survival is poor. (7) Early restenosis and recurrent hospitalization are common. (7)
Since no medical therapy is known to conclusively alter the progression of aortic valve disease, (5) surgical aortic valve replacement (sAVR) (involving sternotomy and cardiopulmonary bypass) has been performed for decades to improve heart function, relieve symptoms, and improve patient survival. sAVR is the gold-standard procedure for the treatment of symptomatic patients with severe AVS and has well-defined indications (8) delineated in the American College of Cardiology/American Heart Association 2006 Practice Guidelines for the Management of Patients with Valvular Heart Disease. (9)
Technology
TAVI has emerged as a less invasive treatment option for patients with AVS who are not candidates for surgery.
PARTNER Trial
The PARTNER trial was the first prospective randomized controlled trial (RCT) to evaluate the safety and effectiveness of TAVI and consists of 2 patient cohorts, both with severe AVS and at high risk for surgery. In cohort A, TAVI was compared with sAVR; in cohort B (in which patients were deemed ineligible for surgery), TAVI was compared with standard treatment (ST) (including balloon valvuloplasty, which was performed in the majority of patients). In cohort A, patients were evaluated to determine eligibility for a transfemoral (TF) approach to TAVI. Those who were eligible were randomized to TF TAVI or sAVR; those who were not eligible were randomized to transapical (TA) TAVI or sAVR.
One-year mortality outcomes showed that TAVI was noninferior to sAVR in patients who were eligible for sAVR (cohort A), and superior to ST in patients who were ineligible for sAVR (cohort B). (10) In cohort A, the risk of cerebrovascular events and vascular injuries was significantly higher in the TAVI group, but the risk of major bleeding was significantly higher in the sAVR group. The risk of paravalvular aortic regurgitation (PAR) was also significantly higher in the TAVI group. In cohort B, the risk of cerebrovascular events, vascular injuries, and major bleeding were significantly higher in the TAVI group than in the ST group.
Follow-up: 2 Years
The results of a 2-year follow-up of the PARTNER trial have recently been published. (11;12) The results are reported based on intention-to-treat analyses; a supplementary document includes results based on “as-treated” analyses.
Health-Related Quality of Life
Health-related quality of life (HRQOL) studies in both cohorts have also recently been published. (13;14) The PARTNER trial used 1 disease-specific instrument (Kansas City Cardiomyopathy Questionnaire [KCCQ]) and 2 generic instruments (Short-Form 12 [SF-12] and EuroQol 5 Domain [EQ-5D]) to measure HRQOL in patients who were treated with TAVI, sAVR, or ST.
Disease-Specific Instrument
Disease-specific measures quantify more clinically relevant domains than generic measures and are often more sensitive to clinical change. The KCCQ is a 23-item, self-administered instrument that helps to assess HRQOL in patients with heart failure. (15) Its subscales quantify physical limitation, social limitation, total symptoms, self-efficacy, and quality of life; the summary scale takes into account the scores from the individual scales. The individual and summary scales are scored from 0 to 100, with higher scores indicating better health status and quality of life.
Generic Instruments
The SF-12 and the EQ-5D are 2 short, self-completed HRQOL instruments that are used increasingly as population health status indicators, owing to their brevity and simplicity. (16)
The SF-12 is a generic multi-item questionnaire that measures perceived physical and mental well-being. It does not target a specific disease and is suitable for use in both general and clinical populations. It is a shorter version of the SF-36 and measures 8 health domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. The questionnaire is used to derive 2 aggregate summary measures—the physical composite score (PCS) and the mental composite score (MCS)—and it contains a mixture of positively and negatively worded responses, so some items must be recoded prior to scoring. The sum of the raw responses is transformed to a score from 0 to 100, with higher scores indicating better health status. Age- and sex-based normative data for PCS and MCS subscales are available for several countries.
In the general population, norms for the SF-12 PCS tend to decline with age, while norms for the MCS remain consistent across age groups; similar findings have been reported for patients living with heart disease or stroke. Lim and Fisher (16) assessed the relative health status of patients with different clinical diagnoses, including heart disease and stroke (they reported on 2,341 of 3,362 patients who returned the questionnaire); the majority of patients were male (61%) and > 75 years of age (74%). In most disease states, the PCS dropped significantly with age in both sexes, while the MCS declined only marginally. In patients with heart disease (ischemic heart disease, acute myocardial infarction, chest pain, and other heart disease) the PCS ranged from 38.7 to 43.0, and the MCS ranged from 45.9 to 46.9.
The EQ-5D health survey is a generic measure of health status for assessing utilities and quality-adjusted life-years (QALYs). Similar to the SF-12, it does not target a specific disease; it is a health utility measure originating from the field of health economics and is 1 of the measures recommended for use in cost-effectiveness analyses. This scale considers multiple independent attributes of an individual’s health to create an indication of overall HRQOL. It measures 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and the endpoints refer to the “best imaginable health state” (1.0) and the “worst imaginable health state” (0.0). It is intended to be a simple questionnaire capable of generating a composite score to reflect the value associated with a given health state.
Evidence-Based Analysis
Research Questions
For high-risk patients who are candidates for surgery:
Is the risk of death following TAVI equal to or less than that following sAVR?
Is TAVI associated with an equal or greater improvement in clinical symptoms compared with sAVR?
What are the adverse events and complications associated with TAVI?
What are the health status and HRQOL of patients following TAVI compared with sAVR?
For high-risk patients who are not candidates for surgery:
Is the risk of death following TAVI less than that following ST?
Is TAVI associated with a greater improvement in clinical symptoms compared with ST?
What are the adverse events and complications associated with TAVI?
What are the health status and HRQOL of patients following TAVI compared with ST?
Research Methods
Literature Search
Search Strategy
A literature search was performed on September 11, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2011, until September 11, 2012. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained.
Inclusion Criteria
English-language full reports
published between January 1, 2011, and September 11, 2012
RCTs
≥ 6 months follow-up
studies investigating clinical outcomes following TAVI in comparison with sAVR (for high-risk patients who are candidates for surgery) or with ST (for high-risk patients who are not candidates for surgery)
studies reporting mortality and/or important cardiovascular outcomes
studies including at least 10 patients
Exclusion Criteria
nonrandomized trials
studies reporting on technical aspects of different prostheses, design of TAVI systems, or techniques for valve implantation
studies reporting on approaches other than TF or TA
studies reporting on combined strategies, such as a combination of TAVI and other cardiac procedures
studies reporting on the implantation of a second valve
studies including fewer than 10 patients
Outcomes of Interest
Primary Outcomes
rate of death
Secondary Outcomes
rate of emergent conversion to surgery
rate of valve embolization
rate of multiple valve insertion
cardiovascular complications: stroke, myocardial infarction, atrial fibrillation, PAR, vascular injuries, need for permanent pacemaker
renal function
improvement in symptoms
improvement in New York Heart Association (NYHA) class
improvement in quality of life
length of hospital stay
length of intensive care unit stay
rehospitalization
Statistical Analysis
RevMan 5.1 and STATA 11 statistical software were used for graphical presentation of data. (17) Since the authors reported all percentages based on Kaplan-Meier estimates at specific time points, we also reported on the proportion of patients who developed adverse events (total number of events divided by the total number of patients). However, only the P values reported by the authors have been used for this report.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the GRADE Working Group criteria. (18) The overall quality was determined to be very low, low, moderate, or high using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that RCTs are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that may raise the quality of evidence were considered: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors. (18) For more detailed information, please refer to the latest series of GRADE articles. (18)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | Very confident that the true effect lies close to the estimate of the effect |
| Moderate | Moderately confident in the effect estimate—the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Confidence in the effect estimate is limited—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very little confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
The database search yielded 1,254 studies published between January 1, 2011, and September 11, 2012 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment.
Four studies (4 RCTs) met the inclusion criteria. The reference lists of the included studies were handsearched to identify any additional potentially relevant studies, but no additional citations were identified. All included studies were related to the PARTNER trial. (11-14) Two studies reported the results of the 2-year follow-up of cohorts A and B, (11;12) and the other 2 reported on the quality of life of patients in cohorts A and B. (13;14)
For each included study, the study design was identified and is summarized below in Table 1, which is a modified version of a hierarchy of study design by Goodman. (19)
Table 1: Body of Evidence Examined According to Study Design.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs Large RCT Small RCT |
4 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls Non-RCT with non-contemporaneous controls Systematic review of non-RCTs with historical controls Non-RCT with historical controls Database, registry, or cross-sectional study Case series Retrospective review, modelling Studies presented at an international conference Expert opinion Total |
4 |
Abbreviation: RCT, randomized controlled trial.
Two-Year Follow-up of the PARTNER Trial
Cohort A
Kodali et al (11) published the results of a 2-year follow up of cohort A.
Death
There were no statistically significant differences between the TAVI and sAVR groups with respect to rate of death from any cause (hazard ratio [HR], 0.90; 95% confidence interval [CI], 0.71-1.15; P = 0.41). Rates of death from any cause were higher with TA TAVI than with TF TAVI (40.4% [Kaplan-Meier estimate 41.4] versus 30.3% [Kaplan-Meier estimate 30.9], respectively).
There were no statistically significant differences between TAVI and sAVR for rate of death due to cardiac causes, a composite index of death from any cause or stroke, or a composite index of death or repeat hospitalization (Figures 1–3).
Figure 1: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TAVI Versus sAVRa.
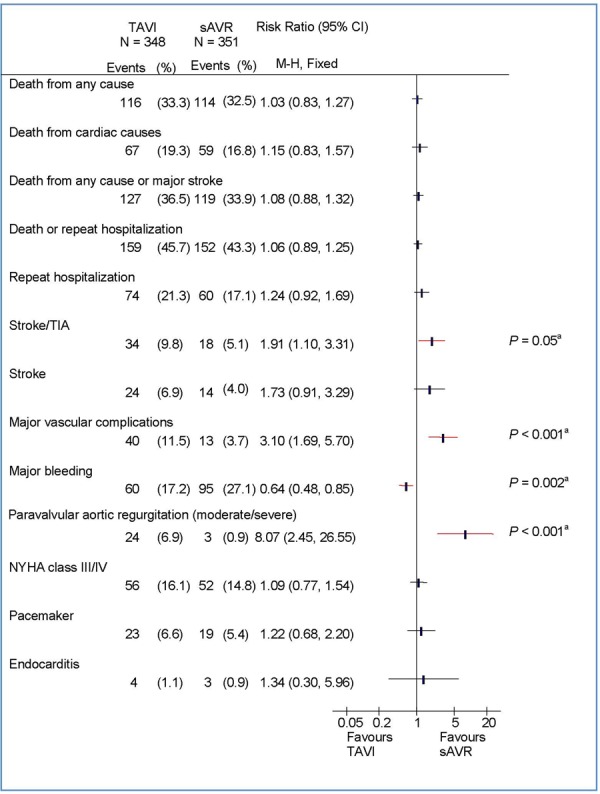
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; NYHA, New York Heart Association; sAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
Figure 3: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TA TAVI Versus sAVRa.
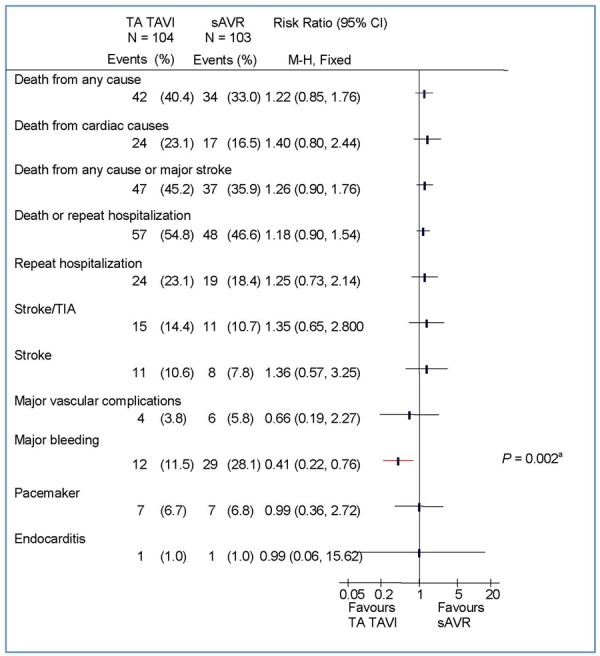
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; sAVR, surgical aortic valve replacement; TA, transapical; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
Stroke
At 2 years, the rate of stroke/transient ischemic attack (TIA) was significantly higher in the TAVI group than in the sAVR group (TAVI, 11.2%; sAVR, 6.5%; P = 0.05 by Kaplan-Meier analysis). In the TF TAVI/sAVR cohort, the difference between TF TAVI and sAVR was statistically significant (TF TAVI, 8.5%; sAVR, 3.4%; P = 0.03 by Kaplan-Meier analysis), but in the TA TAVI/sAVR cohort the difference did not reach significance (TA TAVI, 18.2%; sAVR, 14.1%; P = 0.49 by Kaplan-Meier analysis).
Figure 4 shows rate of stroke/TIA by access site at 1- and 2-year follow-up. The rate of stroke/TIA was higher in the TA TAVI/sAVR cohort than in the TF TAVI/sAVR cohort (12.6% versus 5.3%, respectively). However, it should be noted that the TA TAVI/sAVR cohort had significantly higher rates of cerebral and peripheral vascular disease at baseline and were deemed ineligible for TF TAVI prior to randomization.
Figure 4: Rate of Stroke/TIA With TF TAVI/sAVR and TA TAVI/sAVR at 1- and 2-Year Follow-upa.
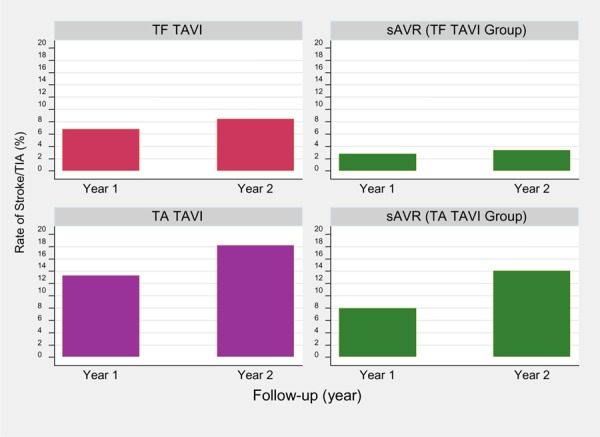
Abbreviations: sAVR, surgical aortic valve replacement; TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TIA, transient ischemic attack.
Based on data from Kodali at al. (11)
Major Vascular Complications
The rate of major vascular complications was higher in the TAVI group than in the sAVR group (TAVI, 11.6%; sAVR, 3.8%; P < 0.001 by Kaplan-Meier analysis). This difference was mainly due to the TF TAVI group, in which a significantly higher rate of vascular injuries was observed (Figures 2–3).
Figure 2: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TF TAVI Versus sAVRa.
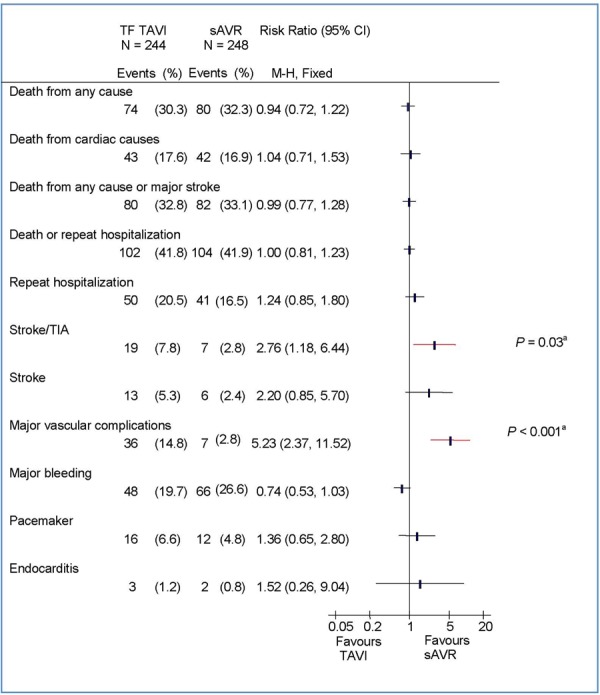
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; sAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TIA, transient ischemic attack.
Major Bleeding
The rate of major bleeding was significantly higher in the sAVR group than in the TAVI group (TAVI, 19.0%; sAVR, 29.5%; P = 0.002 by Kaplan-Meier analysis).
PAR
The rate of moderate to severe PAR was higher in the TAVI group than in the sAVR group, and the difference between the 2 groups was statistically significant (P < 0.001). The presence of paravalvular or total aortic regurgitation (mild/moderate/severe versus none/trace) after TAVI was associated with increased late mortality (HR, 2.11; 95% CI, 1.43-3.10; P < 0.001), and proportional to the severity of the aortic regurgitation. However, even mild aortic regurgitation was reported to be associated with an increased rate of death.
NYHA Class
Mean NYHA class was similar between the TAVI and sAVR groups among survivors (TAVI, 1.72; sAVR, 1.70; P = 0.87).
Other Adverse Events
Rates for endocarditis, renal failure, and the need for a new pacemaker were similar between the TAVI and sAVR groups.
Cohort B
Makkar et al (12) published the results of a 2-year follow up of cohort B.
Death
Kaplan-Meier analysis showed statistically significant differences between TF TAVI and ST with respect to rate of death from any cause (HR, 0.56; 95% CI, 0.43-0.73; P < 0.001), death from cardiac causes (HR, 0.44; 95% CI, 0.32-0.60; P < 0.001), and a composite index of death from any cause or stroke (HR, 0.64; 95% CI, 0.49-0.84; P < 0.001) (Figure 5).
Figure 5: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TF TAVI Versus Standard Treatmenta.
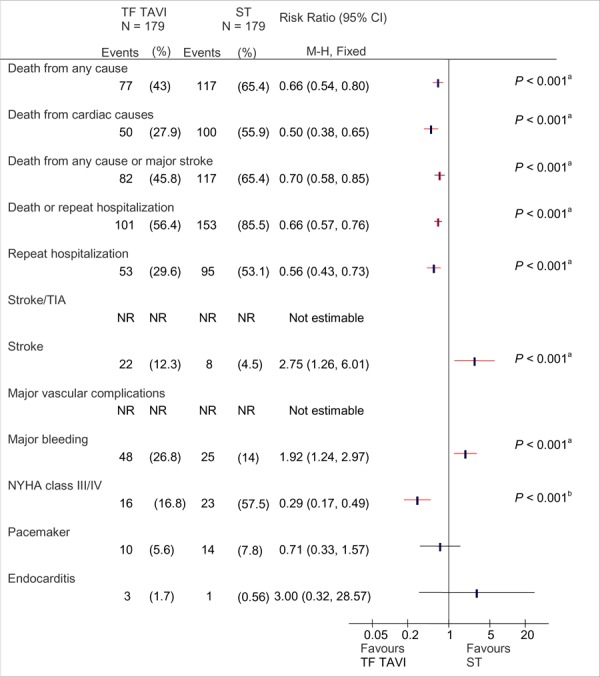
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; NR, not reported; NYHA, New York Heart Association; ST, standard treatment; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TIA, transient ischemic attack.
Based on data from Makkar at al. (12) P values (shown only where significant) are from the Kaplan-Meier analysis as reported by the authors. (12)
Denominators are 95 for TAVI and 40 for ST.
Stroke
The rate of stroke was higher in the TF TAVI group than in the ST group (13.8% versus 5.5%, respectively, by Kaplan-Meier analysis; HR, 2.79; 95% CI, 1.25-6.22; P = 0.009).
TIA
Rates of TIA were not reported.
Major Vascular Complications
Rates of major vascular complications were not reported.
Major Bleeding
The rate of major bleeding was higher in the TF TAVI group than in the ST group, but the difference was not statistically significant (28.9% versus 20.1%, respectively; P = 0.09 by Kaplan-Meier analysis).
PAR
Moderate to severe PAR was present in 10% of patients. Among those in whom echocardiographic data were available at 1- and 2-year follow-up, PAR improved in 42.6%, did not change in 41.0%, and worsened in 16.4%.
There was a nonsignificant trend toward higher cardiac mortality in patients who had moderate or severe PAR than those who had mild PAR or none (moderate/severe, 36.7%; none/mild, 27.0%; P = 0.38).
NYHA Class
More patients in the TF TAVI group had a NYHA class of I or II than patients in the ST group. By Kaplan-Meier analysis, 16.8% of patients in the TF TAVI group and 57.5% of patients in ST group had a NYHA class III or IV, and this difference was statistically significant (P < 0.001).
Other Adverse Events
Rates for endocarditis, renal failure, and the need for a new pacemaker were similar between TF TAVI and ST groups.
HRQOL in the PARTNER Trial
Reynolds et al (13;14) published their findings on HRQOL separately for the 2 cohorts of the PARTNER trial. In both studies, health status and quality of life were assessed at baseline and 1, 6, and 12 months after the intervention using the KCCQ and SF-12 for both cohorts and the EQ-5D for cohort A only. The primary outcome in both studies was the KCCQ summary score.
Cohort A
Reynolds et al (13) compared health status and quality of life in patients treated with TAVI with patients treated with sAVR. Of 699 patients included in cohort A, 71 were excluded from the analysis (no completed questionnaire), leaving a study population of 628 patients. Slightly more data were missing in the sAVR group than in the TAVI group.
Mean baseline scores for the KCCQ summary scale, the SF-12 PCS, the SF-12 MCS, and the EQ-5D were 41.9, 30.2, 47.2, and 0.67, respectively. Since the study identified a significant interaction for the treatment effect and access site, results were reported separately for TF TAVI/sAVR (n = 446) and TA TAVI/sAVR (n = 182). The study reported mean difference from baseline to follow-up.
KCCQ
Overall, the TAVI group had significantly higher KCCQ summary scores than the sAVR group at 1 month (adjusted mean difference [AMD], 5.5; 95% CI, 1.2-9.8; P = 0.01). However, no differences were observed between the 2 groups at 6 months (AMD, -2.6; 95% CI, -6.7 to 1.6; P = 0.22) or 12 months (AMD, -0.5; 95% CI, -4.8 to 3.8; P = 0.82).
In the TF TAVI/sAVR cohort, both groups showed a statistically significant improvement from baseline to 1, 6, and 12 months for the KCCQ summary scale and its subscales (physical limitations, social limitations, total symptoms, and quality of life), although the sAVR group had only a minimal and nonsignificant improvement at 1 month in physical limitation scores. At 1 month, the mean increase in KCCQ summary score was higher in the TF TAVI group than in the sAVR group (TF TAVI 23.7, 95% CI 20.1-27.3; sAVR 12.1, 95% CI 7.4-16.7). The explanation for this observation was that recovery following open-heart surgery is slower than for a less invasive procedure such as TAVI.
In contrast, in the TA TAVI group scores for the physical limitation and social limitation subscales did not demonstrate a significant improvement at 1 month (mean difference from baseline 2.4; 95% CI, -4.9 to 9.7; P = 0.59 for physical limitation; and mean difference from baseline 6.9; 95% CI, -3.2 to 17.0; P = 0.18 for social limitation). The sAVR group in the TA TAVI/sAVR cohort also showed a small and nonsignificant improvement in physical and social limitation scores. However, at 6 and 12 months, the KCCQ summary and subscale scores significantly improved from baseline in both TA TAVI and sAVR groups.
Figure 6 shows the mean change in KCCQ summary scores for the TF TAVI/sAVR and TA TAVI/sAVR cohorts at different follow-up times.
Figure 6: Mean Changes in KCCQ Summary Scores From Baseline to Follow-upa.
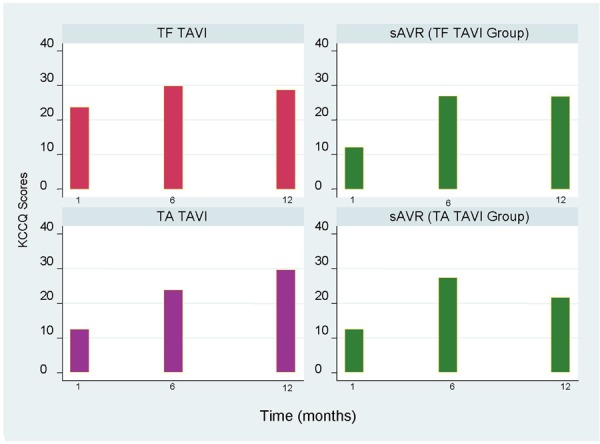
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; sAVR, surgical aortic valve replacement; TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
Based on data from Reynolds et al. (13)
A longitudinal growth curve model showed significant between-group differences in KCCQ summary and subscale scores at 1 month, favouring TF TAVI over sAVR (Table 2). At 6 and 12 months, results were in favour of sAVR, but they were not statistically significant. In the TA TAVI/sAVR group, KCCQ summary scores were in favour of sAVR at 1 month and 6 months, but the differences were significant only at 6 months. At 12 months, the differences between the TA TAVI and sAVR groups were not statistically significant.
Table 4: ICER of TAVI Versus ST in 3-Way Sensitivity Analysesa.
|
Increment in Utility (TAVI Versus ST) |
Difference in Total Cost of TAVI Versus ST | |||
|---|---|---|---|---|
|
$15,233 (Base Case) |
$25,233 | $35,233 | $45,233 | |
| Life Table Mortality After 1 Year for Both TAVI and ST (Base Case) | ||||
| 0 | $37,163 | $61,560 | $85,957 | $110,354 |
| 0.04 | $29,354 | $48,625 | $67,895 | $87,166 |
| 0.08 (base case) | $24,257 | $40,182 | $56,106 | $72,030 |
| 0.12 | $20,669 | $34,237 | $47,805 | $61,373 |
| Mortality After 1 Year of 0.50 for Both ST and TAVI | ||||
| 0 | $68,466 | $134,625 | $200,783 | $266,942 |
| 0.04 | $47,389 | $93,181 | $138,973 | $184,765 |
| 0.08 | $36,234 | $71,248 | $106,261 | $141,274 |
| 0.12 | $29,330 | $57,672 | $86,014 | $114,356 |
| Mortality After 1 Year of 0.50 for ST and 0.30 for TAVI | ||||
| 0 | $30,874 | $52,568 | $74,263 | $95,957 |
| 0.04 | $26,323 | $44,820 | $63,317 | $81,814 |
| 0.08 | $22,942 | $39,063 | $55,183 | $71,304 |
| 0.12 | $20,330 | $34,616 | $48,901 | $63,187 |
Abbreviations: ICER, incremental cost-effectiveness ratio; ST, standard treatment; TAVI, transcatheter aortic valve implantation.
All amounts are in Canadian dollars.
Table 2: Longitudinal Growth Curve Models for KCCQ Summary Scores.
| Follow-up Time | AMD (95% CI) for KCCQ Summary Scores, points | |
|---|---|---|
| TF TAVI/sAVR Cohort | TA TAVI/sAVR Cohort | |
| 1 month | 9.9 (4.9–14.9), P < 0.001 | –5.8 (–13.9 to 2.2), P = 0.15 |
| 6 months | –0.5 (–5.3 to 4.4), P = 0.85 | –7.9 (–15.7 to –0.2), P = 0.04 |
| 12 months | –1.2 (–6.3 to 3.9), P = 0.64 | 0.8 (–7.2 to 8.8), P = 0.85 |
Abbreviations: AMD, adjusted mean difference; CI, confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire; sAVR, surgical aortic valve replacement; TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
From Reynolds et al. (13)
SF-12
In the TF TAVI/sAVR cohort, both groups had a significant improvement in PCS and MCS at all time points, except for MCS scores in the sAVR group at 1 month (no improvement).
In the TA TAVI/sAVR cohort, improvement in PCS scores at 1, 6, and 12 months were all significant for the TA TAVI group. Similar to the KCCQ physical limitation score, the sAVR group did not have a significant improvement in PCS scores at 1 month but did have a significant improvement at 6 and 12 months. MCS scores did not improve significantly at 1 month in either the TA TAVI or sAVR groups, but at 6 and 12 months, both groups showed a significant improvement in MCS scores.
EQ-5D
In the TF TAVI/sAVR cohort, the scores for EQ-5D improved significantly at all time points in the TF TAVI group. Improvement in the sAVR group was significant at 6 and 12 months, but not at 1 month.
In the TA TAVI/sAVR cohort, there was either a nonsignificant decline (TA TAVI) or a nonsignificant improvement (sAVR) at 1 month. Significant improvement was observed in the sAVR group at 6 months, and in the TA TAVI group at 12 months.
Cohort B
The study in cohort B (i.e., patients who were not candidates for sAVR) (14) compared health status and quality of life in patients treated with TF TAVI versus patients who received ST. The study included 358 patients: 179 in the TF TAVI group and 179 in the ST group. Nine patients assigned to TF TAVI did not receive TAVI. In the ST group, 140 patients (78%) underwent balloon valvuloplasty, of which 114 were performed within 1 month of randomization. Death rates in the TF TAVI and ST groups at 1 year were 30.7% and 50.7%, respectively.
Health status and quality of life of patients were assessed using the KCCQ and SF-12 instruments. Patients completed HRQOL questionnaires at baseline (91%), 1 month (82%), 6 months (81%), and 12 months (84%), with higher completion rates among TF TAVI patients (TAVI, 88%; ST, 76%). The 12-month HRQOL scores were available for 61% of patients randomized to TF TAVI and 39% of patients randomized to ST.
Mean baseline scores for the KCCQ summary scale were 36.2 ± 20.5 for the TF TAVI group and 34.4 ± 20.1 for the ST group. Mean baseline scores for the SF-12 PCS and SF-12 MCS were 28.2 ± 7.7 and 44.5 ± 12.2 for TF TAVI and 27.7 ± 6.9 and 45.2 ± 11.0 for ST, respectively. There were no statistically significant differences in baseline scores between the 2 groups.
KCCQ
The study reported mean scores for baseline and all follow-up time points in each treatment group. The KCCQ summary and subscales all improved significantly following TF TAVI across all follow-up time points and were higher than ST at each assessment point. Improvement in the ST group was significant at 1 and 6 months but did not reach statistical significance at 12 months. KCCQ summary scores at baseline and follow-up are shown in Figure 7.
Figure 7: KCCQ Summary Scores at Baseline and Follow-upa.
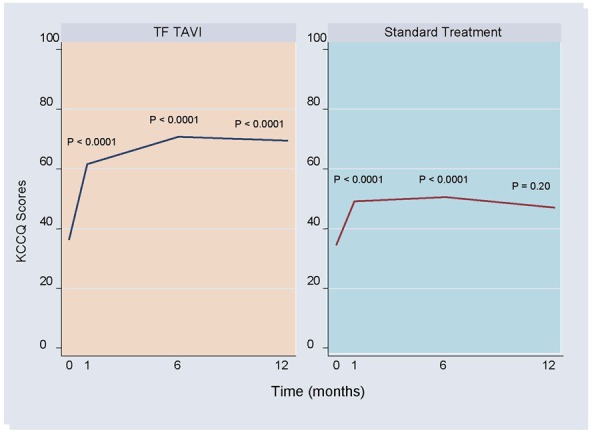
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
Based on data from Reynolds et al. (14) P values are as reported by the authors.
SF-12
PCS and MCS scores both improved significantly from baseline in the TF TAVI group. In the ST group, there was a significant improvement in PCS and MCS scores at 1 month. The improvement in PCS score was still significant at 6 months, but not at 12 months; MCS scores were not significantly different from baseline at 6 and 12 months.
Between-group comparisons from a longitudinal growth curve model showed significantly better HRQOL at 1, 6, and 12 months for patients who underwent TF TAVI compared to patients who received ST, except for MCS scores, which did not differ significantly between the 2 groups at 1 month.
Economic Analysis
Disclaimer: Health Quality Ontario uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province's perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions (CCI) procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Non-hospital: These include physician services costs obtained from the Ontario Schedule of Benefits (OSB), laboratory fees from the Ontario Schedule of Laboratory Fees (OSLF), drug costs from the Ontario Drug Benefit Formulary (ODB), and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Objective
The objective of this analysis was to present an updated economic analysis, incorporating new available evidence on TAVI. All amounts are in Canadian dollars.
Economic Analysis Methods
This updated analysis incorporated the following new evidence in the base-case analyses:
The cost of the transcatheter heart valve was assumed to be $24,000 ($37,606 was used in the original analysis) (Edwards Lifesciences [Canada] Inc., written communication, July 2012.).
For high-risk patients who were candidates for surgery, the difference in EQ-5D-derived health utility at 1 year between TAVI and sAVR was assumed to be 0.01 (-0.01 was used in the 2012 analysis). (13)
For high-risk patients who were not candidates for surgery, the difference in EQ-5D-derived health utility at 1 year between TAVI and ST was assumed to be 0.08 (0.07 was used in the 2012 analysis). (20)
The same risk of stroke for TAVI and ST in inoperable patients and for TAVI and sAVR in operable patients was tested in the sensitivity analyses.
Results
Updated Base-Case Analyses
With the above-mentioned new evidence, the incremental cost-effectiveness ratio (ICER) of TAVI compared with ST in inoperable patients was determined to be $17,898 per life-year (LY) and $24,257 per QALY. For operable patients, TAVI dominated sAVR (i.e., produced more LYs at lower costs) if the outcome was measured using LYs. In contrast, if the outcome was measured using QALYs, TAVI produced fewer QALYs at lower costs than sAVR (i.e., the ICER was $66,985) (Table 3).
Table 3: Updated Base-Case Analysis Resultsa.
| Treatment | Costs | LYs | QALYs | ΔCost/Δ LY | ΔCost/Δ QALY |
|---|---|---|---|---|---|
| Inoperable | |||||
| TAVI | $73,196 | 4.037 | 2.702 | — | — |
| ST | $57,963 | 3.186 | 2.074 | — | — |
| Incremental (TAVI versus ST) | $15,233 | 0.851 | 0.628 | $17,898 | $24,257 |
| Operable | |||||
| TAVI | $69,960 | 4.091 | 2.945 | — | — |
| sAVR | $74,602 | 4.079 | 3.014 | — | — |
| Incremental (TAVI versus sAVR) | −$4,642 | 0.012 | −0.069 | Dominates | $66,985 |
Abbreviations: LY, life-year; QALY, quality-adjusted life-year; sAVR, surgical aortic valve replacement; ST, standard treatment; TAVI, transcatheter aortic valve implantation.
All amounts are in Canadian dollars. Calculations may be inexact due to rounding.
Updated Sensitivity Analyses
In the updated base-case analyses for inoperable patients, the total cost of TAVI was $15,233 higher than that of ST, while the health utility for patients without postsurgical complications in the TAVI group was 0.08 higher than that of the ST group. To assess the sensitivity of the ICER (between TAVI and ST) to a few key parameters—including incremental health utility, incremental cost, and long-term mortality—a wide range of 3-way deterministic sensitivity analyses were conducted. The ICER varied from $20,330 to $266,942 (Table 4). If the risk of stroke beyond 1 year was assumed to be the same for both TAVI and ST, the ICER varied from $12,606 to $239,904.
In the sensitivity analyses for operable patients, ICERs varied widely, from TAVI being dominant to sAVR to TAVI being dominated by sAVR (Table 5). If the risk of stroke beyond 1 year was assumed to be the same for both TAVI and sAVR, the ICER also varied widely, from TAVI being dominant to being dominated.
Table 5: ICER of TAVI Versus sAVR in 3-Way Sensitivity Analysesa.
|
Increment in Utility (TAVI Versus sAVR) |
Difference in Total Cost of TAVI Versus sAVR | |||
|---|---|---|---|---|
|
-$4,642 (Base Case) |
$5,358 | $15,358 | $25,358 | |
| Life Table Mortality After 1 Year for Both TAVI and sAVR (Base Case) | ||||
| 0 | $46,970b | Dominated | Dominated | Dominated |
| 0.01 (base-case) | $66,985b | Dominated | Dominated | Dominated |
| 0.06 | Dominant | $77,904 | $223,313 | $368,722 |
| 0.12 | Dominant | $22,665 | $64,970 | $107,275 |
| Mortality After 1 Year of 0.25 for Both TAVI and sAVR | ||||
| 0 | $182,700a | Dominated | Dominated | Dominated |
| 0.01 | $588,373a | Dominated | Dominated | Dominated |
| 0.06 | Dominant | $39,465 | $141,646 | $243,827 |
| 0.12 | Dominant | $16,842 | $60,448 | $104,054 |
| Mortality After 1 Year of 0.25 for SAVR and 0.24 for TAVI | ||||
| 0 | Dominant | $506,880 | $1,691,211 | $2,875,543 |
| 0.01 | Dominant | $133,845 | $446,574 | $759,303 |
| 0.06 | Dominant | $30,139 | $100,561 | $170,982 |
| 0.12 | Dominant | $15,531 | $51,821 | $88,110 |
Abbreviations: ICER, incremental cost-effectiveness ratio; sAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
All amounts are in Canadian dollars.
The larger ICER means that TAVI is more cost-effective than sAVR (the results are in the southwest quadrant of the cost-effectiveness plane).
Conclusions
The findings of the 2-year follow-up with respect to mortality and adverse events were consistent with those of the 1-year follow-up. TAVI was also associated with improvement in quality of life, although results varied by cohort. Consistent with the 2012 report, TAVI may be cost-effective for patients who are not candidates for surgery.
Acknowledgements
Editorial Staff
Jeanne McKane, CPE, ELS(D)
Medical Information Services
Kellee Kaulback, BA(H), MISt
Appendices
Appendix 1: Literature Search Strategies
TAVI - Evidence Update Literature Search - September 2012
Search date: September 11, 2012
Databases searched: OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, EBSCO CINAHL, Centre for Reviews and Dissemination.
Database: Ovid MEDLINE(R) <1946 to August Week 5 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <September 10, 2012>, Embase <1980 to 2012 Week 36>
Search Strategy:
------------------------------------------------------------
exp Heart Valve Prosthesis Implantation/ or exp Heart Valve Prosthesis/ use mesz (59972)
exp Aorta Valve Replacement/ or exp aorta valve prosthesis/ use emez (13425)
((aorta or aortic) adj2 (replace* or implant* or prosthe* or bioprosthe* or transplant*)).ti,ab. (32607)
avr.ti,ab. (6171)
or/1-4 (77419)
exp Aortic Valve Stenosis/ use mesz (26913)
exp Aorta Valve Stenosis/ use emez (10640)
((supravalvular or subvalvular or aort*) adj2 stenos?s).ti,ab. (26022)
or/6-8 (49939)
exp Surgical Procedures, Minimally Invasive/ use mesz (330958)
exp Minimally Invasive Surgery/ use emez (20819)
(transcatheter* or trans-catheter* or transfemoral or trans-femoral or transapical or trans-apical or percutaneous).ti,ab. (221906)
(minimal* adj3 (surgery or surgeries or surgical or procedure* or invasive)).ti,ab. (78484)
or/10-13 (580573)
5 and 9 and 14 (3520)
(core-valve or corevalve or Cribier-Edwards or Edwards-Sapien or TAVI).ti,ab. (2585)
15 or 16 (4400)
limit 17 to english language (4014)
limit 18 to yr=”2011 -Current” (2069)
remove duplicates from 19 (1495)
Case Report/ use emez (1849271)
limit 20 to (case reports or comment or editorial or letter or note) [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process,Embase; records were retained] (139)
20 not (21 or 22) (1232)
****************************
CRD
| Line | Search | Hits |
|---|---|---|
| 1 | MeSH DESCRIPTOR Heart Valve Prosthesis EXPLODE 1 | 0 |
| 2 | MeSH DESCRIPTOR Heart Valve Prosthesis Implantation EXPLODE ALL TREES |
65 |
| 3 | (((aorta or aortic) adj2 (replace* or implant* or prosthe* or bioprosthe* or transplant*))) |
64 |
| 4 | (avr) | 6 |
| 5 | #1 OR #2 OR #3 OR #4 | 94 |
| 6 | MeSH DESCRIPTOR Aortic Valve Stenosis EXPLODE ALL TREES | 38 |
| 7 | (((supravalvular or subvalvular or aort*) adj2 stenos?s)) | 54 |
| 8 | #6 OR #7 | 61 |
| 9 | MeSH DESCRIPTOR Surgical Procedures, Minimally Invasive EXPLODE ALL TREES |
2710 |
| 10 | ((transcatheter* or trans-catheter* or transfemoral or trans-femoral or transapical or trans-apical or percutaneous)) |
1384 |
| 11 | (minimal* adj3 (surgery or surgeries or surgical or procedure* or invasive)) | 583 |
| 12 | #9 OR #10 OR #11 | 4032 |
| 13 | #5 AND #8 AND #12 | 23 |
| 14 | (core-valve or corevalve or Cribier-Edwards or Edwards-Sapien or TAVI) | 13 |
| 15 | #13 OR #14 | 26 |
| 16 | (#13 OR #14) FROM 2011 TO 2012 | 11 |
Wiley Cochrane
Appendix 2: GRADE Tables
Table A1: GRADE Evidence Profile for TAVI.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations |
Quality |
|---|---|---|---|---|---|---|---|
| Death Rate at 2 Years | |||||||
| 1 (RCT) | No serious limitations | NA | No serious limitations | No serious limitations | NA | NA |
 High High |
| Safety Measures at 2 Years | |||||||
| 1 (RCT) | No serious limitations | NA | No serious limitations | No serious limitations | NA | NA |
 High High |
| HRQOL at 1 Year | |||||||
| 1 (RCT) | No serious limitations | NA | No serious limitations | No serious limitations | NA | NA |
 High High |
Abbreviations: HRQOL, health-related quality of life; No., number; NA, not applicable; RCT, randomized controlled trial; TAVI, transcatheter aortic valve implantation.
Suggested Citation
This report should be cited as follows:
Sehatzadeh S, Doble B, Xie F, Blackhouse G, Campbell K, Kaulback K, Chandra K, Goeree R. Transcatheter aortic valve implantation (TAVI) for treatment of aortic valve stenosis: an evidence update. Ont Health Technol Assess Ser [Internet]. 2013 May;13(1):1-40. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-tavi.pdf.
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/EMBASE, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
About Health Quality Ontario
Health Quality Ontario (HQO) is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. HQO works with clinical experts, scientific collaborators and field evaluation partners to develop and publish research that evaluates the effectiveness and cost-effectiveness of health technologies and services in Ontario.
Based on the research conducted by HQO and its partners, the Ontario Health Technology Advisory Committee (OHTAC) — a standing advisory sub-committee of the HQO Board — makes recommendations about the uptake, diffusion, distribution or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders and policy-makers.
This research is published as part of Ontario Health Technology Assessment Series, which is indexed in CINAHL, EMBASE, MEDLINE, and the Centre for Reviews and Dissemination. Corresponding OHTAC recommendations and other associated reports are also published on the HQO website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, HQO and/or its research partners reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, HQO collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social, and legal issues relating to the intervention assist in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals and organizations wishing to comment on reports and recommendations prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This report was prepared by HQO or one of its research partners for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to HQO. It is possible that relevant scientific findings may have been reported since completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the HQO website for a list of all publications: http://www.hqontario.ca/en/mas/mas_ohtas_mnhtml.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-1251-4 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1: Body of Evidence Examined According to Study Design |
| Table 2: Longitudinal Growth Curve Models for KCCQ Summary Scores |
| Table 3: Updated Base-Case Analysis Resultsa |
| Table 4: ICER of TAVI Versus ST in 3-Way Sensitivity Analysesa |
| Table 5: ICER of TAVI Versus sAVR in 3-Way Sensitivity Analysesa |
| Table A1: GRADE Evidence Profile for TAVI |
List of Figures
| Figure 1: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TAVI Versus sAVRa |
| Figure 2: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TF TAVI Versus sAVRa |
| Figure 3: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TA TAVI Versus sAVRa |
| Figure 4: Rate of Stroke/TIA With TF TAVI/sAVR and TA TAVI/sAVR at 1- and 2-Year Follow-upa |
| Figure 5: Clinical Outcomes at 2 Years by Intention-to-Treat Analysis: TF TAVI Versus Standard Treatmenta |
| Figure 6: Mean Changes in KCCQ Summary Scores From Baseline to Follow-upa |
| Figure 7: KCCQ Summary Scores at Baseline and Follow-upa |
List of Abbreviations
- AMD
Adjusted mean difference
- AVS
Aortic valve stenosis
- CI
Confidence interval
- EQ-5D
EuroQol 5 Domain
- HR
Hazard ratio
- HRQOL
Health-related quality of life
- ICER
Incremental cost-effectiveness ratio
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LY
Life-year
- MCS
Mental composite score
- M-H
Mantel-Haenszel
- NR
Not reported
- NYHA
New York Heart Association
- PAR
Paravalvular aortic regurgitation
- PCS
Physical composite score
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- sAVR
Surgical aortic valve replacement
- SF-12
Short Form-12
- ST
Standard treatment
- TA
Transapical
- TAVI
Transcatheter aortic valve implantation
- TF
Transfemoral
- TIA
Transient ischemic attack
References
- 1.Sehatzadeh S, Doble B, Xie F, Blackhouse G, Campbell K, Kaulback K, et al. Transcatheter aortic valve implantation (TAVI) for treatment of aortic valve stenosis: an evidence-based Analysis (part B) [[updated 2012 May; cited 2012 Oct 1]]. [Internet] Available from: http://www.hqontario.ca/en/eds/tech/pdfs/2012/rev_tavi_may2012.pdf . [PMC free article] [PubMed]
- 2.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011 Mar;8(3):162–72. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997 Mar 1;29(3):630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 4.Leggett M, Otto CM. Aortic valve disease. Curr Opin Cardiol. 1996 Mar;11(2):120–5. doi: 10.1097/00001573-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Zigelman CZ, Edelstein PM. Aortic valve stenosis. Anesthesiol Clin. 2009 Sep;27(3):519–32. doi: 10.1016/j.anclin.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Anvari MS, Boroumand MA, Karimi A, lidoosti M, azdanifard P, hirzad M, et al. Aortic and mitral valve atherosclerosis: predictive factors and associations with coronary atherosclerosis using Gensini score. Arch Med Res. 2009 Feb;40(2):124–7. doi: 10.1016/j.arcmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Mickel MC, Kennedy JW, Alderman EL, Bashore TM, Block PC, et al. Three-year outcome after balloon aortic valvuloplasty. Circulation. 1994 Feb;89(2):642–50. doi: 10.1161/01.cir.89.2.642. Insights into prognosis of valvular aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 8.Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation. 2007;116(11 suppl):1240–5. doi: 10.1161/CIRCULATIONAHA.106.677237. [DOI] [PubMed] [Google Scholar]
- 9.Bonow RO, Carabello BA, Chatterjee K, de Leon ACJ, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006 Aug 1;48(3):e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 11.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 12.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60(6):548–58. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(18):1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 15.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000 Apr;35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 16.Lim LL, Fisher JD. Use of the 12-item short-form (SF-12) Health Survey in an Australian heart and stroke population. Qual Life Res. 1999;8(1-2):1–8. doi: 10.1023/a:1026409226544. [DOI] [PubMed] [Google Scholar]
- 17.Review Manager (RevMan) [Computer program] Version 5.1. Copenhagen (DK): The Nordic Cochrane Centre, The Cochrane Collaboration. 2011.
- 18.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011 Apr;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. 1996. 81p. SBU Report No. 119E. [DOI] [PubMed]
- 20.Neyt M, Van BH. Cost-effectiveness of transcatheter aortic valve replacement: overoptimistic study results and a call for publication of complete trial results. Heart. 2012 Jul;98(13):1031–3. doi: 10.1136/heartjnl-2012-301813. [DOI] [PubMed] [Google Scholar]


