Abstract
Aims
The changes in iron status occurring during the course of heart failure (HF) and the underlying pathomechanisms are largely unknown. Hepcidin, the major regulatory protein for iron metabolism, may play a causative role. We investigated iron status in a broad spectrum of patients with systolic HF in order to determine the changes in iron status in parallel with disease progression, and to associate iron status with long-term prognosis.
Methods and results
Serum concentrations of ferritin, transferrin saturation (Tsat), soluble transferrin receptor (sTfR), and hepcidin were assessed as the biomarkers of iron status in 321 patients with chronic systolic HF [age: 61 ± 11 years, men: 84%, left ventricular ejection fraction: 31 ± 9%, New York Heart Association (NYHA) class: 72/144/87/18] at a tertiary cardiology centre and 66 age- and gender-matched healthy subjects. Compared with healthy subjects, asymptomatic HF patients had similar haematological status, but increased iron stores (evidenced by higher serum ferritin without distinct inflammation, P < 0.01) with markedly elevated serum hepcidin (P < 0.001). With increasing HF severity, patients in advanced NYHA classes had iron deficiency (ID) (reduced serum ferritin, low Tsat, high sTfR), iron-restricted erythropoiesis (reduced haemoglobin, high red cell distribution width), and inflammation (high serum high-sensitivity-C-reactive protein and interleukin 6), which was accompanied by decreased circulating hepcidin (all P < 0.001). In multivariable Cox models, low hepcidin was independently associated with increased 3-year mortality among HF patients (P < 0.001).
Conclusions
Increased level of circulating hepcidin characterizes an early stage of HF, and is not accompanied by either anaemia or inflammation. The progression of HF is associated with the decline in circulating hepcidin and the development of ID. Low hepcidin independently relates to unfavourable outcome.
Keywords: Heart failure, Iron deficiency, Ferritin, Hepcidin, Prognosis
Introduction
Iron deficiency (ID) is frequent among patients with stable systolic heart failure (HF), and has serious unfavourable clinical and prognostic consequences.1–3 Intravenous iron therapy administered in patients with HF and ID lessens the symptoms and improves exercise capacity and quality of life.4–6
However, the sequence of changes in iron status occurring during the natural history of HF and the pathomechanisms triggering ID in these patients still remain enigmatic. Hepcidin as the major regulatory protein of iron metabolism may play a causative role in HF.7–10 The hepatic upregulation of hepcidin has been shown to be centrally involved in the pathogenesis of ID and anaemia associated with chronic inflammatory diseases, including chronic kidney disease (CKD), infections, cancer, autoimmune diseases.7,11–15 Available evidence on detailed iron status in HF is scarce and equivocal.16–20
We set up our study with the following aims: (i) to investigate iron status in a broad spectrum of patients with systolic HF compared with healthy subjects; (ii) to determine the changes in iron status in parallel with increasing HF severity and during clinical follow-up in patients with systolic HF; (iii) to associate iron status with clinical indices, measurements of erythropoiesis, and long-term prognosis in patients with systolic HF.
Methods
Patients with systolic heart failure and healthy subjects
The recruitment phase of the study was conducted among patients with systolic HF who either attended the outpatient clinic or underwent a planned hospitalization (for diagnostic purposes) in a tertiary cardiology centre. The criteria for patients with systolic HF to be included in the study were (i) age ≥18 years; (ii) a documented history of HF of ≥6 months; (iii) left ventricular ejection fraction (LVEF) ≤45% as assessed by echocardiography (performed at the time of the study, using Simpson's planimetric method); (iv) clinical stability and unchanged HF medications for ≥1 month preceding the study.
Healthy subjects were recruited among volunteers, relatives, and colleagues of the staff or patients. The criteria for healthy subjects to be included in the study were (i) age ≥18 years; (ii) neither signs nor symptoms of any cardiovascular disease; (iii) normal echocardiography examination, in particular LVEF ≥50%, performed at the time of the study using Simpson's planimetric method); (iv) absence of any acute (during the previous 6 months) or chronic (at any time in the past) illness and related therapy.
Exclusion criteria included (i) acute coronary syndrome and/or coronary revascularization within the 3 months preceding the study; (ii) unplanned hospitalization due to HF deterioration or any other cardiovascular reason within 1 month preceding the study; (iii) any acute or chronic illness that might influence iron metabolism (including malignancy, infection, severe renal disease requiring dialysis, and haematological diseases); (iv) any anaemia and/or ID treatment either at the time of the study or during the previous 12 months.
The study protocol was approved by the local ethics committee and all subjects gave written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Haematological parameters, indices of iron status, and other laboratory measurements assessed in peripheral blood
In all patients, venous blood samples were taken in the morning following an overnight fast and after at least 15 min of supine rest. Haematological measurements were made in fresh venous blood with EDTA and clotted blood. After centrifuging, the plasma and serum were collected and frozen at −70°C until further laboratory analyses.
Haemoglobin concentration (g/dL), mean corpuscular volume (MCV, fL), and red cell distribution width (RDW, %) were measured using the ADVIA 120 automated system (Siemens, Healthcare Diagnostics, Inc., Deerfield, IL, USA). Anaemia was defined as haemoglobin <12 g/dL in women and <13 g/dL in men.21
The following blood biomarkers reflecting iron metabolism were measured directly: serum concentrations of ferritin (µg/L), iron (µg/dL), and total iron binding capacity (TIBC, µg/dL). Transferrin saturation (Tsat) was calculated as the ratio of serum iron (µg/dL) and TIBC (µg/dL) multiplied by 100 and expressed as a percentage. Serum ferritin was measured using an immunoassay based on electrochemiluminescence with the Elecsys 2010 System (Roche Diagnostics GmbH, Mannheim, Germany). Serum iron and TIBC were assessed using a substrate method with Feren S (Thermo Fisher Scientific, Waltham, MA, USA).
Circulating iron bound to transferrin (expressed as Tsat) reflects the amount of iron available to metabolizing cells.22–24 The interpretation of circulating ferritin levels should always take into account its dual physiological role as the major iron storage molecule and as the acute phase protein, which expression is increased in response to inflammatory stimuli, regardless of the amount of stored iron. However, in the absence of inflammation, circulating ferritin can be considered as a reliable surrogate for the quantity of stored iron.8,22,23,25–27
Serum soluble transferrin receptor (sTfR, mg/L) was measured using immunonephelometry (Siemens Healthcare Diagnostics, Inc.). Increased circulating sTfR (originating from all cells metabolizing iron) is a sensitive indicator of ID and quantitatively reflects tissue iron demand along with the erythroid proliferation rate, but not body iron stores.23,28,29
Serum hepcidin (ng/mL) was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) (BACHEM). This ELISA method was validated with a gold standard for hepcidin assessment, namely liquid chromatography mass spectrometry (LC MS) developed at King's College London, confirming a strong correlation between the measurements performed using the LC MS and the BACHEM assay in patients with CKDs and healthy subjects.30
Plasma level of NT-proBNP (N-terminal pro-B type natriuretic peptide, pg/mL) was measured using an immunoassay based on electrochemiluminescence with the Elecsys 1010/2010 System (Roche Diagnostics GmbH, Mannheim, Germany).
Serum level of high-sensitivity C-reactive protein (hs-C-reactive protein, mg/L) was assessed using kinetic nephelometry (DADE BEHRING, Siemens Healthcare Diagnostics, Inc.).
Serum level of interleukin 6 (IL-6, pg/mL) was measured using a commercially available ELISA (R&D Systems, Minneapolis, MN, USA).
Estimated glomerular filtration rate (GFR, mL/min/1.73 m2) was calculated using the Modification of Diet in Renal Disease equation.31
Study scheme and clinical follow-up
All patients with systolic HF and the healthy subjects were evaluated comprehensively at the time of inclusion for this study. Subsequently, patients with systolic HF were regularly seen by the study investigators in outpatient HF clinics with follow-up ≥12 months in all survivors. Information regarding survival was obtained directly from patients or their relatives, from the HF clinic database or from the hospital system. No patient was lost to follow-up. The primary endpoint was all-cause death. The length of follow-up of survivors and patients in whom an event occurred after 3 years was censored at 1095 days.
Statistical analyses
Continuous variables with a normal distribution [age, body mass index (BMI), LVEF, sodium, GFR, haemoglobin, MCV, iron, TIBC, Tsat] were expressed as means (x) with standard deviations. The remaining continuous variables had a skewed distribution (NT-proBNP, hs-C-reactive protein, IL-6, RDW, ferritin, sTfR, hepcidin) and were expressed as medians with upper and lower quartiles. For further analyses, these variables were log-transformed in order to normalize their distribution. The categorical variables were expressed as numbers with percentages.
The statistical significance of differences between the groups was tested using analysis of variance (ANOVA), Student's t-test, or the χ2 test, where appropriate. The associations between variables were assessed using the univariate Pearson correlatory coefficients or the Spearman rank correlatory coefficients. The statistical significance of paired changes between the first and the second assessments in the longitudinal part of the study was assessed using the paired Student's t-test.
The associations between clinical and laboratory variables and survival were established in patients with systolic HF, using Cox proportional hazards analyses (both univariate and multivariable models). We included the following parameters as potential clinical prognosticators in patients with systolic HF: age, gender, BMI, HF aetiology, New York Heart Association (NYHA) class, LVEF, NT-proBNP (ln), sodium, hs-C-reactive protein (ln), GFR, and the presence of diabetes mellitus. Additionally, the set of haematological parameters and indices of iron status were included as potential prognosticators in this group of patients, namely: haemoglobin, ferritin (ln), Tsat, hepcidin (ln). Owing to a limited number of events (76 deaths), when constructing the multivariable Cox model, we included the following most important clinical prognosticators in HF [age, NYHA class, NT-proBNP (ln), GFR] along with haematological parameters and indices of iron status [haemoglobin, ferritin (ln), Tsat, hepcidin (ln)].
All tests were two-sided. A value of P <0.05 was considered statistically significant. All statistical analyses were performed using Statistica 9.1.
Results
Baseline characteristics of 321 examined patients with systolic HF and 66 healthy subjects are shown in Table 1.
Table 1.
Baseline clinical characteristics, co-morbidities, applied treatment, and implanted devices in 321 patients with systolic heart failure and 66 healthy subjects
| Variables, units | Patients with systolic HF (n = 321) | Healthy subjects (n = 66) |
|---|---|---|
| Clinical characteristics and co-morbidities | ||
| Age, years | 61 ± 11 | 60 ± 11 |
| Male gender | 269 (84%) | 51 (77%) |
| BMI, kg/m2 | 28.0 ± 4.3 | 27.8 ± 4.7 |
| HF aetiology, CAD | 224 (70%) | — |
| NYHA class, I/II/III/IV | 72/144/87/18 (22%/45%/27%/6%) | — |
| LVEF, % | 31 ± 9*** | 64 ± 7 |
| NT-proBNP, pg/mL | 1312 (431–3849)*** | 33 (20–109) |
| Sodium, mmol/L | 141 ± 3** | 142 ± 2 |
| Diabetes mellitus, yes | 111 (35%) | — |
| GFR, mL/min/1.73 m2 | 71.5 ± 20.7*** | 87.3 ± 20.2 |
| hs-C-reactive protein, mg/L | 2.6 (1.3–6.8)*** | 0.9 (0.5–1.3) |
| IL-6, pg/mL | 4.9 (3.7–7.7)*** | 1.4 (1.2–2.0) |
| Treatment | ||
| ACE-I and/or ARB, yes | 300 (94%) | — |
| β-Blocker, yes | 306 (95%) | — |
| Aldosterone antagonist, yes | 106 (33%) | — |
| Loop diuretic, yes | 165 (51%) | — |
| Thiazide diuretic, yes | 123 (38%) | — |
| Digoxin, yes | 86 (27%) | — |
| Statin, yes | 250 (78%) | — |
| Antiplatelet and/or anticoagulant drug, yes | 270 (84%) | — |
| Implanted devices | ||
| ICD, yes | 54 (17%) | — |
| CRT, yes | 10 (3%) | — |
Data are presented as means ± standard deviations, medians (with lower and upper quartiles), or numbers with percentages, where appropriate.
HF, heart failure; BMI, body mass index; CAD, coronary artery disease; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B type natriuretic peptide; GFR, glomerular filtration rate; hs-C-reactive protein, high-sensitivity C-reactive protein; IL-6, interleukin 6; ACE-I, angiotensin-converting enzyme-inhibitor; ARB, angiotensin receptor blocker; ICD, implanted cardioverter defibrillator; CRT, cardiac resynchronization therapy.
**P < 0.01, ***P < 0.001—patients with systolic HF compared with healthy subjects.
Iron status in patients with systolic heart failure according to New York Heart Association class and healthy subjects
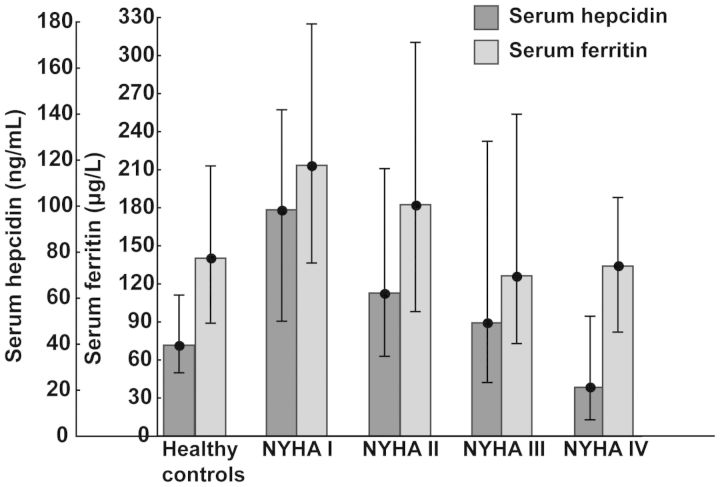
Compared with healthy controls, patients with systolic HF in NYHA class I had similar haematological status (haemoglobin, MCV) (both P> 0.2). The marked differences between patients in NYHA class I and healthy subjects were increased serum levels of ferritin (P < 0.01) and hepcidin (P < 0.001) (Table 2, Figure 1).
Table 2.
Haematological parameters, indices of iron status, and inflammatory markers in 66 healthy subjects and 321 patients with systolic heart failure according to New York Heart Association classification
| Variables, units | Healthy subjects (n = 66) | NYHA I (n = 72) | NYHA II (n = 144) | NYHA III (n = 87) | NYHA IV (n = 18) | P-value for variance among HF patients |
|---|---|---|---|---|---|---|
| Haemoglobin, g/dL | 14.4 ± 1.2 | 14.4 ± 1.2 | 14.2 ± 1.4 | 13.6 ± 1.6 | 13.5 ± 1.8 | <0.001 |
| MCV, fL | 88.9 ± 3.6 | 88.5 ± 4.3 | 89.1 ± 4.3 | 88.1 ± 4.8 | 87.8 ± 5.1 | 0.43 |
| RDW, % | 12.9 (12.5–13.2) | 13.1 (12.7–13.3)* | 13.5 (12.8–14.3) | 14.1 (13.3–15.2) | 14.5 (13.7–18.0) | <0.001 |
| Ferritin, µg/L | 140 (89–213) | 214 (137–325)** | 182 (98–311) | 126 (73–254) | 134 (82–188) | <0.001 |
| Iron, µg/dL | 121 ± 38 | 122 ± 41 | 114 ± 46 | 98 ± 41 | 90 ± 58 | <0.001 |
| TIBC, µg/dL | 279 ± 41 | 298 ± 40** | 308 ± 61 | 319 ± 62 | 330 ± 58 | 0.05 |
| Tsat, % | 43 ± 11 | 41 ± 14 | 38 ± 14 | 32 ± 14 | 27 ± 17 | <0.001 |
| STfR, mg/L | 1.13 (0.98–1.32) | 1.17 (0.98–1.46) | 1.23 (1.05–1.60) | 1.51 (1.05–2.28) | 1.71 (1.52–2.80) | <0.001 |
| Hepcidin, ng/mL | 39.6 (27.6–61.4) | 98.4 (50.0–141.8)*** | 62.2 (34.8–116.3) | 49.3 (23.4–128.2) | 21.3 (7.2–52.2) | <0.001 |
| hs-C-reactive protein, mg/L | 0.9 (0.5–1.3) | 1.54 (1.19–4.02)** | 2.15 (1.30–5.63) | 4.89 (1.41–9.05) | 7.84 (1.24–15.0) | <0.001 |
| IL-6, pg/mL | 1.4 (1.2–2.0) | 3.8 (2.9–5.2)*** | 4.8 (3.4–7.2) | 5.7 (4.5–9.0) | 14.8 (7.1–21.1) | <0.001 |
Data are presented as means ± standard deviations, medians (with lower and upper quartiles), where appropriate.
MCV, mean corpuscular volume; RDW, red cell distribution width; TIBC, total iron binding capacity; Tsat, transferrin saturation; sTfR, soluble transferrin receptor; hs-C-reactive protein, high-sensitivity C-reactive protein; IL-6, interleukin 6.
*P < 0.05, **P < 0.01, ***P < 0.001—healthy subjects compared with asymptomatic patients with systolic HF (NYHA class I).
Figure 1.
Serum levels of hepcidin and ferritin (medians with lower and upper quartiles) in healthy controls and patients with systolic heart failure in subsequent New York Heart Association classes.
With increasing HF severity, assessed by NYHA class, patients developed ID (evidenced by reduced ferritin, low Tsat, high sTfR) and iron-restricted erythropoiesis (reduced haemoglobin, high RDW) (all P < 0.001 for differences across NYHA classes), which was accompanied by a marked decrease in circulating hepcidin (P < 0.001 for differences across NYHA classes) (Table 2).
Among clinical and laboratory indices, the following were associated with low hepcidin level in patients with systolic HF: older age (r = −0.15, P < 0.01), more prevalent ischaemic HF aetiology (t = 2.05, P < 0.05), high plasma NT-proBNP (r = −0.12, P < 0.05), high serum IL-6 (r = −0.33, P < 0.001), low MCV (r = 0.27, P < 0.001), high RDW (r = −0.46, P < 0.001), low serum ferritin (r = 0.57, P < 0.001), low Tsat (r = 0.14, P < 0.05), increased TIBC (r = −0.16, P < 0.01), and high sTfR (r = −0.22, P < 0.001).
Serum hepcidin and ferritin, and the presence of anaemia in patients with systolic heart failure
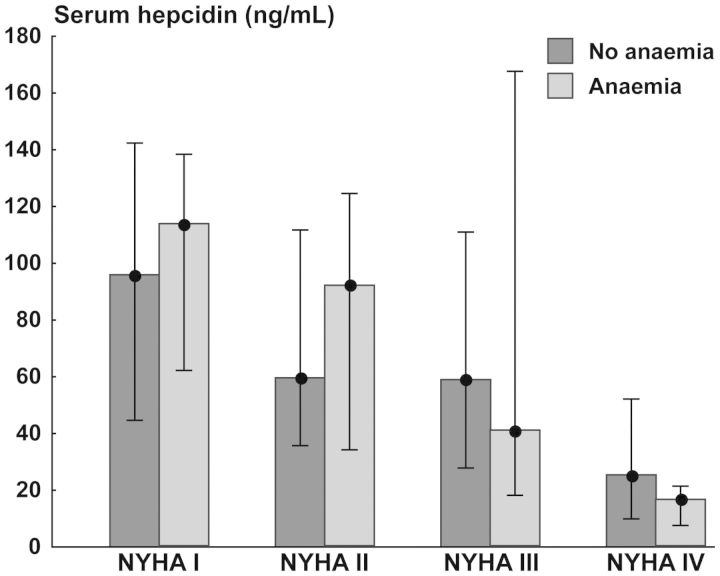
Anaemia was found in 60 (19%) of patients with systolic HF. There were no differences in serum hepcidin between anaemic and non-anaemic patients with systolic HF in subsequent NYHA classes (the results of ANOVA: for NYHA class F = 9.28, P < 0.001; for anaemia F = 0.01, P = 0.93; for interactions F = 0.67, P = 0.57) (Figure 2), as well as there was no correlation between serum hepcidin and haemoglobin level (P> 0.2). Similar to serum hepcidin, serum ferritin was associated with neither the presence of anaemia nor haemoglobin level in patients with systolic HF, regardless of NYHA class (all P> 0.2).
Figure 2.
Serum hepcidin (medians with lower and upper quartiles) in patients with systolic heart failure in subsequent New York Heart Association classes, split into those with and without anaemia.
Serum hepcidin and ferritin, and the proinflammatory activation in patients with systolic heart failure
Proinflammatory activation was associated with HF severity, as evidenced by increased serum levels of hs-C-reactive protein and IL-6 in patients with systolic HF in subsequent NYHA classes (both P < 0.001) (Table 2).
Neither serum hepcidin nor serum ferritin correlated with serum hs-C-reactive protein, when the associations were sought in all patients with systolic HF, and separately in those in NYHA classes I–II and in those in NYHA classes III–IV (all P> 0.2).
Both serum hepcidin and serum ferritin were inversely related to serum IL-6: in all patients with systolic HF (r = −0.33, P < 0.001, and r = −0.31, P < 0.01, respectively, for serum hepcidin and serum ferritin), in those in NYHA classes I–II (r = −0.35, P < 0.01, and r = −0.27, P < 0.05), and in those in NYHA classes III–IV (r = −0.35, P < 0.05, and r = −0.32, P = 0.05).
Longitudinal changes in iron status in asymptomatic patients with systolic heart failure
Patients with systolic HF in NYHA class I at baseline were invited after ≥12 months for a second comprehensive assessment, to examine the changes over time in clinical status, echocardiography, haematological parameters, and indices of iron status, provided they were alive, experienced no episode of decompensation during follow-up and agreed to participate.
Out of 72 patients in NYHA class I at baseline, 21 (19 men, mean age: 57 ± 10 years) were re-assessed, after a mean follow-up of 28 ± 7 months, 6 of whom remained in NYHA class I and 15 deteriorated to NYHA class II. The baseline values of LVEF, NT-proBNP, sodium, GFR, haemoglobin, ferritin, iron, TIBC, Tsat, sTfR, hepcidin, and hs-C-reactive protein did not differ between the whole group of 72 patients in NYHA class I and those 21 subjects included in the longitudinal part of the study (all P> 0.1).
During follow-up, the following parameters did not change: LVEF, NT-proBNP, sodium, hs-C-reactive protein, GFR, haemoglobin, ferritin, iron, TIBC, and Tsat (all P> 0.2). However, these patients developed a marked decrease in circulating hepcidin from 111.3 (52.3–141.3) to 39.5 (22.7–64.8) ng/mL (P < 0.01) along with an increase in serum sTfR from 1.25 (1.14–1.62) to 1.48 (1.00–2.20) mg/L (P = 0.05).
There were no associations between the decline in serum hepcidin and the changes in haemoglobin and serum hs-C-reactive protein during the clinical follow-up (P> 0.2), but the decrease in serum hepcidin was related to an increase in serum sTfR (r = −0.54, P < 0.05).
Indices of iron status and survival in patients with systolic heart failure
The mean duration of follow-up in all 321 patients with HF was 28 ± 11 months (median: 36 months); time to death was 14 ± 10 months (median: 12 months). In the whole group, the 3-year survival rate was 76.3% (95% CI: 71.4–81.2%) (all 76 deaths were cardiovascular).
Univariate analyses
In univariate Cox proportional hazard regression models, the following clinical variables were associated with higher mortality during the 3-year follow-up in patients with systolic HF: higher NYHA class, lower LVEF, higher NT-proBNP, lower sodium, higher hs-C-reactive protein, and lower GFR (all P < 0.05, Table 3).
Table 3.
Prognosticators of 3-year mortality in 321 patients with systolic heart failure (univariate and multivariable Cox proportional hazard regression models)
| Prognosticators, units | Univariate models |
Multivariable model |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, 5 years | 0.99 | 0.89–1.09 | 0.77 | 0.98 | 0.96–1.00 | 0.12 |
| Gender, men vs. women | 1.92 | 0.88–4.17 | 0.10 | — | — | — |
| BMI, 1 kg/m2 | 0.95 | 0.90–1.01 | 0.09 | — | — | — |
| HF aetiology, CAD vs. non-CAD | 0.71 | 0.45–1.13 | 0.15 | — | — | — |
| NYHA class, III–IV vs. I–II | 3.45 | 2.18–5.44 | <0.001 | 1.42 | 0.81–2.51 | 0.22 |
| LVEF, 1% | 0.93 | 0.91–0.96 | <0.001 | — | — | — |
| NT-proBNP, 1 ln pg/mL | 1.78 | 1.49–2.12 | <0.001 | 1.50 | 1.20–1.87 | <0.001 |
| Sodium, 1 mmol/L | 0.87 | 0.82–0.92 | <0.001 | — | — | — |
| hs-C-reactive protein, 1 ln mg/L | 1.80 | 1.37–2.36 | <0.001 | — | — | — |
| Diabetes mellitus, yes vs. no | 1.23 | 0.78–1.95 | 0.37 | — | — | — |
| GFR, 5 mL/min/1.73 m2 | 0.94 | 0.89–0.99 | 0.03 | 0.99 | 0.93–1.06 | 0.79 |
| Haemoglobin, g/dL | 0.80 | 0.69–0.92 | 0.002 | 0.96 | 0.82–1.12 | 0.61 |
| Ferritin, 1 ln µg/L | 0.59 | 0.46–0.77 | <0.001 | 0.93 | 0.64–1.34 | 0.68 |
| Tsat, 1% | 0.95 | 0.94–0.97 | <0.001 | 0.97 | 0.95–0.99 | 0.01 |
| Hepcidin, 1 ln ng/mL | 0.54 | 0.45–0.67 | <0.001 | 0.64 | 0.49–0.82 | <0.001 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; HF, heart failure; CAD, coronary artery disease; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B type natriuretic peptide; hs-C-reactive protein, high-sensitivity C-reactive protein; GFR, glomerular filtration rate.
Also in univariate models, all analysed haematological parameters and indices of iron status were associated with 3-year mortality rates in patients with systolic HF (all P < 0.01, Table 3).
Multivariable analyses
In the multivariable Cox proportional hazard regression model, after adjustment for most important prognosticators in HF and measures of haematological and iron status, low circulating hepcidin remained independent prognostic factors of higher mortality during the 3-year follow-up in patients with systolic HF (P < 0.001) (Table 3).
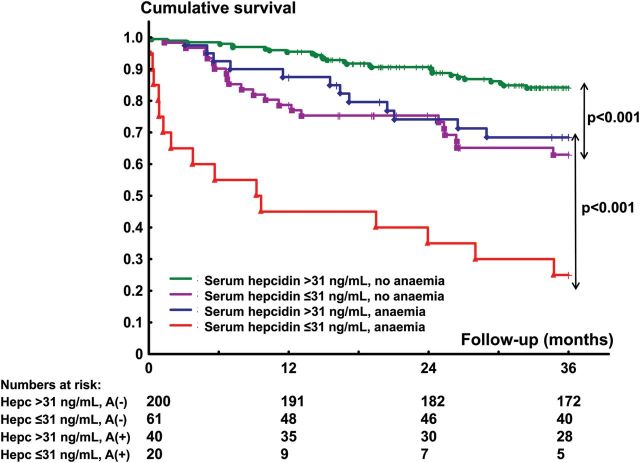
Low serum hepcidin (≤31 ng/mL, lower quartile) was associated with an increased 3-year mortality both in non-anaemic (HR = 2.89, 95% CI: 1.63–5.05, P < 0.001) and anaemic (HR = 3.94, 95% CI: 1.84–8.45, P < 0.001) patients with systolic HF (Figure 3).
Figure 3.
Kaplan–Meier curves depicting the 3-year cumulative survival rates separately in anaemic and non-anaemic patients with systolic heart failure, split into those with and without low circulating hepcidin (≤31 ng/mL, lower quartile).
Discussion
The major findings of our study are the following: (i) deranged iron status was detectable at an early stage of systolic HF, as demonstrated by elevated serum ferritin (reflecting an increased amount of stored iron in the absence of distinct inflammation) and hepcidin levels, (ii) the progression of systolic HF was associated with the development of ID and iron-restricted erythropoiesis in parallel with a gradual decline in circulating hepcidin, and (iii) in patients with systolic HF, circulating hepcidin levels were related neither to anaemia nor to proinflammatory activation.
As hepcidin is commonly and predominantly acknowledged as the key regulator of iron metabolism, an analysis of the pattern of its changes may allow one to understand the pathomechanisms leading to ID, which characterizes the HF syndrome. Hepcidin, upon binding to the only known cellular iron export protein, ferroportin, results in a ferroportin degradation and the blockage of cellular iron egress.8,10 In this way, duodenal iron absorption is decreased and retention of iron in the reticuloendothelial system occurs, thus reducing both circulating iron concentrations and iron availability to target tissues.8,10,32 The physiological role of hepcidin is, however, broader than orchestrating systemic iron metabolism and also comprises control of efficient iron-replete erythropoiesis, and anti-microbial innate immune response.7–10,33,34 Thus, the hepatic production of hepcidin is controlled in feedback loops through three groups of molecules originating, respectively, from iron-sensing systems (increased circulating iron concentrations induce hepcidin expression and release into the circulation, whereas systemic ID causes the opposite effects), inefficient erythropoiesis (anaemia and/or hypoxia can inhibit hepatic production of hepcidin), and inflammatory processes.7–10,33,34
Of note, inflammatory stimuli, such as IL-6 and lipopolysaccharide, are able to induce the hepatic expression of hepcidin, and this mechanism seems to be responsible for the development of functional ID and anaemia in patients with chronic inflammatory diseases.7–10 As HF is accompanied by a chronic inflammatory state with increased circulating proinflammatory molecules,35,36 we have anticipated that a proinflammatory stimulus may be crucial for an increased hepatic production of hepcidin with further clinical consequences (ID, anaemia). Interestingly, our data seem to be rather contradictory, as in patients with systolic HF, high serum hepcidin was associated with low circulating proinflammatory markers (IL-6 and hs-C-reactive protein), and serum hepcidin was related to the presence of neither anaemia nor haemoglobin level (regardless whether these analyses were performed separately in NYHA classes I–II or III–IV). These findings seem to be opposing to the prevalent literature and deserve to be discussed.
At the early stage of HF, both the inflammatory stimuli and the stimuli associated with compromised erythropoiesis seem to be of a minor importance in the regulation of hepatic hepcidin expression. Thus, high circulating hepcidin levels can be interpreted predominantly as a response to iron excess, which, in the absence of proinflammatory activation, is evidenced by elevated serum ferritin. As mentioned before, plethora of biological effects of hepcidin also comprises triggering an anti-inflammatory response.37,38 In the experimental settings, pre-treatment with intravenous hepcidin or the induction of hepatic hepcidin expression prevents the inflammatory response due to the exposure to lipopolysaccharide.37,38 In this context, an inverse relationship between proinflammatory markers and circulating hepcidin levels may result from the anti-inflammatory properties of the latter.
In our study, circulating hepcidin did not differ between anaemic and non-anaemic subjects, and there was no relationship between the decline in serum hepcidin and the change in either haemoglobin or serum hs-C-reactive protein during the progression of HF. The results of two small studies addressing the analogous associations in patients with HF are equivocal.18,19 Divakaran et al.18 did not find any differences in either serum or urine hepcidin in HF patients with and without anaemia (97 examined subjects in total), whereas in the study of Matsumoto et al.,19 36 anaemic patients with HF had reduced serum hepcidin compared with 25 non-anaemic subjects, and serum hepcidin did not correlate with serum IL-6 in patients with HF.
We believe that we have been able to describe a novel adaptive mechanism, triggered at the initial phase of HF, and protecting against potentially toxic effects of iron excess. Biological properties of hepcidin may be advantageous. The limitation of intestine iron absorption and iron sequestration decreases the labile iron pool, which further reduces unfavourable effects of iron excess (generation of free radicals), whereas the inhibition of innate immune response may prevent the development of inflammatory reactions. Both oxidative stress and inflammation are crucial mechanisms underlying progression of the cardiovascular damage; hence, their prevention due to the upregulation of hepcidin could be considered as an adaptive response. As the regulation of hepatic expression of hepcidin is very complex and not yet fully understood, other factors, not assessed here, may also be involved. In the longer perspective, however, an upregulation of hepcidin blocks iron absorption and release, and leads to the development of ID, which in turn unfavourably affects the myocardium itself, peripheral muscles, and the haematopoietic system, ultimately causing the progression of the disease.
Iron deficiency itself inhibits hepcidin expression and its release into the circulation,8–10 which explains a gradual reduction of serum hepcidin in the natural course of HF and accompanying depleted iron stores (low serum ferritin), negative tissue iron balance (low Tsat, high serum sTfR), and iron-restricted erythropoiesis (reduced haemoglobin, high RDW). Interestingly, low serum hepcidin appeared to be a strong and independent prognosticator of an increased 3-year mortality in patients with systolic HF, regardless of their clinical, haematological, and iron status. Our findings are in accordance with data from small studies showing that HF patients have low serum and urine hepcidin, which is accompanied by depleted body iron as reflected by reduced serum ferritin.17–19
As the liver is an established major source of circulating hepcidin,7–10 it is the most likely origin of markedly elevated serum hepcidin in asymptomatic HF patients. There is an alternative (so far hypothetical) theory about an extra-hepatic origin of circulating hepcidin in HF (e.g. from ischaemic and/or hypoperfused myocardium). There is evidence that myocardial hepcidin expression is increased in experimental models of myocardial ischaemia, myocarditis, and HF accompanying CKD.39–41 However, whether an increased expression of hepcidin in the diseased myocardium could significantly contribute to the amount of the circulating hepcidin pool in the clinical setting of HF or rather contribute to ID at the local myocardial level remains largely unknown.
It would be prudent to confront deranged iron status in HF with the abnormalities in iron metabolism seen in chronic inflammatory diseases.7,11–15 In these patients, high circulating hepcidin levels are typically found in advanced stages of the disease, e.g. in patients with CKD requiring dialysis,11,12,15 in septic patients with the systemic inflammatory response syndrome,14 and in patients with rheumatoid arthritis of a long duration,13 being also accompanied by inflammation, ID, and anaemia. On the contrary, our results demonstrate, for the first time ever, that high circulating hepcidin characterizes an early stage of systolic HF, with the subsequent decline along with the progression of HF and development of ID. High circulating hepcidin in patients with systolic HF was related to the presence of neither augmented inflammation nor anaemia. We hypothesize that the pathophysiological mechanisms underlying ID in HF seem to markedly differ from those demonstrated in chronic inflammatory diseases. Therefore, diagnostic algorithms and therapeutic approaches specific for HF need to be further developed and studied.
Study limitations
For the description of iron status in patients with HF, we have applied several biomarkers assessed in peripheral blood.22–24,28,29 Their interpretation has been based on the experience of nephrologists and haematologists,22–24,28,29 and the reader should be aware that in patients with HF they have never been validated with a ‘gold standard’ (iron amount in bone marrow).
The observational character of the study needs to be acknowledged, and the pilot longitudinal data allow us only to hypothesize about the sequence of changes in iron status during the natural history of systolic HF. The limited number of patients included in the longitudinal part of our project needs to be considered as the limitation of the corresponding analyses. They should be interpreted with caution, and serve as hypothesis-generating data. As we have not investigated mechanisms underlying iron excess observed at an early stage of HF, we are not able to explain this phenomenon. We also still do not have the data to clearly explain markedly declining levels of circulating hepcidin along with the progression of the disease. Further mechanistic studies are needed to address underlying pathophysiological mechanisms.
Conclusion
Increased level of circulating hepcidin characterizes an early stage of HF, and is not accompanied by either anaemia or inflammation. The progression of HF is associated with the decline in circulating hepcidin and the development of ID. Low hepcidin relates to unfavourable outcome.
Funding
This research was financially supported by The National Science Centre (Poland) (grant no. NN 519 580838).
Conflict of interest: E.A.J. reports receiving honoraria for lectures and participation in advisory board from Vifor Pharma and related travel/accommodation expenses covered by Vifor Pharma. S.D.A. reports receiving consulting fees from Alere, Amgen, Brahms GmbH, Abbott laboratories, Takeda & Noxxon, Vifor Pharma; honoraria from Alere, Amgen, BRAHMS GmbH, Vifor Pharma, and research support from BRAHMS GmbH and Vifor Pharma. P.P. reports receiving consulting fees from Vifor Pharma and Amgen. Inc.; honoraria from Vifor Pharma; and travel/accommodation expenses covered by Vifor Pharma and Amgen, Inc. H.A. received speaking honoraria from Merck and served as an advisor for Cubist Pharmaceuticals and Novartis. SvH received speaker's honoraria and travel support from Vifor Pharma and BRAHMS GmbH and research support from BRAHMS GmbH. G.W. received lecture fees from Vifor and Pharmacosmos. JMcM's employer, Glasgow University, received payment for his time spent as an Executive Committee member for the TREAT and RED-HF trials sponsored by Amgen Inc and he received travel and accommodation expenses related to Executive Committee meetings. All other authors report no conflict of interest related to the content of this manuscript.
References
- 1.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 2.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P for the FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 5.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J. 2013;34:30–38. doi: 10.1093/eurheartj/ehr504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–693. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55:726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Ding XQ, Zou JZ, Liu ZH, Jiang SH, Chen YM. Serum hepcidin in haemodialysis patients: associations with iron status and microinflammation. J Int Med Res. 2011;39:1961–1967. doi: 10.1177/147323001103900542. [DOI] [PubMed] [Google Scholar]
- 12.Jairam A, Das R, Aggarwal PK, Kohli HS, Gupta KL, Sakhuja V, Jha V. Iron status, inflammation and hepcidin in ESRD patients: the confounding role of intravenous iron therapy. Indian J Nephrol. 2010;20:125–131. doi: 10.4103/0971-4065.70840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Khalek MA, El-Barbary AM, Essa SA, Ghobashi AS. Serum hepcidin: a direct link between anemia of inflammation and coronary artery atherosclerosis in patients with rheumatoid arthritis. J Rheumatol. 2011;38:2153–2159. doi: 10.3899/jrheum.110339. [DOI] [PubMed] [Google Scholar]
- 14.van Eijk LT, Kroot JJ, Tromp M, van der Hoeven JG, Swinkels DW, Pickkers P. Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: an observational study. Crit Care. 2011;15:R9. doi: 10.1186/cc9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rumjon A, Sarafidis P, Brincat S, Musto R, Malyszko J, Bansal SS, Macdougall IC. Serum hemojuvelin and hepcidin levels in chronic kidney disease. Am J Nephrol. 2012;35:295–304. doi: 10.1159/000336528. [DOI] [PubMed] [Google Scholar]
- 16.Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. doi: 10.1016/j.jacc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 17.van der Putten K, Jie KE, van den Broek D, Kraaijenhagen RJ, Laarakkers C, Swinkels DW, Braam B, Gaillard CA. Hepcidin-25 is a marker of the response rather than resistance to exogenous erythropoietin in chronic kidney disease/chronic heart failure patients. Eur J Heart Fail. 2010;12:943–950. doi: 10.1093/eurjhf/hfq099. [DOI] [PubMed] [Google Scholar]
- 18.Divakaran V, Mehta S, Yao D, Hassan S, Simpson S, Wiegerinck E, Swinkels DW, Mann DL, Afshar-Kharghan V. Hepcidin in anemia of chronic heart failure. Am J Hematol. 2011;86:107–109. doi: 10.1002/ajh.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Akahori H, Sakoda T, Ohyanagi M, Tomosugi N, Masuyama T. Iron regulatory hormone hepcidin decreases in chronic heart failure patients with anemia. Circ J. 2010;74:301–306. doi: 10.1253/circj.cj-09-0663. [DOI] [PubMed] [Google Scholar]
- 20.Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–826. doi: 10.1093/eurheartj/ehs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanc B, Finch CA, Hallberg L. Nutritional anaemias. Report of a WHO scientific group. WHO Tech Rep Ser. 1968;405:1–40. [PubMed] [Google Scholar]
- 22.Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1(Suppl. 1):S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 23.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 24.Briggs C. Quality counts: new parameters in blood cell counting. Int J Lab Hematol. 2009;31:277–297. doi: 10.1111/j.1751-553x.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800:760–769. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hintze KJ, Theil EC. Cellular regulation and molecular interactions of the ferritins. Cell Mol Life Sci. 2006;63:591–600. doi: 10.1007/s00018-005-5285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23:95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345–352. [PubMed] [Google Scholar]
- 29.Skikne BS. Serum transferrin receptor. Am J Hematol. 2008;83:872–875. doi: 10.1002/ajh.21279. [DOI] [PubMed] [Google Scholar]
- 30.Rumjon A, Bansal SS, Malyszko J, Macdougall IC. Intra-individual variability of hepcidin in haemodialysis patients using mass spectrometry and ELISA. J Am Soc Nephrol. 2011;22(Suppl. A):481A–482A. doi: 10.1093/ndt/gfs164. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 32.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT, Wroblewski VJ, Wurz E, Datz C, Weiss G. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure – pathophysiological links. Cardiovasc Res. 2006;70:434–445. doi: 10.1016/j.cardiores.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 36.von Haehling S, Jankowska EA, Anker SD. Tumour necrosis factor-alpha and the failing heart: pathophysiology and therapeutic implications. Basic Res Cardiol. 2004;99:18–28. doi: 10.1007/s00395-003-0433-8. [DOI] [PubMed] [Google Scholar]
- 37.Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118:736–746. doi: 10.1182/blood-2011-02-337212. [DOI] [PubMed] [Google Scholar]
- 38.De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, Daynes RA, Kushner JP, Li D, Ward DM, Kaplan J. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395–2405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonis G, Mueller K, Schwarz P, Wiedemann S, Adler G, Strasser RH, Kulaksiz H. The iron-regulatory peptide hepcidin is upregulated in the ischemic and in the remote myocardium after myocardial infarction. Peptides. 2010;31:1786–1790. doi: 10.1016/j.peptides.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Isoda M, Hanawa H, Watanabe R, Yoshida T, Toba K, Yoshida K, Kojima M, Otaki K, Hao K, Ding L, Tanaka K, Takayama T, Kato K, Okura Y, Kodama M, Ota Y, Hayashi J, Aizawa Y. Expression of the peptide hormone hepcidin increases in cardiomyocytes under myocarditis and myocardial infarction. J Nutr Biochem. 2010;21:749–756. doi: 10.1016/j.jnutbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Toblli JE, Cao G, Rivas C, Kulaksiz H. Heart and iron deficiency anaemia in rats with renal insufficiency: the role of hepcidin. Nephrology. 2008;13:636–645. doi: 10.1111/j.1440-1797.2008.01019.x. [DOI] [PubMed] [Google Scholar]