Abstract
Aims
People whose birthweights were towards the lower end of the normal range are at increased risk of coronary heart disease. This is attributed to foetal programming through malnutrition, but the cause of the malnutrition is unknown.
Methods and results
We studied 6975 men born in Helsinki during 1934–44. Their size at birth was recorded. Babies who later developed coronary heart disease tended to have a low ponderal index (birthweight/length3). Three different placental phenotypes predicted the disease. In primiparous mothers who were short, having below median height, the hazard ratio for the disease was 1.14 (95% confidence interval 1.08–1.21, P< 0.0001) for each centimetre increase in the difference between the length and breadth of the placental surface. In tall mothers whose body mass index was above the median, the hazard ratio was 1.25 (1.10–1.42, P= 0.0007) per 40 cm2 decrease in the surface area. In tall mothers whose body mass index was below the median, the hazard ratio was 1.07 (1.02–1.13, P= 0.01) per 1% increase in the placental weight/birthweight ratio.
Conclusions
Three different combinations of maternal and placental size predicted coronary heart disease. The mother's body size determines the availability of nutrients and is linked to the development and function of the placenta, reflected in its shape and size. We speculate that variations in three processes of normal placental development lead to foetal malnutrition. The processes are (i) implantation and spiral artery invasion, (ii) growth of the chorionic surface, and (iii) compensatory expansion of the chorionic surface.
Keywords: Foetal programming, Birthweight, Maternal body size, Placental size, Coronary disease, Placenta, Epidemiology
Introduction
People who, although born at term, had birthweights towards the lower end of the normal range are at increased risk of coronary heart disease.1–4 This is thought to reflect foetal programming, the process whereby malnutrition in utero, and consequent small body size at birth, alters gene expression and leads to lifelong changes in the body's organs and systems.5 A baby's nutrition depends not only on its mother's diet during pregnancy but also on her lifetime nutrition and metabolism, which are reflected in her height and weight.6
A baby's nutrition also depends on the placenta's ability to transport nutrients to it from its mother's blood.7 Placental weight correlates with birthweight and placental size is therefore related to its function.8 In some circumstances a foetus that is undernourished in mid-gestation can expand the placental surface to extract more nutrients from the mother.9,10 This leads to a high placental weight in relation to birthweight. Both low placental weight and high placental weight in relation to birthweight have been shown to predict coronary heart disease.11,12 This indicates that placental size is linked to the foetal programming of coronary heart disease.
Placental weight is a crude marker of placental size because it does not distinguish the size of the surface from the thickness. Growth of the surface reflects recruitment of maternal spiral arteries while thickness reflects the depth of invasion of the maternal endometrium.13 Recruitment of sufficient spiral arteries, and adequate invasion of the recruited arteries are both pre-requisites for normal placental function. We have been able to examine, for the first time, how the size and shape of the placental surface relate to coronary heart disease. The Helsinki Birth Cohort comprises men and women born during 1934–44, for whom the size of the placental surface was routinely recorded.4,10 A priori it is likely that the contribution of placental size to foetal programming will depend on the mother's nutritional history, reflected in her body size. We have therefore examined the effects of the placental surface within different categories of the mother's body size. We hypothesized that the association between low birthweight and coronary heart disease reflects combinations of maternal body size and placental size that restrict foetal nutrition and growth and programme the foetus.
Methods
The Helsinki Birth Cohort includes 6975 men who were born in the city during 1934–44 and went to child welfare clinics. They were born in either the University Central Hospital or in the Maternity Hospital. Details of the birth records have been described.4 They include the mother's height and weight in late pregnancy, her age and parity, and the date of her last menstrual period. The weight and length of the baby were recorded, and we calculated the ponderal index (birthweight/length3). The records also include the weight of the placenta, together with the maximal so-called ‘diameter’ of the surface and a lesser ‘diameter’ bisecting it at right angles.10 We refer to these diameters as the length and breadth of the surface. They were measured because, in the past, it was recognized that the placental surface is more oval than circular and the two diameters were used routinely to describe this.14 The placental measurements were correlated, r = 0.58 for length and breadth, 0.50 for weight and length, and 0.45 for weight and breadth. Assuming an elliptical surface, we estimated the surface area of the placenta as length × breadth × π/4. We used the difference between the length and breadth to determine the degree of ovality of the placental surface. Assuming a constant density we estimated the thickness of the placenta as weight divided by area.
We used the father's occupation to define the mother's social class. Based on a classification from Statistics Finland fathers were grouped into upper and lower-middle class and manual workers. The men's own occupations, recorded at successive 5-year censuses from 1970 to 2000, were obtained from Statistics Finland, who grouped them into four categories—higher official, lower official, self-employed, and manual worker. We used the highest category attained. Using the personal identification number assigned to each Finnish citizen, we identified all hospital admissions and deaths from coronary heart disease among the men during 1971–2003. In ascertaining coronary heart disease, we used the international classification of diseases (ICD) codes that define myocardial infarction and ischaemic heart disease: ICD-8 and ICD-9 codes 410-414 and in ICD-10 codes I21-I25. All hospital discharges in Finland are recorded in the national hospital discharge register, and all deaths are recorded in the national mortality register. In all, 655 men had either been admitted or had died from coronary heart disease. The equivalent figure for women was 166 and we therefore restricted our analyses to men.
Statistical methods
The endpoint for our survival analysis was the first admission to hospital with coronary heart disease, or death from the disease. Men were censored in the analysis when they migrated from Finland or died. The median age at the end of follow-up was 60.8 years (interquartile range 58.4–63.2). We used a Cox proportional hazards model to calculate the hazard ratios for coronary heart disease for a unit increase in the measurements of maternal, placental, and birth size, and the length of gestation. To determine the statistical significance of trends in coronary heart disease, the measurements were analysed as continuous variables although they are presented in the tables as groups. The significance tests were two sided and we used P= 0.05 as the threshold for statistical significance. In previous analyses of hypertension, we divided the mothers around their median height as associations with placental size differed in short and tall mothers.10 We used the same division in the current analyses, in which we also subdivided the mothers according to their median body mass index in pregnancy. We also examined primiparous and multiparous mothers separately because the processes of placentation are different in first and later pregnancies. Our software was SPSS version 15.
Results
There were 655 cases of coronary heart disease, 211 of whom had died. Table 1 shows the mean size of the mothers, placentas, and newborn babies. Neither the mother's height, weight, age nor parity predicted coronary heart disease, and the disease was unrelated to the length of gestation. Table 2 shows hazard ratios for coronary heart disease according to the men's body size and placental size at birth. The disease was associated with low birthweight (data not shown, P for trend = 0.0006) and more strongly with low ponderal index (Table 2). Hazard ratios increased with reduced breadth of the placental surface (P= 0.01). There was no similar trend with the length of the surface and hazard ratios therefore increased as the difference between the length and breadth increased, that is, as the placental surface became more oval (Table 2).
Table 1.
Mean (SD) size of mothers, placentas, and babies
| Mothers | |
| Height (cm) | 159.8 (5.8) |
| Weight (kg) | 67.0 (8.2) |
| Body mass index (kg/m2) | 26.2 (2.9) |
| Placentas | |
| Weight (g) | 651 (120) |
| Length (cm) | 19.5 (2.1) |
| Breadth (cm) | 17.0 (2.2) |
| Area (cm2) | 262 (55) |
| Length minus breadth (cm) | 2.6 (2.0) |
| Babies | |
| Birthweight (g) | 3475 (480) |
| Placental/birthweight (%) | 18.8 (3.0) |
| Length (cm) | 50.6 (1.9) |
| Ponderal index (kg/m3) | 26.7 (2.3) |
Table 2.
Hazard ratios for coronary heart disease according to neonatal measurements and mother's height
| Neonatal measurement | All mothers |
Mother's height ≤ 160 cm |
Mother's height > 160 cm |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | Cases/men | HR (95% CI) | Cases/men | HR (95% CI) | Cases/men | |
| Ponderal index (kg/m3) | ||||||
| ≤25 | 1.5 (1.1–1.9) | 165/1386 | 1.3 (0.9–1.8) | 95/814 | 1.7 (1.2–2.5) | 70/572 |
| −26.5 | 1.4 (1.1–1.8) | 183/1570 | 1.4 (1.0–2.0) | 115/884 | 1.4 (0.9–2.0) | 68/686 |
| −28.5 | 1.2 (1.0–1.5) | 204/2024 | 1.1 (0.8–1.5) | 109/1091 | 1.4 (1.0–2.1) | 95/933 |
| >28.5 | 1.0 (baseline) | 99/1252 | 1.0 (baseline) | 58/647 | 1.0 (baseline) | 41/605 |
| P for trend | <0.001 | 0.01 | 0.007 | |||
| Placental weight (kg) | ||||||
| ≤0.55 | 1.2 (1.0–1.6) | 151/1378 | 1.2 (0.9–1.6) | 100/857 | 1.2 (0.8–1.8) | 51/521 |
| −0.65 | 1.1 (0.9–1.4) | 208/2107 | 0.9 (0.7–1.3) | 111/1185 | 1.3 (0.9–1.9) | 97/922 |
| −0.75 | 1.3 (1.0–1.6) | 190/1660 | 1.3 (0.9–1.8) | 112/872 | 1.3 (0.9–1.8) | 78/788 |
| >0.75 | 1.0 (baseline) | 105/1120 | 1.0 (baseline) | 56/548 | 1.0 (baseline) | 49/572 |
| P for trend | 0.1 | 0.5 | 0.1 | |||
| Placental area (cm2) | ||||||
| ≤225 | 1.2 (1.0–1.5) | 168/1389 | 1.0 (0.8–1.3) | 100/827 | 1.5 (1.1–2.1) | 68/562 |
| −255 | 0.9 (0.8–1.2) | 159/1679 | 0.8 (0.6–1.1) | 92/954 | 1.1 (0.8–1.6) | 67/725 |
| −295 | 1.1 (0.9–1.4) | 174/1589 | 1.0 (0.7–1.3) | 96/866 | 1.4 (1.0–1.9) | 78/723 |
| >295 | 1.0 (baseline) | 152/1585 | 1.0 (baseline) | 91/799 | 1.0 (baseline) | 61/786 |
| P for trend | 0.1 | 0.6 | 0.08 | |||
| Length minus breadth (cm) | ||||||
| ≤1 | 1.0 (baseline) | 184/1972 | 1.0 (baseline) | 103/1110 | 1.0 (baseline) | 81/862 |
| −2 | 1.1 (0.9–1.4) | 181/1758 | 1.2 (0.9–1.6) | 103/954 | 1.0 (0.7–1.4) | 78/804 |
| −3 | 1.3 (1.0–1.6) | 126/1023 | 1.2 (0.9–1.7) | 66/561 | 1.3 (0.9–1.8) | 60/462 |
| >3 | 1.1 (0.9–1.4) | 162/1489 | 1.4 (1.1–1.9) | 107/821 | 0.8 (0.6–1.1) | 55/668 |
| P for trend | 0.02 | <0.001 | 0.8 | |||
| Placental/birthweight (%) | ||||||
| ≤16.5 | 1.0 (baseline) | 119/1322 | 1.0 (baseline) | 73/719 | 1.0 (baseline) | 46/603 |
| −18.5 | 1.2 (1.0–1.5) | 189/1780 | 1.1 (0.8–1.5) | 109/972 | 1.3 (0.9–1.9) | 80/808 |
| −20.5 | 1.1 (0.9–1.4) | 166/1583 | 1.1 (0.8–1.5) | 99/882 | 1.2 (0.8–1.8) | 67/701 |
| >20.5 | 1.2 (1.0–1.5) | 180/1580 | 1.1 (0.8–1.5) | 98/889 | 1.4 (1.0–2.0) | 82/691 |
| P for trend | 0.5 | 0.8 | 0.2 | |||
HR, hazard ratio; CI, confidence interval.
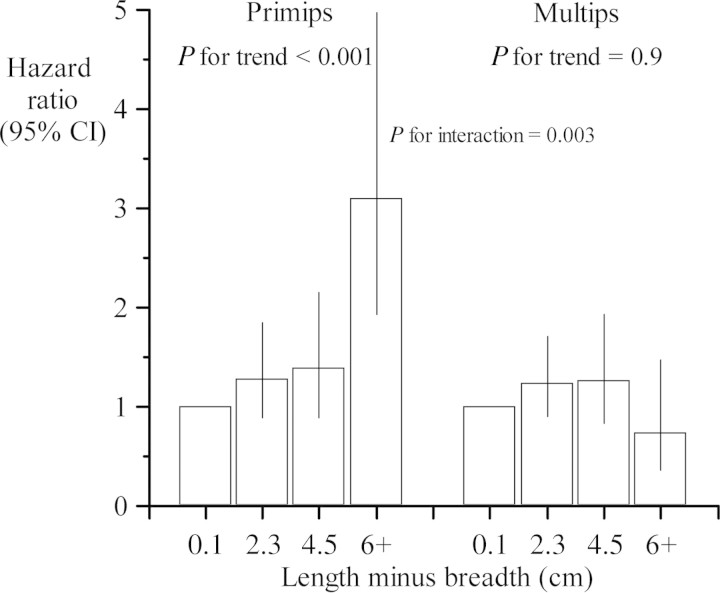
We divided the men around the median height of their mothers (160 cm). Table 2 shows that the trend in coronary heart disease with an oval placental surface was confined to men with short mothers. The interaction between the effects of ovality and mother's height was statistically significant (P for interaction = 0.03). We examined whether the effect of ovality varied with the mother's parity. Table 3 and Figure 1 show that the effect was confined to men whose mothers were primiparous. In these men, the hazard ratio for coronary heart disease was 1.14 (95% confidence interval 1.08–1.21, P< 0.0001) for each centimetre increase in the difference between the length and breadth of the placental surface. The interaction between the effects of ovality and mother's parity was strongly statistically significant (Table 3). The interaction was unchanged by exclusion of pregnancies complicated by pre-eclampsia.
Table 3.
Hazard ratios for coronary heart disease in men born to short mothers, with height ≤ 160 cm, according to parity and the difference in placental length and breadth
| Length minus breadth (cm) | Parity |
|||
|---|---|---|---|---|
| Primiparous |
Multiparous |
|||
| Cases/men | HR (95% CI) | Cases/men | HR (95% CI) | |
| 0,1 | 44/497 | 1.0 (baseline) | 62/641 | 1.0 (baseline) |
| 2,3 | 79/725 | 1.3 (0.9–1.8) | 96/816 | 1.2 (0.9–1.7) |
| 4,5 | 36/300 | 1.4 (0.9–2.1) | 34/289 | 1.3 (0.8–1.9) |
| ≥6 | 29/117 | 3.1 (1.9–5.0) | 9/129 | 0.7 (0.4–1.5) |
| P for trend | <0.001 | 0.9 | ||
| P for interaction | 0.003 | |||
HR, hazard ratio; CI, confidence interval.
Figure 1.
Hazard ratios for coronary heart disease according to the difference between the length and breadth of the placental surface, in men born to short mothers.
We further subdivided the mothers around their median body mass index in pregnancy (26 kg/m2). Among men whose mothers were short, the effects of an oval placenta on later coronary heart disease did not differ in the two maternal body mass index groups. Table 4 shows, however, that among men whose mothers were tall there were different associations between placental size and coronary heart disease in the two body mass index groups. In men whose mother's body mass index was above the median, coronary heart disease was associated with low placental weight and small placental area (Table 4). In these men the hazard ratio was 1.25 (1.10–1.42, P= 0.0007) per 40 cm2 decrease in the surface area. We examined the effects of low placental area across the entire range of mother's body mass index. The effects of low area on the risk of coronary heart disease rose progressively with increasing maternal body mass index (P for interaction = 0.01).
Table 4.
Hazard ratios for coronary heart disease in men born to tall mothers, with height > 160 cm, according to neonatal measurements and mother's body mass index
| Neonatal measurement | Mother's BMI ≤ 26 kg/m2 |
Mother's BMI > 26 kg/m2 |
||
|---|---|---|---|---|
| HR (95% CI) | Cases/men (135/1536) | HR (95% CI) | Cases/men (140/1275) | |
| Ponderal index (kg/m3) | ||||
| ≤25 | 1.8 (1.0–3.1) | 41/357 | 1.9 (1.1–3.2) | 29/215 |
| −26.5 | 1.3 (0.7–2.3) | 31/380 | 1.6 (0.9–2.7) | 37/306 |
| −28.5 | 1.4 (0.8–2.5) | 45/505 | 1.5 (0.9–2.4) | 50/428 |
| >28.5 | 1.0 (baseline) | 18/288 | 1.0 (baseline) | 23/317 |
| P for trend | 0.05 | 0.01 | ||
| Placental weight (g) | ||||
| ≤550 | 0.8 (0.4–1.3) | 26/335 | 2.2 (1.3–4.0) | 25/186 |
| −650 | 0.9 (0.6–1.5) | 49/526 | 1.9 (1.2–3.2) | 48/396 |
| −750 | 0.8 (0.5–1.4) | 34/416 | 1.8 (1.1–3.1) | 44/372 |
| >750 | 1.0 (baseline) | 26/254 | 1.0 (baseline) | 23/318 |
| P for trend | 0.5 | 0.002 | ||
| Placental area (cm2) | ||||
| ≤225 | 1.0 (0.6–1.7) | 30/339 | 2.2 (1.4–3.7) | 38/223 |
| −255 | 1.0 (0.6–1.6) | 36/427 | 1.3 (0.8–2.2) | 31/298 |
| −295 | 1.1 (0.7–1.9) | 36/379 | 1.7 (1.0–2.7) | 42/344 |
| >295 | 1.0 (baseline) | 32/380 | 1.0 (baseline) | 29/406 |
| P for trend | 0.5 | <0.001 | ||
| Length minus breadth (cm) | ||||
| ≤1 | 1.0 (baseline) | 38/474 | 1.0 (baseline) | 43/388 |
| −2 | 1.0 (0.7–1.6) | 36/439 | 1.0 (0.6–1.5) | 42/365 |
| −3 | 1.5 (0.9–2.4) | 32/257 | 1.2 (0.7–1.9) | 28/205 |
| >3 | 0.9 (0.6–1.5) | 28/355 | 0.7 (0.4–1.1) | 27/313 |
| P for trend | 0.9 | 0.8 | ||
| Placental/birth weight (%) | ||||
| ≤16.5 | 1.0 (baseline) | 23/317 | 1.0 (baseline) | 23/286 |
| −18.5 | 0.9 (0.5–1.6) | 29/439 | 1.8 (1.1–2.9) | 51/369 |
| −20.5 | 1.1 (0.7–1.9) | 32/383 | 1.3 (0.8–2.2) | 35/318 |
| >20.5 | 1.8 (1.1–3.0) | 51/392 | 1.1 (0.6–1.8) | 31/299 |
| P for trend | 0.01 | 0.5 | ||
HR, hazard ratio; CI, confidence interval; BMI, body mass index.
In men whose mother's body mass index was below the median (Table 4), coronary heart disease was related to a high ratio of placental weight to birthweight and a high ratio of placental area to birthweight (P= 0.02). In these men the hazard ratio was 1.07 (1.02–1.13, P= 0.01) per 1% increase in the placental weight/birthweight ratio. In none of the three placental phenotypes that predicted coronary heart disease (Tables 2 and 4) was the disease associated with placental thickness.
Interactions with birthweight
We examined whether the effects of placental size on later coronary heart disease interacted with the effects of birthweight. Treating birthweight as a continuous measure, there were no interactions between any placental measurement and birthweight.
Social class
Placental size was not related to the mother's social class or to the men's own social class. The associations between placental size and coronary heart disease were little changed by adjustment for either social class.
Discussion
We found that three combinations of maternal body size and placental size predicted later coronary heart disease in men. The disease was associated with (i) an oval placental surface in short mothers, (ii) a small placental surface in tall mothers whose body mass index was above the median, and (iii) a large placental surface in relation to birthweight in tall mothers whose body mass index was below the median. These associations were independent of the men's social class or the social class of the family into which they were born.
In each combination of maternal body size and placental size the babies that later developed coronary heart disease tended to have a low ponderal index, indicating that they were undernourished.7 The three maternal–placental phenotypes may reflect three different circumstances in which a foetus becomes undernourished, and coronary heart disease is programmed. A foetus may be undernourished because the mother's nutritional state and metabolism, which are reflected in her body size, limit the availability of nutrients at the placental surface. Alternatively, the mother's nutritional state and metabolism may adversely affect the development and function of the placenta. Thus, the predictions of coronary heart disease by placental size and shape are conditioned by the mother's body size. For this reason, as we have previously found in analyses of hypertension,10 analyses of the association between placental measurements and coronary heart disease required subgroups of mothers defined by their body size.
Oval placental surface
We have previously shown that the placentas of babies born after pregnancies complicated by pre-eclampsia had a more oval-shaped surface than those from normotensive pregnancies.15 Pre-eclampsia was associated with reduced breadth of the surface but not reduced length. We concluded that disruption of implantation, such as occurs in pre-eclampsia,16 has a greater effect on growth along the breadth and therefore results in an oval-shaped surface.
The association between an oval placenta and coronary heart disease was confined to men whose mothers were primiparous (Figure 1). Pre-eclampsia is largely confined to first pregnancies.16 The processes of early placentation, which include remodelling of the spiral arteries, differ in first and later pregnancies.17 Spiral arteries being remodelled for the first time differ from those that have been remodelled before. We conclude that an oval placental surface is a marker of disrupted early placentation that occurs in first pregnancies, leads to foetal undernutrition and programmes coronary heart disease. An oval placenta only predicted coronary heart disease in men with short mothers, who are known to be less metabolically competent than tall mothers, having lower rates of protein synthesis during pregnancy.18 Their foetuses are, therefore, more vulnerable to undernutrition if placental development is disrupted.
Small placental surface
In the second placental phenotype, the surface area and weight of the placenta were reduced. In normal pregnancy placental growth precedes foetal growth and restriction of placental growth jeopardizes the nutrition of the foetus in mid-gestation.8,13 Placental growth depends on the structure and function of the mother's uterine wall, which is established during her early development. Foetal growth depends on the availability of nutrients. Restricted placental growth may, paradoxically, have a greater effect in babies who are growing rapidly because their mothers are well nourished. In the present study, small placental area predicted coronary heart disease in mothers who were tall and whose body mass index in pregnancy was above the median. These are likely to have been the best-nourished mothers. We therefore speculate that their foetuses were able to grow rapidly but the placenta restricted their growth in mid-gestation so that they had a low ponderal index at birth.
Large placental surface in relation to birthweight
In the third placental phenotype, the weight and surface area of the placentas were large in relation to birthweight. If ewes are put in poor pasture in mid-pregnancy, so that they become undernourished, the foetus responds by expanding the area of the placenta to extract more nutrients from the mother.9 If the ewes are returned to good pasture in late gestation, this placental expansion results in a larger lamb than there would otherwise have been. This is profitable for the farmer, and manipulation of placental size by changing the pasture of pregnant ewes is standard practice in sheep farming. There is evidence that similar compensatory placental expansion occurs in humans.10 Compensatory expansion may be beneficial in some circumstances but if the compensation is inadequate, and the foetus continues to be undernourished, the need to share its nutrients with an enlarged placenta may become a metabolic burden, and the foetus may become thin. In sheep, compensatory expansion can only occur if the ewe was well nourished at the time of mating.9 Our findings are consistent with this in that the association between coronary heart disease and a large placental area in relation to birthweight only occurred in men whose mothers were tall, but whose body mass index in pregnancy was below the median. The mother's tall stature indicates good nutrition before pregnancy, while their low body mass index may indicate poor nutrition during pregnancy.19
Foetal undernutrition and heart development
There are a number of ways in which placental size and foetal undernutrition could initiate coronary heart disease. Foetal undernutrition, reflected in low birthweight, is known to be associated with structural abnormalities in the myocardium of children.20 It also predicts a number of coronary risk factors in adult life, including hypertension, type 2 diabetes, and the metabolic syndrome.5 An oval placental surface may be a marker of foetal undernutrition throughout gestation and, depending on the mother, may be associated with these risk factors. A small placental surface has direct effects on the development of the heart in sheep. It suppresses foetal myocyte proliferation and depresses the rate of cardiomyocyte maturation.21,22 At birth the heart contains an inadequate endowment of cardiomyocytes. As the number of coronary microvessels is linked to myocyte number, the heart also has an inadequate coronary tree. These are lifelong changes. Consistent with this, in the Helsinki Birth Cohort reduced placental area is associated with chronic heart failure in later life.23 Compensatory expansion of the placental surface occurs in sheep during mid-gestation and any adverse metabolic consequences for the foetus are evident in late gestation. In humans, a high placental weight in relation to birthweight is associated with hypertension in later life.24,25 Late gestation is a critical period for renal development during which nephron number is established for life, and reduced nephron number has been proposed as a cause of hypertension.26
Limitations of the study
The placental measurements in our study were made during routine obstetric practice 70 years ago. Routine measurements of the placental diameters ceased in Helsinki in the 1970s. We have discussed the simple procedures used with people who worked as midwives at that time. The quality of the measurements was not routinely checked, just as there are no routine checks of other clinical measurements, such as blood pressure. The mean placental weight in our study was above the median recorded in a recent series of deliveries in Europe.27 One explanation for this could be that the cord and membranes were not trimmed prior to weighing. This would make our estimates of placental thickness less accurate. Measurement errors would tend to diminish associations between placental size and coronary heart disease in later life. The mothers in our study were weighed in late gestation and we are not able to distinguish the effects of pre-pregnancy body mass from those of weight gain in pregnancy. Our study was restricted to people who had visited child welfare clinics. Although the majority of children visited these clinics, which were free, the visits were voluntary. The people in our study may not be representative of all people now living in Helsinki, although at birth their social class distribution was similar to that in the city as a whole.4 We identified men with coronary heart disease using the national hospital discharge and mortality registers. The validity of these registers has been established.28,29 Our ascertainment of coronary heart disease began in 1970 when the hospital admission register was established. We may therefore have omitted a few men who developed the disease at an unusually young age. The findings in our study cannot be extended to women among whom coronary heart disease is associated with short length at birth rather than thinness.4 We do not have data on the standard coronary risk factors among the men, and therefore cannot determine the extent to which the associations between coronary heart disease and maternal and placental size are mediated through these risk factors.
Conclusions
Three combinations of maternal body size and placental size predicted later coronary heart disease in men. The disease was associated with (i) an oval placental surface in short mothers, (ii) a small placental surface in tall mothers whose body mass index was above the median, and (iii) a large placental surface in relation to birthweight in tall mothers whose body mass index was below the median. In each combination the babies that later developed coronary heart disease tended to have a low ponderal index, indicating that they were undernourished. We speculate that variations in three processes of normal placental development lead to foetal malnutrition and programme coronary heart disease. We suggest that mother's body size determines whether a placental phenotype programmes coronary heart disease because it reflects the availability of nutrients at the placental surface, and because it affects the development and function of the placenta.
Funding
This work was supported by the British Heart Foundation, the Academy of Finland, the Paivikki and Sakari Sohlberg Foundation, the Finnish Diabetes Research Foundation, the Finnish Foundation for Cardiovascular Research, the Finnish Medical Society Duodecim, Yrjo Jahnsson Foundation, Finska Lakaresällskapet, and the M. Lowell Edwards Endowment.
Conflict of interest: none declared.
References
- 1.Barker DJP, Osmond C, Winter PD, Margetts BM, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 2.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 3.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Br Med J. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJP. Fetal origins of coronary heart disease. Br Med J. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson AA. All that glitters. Br Nutr Foundation Annual Lecture. Nutr Bull. 2000;25:11–24. [Google Scholar]
- 7.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Sibley CP. The pregnant woman. In: Case RM, Waterhouse JM, editors. Human Physiology: Age, Stress, and the Environment. Oxford: Oxford University Press; 1994. pp. 3–27. [Google Scholar]
- 9.McCrabb GJ, Egan AR, Hosking BJ. Maternal undernutrition during mid-pregnancy in sheep; variable effects on placental growth. J Agric Sci. 1992;118:127–132. [Google Scholar]
- 10.Barker DJP, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJP. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. Br Med J. 1997;315:837–840. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martyn CN, Barker DJP, Osmond C. Mothers pelvic size, fetal growth and death from stroke in men. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton WJ, Boyd JD, Mossman HW. Human Embryology. 4th edn. London: McMillan Press; 1976. [Google Scholar]
- 14.Anderson MC. Lessons in Midwifery for Nurses and Midwifes. London: A & C Black; 1930. [Google Scholar]
- 15.Kajantie E, Barker DJP, Osmond C, Kajantie E, Eriksson JG. In pre-eclampsia the placenta grows slowly along its minor axis. Int J Dev Biol. 2010;54:469–473. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001;357:53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 17.Burton G, Barker DJP, King A, Thornburg K, editors. The Placenta in Developmental Programming. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 18.Duggleby SL, Jackson AA. Relationship of maternal protein turnover and lean body mass during pregnancy and birth length. Clin Sci. 2001;101:65–72. [PubMed] [Google Scholar]
- 19.Tanner JM. Fetus into Man. Ware: Castlemead; 1989. [Google Scholar]
- 20.Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A, Gratacós E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121:2427–2436. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- 21.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- 22.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep. cardiomyocytes. J Physiol. 2007;580:639–648. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker DJP, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson J. The early origins of chronic heart failure: impaired placentation and initiationof insulin resistance in childhood. Eur J Heart Fail. 2010;12:819–825. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJP, Bull AR, Osmond C, Simmonds S. Fetal and placental size and risk of hypertension in adult life. Br Med J. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson J, Forsen T, Toumilheto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 26.Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:700–707. doi: 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- 27.Burkhard T, Schaffer L, Schneider S, Zimmerman R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight–birth weight ratios. Eur J Obstet Gynaecol. 2006;128:248–252. doi: 10.1016/j.ejogrb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Räihä P, Kärjä-Koskenkari P, Mähönen M, Niemelä M, Kuulasmaa K, Palomäki P, Mustonen J, Lehtonen A, Arstila M, Vuorenmaa T, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesäniemi YA, Pyörälä K, Salomaa V. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–137. doi: 10.1097/00149831-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rapola JM, Virtamo J, Korhonen P, Haapakoski J, Hartman AM, Edwards BK, Hecinonen OP. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997;13:133–138. doi: 10.1023/a:1007380408729. [DOI] [PubMed] [Google Scholar]