Autologous transplantation of pulp stem cells with granulocyte-colony stimulating factor (G-CSF) in a dog pulpectomized tooth yielded better effects than transplantation of G-CSF or pulp stem cells alone. The combinatorial trophic effects of pulp stem cells and G-CSF are of immediate utility for pulp/dentin regeneration, demonstrating the prerequisites of safety and efficacy critical for clinical applications.
Keywords: Autologous stem cell transplantation, G-CSF, Mesenchymal stem cells, Preclinical trials, Dog model, Stem cell culture, Tissue regeneration
Abstract
Treatment of deep caries with pulpitis is a major challenge in dentistry. Stem cell therapy represents a potential strategy to regenerate the dentin-pulp complex, enabling conservation and restoration of teeth. The objective of this study was to assess the efficacy and safety of pulp stem cell transplantation as a prelude for the impending clinical trials. Clinical-grade pulp stem cells were isolated and expanded according to good manufacturing practice conditions. The absence of contamination, abnormalities/aberrations in karyotype, and tumor formation after transplantation in an immunodeficient mouse ensured excellent quality control. After autologous transplantation of pulp stem cells with granulocyte-colony stimulating factor (G-CSF) in a dog pulpectomized tooth, regenerated pulp tissue including vasculature and innervation completely filled in the root canal, and regenerated dentin was formed in the coronal part and prevented microleakage up to day 180. Transplantation of pulp stem cells with G-CSF yielded a significantly larger amount of regenerated dentin-pulp complex compared with transplantation of G-CSF or stem cells alone. Also noteworthy was the reduction in the number of inflammatory cells and apoptotic cells and the significant increase in neurite outgrowth compared with results without G-CSF. The transplanted stem cells expressed angiogenic/neurotrophic factors. It is significant that G-CSF together with conditioned medium of pulp stem cells stimulated cell migration and neurite outgrowth, prevented cell death, and promoted immunosuppression in vitro. Furthermore, there was no evidence of toxicity or adverse events. In conclusion, the combinatorial trophic effects of pulp stem cells and G-CSF are of immediate utility for pulp/dentin regeneration, demonstrating the prerequisites of safety and efficacy critical for clinical applications.
Introduction
Dental pulp has multiple functions in the homeostasis of teeth, such as regulation of inflammation, control of pulp defense, and a source of stem cells for dental regeneration and repair. Maintenance of the function of pulp tissue is critical for longevity of teeth and attendant enhancement of quality of life. Dental caries with associated pulpitis is one of the most challenging public health issues. Deep caries and pulp exposure have been treated by pulp capping or pulp amputation to conserve pulp tissue. To date, however, success has been very limited, resulting in early loss of dental pulp, followed by tooth fracture and/or periapical disease and final loss of teeth. Thus, a potential stem cell-based therapy has been developed to regenerate the dentin-pulp complex for conservation and total restoration of the structure and function of a tooth. We have established a potential cell therapy for pulp regeneration in a canine pulpitis model harnessing autologous dental pulp-derived mesenchymal stem cells, CD31− side population (SP) cells, or CD105+ cells together with stromal cell-derived factor 1 (SDF-1) [1, 2]. The efficacy and safety of this therapy for pulp regeneration must be examined prior to initiating clinical evaluation. Since CD31− SP cells have to be labeled with the DNA-binding dye Hoechst 33342 and isolated by flow cytometry, it is difficult to evaluate safety. Alternatively, isolation of CD105-positive cells by the magnetic antibody bead method is cost-effective. Therefore, we have recently developed a novel method for cell isolation and processing to obtain clinical-grade human pulp stem cells from a small amount of pulp tissue based on good manufacturing practice (GMP)-grade guidelines [3]. The isolated human pulp stem cells expressed higher levels of CD105, CXCR4, and granulocyte colony-stimulating factor receptor (G-CSFR) compared with unfractionated total pulp cells, and they showed stronger angiogenic and neurogenic properties. Transplantation of the human pulp stem cells with a scaffold of the tooth root in ectopic sites induced pulp regeneration in an immunodeficient mouse, demonstrating their potential utility for pulp regeneration (our unpublished data). In addition to pulp stem cells, GMP-grade migration/homing factors for transplantation must be evaluated. The combinatorial effect of SDF-1 and pulp CD105+ cells on accelerated pulp regeneration has been suggested by the homing of stem cells expressing CXCR4 by the SDF-1-CXCR4 axis during pulp regeneration [2]. SDF-1, however, is not available in GMP grade. Granulocyte-colony stimulating factor (G-CSF) also has a migratory effect on pulp stem cells similar to that of SDF-1 [4]. G-CSFR-positive cells represented 59% of the human pulp stem cells (Murakami M, Horibe H, Iohara K et al., manuscript in preparation). Thus, G-CSF, which stimulates migration of bone marrow mesenchymal stem cells, is based on their expression of G-CSFR [5] and may be a potential alternative to SDF-1 for pulp regeneration. Transplantation of stem cells derived from other tissues, bone marrow stromal cells, neuronal stem cells, and amniotic fluid stem cells, with G-CSF into the spinal cord injury results in a better functional and morphological recovery with augmentation of nerve regeneration. The synergistic/additive effect of stem cells and G-CSF is involved in the suppression of apoptotic death in transplanted stem cells and in the attenuation of inflammatory response [6–8]. We therefore set out to harness pulp stem cells in combination with G-CSF for regeneration of teeth.
Accumulating data have demonstrated the therapeutic effects of mesenchymal stem cells (MSCs) in animal models of various diseases, and a number of clinical trials have been conducted to test the safety and efficacy of MSCs [9]. There have been no preclinical reports, however, to support the potential use of the pulp stem cells for treatment of pulpitis in clinical trials. Thus, in this study, pulp stem cells were isolated in a totally enclosed system in a GMP-compliant facility. We examined the karyotype, safety, and efficacy of the pulp stem cells. Autologous pulp stem cells were transplanted in canine pulpectomized teeth in combination with GMP-grade G-CSF to establish preclinical feasibility, safety, and efficacy of pulp regeneration in a canine pulpitis model. The results of this investigation will demonstrate the standardization and implementation of regulatory aspects of stem cell therapies in clinical and translational endodontics.
Materials and Methods
This study was approved by the animal care and use committees of the National Center for Geriatrics and Gerontology, Research Institute, and Aichi-gakuin University. All experiments were conducted using the strict guidelines of DNA Safety Programs.
Cell Isolation and Culture
Freshly extracted upper canine teeth from 8–10-month-old beagle dogs (Kitayama Labes, Ina, Japan, http://www.labes.co.jp) were soaked in Hanks' balanced salt solution (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) after a longitudinal cut was made, and they were transported to a GMP-compliant facility in a container (Taiyo Kogyo, Tokyo, Japan, http://www.taiyokogyo.co.jp) within 1 hour under strict control of temperature (Testo, Yokohama, Japan, http://www.testo.jp). Dental pulp tissues were separated from the teeth, minced into pieces in a totally enclosed system of the Isolator (Panasonic Healthcare, Tokyo, Japan, http://panasonic.co.jp/hcc), and enzymatically digested in 0.04 mg/ml Liberase (Roche, Mannheim, Germany, http://www.roche.com) for 30 minutes at 37°C. The isolated pulp cells were plated at 2–6 × 104 cells on 35-mm dishes (Asahi Technoglass, Funabashi, Japan, http://www.atgc.co.jp) in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) supplemented with 10% autologous canine serum (autoserum). They were detached by incubation with TrypLE Select (Invitrogen) prior to 70% confluence (additional details are given in the supplemental online data).
We next isolated pulp stem cells by a novel method we devised using G-CSF-induced stem cell mobilization [3] (additional details are given in the supplemental online data). Isolated pulp stem cells and unfractionated total pulp cells at 60%–70% confluence were detached by incubation with TrypLE Select (Invitrogen) and subcultured at a 1:3 dilution into cell culture flasks (25 cm2 and, further, 75 cm2) (Asahi Technoglass) at 1 × 104 cells per cm2 in DMEM (Sigma-Aldrich) supplemented with 10% autoserum according to a standard operational procedure under strict GMP conditions. The cells were suspended at a cell concentration of 1 × 106 cells per milliliter in an extracellular cryoprotectant, CP-1 (Kyokuto Pharmaceutical Industrial, Tokyo, Japan, http://www.kyokutoseiyaku.co.jp/english) that was gradually decreased in temperature to −40°C at a rate of −2°C/minute and further to −80°C at a rate of −10°C/minute for cryopreservation according to freezing protocol in a Program Deep Freezer (Strex, Osaka, Japan, http://www.strex.co.jp).
Characterization of Pulp Stem Cells
The phenotype of pulp stem cells was further characterized by flow cytometry at the seventh passage of culture compared with total pulp cells after immunolabeling with antigen surface markers (additional details are given in the supplemental online data). To examine expression of angiogenic/neurotrophic factors, real-time reverse transcription-polymerase chain reaction analysis was also performed [2] (additional details are given in the supplemental online data). The multilineage differentiation potential of pulp stem cells at the seventh passage was compared with that of total pulp cells as described previously [10]. Proliferation and migration activities with G-CSF were examined (additional details are given in the supplemental online data).
Bacteria, Mycoplasma, Endotoxin Tests, Q-Banding Karyotype Analysis, and Tumorigenicity Assay of Pulp Stem Cells
Additional details are given in the supplemental online data.
Preclinical Efficacy Test of Pulp Regeneration
For preclinical efficacy assessment, an experimental model of pulp regeneration was established in the permanent teeth with complete apical closure in dogs at 9–11 months of age [2]. The whole pulp tissue was removed, and the root canals were enlarged to open the apical foramen to 0.6 mm in width in incisors. A total of 72 teeth from 18 dogs were randomly divided into six groups for autologous transplantation and morphological analysis: group I, pulp stem cells and G-CSF (Neutrogin; Chugai Pharmaceutical, Tokyo, Japan, http://www.chugai-pharm.co.jp) with a clinical-grade atelocollagen scaffold (Koken, Tokyo, Japan, http://www.kokenmpc.co.jp); group II, unfractionated total pulp cells and G-CSF; group III, pulp stem cells only; group IV, unfractionated total pulp cells; group V, G-CSF only; and group VI, collagen only (additional details are given in the supplemental online data).
Routine histology with hematoxylin and eosin staining, in situ hybridization, immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and microarray analysis was performed in the regenerated pulp tissue. Functional revascularization, vitality, reinnervation/neurogenesis, and x-ray analysis (Morita, Osaka, Japan, http://japan.morita.com) was also performed in the regenerated pulp tissue (additional details are given in the supplemental online data).
Combinatorial Effect of the Conditioned Medium of Pulp Stem Cells With G-CSF In Vitro
The migratory effect, antiapoptotic effect, proliferative effect, immunomodulatory effect, and neurite extension effect of the conditioned medium of pulp stem cells with G-CSF were compared with those of the conditioned medium only and G-CSF only (additional details are given in the supplemental online data).
Statistical Analyses
Data are reported as means ± SD. p values were calculated using Student's t test and Tukey's multiple comparison test method in SPSS 21.0 (IBM, Armonk, NY, http://www.ibm.com).
Results
Isolation and Characterization of Stem Cells From Pulp Tissue
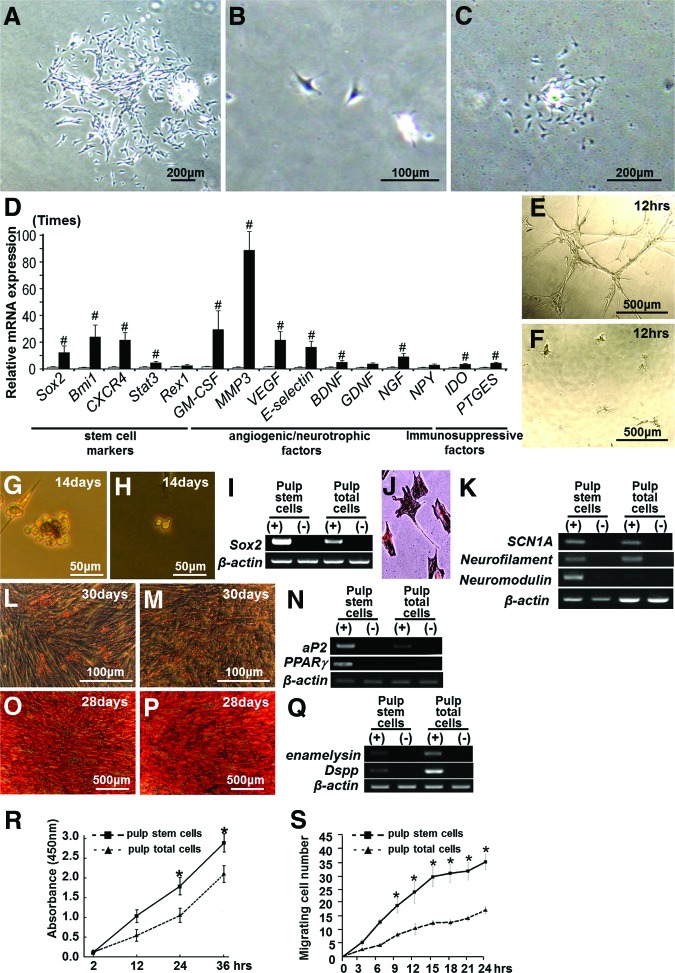
Clinical-grade canine pulp stem cells were isolated by a novel method using G-CSF-induced stem cell mobilization in the isolator, and colony formation assays demonstrated that the isolated cells accounted for 5%–7% of primary total pulp cells (Fig. 1A). The pulp stem cells were stellate with long processes (Fig. 1B). Clonal culture of pulp stem cell yielded a colony in 10 days, documenting the colony-forming ability of these cells (Fig. 1C). Limiting dilution analysis at the third-passage culture showed that the ratio of colony-forming units in pulp stem cells was approximately 90%, whereas the ratio in unfractionated total pulp cells was approximately 75%.
Figure 1.
Isolation of pulp stem cells from canine adult total pulp cells. (A): Primary total pulp cells on day 7. (B): Pulp stem cells on day 2. (C): Pulp stem cells forming a colony on day 10. (D): Real-time reverse transcription-polymerase chain reaction analysis of the relative expression of stem cell markers and angiogenic/neurotrophic factors in pulp stem cells compared with those in total pulp cells. #, p < .01 versus total pulp cells. The experiment was repeated four times, and one representative experiment is presented. (E–Q): Multilineage differentiation potential of pulp stem cells and total pulp cells. (E, G, J, L, O): Pulp stem cells. (F, H, M, P): Total pulp cells. (E, F): Angiogenic potential on Matrigel. (G, H): Neurosphere formation. (I): Sox2 mRNA expression in neurospheres. (J, K): Neurogenic potential 14 days after induction of dissociated neurosphere cells. (J): Immunostaining with neurofilament. (K): Scn1A, neurofilament, and neuromodulin mRNA expression. (L–N): Adipogenic potential. (L, M): Oil Red O staining. (N): Expression of aP2 mRNA. (O–Q): Odontogenic potential. (O, P): Alizarin red staining. (Q): Expression of enamelysin and Dspp mRNA. (R): Proliferation activity with granulocyte colony-stimulating factor (G-CSF). Data are expressed as means ± SD of four determinations. Note that pulp stem cells had significantly higher proliferation activity compared with total pulp cells (*, p < .01). (S): The migration activity with G-CSF in TAXIScan-FL. Data are expressed as means ± SD of three determinations. Note that pulp stem cells had significantly higher chemotactic activity compared with total pulp cells (*, p < .01). Abbreviations: Dspp, dentin sialophosphoprotein; GM-CSF, granulocyte macrophage colony-stimulating factor; VEGF, vascular endothelial growth factor.
To characterize the “stemness” of pulp stem cells, cell surface antigen markers were examined by flow cytometry and compared with those for unfractionated total pulp cells. Pulp stem cells and total pulp cells were positive for CD29, CD44, CD73, and CD90 and negative for CD31 at the seventh passage, which are minimal positive criteria for mesenchymal stem cells. It is noteworthy that, as expected, the percentages of pulp stem cells expressing CD105, CXCR4, and G-CSFR were higher than those of total pulp cells (Table 1). The mRNA expression levels of stem cell markers Sox2, Bmi1, CXCR4, Stat3, and Rex1 were 3–29 times higher in pulp stem cells than in unfractionated total pulp cells, indicating enrichment of stem cells and establishing the properties of pulp stem cells. Angiogenic factors and/or neurotrophic factors GM-CSF (granulocyte macrophage colony-stimulating factor), MMP3 (matrix metalloproteinase 3), VEGF, E-selectin, BDNF, GDNF, and NGF were expressed at levels 3–87 times higher in pulp stem cells than in total pulp cells (Fig. 1D). Immunosuppressive factors Prostaglandin E2 (PG) and indoleamine-2,3-dioxygenase (IDO) were expressed at levels 3 times higher in pulp stem cells than in unfractionated total pulp cells (Fig. 1D).
Table 1.
Expression of cell surface markers in pulp stem cells compared with unfractionated total pulp cells
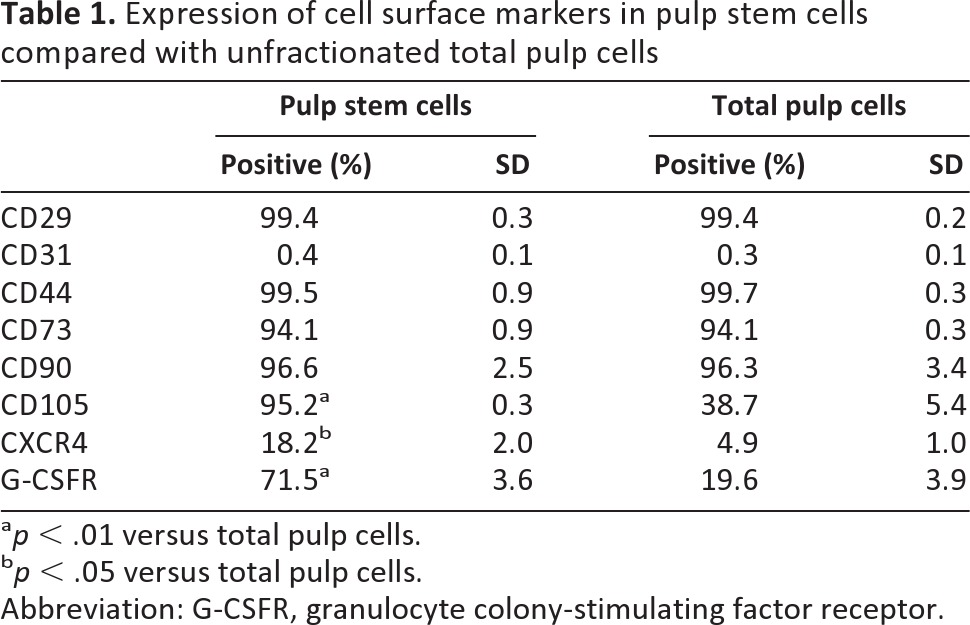
ap < .01 versus total pulp cells.
bp < .05 versus total pulp cells.
Abbreviation: G-CSFR, granulocyte colony-stimulating factor receptor.
Differentiation of pulp stem cells into endothelial cells (Fig. 1E), neuronal cells (Fig. 1G, 1I–1K), adipose cells (Fig. 1L, 1N), and odontoblast lineage cells (Fig. 1O, 1Q) was demonstrated. The G-CSF-induced proliferation was significantly higher in pulp stem cells than in unfractionated total pulp cells (Fig. 1R). Migration activity with G-CSF shown in TAXIScan-FL (ECI, Inc., Kanagawa, Japan, http://effec564.rsjp.net/english/) was much higher in pulp stem cells than that in total pulp cells (Fig. 1S).
Safety Evaluation
The expanded cells were characterized to evaluate the sterile nature, viability, and karyotype before transplantation. After in vitro expansion for seven passages and cryopreservation, pulp stem cells showed no bacterial, fungal, mycoplasma, endotoxin, or virus contamination (supplemental online Table 1). It is noteworthy that there were no chromosomal abnormalities/aberrations in the karyotype (supplemental online Fig. 1A). Furthermore, injection of pulp stem cells in intratesticular sites in immunodeficient mice resulted in no teratoma formation hematological abnormality (supplemental online Fig. 1B).
Pulp Regeneration After Transplantation of Pulp Stem Cells With G-CSF in the Root Canal
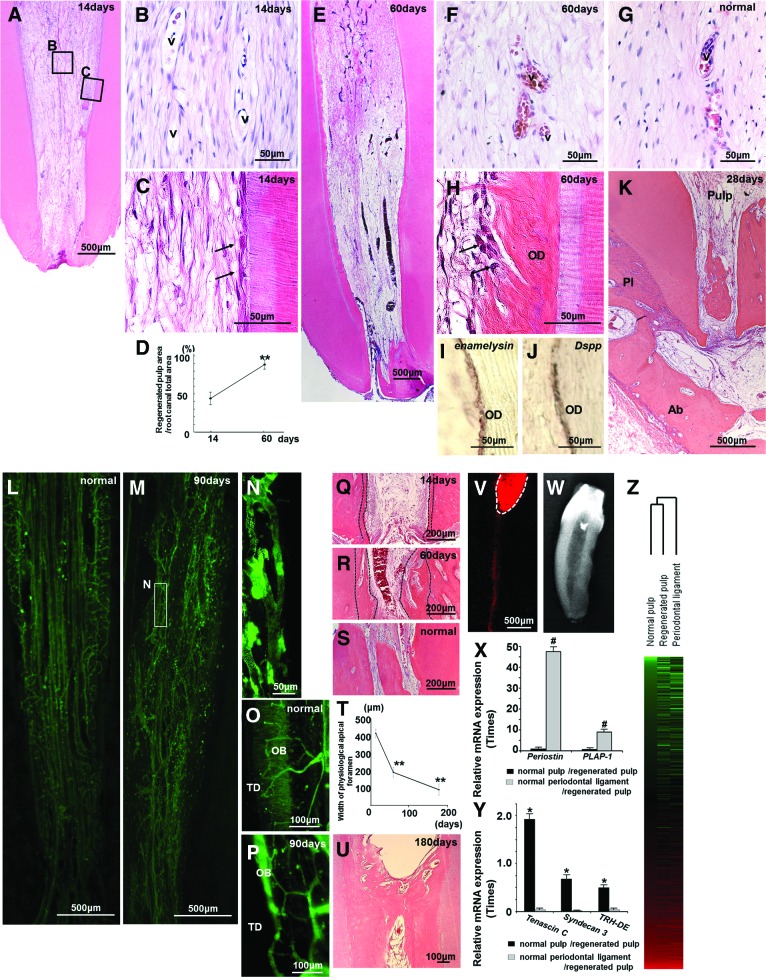
We next evaluated the efficacy of autologous transplantation of clinical-grade pulp stem cells and G-CSF into the root canals of mature teeth after pulpectomy in dogs (Figs. 2–4). Pulp-like loose connective tissue with vasculature was regenerated 14 days after autologous transplantation of pulp stem cells with G-CSF (Fig. 2A, 2B). Odontoblast-like cells attached to the dentinal wall in the root canal (Fig. 2C). The pulp-like tissue differentiated further to the cementum-enamel junction below the cement filling 60 days after transplantation of pulp stem cells with G-CSF (Fig. 2E), indicating a coverage of more than 90% of the total area of the root canal (Fig. 2D). Cell morphology, cell density, and architecture of the extracellular matrix were similar to those of the normal pulp (Fig. 2F, 2G). It is noteworthy that dentin formation was observed along the dentinal wall (Fig. 2H). The odontoblasts lining the dentinal wall were positive for enamelysin/matrix metalloproteinase (MMP) 20 (Fig. 2I) and Dspp (dentin sialophosphoprotein) (Fig. 2J), two markers for odontoblasts. Infiltration of inflammatory cells was not observed at all in the periapical region, such as the periodontal ligament and alveolar bone 30 days after transplantation. In addition, there was no internal or external resorption of the tooth (Fig. 2K).
Figure 2.
Regeneration of pulp tissue after autologous transplantation of pulp stem cells with granulocyte colony-stimulating factor in pulpectomized teeth of dogs. (A–C, E, F, H–K, M, N, P–R, U–W): Regenerated pulp tissue. (G, L, O, S): Normal pulp tissue. (D): Ratio of newly regenerated area to root canal area on days 14 and 60. Data are means ± SD of five determinations (**, p < .001). (C, H): Odontoblastic cells (arrows) lining to newly formed osteodentin/tubular dentin along with the dentin. (I, J): In situ hybridization analyses of odontoblastic differentiation markers enamelysin (I) and Dspp (J) on day 60. (K): Periapical region on day 28, indicating no infiltration of inflammatory cells and no internal/external resorption of the tooth. (L–N): Three-dimensional images of vascularization by whole mount immunostaining with BS-1 lectin. (N): Higher magnification of (M). (O, P): Three-dimensional images of innervation by whole mount immunostaining with Protein Gene Product 9.5 (PGP9.5) (Q–S): The apical part. (T): Gradual decrease in width of the physiological apical foramen. Data represent means ± SD of five determinations (**, p < .001). (U): The coronal part of the root canal. Note thick formation of regenerated dentin. (V): DiI labeling from the upper part of the regenerated pulp on day 21. Note the inferior alveolar nerve connecting to the regenerated pulp (white dotted line), suggesting reinnervation. (W): X-ray photograph showing apical closure on day 180. (X, Y): Relative mRNA expression of biomarkers for periodontal ligament (X) and pulp (Y) by real-time reverse transcription-polymerase chain reaction analyses. #, p < .01 versus normal pulp/regenerated pulp. *, p < .01 versus normal periodontal ligament/regenerated pulp. The experiments were repeated four times, and one representative experiment is presented. (Z): Hierarchical cluster analysis of gene expression patterns among normal pulp, regenerated pulp, and periodontal ligament. A total of 13,286 probe sets are displayed. Red indicates high expression level, green indicates low expression level, and black indicates median expression level. Abbreviations: Ab, alveolar bone; OB, odontoblastic layer; OD, osteodentin/tubular dentin; Pl, periodontal ligament; TD, tubular dentin; v, vessels.
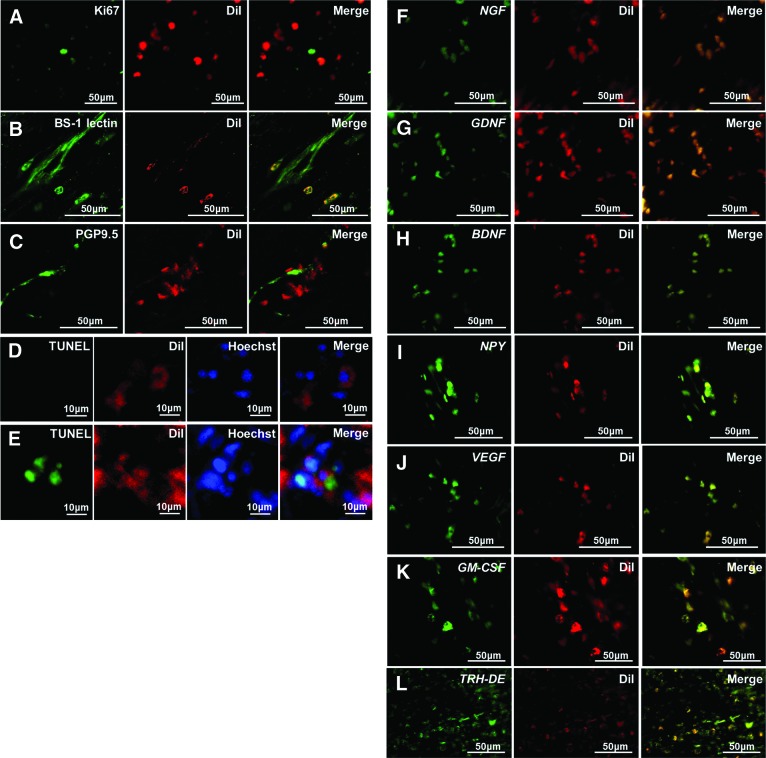
Figure 3.
Localization and gene expression in regenerated pulp tissue on day 14 by confocal laser microscopic analysis. (A–D, F–L): Transplantation of pulp stem cells with granulocyte colony-stimulating factor. (E): Transplantation of pulp stem cells only. (A–E): Immunohistochemical staining of Ki67 (A), BS-1 lectin (B), PGP9.5 (C), and TUNEL (D, E). (F–L): In situ hybridization analysis of mRNA of NGF (F), GDNF (G), BDNF (H), NPY (I), VEGF (J), GM-CSF (K), and pulp biomarker TRH-DE (L). Red indicates DiI-labeled transplanted cells. The experiment was repeated three times and yielded similar results. Abbreviations: GM-CSF, granulocyte macrophage colony-stimulating factor; PGP9.5, Protein Gene Product 9.5; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; VEGF, vascular endothelial growth factor.
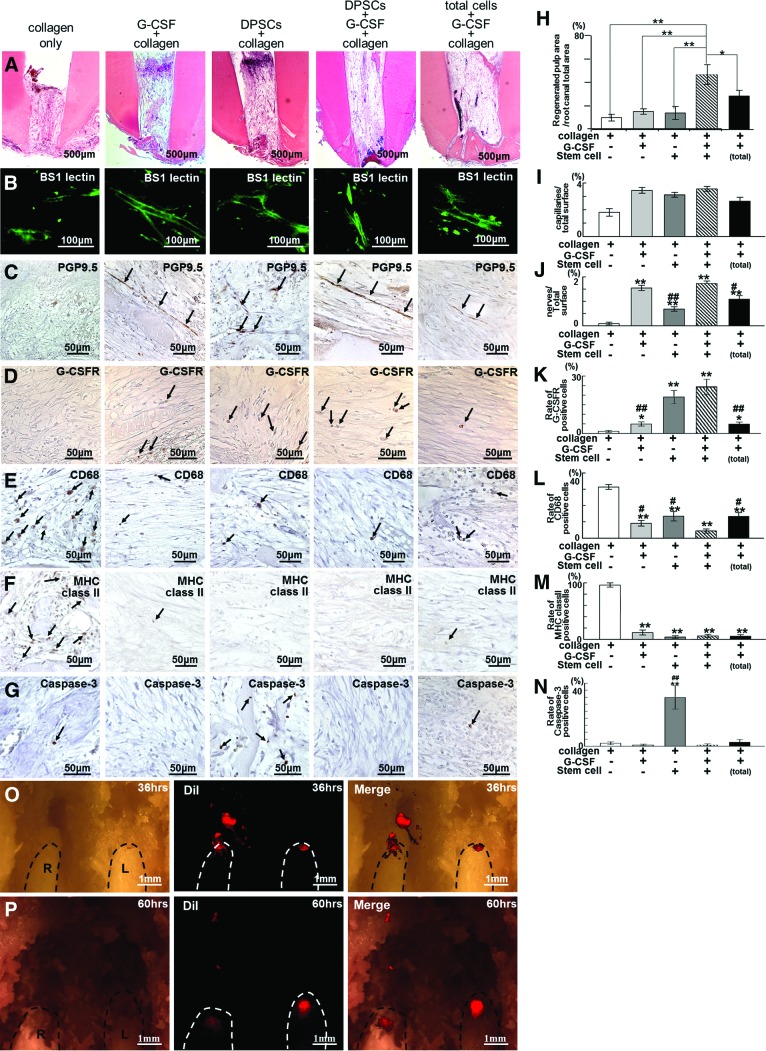
Figure 4.
Combinatorial effect of G-CSF and pulp stem cells in regenerated pulp tissue. (A–N): Transplantation of pulp stem cells and G-CSF with collagen was compared with transplantations of collagen only, G-CSF with collagen only, pulp stem cells with collagen only, and total pulp cells and G-CSF with collagen on day 14. (A): Hematoxylin and eosin staining. (B–G, I–N): Immunohistochemical analysis of BS-1 lectin (B, I), PGP9.5 (C, J), G-CSFR (D, K), CD68 (E, L), MHC class II (F, M), and Caspase-3 (G, N). (H–N): Morphometric statistical analyses. Data are means ± SD of five determinations. The experiment was repeated three times. (H): Ratio of newly regenerated area to root canal area. *, p < .01; **, p < .001 versus stem cells with G-CSF transplantation. (I, J): Ratio of positively stained area in a frame composed of 310 μm × 240 μm in size. ##, p < .01; #, p < .05 versus stem cells with G-CSF transplantation. **, p < .01; *, p < .05 versus collagen only. (K–N): Ratio of positively stained cell number to total cell number in a frame 310 × 240 μm in size. Microscopic digital images of six sections every 120 μm were scanned in the frame. ##, p < .01; #, p < .05 versus stem cells with G-CSF transplantation. **, p < .01; *, p < .05 versus collagen only. (O, P): Migration analysis of pulp stem cells transplanted with G-CSF in the upper left central incisor (L) and without G-CSF in the right central incisor (R) 36 and 60 hours after transplantation. Dotted line indicates incisors. Note that the DiI-labeled transplanted pulp cells (red) were migrating from the root canal of the right incisor (R) and were dispersed in the periapical region and alveolar bone, although the cells were localized in the root canal in the left incisor (L). Abbreviations: DPSC, dental pulp stem cells; G-CSF, granulocyte colony-stimulating factor; G-CSFR, granulocyte colony-stimulating factor receptor; L, left central incisor; MHC, major histocompatibility complex; PGP9.5, Protein Gene Product 9.5; R, right central incisor.
The three-dimensional image of vascularization in the regenerated tissue was also similar in density and orientation to that of the normal pulp (Fig. 2L–2N). Nerve fibers positively stained by Protein Gene Product 9.5 (PGP9.5) antibody invaded the newly regenerated tissues, formed a dense subodontoblastic plexus, and terminated in the odontoblastic layer as in the normal pulp, indicating neurogenesis/reinnervation of the regenerated tissue (Fig. 2O, 2P). In the apical part of the root canal, the physiological apical foramen gradually decreased in diameter by additional formation of dentin and cementum (Fig. 2Q–2T). In the coronal part of the root canal, thick dentinal bridge formation was observed on the surface of the regenerated pulp tissue on day 180 (Fig. 2U). DiI labeling on the regenerated pulp in the lower third incisor in vivo showed the inferior alveolar nerve connecting to the neuronal process in the regenerated pulp, suggesting reinnervation (Fig. 2V). Dental radiographical examination on day 180 showed complete obliteration of the enlarged apical portion following pulpectomy and lateral and coronal dentin formation (Fig. 2W).
Pulp Regeneration Confirmed by Gene Expression Analyses
To further confirm that pulp-like tissue regenerated with clinical-grade pulp stem cells and that G-CSF is identical to normal functional pulp tissue, specific markers for the periodontal ligament and pulp tissue, respectively, were used. The mRNA expression levels of periostin and asporin/periodontal ligament-associated protein 1 (PLAP-1) were much higher (47.5-fold and 8.9-fold, respectively) in the normal periodontal ligament than in the regenerated tissue on day 28 (Fig. 2X). The expression levels of tenascin C, syndecan 3, and TRH-DE, known to be highly expressed in pulp, were 62.5 times, 7.7 times, and 5.0 times higher in the regenerated tissue than in the periodontal ligament, although the expression levels in the regenerated tissue were similar to those in normal pulp (Fig. 2Y). Hierarchical clustering based on Affymetrix (Santa Clara, CA, http://www.affymetrix.com) data indicated that a gene expression pattern in regenerated tissue was more similar to that in normal pulp tissue compared with that in periodontal ligament (Fig. 2Z). Thus, gene expression analyses demonstrated that the regenerated tissue was identical to true functional dental pulp, and thus the efficacy of the pulp stem cell therapy in the preclinical trial was firmly established.
Combinatorial Effects of G-CSF and Pulp Stem Cells in Regeneration of Pulp Tissue
Confocal laser microscopic analyses in cryosections demonstrated that the DiI-labeled transplanted pulp stem cells with G-CSF were not stained with Ki67, a proliferation marker, on day 14. (Fig. 3A). The DiI-labeled transplanted pulp stem cells were in the vicinity of the newly formed capillaries and outgrowth neurites, implicating their trophic role in angiogenesis and reinnervation (Fig. 3B, 3C). The proportions of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells in the DiI-labeled transplanted cells was significantly lower in the case of transplantation of pulp stem cells with G-CSF compared with in the case of transplantation of pulp stem cells alone (0.5% and 40.0%, respectively) (Fig. 3D, 3E), suggesting an antiapoptotic effect of G-CSF on the transplanted pulp stem cells. On the other hand, the proportions of TUNEL-positive cells in migrating endogenous cells in the case of transplantation of pulp stem cells with G-CSF and in the case of transplantation of pulp stem cells alone were 0.6% and 0.4%, respectively. High expression levels of angiogenic/neurotrophic factors, including NGF, GDNF, BDNF, neuropeptide Y, VEGF, and GM-CSF, by the DiI-labeled transplanted pulp stem cells were found by in situ hybridization (Fig. 3F–3K), demonstrating their potent trophic effects. TRH-DE, a pulp biomarker, was expressed in all of the transplanted pulp stem cells and in some of the migrating endogenous cells (Fig. 3L).
The regenerated area was significantly larger in the case of transplantation of pulp stem cells with G-CSF than in the case of transplantation of collagen only, stem cells alone, or G-CSF alone (4.6-fold, 3.1-fold, and 3.3-fold increase, respectively). Transplantation of total pulp cells with G-CSF also resulted in less regenerated pulp tissue (0.6-fold) than transplantation of pulp stem cells with G-CSF (Fig. 4A, 4H). There was little difference in BS-1 lectin staining after all transplantations except for transplantation of collagen only (Fig. 4B, 4I). Longer neurite outgrowth was observed in transplantation of pulp stem cells with G-CSF (Fig. 4C, 4J).
There were more G-CSFR-positive cells in transplantation of pulp stem cells with G-CSF, pulp stem cells alone, and G-CSF alone compared with collagen only (Fig. 4D, 4K), suggesting that exogenous G-CSF and trophic factors released from the transplanted stem cells promote migration of G-CSFR-positive stem cells from the surrounding tissue such as the periodontal ligament, bone marrow, and blood vessels in the whole body. The numbers of CD68 and major histocompatibility complex class II-positive cells were fewer in transplantation of pulp stem cells with G-CSF, pulp stem cells alone and G-CSF alone compared with collagen only (Fig. 4E, 4F, 4L, 4M). In addition, there were significantly fewer Caspase-3-positive cells in transplantations of pulp stem cells with G-CSF and G-CSF alone compared with pulp stem cells alone (Fig. 4G, 4N), suggesting an antiapoptotic effect of G-CSF. The migratory and immunosuppressive effects of pulp stem cells with G-CSF were significantly greater than those of total pulp cells with G-CSF (Fig. 4K, 4L). Furthermore, the number of CD68-positive cells in transplantation of pulp stem cells with G-CSF was lower compared with pulp stem cells alone and G-CSF alone (Fig. 4L), suggesting combinatorial effects of pulp stem cells and G-CSF on immunosuppression. The in vivo results demonstrate that pulp stem cells together with G-CSF have a combinatorial influence on pulp regeneration that is based on stem cell migration, neurite outgrowth, immunosuppression, and inhibition of cell death.
To address the migratory effect of G-CSF, DiI-labeled pulp stem cells were transplanted with and without G-CSF. When pulp stem cells were transplanted without G-CSF into the root canal, the transplanted cells were dispersed in the apical periodontal tissue and the alveolar bone 36 hours after transplantation (Fig. 4O) and had declined in number after 60 hours, indicating their migration in the absence of G-CSF into the surrounding tissue from the root canal (Fig. 4P). On the other hand, when pulp stem cells were transplanted together with G-CSF, the transplanted cells remained in the root canal even after 60 hours (Fig. 4P), suggesting a localized effect of G-CSF on G-CSFR-positive transplanted pulp stem cells in the root canal.
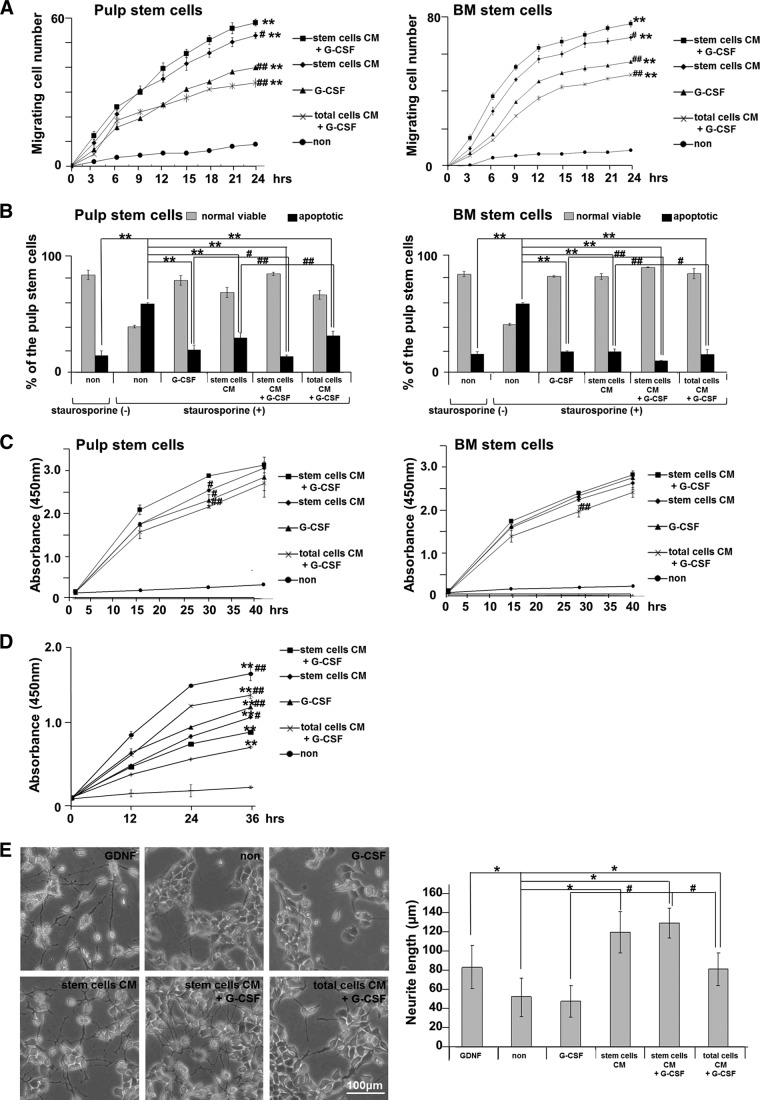
Stimulation by the conditioned medium (CM) of pulp stem cells together with G-CSF was further examined in both cultures of pulp CD31− SP cells and bone marrow CD31− SP cells. The CM of pulp stem cells with G-CSF had higher levels of proliferation activity, migration activity, and antiapoptotic activity compared with the CM of pulp stem cells only, G-CSF only, or CM of total pulp cells with G-CSF in both cultures (Fig. 5A–5C). A greater immunosuppressive effect of the CM of pulp stem cells with G-CSF was also demonstrated by mixed lymphocyte reaction assay (Fig. 5D). A stimulatory effect of the CM of pulp stem cells on neurite outgrowth was also shown in human neuroblastoma TGW cells (Japanese Collection of Research Bioresources Cell Bank, Tokyo, Japan, http://cellbank.nibio.go.jp). G-CSF, however, had little effect on neurite outgrowth (Fig. 5E). G-CSF significantly enhanced the migratory, antiapoptotic, and immunosuppressive effects of the CM of pulp stem cells, indicating a combinatory effect of the CM and G-CSF.
Figure 5.
Combinatorial effect of G-CSF and CM of pulp stem cells. (A–D): In pulp CD31− side population (SP) cells and bone marrow CD31− SP cell cultures. (A): Migration activity during a 24-hour period of culture was analyzed by TAXIScan-FL. **, p < .001 versus control. #, p < .05; ##, p < .01 versus CM of pulp stem cells with G-CSF. (B): Relative percentages of viable and apoptotic cells analyzed by flow cytometry. In the presence of 100 nM staurosporine, CM of pulp stem cells with G-CSF had a significantly stronger antiapoptotic effect compared with CM of pulp stem cells only, G-CSF only, or CM of total pulp cells with G-CSF (#, p < .05; ##, p < .01). **, p < .01 versus only staurosporine-treated. (C): Proliferation activity at 2, 16, 32, and 40 hours of culture. CM of pulp stem cells with G-CSF had a significantly stronger proliferative effect compared with CM of pulp stem cells only, G-CSF only, or CM of total pulp cells with G-CSF (#, p < .05; ##, p < .01) at 30 hours of culture. (D): Mixed lymphocyte reaction assay. Note that the CM of pulp stem cells with G-CSF had a significantly stronger immunomodulatory effect compared with the CM of pulp stem cells only, G-CSF only, or the CM of total pulp cells with G-CSF (#, p < .01; ##, p < .001). **, p < .001 versus control. (E): Neurite outgrowth was not seen in the presence of G-CSF only in TGW cell culture. The CM of pulp stem cells with G-CSF had a significantly stronger effect compared with the CM of pulp stem cells only, G-CSF only, or the CM of total pulp cells with G-CSF (#, p < .01). *, p < .001 versus untreated control. Data are expressed as means ± SD of four determinations. Each experiment was repeated three times, and one representative experiment is presented. Abbreviations: BM, bone marrow; CM, conditioned medium; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; non, nontreated; PBMC, peripheral blood mononuclear cell; SP, side population.
Safety Evaluation of the Pulp Stem Cell Therapy
Toxicology assessment showed no adverse effects on appearance, clinical signs, food consumption, and body weight after autologous transplantation of clinical-grade pulp stem cells (5 × 105) and G-CSF embedded in a collagen scaffold into pulpectomized teeth in dogs. Serum and urine chemistry parameters showed values within normal ranges for 4 weeks after stem cell transplantation. Necropsy and histopathological examinations revealed no abnormalities due to transplantation in any organ or tissue of the dogs at day 28.
Functional Recovery of Regenerated Pulp Tissue
Laser Doppler analysis showed that there was no significant difference in pulpal blood flow in regenerated pulp tissue compared with that in normal pulp tissue 90 days after transplantation (1.8 ± 0.2 and 1.9 ± 0.1 ml/minute per 100 g, respectively), indicating complete functional angiogenesis/vasculogenesis. Assessment of pulp vitality by pulp testing (Vitality Scanner 2006; SybronEndo, Orange, CA, http://www.sybronendo.com) in regenerated pulp tissue demonstrated a positive response on day 60 and day 180, suggesting functional reinnervation/neurogenesis in the regenerated pulp tissue.
Discussion
The present investigation represents the first preclinical demonstration of the efficacy and safety after transplantation of clinical-grade pulp stem cells together with G-CSF for dentin-pulp regeneration in the pulpectomized tooth in dogs. An increasing number of clinical trials focusing on a variety of diseases, including different types of ischemic diseases and neurological disorders, have shown that the therapeutic effects of MSCs depend not only on their differentiation ability to repair damaged tissue but also on their potency to modulate the local environment, activate endogenous progenitor cells, and secrete various factors [11, 12]. However, the detailed mechanisms of these effects are far from clear, and scientific evidence of the safety and efficacy of stem cell therapies must be established in appropriate animal models before clinical application in humans. Our previous study [2] has demonstrated the utility of a canine model of pulpectomy for pulp regeneration, which is similar to that in humans in various aspects including tooth morphology, biology, and physiology, harnessing autologous pulp-derived CD105+ stem cells with SDF-1. Thus, in this preclinical study, clinical-grade canine pulp stem cells were isolated by our novel method using G-CSF-induced chemotaxis through a chemically embellished membrane, and the biological characteristics of the pulp stem cells that contribute to the therapeutic effects on angiogenesis/vasculogenesis, neurogenesis, and pulp regeneration were elucidated. The results demonstrate the following three critical criteria: (a) high migratory activity in order to migrate to the apical part before vascularization in the pulpectomized root canal; (b) high expression of multiple trophic factors in order to stimulate migration and proliferation of endogenous stem/progenitor cells from adjacent tissues and vessels, to enhance angiogenesis and reinnervation, and to inhibit apoptosis; and (c) high immunomosuppressive and immunomodulatory properties. The pulp stem cells are more advantageous than the unfractionated total pulp cells, resulting in significantly less inflammation and apoptosis, significantly larger volume of regenerated pulp tissue, and higher density of angiogenesis and neurogenesis in vivo.
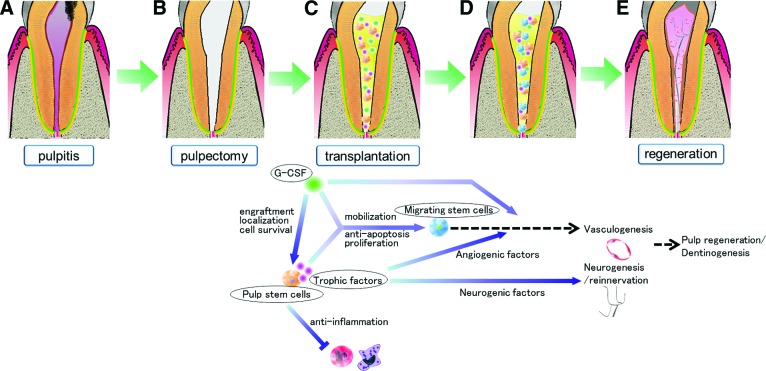
G-CSF has been approved by the Pharmaceuticals and Medical Devices Agency, Japan, and the U.S. Food and Drug Administration for decreasing the incidence of infection. G-CSF has been used extensively for several decades to mobilize CD34+ hematopoietic stem cells in neutropenic patients for reconstitution of bone marrow [13, 14]. It has been shown to be safe with only a few well-described side effects. G-CSF has a therapeutic potential in neuroprotective action attributed to its anti-inflammatory and antiapoptotic effects and regenerative activity induced by neurogenesis and angiogenesis [15] in experimental stroke models [16–18], retinal ganglion cell axotomy models [19], and spinal cord injury models [20, 21]. G-CSF is also critically involved not only in mobilizing bone marrow-derived stem cells into peripheral blood but also in functional recovery, cardiac muscle regeneration, acceleration of healing, and direct protection for cardiomyocytes in acute myocardial infarction [22–25]. Beneficial effects of local application of G-CSF on recovery of blood flow and an increase in the number of capillaries have been shown in ischemic hind limb models for peripheral vascular disease, indicating its direct enhancement of endothelial cell migration and vessel formation [26]. Treatment of stroke patients with G-CSF also results in a positive functional and potentially structural effect [27–29]. Recent experimental studies on combined strategies (i.e., transplantation of neural stem cells, bone marrow stromal cells, or amniotic fluid mesenchymal stem cells together with local application of G-CSF) have shown augmentation of peripheral nerve regeneration [30], spinal cord regeneration [6, 7], and cerebral ischemia recovery [31], suggesting that the positive effect is attributed to increased proliferation of progenitor cells, suppression of apoptotic death of transplanted stem cells, attenuation of inflammatory response, and induction of extrinsic and endogenous neurogenesis. The safety and efficacy of the use of G-CSF with autologous bone marrow stem/progenitor cells for improvement of cardiac function and symptoms in heart failure patients have recently been investigated in a clinical trial [32]. In the present in vitro study, G-CSF showed stimulatory effects on migration, antiapoptosis, and proliferation in pulp and bone marrow stem cell culture and on immunosuppression and neurite outgrowth. These results are consistent with some reports showing neuroprotective effects in vitro attributed to an anti-inflammatory effect, an antiapoptotic effect on neurons partially mediated by the phosphatidylinositol 3-kinase/Akt potential pathway, and induced differentiation of neuronal stem cells [33, 34]. The stimulatory effect of G-CSF with secretory factors from pulp stem cells on these properties was also elucidated in pulp and bone marrow stem cell culture. It was further demonstrated in pulp stem cell transplantation that pulp regeneration was enhanced by G-CSF. This may be due to greater localization of transplanted pulp stem cells in the root canal without migration to tissue surrounding the tooth, greater migration of G-CSFR-positive cells, less inflammation, lower apoptosis, higher density of vascularization, and longer extension of neurites. The transplanted pulp stem cells expressed neurotrophic factors, including NGF, GDNF, and BDNF, in the regenerated pulp tissue. Neurotrophic factors (NGF in particular but also GDNF) provided by pulp stem cells are the main factors responsible for the innervation of pulp [35]. BDNF influences the terminal arborization pattern, the biochemical phenotype of neurons, and/or the survival of some subpopulations of trigeminal ganglion neurons that are engaged [35]. These results indicated endogenous effects of trophic factors from the transplanted pulp stem cells and exogenous effects of transplanted G-CSF on pulp regeneration, including creation of a favorable environment for migration of stem cells, inhibition of apoptosis and promotion of cell survival, suppression of inflammation, and induction of angiogenesis and neurogenesis (schematic diagrams are shown in Fig. 6).
Figure 6.
Schematic diagrams demonstrating the mechanism of pulp regeneration after transplantation of pulp stem cells with G-CSF in a canine pulpitis model in permanent mature teeth. (A): Pulpitis model. (B): Whole pulp removal and enlargement of apical foramen, 0.6 mm in width. (C): Irrigation and filling with pulp stem cells and G-CSF together with collagen scaffold. (D): Combinatorial effect of pulp stem cells and G-CSF. (E): Complete pulp regeneration. Abbreviation: G-CSF, granulocyte colony-stimulating factor.
The critical challenge and ultimate goal for pulp/dentin regeneration is functional recovery of the tooth. Innervation of the pulp has a critical role in the homeostasis of dental pulp. The pulp defense mechanism in which immune and inflammatory cells invade sites of injury in the pulp is stimulated by sensory nerves [36]. Sensory denervation results in rapid necrosis of pulp because of impaired blood flow and extravasation of immune cells [37, 38]. Reinnervation/neurogenesis leads to recovery in coronal dentin [36]. The results of the present study showing a positive reaction in electric pulp testing and DiI-labeled nerves extending to the trigeminal ganglion indicated that regenerated tissue can transmit sensory signals perceived as pain, the sensory nerves of which are derived from the trigeminal ganglion, including nociceptive axons. This reinnervation/neurogenesis might contribute to angiogenesis, extravasation of immune cells, and regulation of inflammation to minimize initial damage, maintain pulp tissue, and strengthen pulpal defense mechanisms [39]. The present study demonstrated the recovery of blood flow in the regenerated pulp analyzed by a laser Doppler blood flowmeter at 2 months after transplantation to the same level as that in normal pulp. This vascularization may play an important role in regulation of inflammation and subsequent regeneration of pulp and dentin [39]. An intimate association of the neural elements with vascular supply in the regenerated pulp as in normal pulp [40] suggested interplay of neural and vascular elements and involvement in pulp homeostasis in the regenerated pulp. Furthermore, the present findings of obliteration of the enlarged apical portion following pulpectomy and lateral dentin formation indicated an advantage for preventing tooth fracture. Thick coronal dentin formation with mechanical integrity of the native dentin-pulp complex also prevents coronal microleakage leading to secondary caries. Thus, this complete regeneration of pulp tissue can possibly prolong the life of teeth.
The production of clinical-grade pulp stem cells identified stable quality; more than 95% viability at release; positive expression of CD29, CD44, CD73, CD90, and CD105 cell surface markers and negative expression of the CD31 cell surface marker; and microbiological safety. Potential side effects linked to genomic instability driving transformation and senescence or decrease in cell function were not detected at the 30th passage of human pulp stem cell culture [41]. There was little change in cell phenotype, biological characteristics, or properties that contributed to the therapeutic effects after prolonged ex vivo culture, indicating stability of the pulp stem cells, consistent with results of other studies showing that MSCs are not prone to genetic instability and do not easily transform during the normal culture process [42]. To examine the safety of human MSCs from tumorigenicity in the cell-based therapy, we have to discriminate between cells that have the capacity to form tumors and cells that do not. In this study, canine pulp stem cells were xenogenously transplanted into NOD/SCID mice [43], in which T and B cells are absent and NK cell activity is reduced, resulting in no tumor formation in the mice. However, residual NK cell activity might interfere with engraftment efficiency for transplanted human cells in the NOD/SCID mice [44, 45]. Recently, NOD/Shi-scid IL2Rgnull (NOG) mice (T, B, and NK cell-defective) have been developed that have a higher susceptibility to xenotransplanted tumors [46]. In this study, however, canine pulp stem cells were further autologously transplanted in the emptied root canals to evaluate their safety in tumorigenicity as a more sensitive assay. It is noteworthy that no tumors were formed in any of the tissues or organs for the 3-month duration of the study in dogs by toxicological and histopathological analyses. This condition is the same as that of our recently proposed clinical trial in humans. Based on these results of preclinical trials, scientific evidence of the safety of pulp stem cell therapy for pulp regeneration has been presented before clinical application.
Conclusion
This investigation demonstrates the safety of clinical-grade pulp stem cells and their potential for pulp and dentin regeneration when used with G-CSF and collagen scaffolds in a canine pulpectomy model. The current study establishes that preclinical efficacy and safety evaluation has elucidated versatile combinatorial functions of pulp stem cells with G-CSF to induce angiogenesis, neurogenesis, and pulp regeneration. These advances will provide a framework for further standardization and implementation of regulatory rules for emerging stem cell therapies in clinical and translational endodontics.
Supplementary Material
Acknowledgments
We thank Masaaki Shimagaki from Toray Industry Inc. for supplying the chemically embellished transmembrane. We also thank Dr. Toyoda Masashi (Tokyo Metropolitan Institute of Gerontology), Dr. Akifumi Matsuyama (Foundation for Biomedical Research and Innovation), and Dr. Hiroki Tanabe from Asahikawa Medical College for establishment of the standard operational procedure and Dr. Yasushi Satoh (National Defense Medical College) for technical assistance. This work was supported by the Budget for Promoting Science and Technology in Japan, directly following the policy of the Council for Science and Technology Policy chaired by the Prime Minister (M.N.) and the Research Grant for Longevity Sciences (23-10) from the Ministry of Health, Labor and Welfare (M.N.).
Author Contributions
K.I.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; M.M., N.T., and Y.O.: collection of data, data analysis; M.I., R.I., and S.U.: collection of data; H.N. and K.M.: provision of study material; M.N.: conception and design, financial support, provision of study materials, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Ishizaka R, Iohara K, Murakami M, et al. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–2118. doi: 10.1016/j.biomaterials.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 2.Iohara K, Imabayashi K, Ishizaka R, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima M, Iohara K, Yamada K, et al., inventors. National Center for Geriatrics and Gerontology, Japan, assignee. Membrane-separation-type culture device, membrane-separation-type culture kit, stem cell separation method using same, and separation membrane. Japan patent WO2012133803 A1. PCT/JP2012/058637. 2012 Oct 4;
- 4.Iohara K, Zheng L, Wake H, et al. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells. 2008;26:2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 5.Ponte AL, Ribeiro-Fleury T, Chabot V, et al. Granulocyte-colony-stimulating factor stimulation of bone marrow mesenchymal stromal cells promotes CD34+ cell migration via a matrix metalloproteinase-2-dependent mechanism. Stem Cells Dev. 2012;21:3162–3172. doi: 10.1089/scd.2012.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan HC, Cheng FC, Lai SZ, et al. Enhanced regeneration in spinal cord injury by concomitant treatment with granulocyte colony-stimulating factor and neuronal stem cells. J Clin Neurosci. 2008;15:656–664. doi: 10.1016/j.jocn.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Zhang HT, Jiang XD, et al. Combination of bone marrow stromal cell transplantation with mobilization by granulocyte-colony stimulating factor promotes functional recovery after spinal cord transection. Acta Neurochir (Wien) 2009;151:1483–1492. doi: 10.1007/s00701-009-0402-6. [DOI] [PubMed] [Google Scholar]
- 8.Pan HC, Yang DY, Ho SP, et al. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, natto. J Biomed Sci. 2009;16:75–87. doi: 10.1186/1423-0127-16-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19–28. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iohara K, Zheng L, Ito M, et al. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 11.Tögel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Phisiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 13.Begley CG, Lopez AF, Nicola NA, et al. Purified colony-stimulating factors enhance the survival of humanneutrophils and eosinophils in vitro: A rapid and sensitive microassay for colony-stimulating factors. Blood. 1986;68:162–166. [PubMed] [Google Scholar]
- 14.Metcalf D. The colony stimulating factors. Discovery, development, and clinical applications. Cancer. 1990;65:2185–2195. doi: 10.1002/1097-0142(19900515)65:10<2185::aid-cncr2820651005>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Solaroglu I, Cahill J, Jadhav V, et al. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- 16.Schäbitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson's disease: Focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord. 2003;18:408–414. doi: 10.1002/mds.10385. [DOI] [PubMed] [Google Scholar]
- 17.Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 18.Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- 19.Frank T, Schlachetzki JC, Göricke B, et al. Both systemic and local application of granulocyte-colony stimulating f actor (G-CSF) is neuroprotective after retinal ganglion cell axotomy. BMC Neurosci. 2009;10:49–59. doi: 10.1186/1471-2202-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitzer C, Klussmann S, Krüger C, et al. The hematopoietic factor granulocyte-colony stimulating factor improves outcome in experimental spinal cord injury. J Neurochem. 2010;113:930–942. doi: 10.1111/j.1471-4159.2010.06659.x. [DOI] [PubMed] [Google Scholar]
- 21.Dittgen T, Pitzer C, Plaas C, et al. Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS One. 2012;7:e29880. doi: 10.1371/journal.pone.0029880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minatoguchi S, Takemura G, Chen XH, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuka M, Takano H, Zou Y, et al. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–853. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 24.Harada M, Kumemura H, Yanagimoto C, et al. Vascular endothelial growth factor is involved in angioedema associated with eosinophilia. Kurume Med J. 2005;52:89–91. doi: 10.2739/kurumemedj.52.89. [DOI] [PubMed] [Google Scholar]
- 25.Kuethe F, Krack A, Fritzenwanger M, et al. Treatment with granulocyte-colony stimulating factor in patients with acute myocardial infarction. Evidence for a stimulation of neovascularization and improvement of myocardial perfusion. Pharmazie. 2006;61:957–961. [PubMed] [Google Scholar]
- 26.Lee M, Aoki M, Kondo T, et al. Therapeutic angiogenesis with intramuscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler Thromb Vasc Biol. 2005;25:2535–2541. doi: 10.1161/01.ATV.0000190609.28293.17. [DOI] [PubMed] [Google Scholar]
- 27.Schäbitz WR, Schneider A. Developing granulocyte-colony stimulating factor for the treatment of stroke: Current status of clinical trials. Stroke. 2006;37:1654. doi: 10.1161/01.STR.0000227299.62106.0e. [DOI] [PubMed] [Google Scholar]
- 28.Shyu WC, Lin SZ, Lee CC, et al. Granulocyte colony-stimulating factor for acute ischemic stroke: A randomized controlled trial. CMAJ. 2006;174:927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boy S, Sauerbruch S, Kraemer M, et al. Mobilisation of hematopoietic CD34+ precursor cells in patients with acute stroke is safe: Results of an open-labeled nonrandomized phase I/II trial. PLoS One. 2011;6:e23099. doi: 10.1371/journal.pone.0023099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan HC, Wu HT, Cheng FC, et al. Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem Biophys Res Commun. 2009;382:177–182. doi: 10.1016/j.bbrc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XM, Du F, Yang D, et al. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC Neurosci. 2011;12:61–70. doi: 10.1186/1471-2202-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo C, Saunders N, Locca D, et al. Ficoll-Paque versus Lymphoprep: A comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen Med. 2009;4:689–696. doi: 10.2217/rme.09.44. [DOI] [PubMed] [Google Scholar]
- 33.Schneider A, Krüger C, Steigleder T, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solaroglu I, Tsubokawa T, Cahill, et al. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried K, Lillesaar C, Sime W, et al. Target finding of pain nerve fibers: Neural growth mechanisms in the tooth pulp. Physiol Behav. 2007;92:40–45. doi: 10.1016/j.physbeh.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Fristad I, Kvinnsland IH, Jonsson R, et al. Effect of intermittent long-lasting electrical tooth stimulation on pulpal blood flow and immunocompetent cells: A hemodynamic and immunohistochemical study in young rat molars. Exp Neurol. 1997;146:230–239. doi: 10.1006/exnr.1997.6523. [DOI] [PubMed] [Google Scholar]
- 37.Byers MR, Taylor PE. Effect of sensory denervation on the response of rat molar pulp to exposure injury. J Dent Res. 1993;72:613–618. doi: 10.1177/00220345930720031001. [DOI] [PubMed] [Google Scholar]
- 38.Olgart L, Kostouros GD, Edwall L. Local actions of acetylcholine on vasomotor regulation in rat incisor pulp. Acta Physiol Scand. 1996;158:311–316. doi: 10.1046/j.1365-201X.1996.69319000.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 40.Iijima T, Zhang JQ. Three-dimensional wall structure and the innervation of dental pulp blood vessels. Microsc Res Tech. 2002;56:32–41. doi: 10.1002/jemt.10007. [DOI] [PubMed] [Google Scholar]
- 41.Iohara K, Murakami M, Takei Y, et al. Quality assurance of clinical grade pulp stem cells manufactured in GMP-compliant facility. Jpn J Conser Dent. 2013;56:121–129. [Google Scholar]
- 42.Philippe B, Luc S, Valérie PB, et al. Culture and use of mesenchymal stromal cells in Phase I and II clinical trials. Stem Cells Int. 2010;2010:503–511. doi: 10.4061/2010/503593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 44.Tanaka Y, Koyanagi Y, Tanaka R, et al. Productive and lytic infection of human CD4+ type 1 helper T cells with macrophage-tropic human immunodeficiency virus type 1. J Virol. 1997;71:465–470. doi: 10.1128/jvi.71.1.465-470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshino H, Ueda T, Kawahata M, et al. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 2000;26:1211–1216. doi: 10.1038/sj.bmt.1702702. [DOI] [PubMed] [Google Scholar]
- 46.Machida K, Suemizu H, Kawai K, et al. Higher susceptibility of NOG mice to xenotransplanted tumors. J Toxicol Sci. 2009;34:123–127. doi: 10.2131/jts.34.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.