Abstract
These studies illustrate synthetic paths to covalently attach T1 and φ11 bacteriophages (phages) to inert polymeric surfaces while maintaining bacteriophage’s biological activities capable of killing deadly human pathogens. The first step involved formation of acid (COOH) groups on polyethylene (PE) and polytetrafluoroethylene (PTFE) surfaces using microwave plasma reactions in the presence of maleic anhydride, followed by covalent attachment of T1 and φ11 species via primary amine groups. The phages effectively retain their biological activity manifested by a rapid infection with their own DNA and effective destruction of Escherichia coli and Staphylococcus aureus human pathogens. These studies show that simultaneous covalent attachment of two biologically active phages effectively destroy both bacterial colonies and eliminate biofilm formation, thus offering an opportunity for an effective combat against multi-bacterial colonies as well as surface detections of other pathogens.
The majority of interactions between biologically active species and synthetic materials are inherently non-favorable. However, formation of biofilms represent an important and unwelcome exception resulting from the attachment of bacteria to a synthetic surface, leading to the formation of a complex biofilm community that is often encased on a surface of polymeric materials in contact with blood. Microbial biofilms are very resilient communities that resist removal by chemical or physical means because their residents, bacterial cells, are capable of adhering to a variety of biotic and abiotic surfaces - and continue to grow biofilms as long as the nutrients become available. In the context of human health, biofilm are responsible for the vast majority of deadly infections including medical-device associated diseases. Because they often become resistant to antibiotics or host defenses, the use of antibiotics may be uneffective to many pathogens, making conventional therapies troublesome.1
In view of these considerations, new approaches are needed to address infections on various fronts, but in particular surface medical-device associated infections. They may include simple catheters, implants, stents, monitoring devices, to name just a few. The first logical step has led to the development of novel drugs, but surface modifications with drugs bring another level of challenges associated with their attachment, long-term effectiveness, and maintenance. Among anti-biofilm formation strategies, covalent attachments of antibiotics or antimicrobial agents to polymeric surfaces have been somewhat successful with relatively longer durations, but still long-term activities might be limited.2,3 Ideally, one would like to create stimuli-responsive attributes on polymeric surfaces, where a surface remains silent unless external stimulus triggers desirable responses. In essence, the goal is to prevent biofilm formation from developing at its inception.
An alternative approach of fighting microbial wars on polymeric or other surfaces is to utilize bacterial viruses (or bacteriophages) that attach specifically to their target host bacteria, inject their genetic material, reproduce inside the host, kill the host, and release their progeny. Although the first observations of lytic phages to cure infectious diseases go back to the end of the19th 4 and early 20th Centuries,5–7 it was not until 1960s, where prophylaxis and treatment of bacterial infections appeared favorable choice with a remarkable recovery efficacy above 92%.8
Later on, the use of bacteriophages was exploited by physical mixing with or physisorbed on polymers (Nylon™),9, 10 glass,11 and gold.12 Although several reports claimed that surface plasmon resonance (SPR) measurements are capable of measuring covalent attachment of bacteriophages, proteins, and other bioactive macromolecules to gold surfaces, the increase of the refractive index and subsequent increase of the surface plasmon waves in SPR does not justify covalent attachment. These data showed that the thickness of deposited layers increases, thus drawing conclusions regarding the type of bonding is premature. The unique attributes of bacteriophages are their host-dependent reproduction - bacteriophages will remain silent until they find a specific bacterium, thus minimizing safety concerns associated with excessive concentration levels. Furthermore, due to evolution with their hosts and host-specificity, there are numerous bacteriophages for each bacterium.
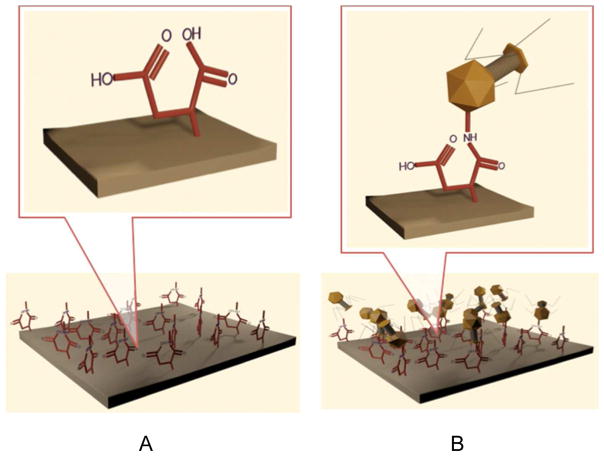
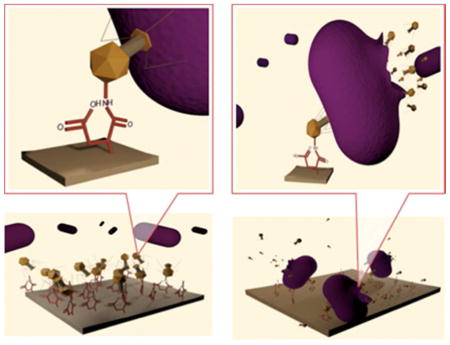
Taking advantage of the ability of bacteriophages to recognize a host bacterium and their ability to kill specific hosts, we covalently attached T1 and φ11 phages onto surfaces of polytetrafluoroethylene (PTFE) and ultra high molecular weight polyethylene (PE). The premise behind this approach is the covalent anchoring the bacteriophages to polymer surfaces while maintaining their biological activity. Figure 1, A–D, depicts a sequence of steps leading to the formation and subsequent destruction of the bacteria attempting to form biofilms on the phage-modified polymeric substrates. The first step (Figure 1, A) involves the formation of reactive acid groups on polymeric substrates3 accomplished by simple and clean microwave plasma reactions in the presence of maleic anhydride, followed by covalent attachment of T1 phages via acid-amine reactions leading to amide linkages. Supporting Documents provide spectroscopic evidence for these reactions.
Figure 1.
A: Covalent attachment of acid groups to polymeric surfaces; B: Reactions of NH2 groups of T1 with polymer surface acid groups.
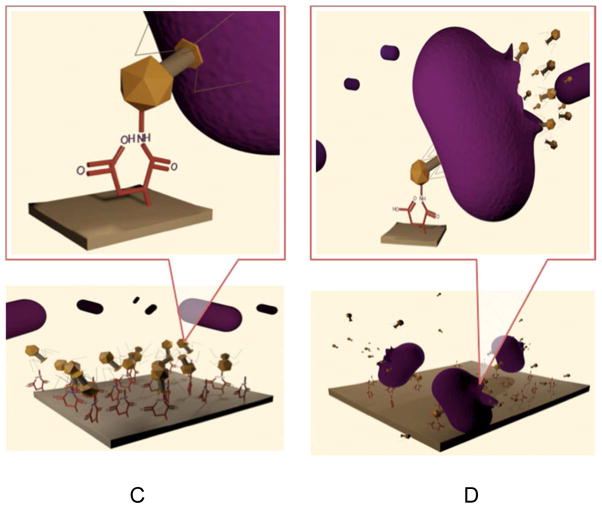
C: Attachment of phages to bacteria and injection of DNA; D: Replication of DNA and destruction of bacteria.
When bacteria attempt to adhere to the surface of the phage-modified polymer substrate, the phage attaches specifically to an external structure of the bacterium (e.g. lipopolysaccharide or protein) and injects its genetic material into its target (Figure 1, C). Upon completion of this process, the phage DNA is replicated by the bacterial host machinery, several capsid proteins are produced and assembled, phage DNA is packaged and the bacteriophage progeny are released by lysis of the bacterial host. Depending on the particular bacteriophage, the progeny can be up to 200 for each individual infection. Perhaps the most significant advantage of using bacteriophages comes from the fact that each member of the progeny is capable of infecting more bacteria and releasing a progeny of its own. This amplification effect continues until all bacteria cells are killed. Of course, one can envision potential challenges associated with the control of the bacteriophage population, especially when utilized in therapy, which needs to be carefully adjusted.
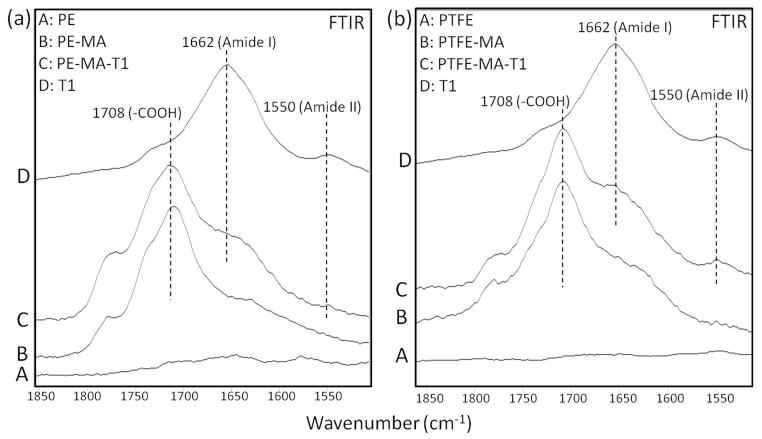
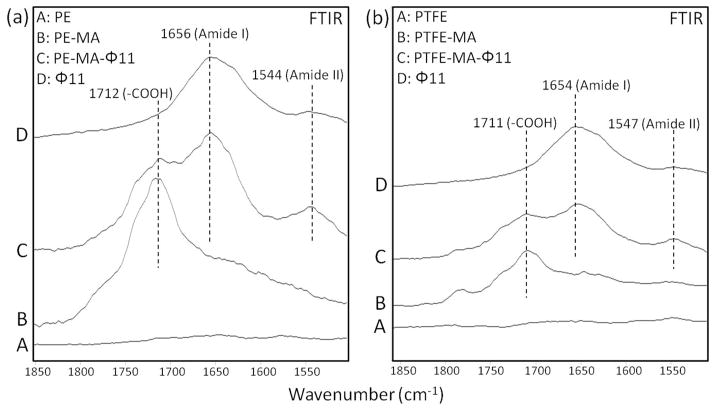
While Supporting Documents provide specifics related to the T1 and φ11 phages preparation, verification of chemical reactions leading to T1 and φ11 covalent attachments are shown in Figures 2 and 3, respectively. Traces A, B, C, and D of Figures 2 and 3 illustrate ATR-FTIR spectra recorded from PE (a) and PTFE (b) surfaces, respectfully. Traces B represent the spectra of maleic anhydride plasma modified PE-MA and PTFE-MA surfaces. When T1 or φ11 phages are reacted to PE-MA and PTFE-MA, the spectra shown in Traces C are obtained. As seen, two characteristic bands at 1662 and 1550 cm−1 due to Amide I and II bands characteristics of the T1 and φ11 outer functionalities are detected in Figure 2, which are due to covalent attachment of bacteriophage T1 to acid-functionalized PE as well as PTFE surfaces. In the same manner, Figure 3, (a) and (b), illustrates ATR-FTIR spectra of PE (a) and PTFE (b) surfaces, with two bands at 1655 and 1545 cm−1 due to Amide I and II bands characteristic of φ11 functionalities. For comparison, Traces D in Figures 2 and 3, (a) and (b), illustrate ATR-FTIR spectra of T1 and φ11.
Figure 2.
A: ATR-FTIR spectra of PE (a) and PTFE (b) surfaces; B: after plasma reactions on PE and PTFE surfaces in the presence of maleic anhydride; C: after T1 phage covalent attachment to MA modified surfaces; D: Reference spectrum of T1 phage (at 20% scale).
Figure 3.
A: ATR-FTIR spectra of PE (a) and PTFE (b) surfaces; B: after plasma reactions on PE and PTFE surfaces in the presence of maleic anhydride; C: after φ11 phage covalent attachment to MA modified surface; D: Reference spectrum of φ11 phage (at 20% scale).
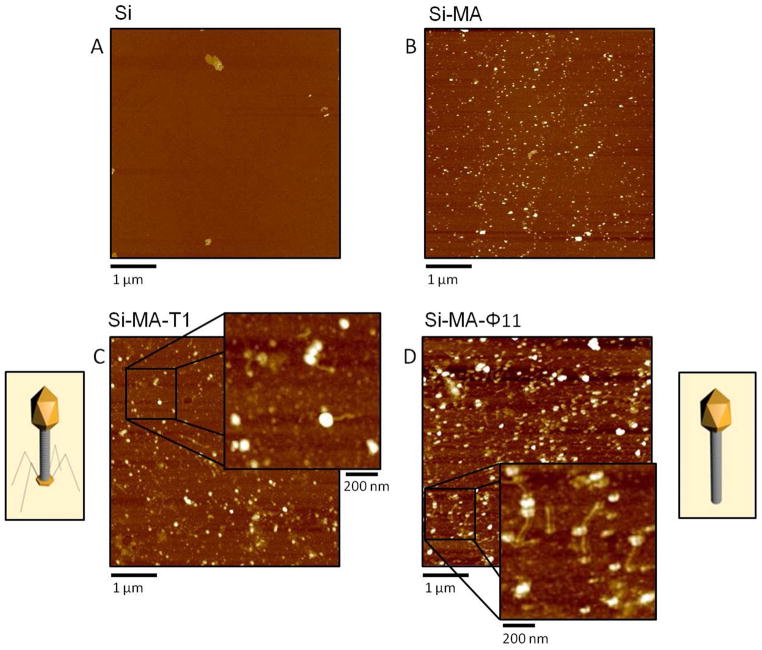
In order to visualize the presence of phages on surfaces we utilized atomic force microscopy (AFM). Since polymer surface roughness profiles quantitatively expressed as root mean squared (RMS)*are typically in the range RMS = 20–80, it is more convenient to utilize smooth surfaces, such as Si (RMS<1). For that reason the surface reactions were employed on Si surfaces and the results of these experiments summarized in the Supporting Documents confirm again covalent attachment of T1 and φ11 phages. To visually assess the presence of T1 and φ11 phages on surfaces, AFM images were collected after each step, and Figure 4, A, B, C and D illustrate the height images collected from Si wafer surfaces after each step. Figure 4, A depicts the heights image of the Si wafer surface, while B illustrates the height image after covalent attachment of maleic anhydride via microwave plasma. Figure 4, C and D confirms the presence of T1 and φ11 phages on surfaces, respectfully, while inserts of C and D visually illustrate shapes corresponding to T1 and φ11 morphologies and size. Using AFM data shown in Figure 4, we quantified the mean number of phages per μm2 using image analysis. The mean average number of T1 and φ11 phages per μm2 is 5.8 ± 1.7 and 10.8 ± 1.0, respectively.
Figure 4.
A: AFM height image of Si wafer surface; B: Height image of Si-MA surface; C: AFM height image of T1 bacteriophages attached to Si-MA surfaces; D: Height image of Si-MA surfaces with covalently attached φ11 phages.
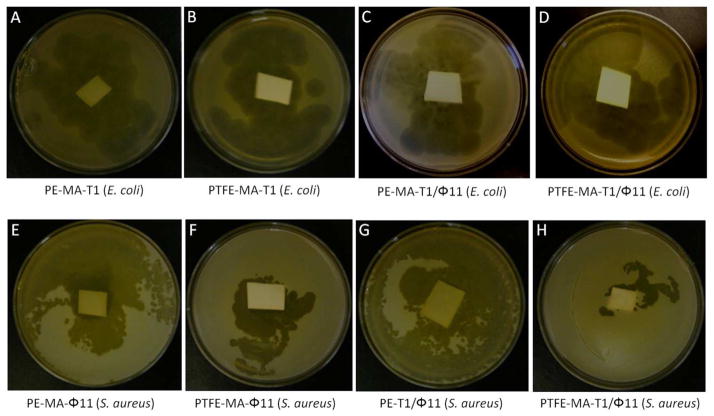
The antimicrobial effectiveness of T1 and φ11 phages covalently attached to PTFE and PE surfaces against E. coli and S. aureus was confirmed using plaque formation assays. As shown in Figure 5, A and B, PE and PTFE surfaces with covalently attached T1 phages kill E. coli bacteria, as manifested by the clear zone around each substrate. Similarly, Figure 5, E and F, show the same PE and PTFE surfaces with covalently attached φ11 phages that kill S. aureus bacteria, which are also detected as the clear zone surrounding each substrate. To achieve dual antimicrobial protection, a 1:1 mixture of T1 and φ11 phages were covalently affixed to PE and PTFE surfaces. The efficacy of these surfaces against bacteria are illustrated in Figure 5, C and D, for PE and PTFE with T1 and φ11 phages against E. coli, as well as Figure 5, G and H, for the same surfaces against S. aureus bacteria. As shown, the effectiveness of T1 and φ11 phages covalently attached to PE and PTFE surfaces is apparent. The effectiveness of T1 and Phi11 phages covalently attached to PE and PTFE surfaces is manifested by the formation of plaques on and around be polymer specimen surfaces that exhibit “clear zone” appearance, indicating destroyed microbial films. It should be also noted that although the objective of these studies was not to determine the longevity of phage-modified surfaces, these experiments indicated that the surfaces retain their continuous phage activity for several months as long as surfaces are exposed to high humidity or aqueous environment conditions.
Figure 5.
Plaque formation assays for covalently attached T1 phages on A: PE and B: PTFE surfaces against E. coli; C: PE and D: PTFE with covalently attached 1:1 mixture of T1 and φ11 phages against E. coli. Plaque formation assays for covalently attached φ11 phages on E: PE and F: PTFE surfaces against S. aureus; G: PE and H: PTFE with covalently attached 1:1 mixture of T1 and φ11 phages against S. aureus.
Summary
These studies established a universal approach of covalent attachment of bacteriophages to inert PE and PTFE polymeric surfaces. The reactions can be conducted on almost any surface and, although the limiting factor can be the maintenance of biological activity of phages, these studies show that covalent attachment of T1 and φ11 does not adversely affect the phages biological activities. Multiple venues for polymer surface modifications combined with over a thousand individual phage species, with hundreds of different strengths, provide endless possibilities of creating surfaces capable of killing specific bacteria. Although several synthetic approaches to combat biofilm formation were exploited, the use of bacteriophages to kill human pathogens anchored to synthetic surfaces show a promising and effective means of combating antibiotic-resistant infections.
Supplementary Material
Acknowledgments
The authors (HAP and MWU) thank the National Science Foundation for partial support of these studies. GSS and MOE acknowledge the support by the Mississippi INBRE (P20RR016476) funded by the National Institute of General Medical Sciences, National Institutes of Health.
Footnotes
RMS=√(1/nΣyi2; where n represents the number of equally spaced points along the trace, and yi is the vertical distance from the mean ith data point.
Supporting Information Available. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Webb GF, D’Agata EMC, Magal P, Ruan S. A model of antibiotic-resistant bacterial epidemics in hospitals. PNAS. 2005;102:13343. doi: 10.1073/pnas.0504053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aumsuwan N, Heinhorst S, Urban MW. Antibacterial surfaces on expanded polytetrafluoroethylene; penicillin attachment. Biomacromolecules. 2007;8:713. doi: 10.1021/bm061050k. [DOI] [PubMed] [Google Scholar]
- 3.Gaboury SR, Urban MW. Microwave plasma reactions of solid monomers with silicone elastomer surfaces: a spectroscopic study. Langmuir. 1993;9:3225. [Google Scholar]
- 4.Hankin EH. L’action bactericide des eaux de la Jumma et du Gange sur le vibrion du cholera. Ann Inst Pasteur. 1896;10:511. [Google Scholar]
- 5.D’Herelle F. Sur un microbe invisible antagoniste des bacilles dysenteriques. C R Acad Sci. 1917;165:373. [Google Scholar]
- 6.Twort FW. An investigation on the nature of ultramicroscopic viruses. Lancet. 1915:ii, 1241. [Google Scholar]
- 7.Stone R. Stalin’s forgotten cure. Science. 2002;298:728. doi: 10.1126/science.298.5594.728. [DOI] [PubMed] [Google Scholar]
- 8.Sulakvelidze A, Alavidze Z, Morris JGJ. Bacteriophage therapy. Antimicrobial Agents and Chemotherapy. 2001;45:649. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chkhaidze JD, Imedashvili NE. The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin Exp Dermatol. 2005;30:23. doi: 10.1111/j.1365-2230.2004.01600.x. [DOI] [PubMed] [Google Scholar]
- 10.Markoishvili K, Tsitlanadze G, Katsarava R, Morris JGJ, Sulakvalidze A. A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promice in management in infected venous stasis ulcers and other poorlt healing woulds. Int J Dermatol. 2002;41:453. doi: 10.1046/j.1365-4362.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinidoust Z, Van de Ven TGM, Tufenkji N. Bacterial capture efficiency and antimicrobial activity of phage-functionalized model surfaces. Langmuir. 2011;27:5472. doi: 10.1021/la200102z. [DOI] [PubMed] [Google Scholar]
- 12.Yang Li-Mei C, Tam Phillip Y, Murray Benjamin J, McIntire Theresa M, Overstreet Cathie M, Weiss Gregory A, Penner RM. Virus Electrodes for Universal Biodetection. Analytical Chemistry. 2006;78:3265. doi: 10.1021/ac052287u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.