Abstract
Limbic endocannabinoid signaling is known to be sensitive to chronic stress; however, studies investigating the impact of prolonged exposure to glucocorticoid hormones have been limited by the concurrent exposure to the stress of daily injections. The present study was designed to examine the effects of a noninvasive approach to alter plasma corticosterone (CORT) on the endocannabinoid system. More precisely, we explored the effects of a 4-week exposure to CORT dissolved in the drinking water of mice (100 μg/ml) and measured cannabinoid CB1 receptor binding, endocannabinoid content, activity of the endocannabinoid degrading enzyme free fatty acid amide hydrolase (FAAH), and mRNA expression of both the CB1 receptor and FAAH in both the hippocampus and amygdala. Our data demonstrate that CORT decreases CB1 receptor binding site density in both the hippocampus and amygdala and also reduced anandamide (AEA) content and increased FAAH activity within both structures. These changes in both CB1 receptor binding and FAAH activity were not accompanied by changes in mRNA expression of either the CB1 receptor or FAAH in either brain region. Interestingly, our CORT delivery regimen significantly increased 2-AG concentrations within the hippocampus, but not the amygdala. Collectively, these data demonstrate that the confounder of injection stress is sufficient to conceal the ability of protracted exposure to glucocorticoids to reduce CB1 receptor density and augment AEA metabolism within limbic structures.
Glucocorticoids are the final mediators of the hypothalamic pituitary adrenal (HPA) axis and play a crucial role in mounting the adaptive response to stress. Stress, defined as a state of threatened homeostasis following exposure to extrinsic or intrinsic adverse forces, mobilizes a vast number of physiologic and behavioral responses that constitute the adaptive stress response that aim to restore disturbed equilibrium (McEwen, 2007). The primary means of HPA axis regulation occurs through a well characterized negative feedback loop where glucocorticoids suppress ongoing HPA activity at the hypothalamic and pituitary levels; however, forebrain and hindbrain regions have shown to regulate the HPA axis under both basal and stress conditions (Herman et al., 2003; Pecoraro et al., 2006).
Accumulating evidence has demonstrated that glucocorticoids induce endocannabinoid signaling and, in turn, endocannabinoids regulate glucocorticoid secretion through both local and distal regulation of HPA axis activity (Hill and McEwen, 2010; Hill et al., 2010c; Steiner and Wotjak, 2008). The endocannabinoid system (ECS) was originally characterized as the neuronal system with which the psychoactive constituent of cannabis Δ9-tetrahydrocannabinol (THC) interacted to exert its effects on physiology and behavior. The system comprises of two subtypes: the cannabinoid type 1 (CB1) and type 2 (CB2) signaling receptors (Matsuda et al., 1990; Munro et al., 1993). The system also comprises the endogenous ligands of both receptors, anandamide (AEA) and 2-arachidonoylgycerol (2-AG), as well as the enzymes for ligand biosynthesis and degradation, such as the fatty acid amide hydrolase (FAAH) (Devane et al., 1992; Sugiura et al., 1995; Deutsch et al., 2002).
Interestingly, following conditions of chronic stress endocannabinoid signaling appears to breakdown, as chronic stress has been found to reduce both endocannabinoid content and receptor density throughout limbic structures (reviewed in Gorzalka et al., 2008). However, the extent to which glucocorticoids contribute to the effects of chronic stress on limbic endocannabinoid signaling is unclear. In rats it has been shown that chronic corticosterone injections recapitulate the effects of chronic stress on CB1 receptor expression in the hippocampus; however, no changes in endocannabinoid content were seen (Hill et al., 2008a). However, given the stressful nature of chronic injections, the possibility exists that injection stress could have occluded changes induced by corticosterone administration. As such, the aim of the current study was twofold: (1) to determine if noninvasive administration of glucocorticoids modulates limbic endocannabinoid in a similar fashion to chronic stress; (2) to extend the findings obtained in rats to mice.
EXPERIMENTAL PROCEDURES
Animals
Adult male mice (C57/BL6; 19–21 g at receiving) from Charles River Laboratories (Kingston, NY, USA) were group housed (n=5/cage) in standard cages (28.5×17×13 cm3), on a 12-h light, 12-h dark cycle (lights off at 1800 h). A 2 lux red light allowed for animal maintenance in the dark phase. Temperature in the room was maintained at 21±2 °C. Mice were allowed to acclimate to the facility for 7 days before the beginning of all experimentation. After the acclimation phase, water was replaced with a solution containing 100 μg/ml free corticosterone (CORT; Sigma, St. Louis, MO, USA) or 1% EtOH vehicle alone as previously described (Karatsoreos et al., 2010). Animals were weighed once a week during cage change, at which time solutions were replaced, and otherwise left undisturbed. After the 4-week CORT treatment, animals were killed by rapid decapitation, brains were removed, and the hippocampus and amygdala were dissected over dry ice and stored at −80 °C until analysis. Three separate cohorts of animals were generated, one for membrane preparation (FAAH assays and CB1 receptor binding), one for endocannabinoid analysis, and one for mRNA analysis. From the third group, trunk blood was collected in BD Vacutainer K3 EDTA-coated glass tubes (VWR, West Chester, PA, USA), placed on ice, and centrifuged at 1500 rpm for 15 min at 4 °C. Plasma was removed and stored at −80 °C until used for analyses. The Rockefeller University Institutional Animal Care and Use Committee approved all experimental procedures involving animals.
Membrane preparation
Membranes were collected from isolated brain regions by homogenization of frozen tissue in 10 volumes of TME buffer (50 mM Tris–HCl, pH 7.4; 1 mM EDTA, and 3 mM MgCl2). Homogenates were centrifuged at 18,000×g for 20 min, and the resulting pellet, which contains a crude membrane fraction, was resuspended in 10 volumes of TME buffer. Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA, USA).
CB1 receptor radioligand binding assay
CB1 receptor agonist binding parameters were determined through radioligand binding using a Multiscreen Filtration System with Durapore 1.2-μM filters (Millipore, Bedford, MA, USA). Incubations (total volume=0.2 ml) were carried out using TME buffer containing 1 mg/ml bovine serum albumin (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.1, 0.25, 0.5, 1.0, 1.5, or 2.5 nM [3H]-CP 55,940, a cannabinoid CB1 receptor agonist. Ten μM Δ9-tetrahydrocannabinol was used to determine nonspecific binding. The dissociation constant (KD) and binding site density (Bmax) values were determined by nonlinear curve fitting of specific binding data to the single site binding equation using GraphPad Prism (San Diego, CA, USA).
FAAH activity assay
FAAH activity was measured as the conversion of AEA labeled with [3H] in the ethanolamine portion of the molecule ([3H]AEA; Omeir et al., 1995) to [3H]ethanolamine as reported previously (Hillard et al., 1995). Membranes were incubated in a final volume of 0.5 ml of TME buffer (50 mM Tris–HCl, 3.0 mM MgCl2, and 1.0 mM EDTA, pH 7.4) containing 1.0 mg/ml fatty acid-free bovine serum albumin and 0.2 nM [3H]AEA. Isotherms were constructed using eight concentrations of AEA between 10 nM and 10 μM. Incubations were carried out at 37 °C and were stopped with the addition of 2 ml of chloroform/methanol (1:2). After standing at ambient temperature for 30 min, 0.67 ml of chloroform and 0.6 ml of water were added. Aqueous and organic phases were separated by centrifugation at 1000 rpm for 10 min. The amount of [3H] in 1 ml of the aqueous phase was determined by liquid scintillation counting, and the conversion of [3H]AEA to [3H]ethanolamine was calculated. The binding affinity of AEA for FAAH (Km) and maximal hydrolytic activity of FAAH (Vmax) values for this conversion were determined by fitting the data to the Michaelis–Menton equation using Prism.
Endocannabinoid extraction and analysis
Brain regions were subjected to a lipid extraction process as described previously (Hill et al., 2010a). Tissue samples were weighed and placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 84 pmol of [2H8]anandamide and 186 pmol of [2H8]2-AG. Tissue was homogenized with a glass rod and sonicated for 30 min. Samples were incubated overnight at −20 °C to precipitate proteins, then centrifuged at 1500×g to remove particulates. The supernatants were removed to a new glass tube and evaporated to dryness under N2 gas. The samples were resuspended in 300 μl of methanol to recapture any lipids adhering to the glass tube and dried again under N2 gas. Final lipid extracts were suspended in 20 μl of methanol and stored at −80 °C until analysis. The contents of the two primary endocannabinoids AEA and 2-AG within lipid extracts in methanol from brain tissue were determined using isotope-dilution, liquid chromatography-mass spectrometry as described previously (Patel et al., 2005a).
Quantitative (real-time) RT-PCR analysis
Dissected hippocampus and amygdala brain regions from control and CORT-treated mice were separately homogenized in 1 ml of QIAzol® (Qiagen, Valencia, CA, USA). Total RNA was extracted using an RNAeasy Mini Kit (Qiagen) according to manufacturer’s recommendations. DNAse digestion, to remove contaminating genomic DNA, was also completed using the kit and manufacturer’s protocol. After extraction and digestion, the purity and concentration of RNA were determined by Nano Drop 260/280 ratios (NanoDrop Technologies, Wilmington, DE, USA). All samples tested had a purity of 1.7–2.0. Two micrograms of total RNA, as evaluated by NanoDrop, was reverse transcribed using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) using the manufacturer’s recommendations. Target gene expression was quantified with gene-specific primers and Power SYBR Green master mix (ABI) using Applied Biosystems 7900HT Sequence Detection System at 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s. Each sample was done in triplicate, and each reaction was repeated at least once to ensure reproducibility. Raw threshold cycle (Ct) values were obtained from the Sequence Detection Systems 2.0 software (Applied Biosystems). An average cycle threshold value (Ct) was calculated from triplicate results for each gene. Threshold values were normalized to the housekeeping gene GAPDH to provide ΔCt values. Fold change for each gene were then calculated using the formula 2-ΔCt. The following primers were used: CB1R 5′-GGTTCTGATCCTGGTGGT-GTTGAT-3′ and 5′-CCGATGAGACAACAGACTTCT-3′; FAAH 5′-TGTGTGGTGGTGCAGGTACT-3′, 5′-CTGCACTGCTGTCTGTC-CAT-3′; and GAPDH, 5′-ATGACATCAAGAAGGTGGTG-3′, 5′-CATACCAGGAAATGAGCTTG-3′.
Corticosterone measurements
Plasma CORT concentrations were measured using a commercially available radioimmunoassay (MP Biomedicals, Inc., Solon, OH, USA). Samples were analyzed in duplicate and results are reported as ng/ml. The assay provided an intra-assay coefficient of variation of 8%, with a lower limit of detectability of 15.9 ng/ml.
Statistics
Biochemical analysis of the effects of corticosterone on various parameters of the endocannabinoid system was analyzed using independent t-tests. Significance for all tests was established at P<0.05. All data in the figures and tables are presented as mean values±standard error.
RESULTS
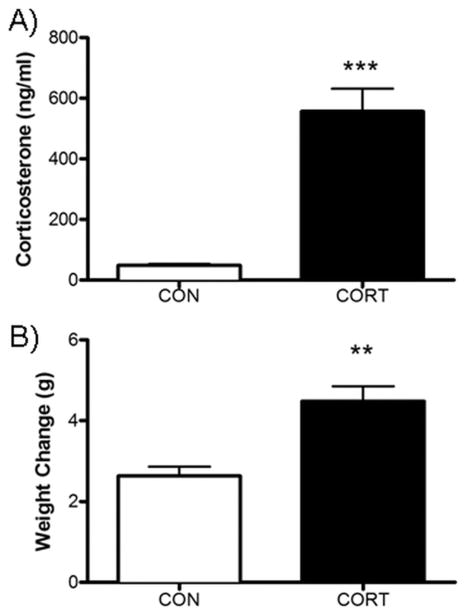
Chronic exposure to CORT in the drinking water resulted in a significant increase in the concentration of plasma corticosterone measured in blood obtained following 4 weeks of sustained CORT exposure [t(8)=6.705, P<0.01; Fig. 1A). These data are consistent with our previous report that demonstrates elevations in circulating CORT at all points of the circadian cycle with this dosage (Karatsoreos et al., 2010). Additionally, mice that were exposed to CORT exhibited an increase in body weight relative to control animals [t(6)=4.50, P<0.01; Fig. 1B), replicating our previous report that protracted exposure to CORT in the drinking water produces an obese phenotype (Karatsoreos et al., 2010).
Fig. 1.
Chronic CORT treatment (100 μg/ml) results in (A) 10-fold elevation of plasma CORT (blood samples during light phase) and (B) a significant increase in weight gain across the 28 d of CORT exposure. ** Signifies P<.005.
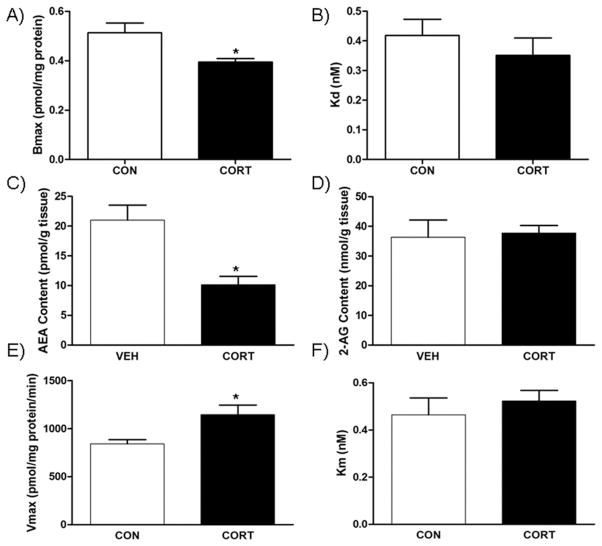
In the amygdala, chronic CORT treatment resulted in a decrease in the Bmax [t(4)=2.812, P<.05; Fig. 2A], but no significant difference in the KD [t(4)=0.84, P>.05; Fig. 2B] for [3H]-CP 55,940 compared with those exposed to vehicle alone. Chronic exposure to CORT significantly decreased amygdalar content of the endocannabinoid AEA [t(7)=3.641, P<.01; Fig. 2C]; however, there was no change in concentration of 2-AG in mice treated with CORT compared to those receiving vehicle [t(7)=0.3967, P>.05; Fig. 2D]. The fatty acid ethanolamides palmitoylethanolamine (PEA) [t(7)=2.191, P<.05; Table 1] and oleoylethanolamine (OEA) [t(7)=2.198, P<.05; Table 1] were also decreased in the amygdala of mice exposed to chronic CORT compared to vehicle. The Vmax for AEA hydrolysis by membranes isolated from the amygdala of mice exposed to chronic CORT was significantly increased compared to membranes from vehicle-exposed mice [t(4)=2.858, P<.05; Fig. 2E]. There was no significant difference in Km for AEA hydrolysis between the two groups [t(4)=0.6917, P>.05; Fig. 2F].
Fig. 2.
Effect of chronic corticosterone (CORT; 100 μg/ml) on endocannabinoid parameters in the amygdala. (A) The binding site density (Bmax.) of the CB1 receptor was reduced by CORT treatment; (B) however, there was no effect on the binding affinity (Kd) of the CB1 receptor. (C) CORT treatment reduced the tissue content of endocannabinoid anadamide (AEA), but (D) had no effect on the endocannabinoid 2-AG. (E) Consistent with the reduction in AEA content, CORT treatment increased the maximal hydrolytic activity (Vmax.) of the enzyme for AEA degradation fatty acid amide hydrolase (FAAH); (F) but had no effect on the binding affinity of AEA for FAAH. * Significantly different from control (P<.05), ** (P=.0024 and P=.008), *** (P=.0001) (n=7–8/group for endocannabinoid quantification; n=4 for CB1 receptor binding parameters and enzyme activity).
Table 1.
The effects of chronic corticosterone administration on the tissue content of palmitoylethanolamine and oleoylethanolamine in the amygdala and hippocampus
| Control | CORT | |
|---|---|---|
| Amygdala | ||
| PEA (pmol/g tissue) | 103.6±15.0 | 67.7±3.1* |
| OEA (pmol/g tissue) | 52.3±8.7 | 31.2±2.5* |
| Hippocampus | ||
| PEA (pmol/g tissue) | 161.4±14.1 | 111.9±5.5* |
| OEA (pmol/g tissue) | 93.5±6.1 | 49.6±2.8* |
Twenty-eight day administration of corticosterone (CORT) in the drinking water reduced the tissue content of the fatty acid ethanol-amide molecules palmitoylethanolamine (PEA) and oleoylethanolamine (OEA) within both the hippocampus and amygdala.
Significantly different from control (P<.05). Values denoted are means ± SEM. For all treatment conditions, n=7–10.
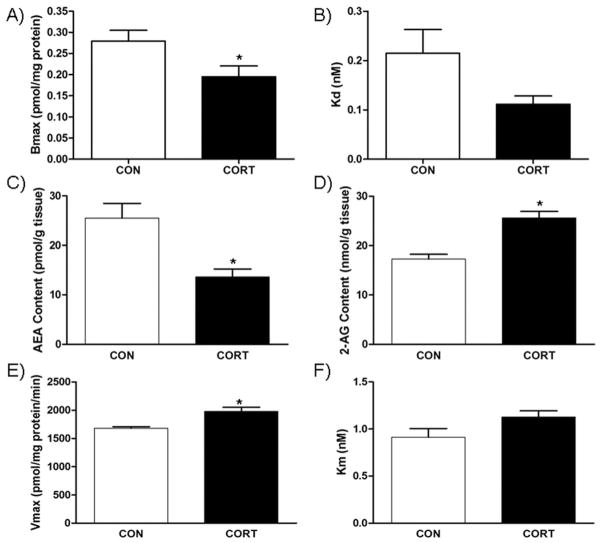
In the hippocampus, membranes isolated from mice treated with chronic CORT exhibited a significant decrease in [3H]-CP 55,940 Bmax [t(5)=2.336, P<.05; Fig. 3A], without a significant change in the KD [t(5)=2.031, P>.05; Fig. 3B] compared to the vehicle-treated mice. Chronic CORT treatment resulted in a significant reduction in AEA content [t(10)=3.534, P<.05; Fig. 3C], while there was a large increase in 2-AG content [t(10)=4.979, P<.0001; Fig. 3D] in the hippocampus. As with AEA, PEA [t(10)=3.264, P<.05; Table 1] and OEA [t(10)=6.611, P<.0001; Table 1] also show significant decrease in the hippocampus as a result of chronic CORT treatment. Consistent with reductions in NAEs in the hippocampus, there was an increase in the Vmax for AEA hydrolysis in hippocampal membranes from CORT-treated mice [t(4)=3.902, P<.05; Fig. 3E] and no change in Km [t(4)=1.905, P>.05; Fig. 3F] compared to vehicle-treated mice.
Fig. 3.
Effect of chronic corticosterone (CORT; 100 μg/ml) on endocannabinoid parameters in the hippocampus. (A) The binding site density (Bmax.) of the CB1 receptor was reduced by CORT treatment; (B) however, there was no effect on the binding affinity (Kd) of the CB1 receptor. (C) CORT treatment reduced the tissue content of endocannabinoid anadamide (AEA), but (D) increased the content of the other endocannabinoid 2-AG. (E) Consistent with the reduction in AEA content, CORT treatment increased the maximal hydrolytic activity (Vmax.) of the enzyme for AEA degradation fatty acid amide hydrolase (FAAH); (F) but had no effect on the binding affinity of AEA for FAAH. * Significantly different from control (P<0.05), ** (P=.003); (n=9–10/group for endocannabinoid quantification, n=4 for CB1 receptor binding parameters and enzyme activity).
To quantify FAAH and CB1R expression under conditions of chronic CORT, we used quantitative real-time RT-PCR and mRNA extracted from the hippocampal and amygdalar brain regions from CORT and vehicle-treated mice. Treatment with CORT resulted in no significant change compared to vehicle-treated in mRNA for FAAH (hippocampus: t(6)=1.28, P>0.05; amygdala: t(5)=0.278, P>0.05) or the CB1 receptor (hippocampus: t(6)=0.783, P>0.05; amygdala: t(7)=0.286, P>0.05) (see Table 2).
Table 2.
The effects of chronic corticosterone administration on gene expression of the cannabinoid receptor and fatty acid amide hydrolase
| Control | CORT | |
|---|---|---|
| Amygdala | ||
| CB1 receptor | 1.08±0.35 | 1.25±0.53 |
| FAAH | 1.10±0.18 | 1.19±0.25 |
| Hippocampus | ||
| CB1 receptor | 1.21±0.33 | 1.58±0.25 |
| FAAH | 1.22±0.19 | 1.86±0.59 |
Twenty-eight day administration of corticosterone (CORT) in the drinking water had no effect on the expression of mRNA for either the cannabinoid CB1 receptor or fatty acid amide hydrolase (FAAH) within either the hippocampus and amygdala. Values denoted are means±SEM and are expressed as fold changes of mRNA expression.
DISCUSSION
In this study, we demonstrated that chronic CORT delivered via the drinking water resulted in a significant decrease in the density of [3H]-CP 55,940 binding sites in the hippocampus and amygdala, suggestive of a decrease in CB1 receptor function. Chronic CORT in the drinking water also resulted in a significant reduction in the concentration of AEA within both of these brain regions. We also found that the Vmax. for hydrolysis of AEA by membranes from both brain regions, which reflects the activity of FAAH (Patel et al., 2005a), was increased suggesting that chronic CORT likely reduces AEA through an increase in metabolism as opposed to reductions in synthesis (although this was not directly tested and thus still remains a possibility). In addition, chronic CORT treatment increased 2-AG concentrations in the hippocampus, but not the amygdala, compared to vehicle-treated mice.
Our finding that chronic CORT decreased CB1 receptor density in the hippocampus is similar to the effects of chronic stress on the same parameter in hippocampus (Hill et al., 2005b, 2008b; Reich et al., 2009). However, in contrast to our earlier finding that chronic stress did not affect CB1 receptor binding in the amygdala of rats (Hill et al., 2005a), we found in the present study that chronic CORT treatment did reduce CB1 receptor agonist binding in the amygdala of mice. We have shown previously that administration of CORT to mice via the drinking water as done in this study results in circulating CORT concentrations equivalent to that evoked by stress (Karatsoreos et al., 2010). However, during exposure to stress, CORT concentrations increase in a pulsatile and transient manner, while the CORT administration protocol used herein produces a sustained increase in circulating CORT. As a result, we hypothesize that CORT administration in the drinking water results in higher concentrations of CORT in the brain than stress. We further hypothesize that stress-induced increases in CORT are sufficient to decrease CB1 receptor binding site density in the hippocampus but not in the amygdala. However, sustained increases in CORT also decrease CB1 receptor binding site density in the amygdala, suggesting similar regulatory mechanisms occur in both brain regions, but sensitivity to CORT differs. This possibility is consistent with the fact that the hippocampus exhibits a greater density of glucocorticoid receptors than the amygdala (Ahima and Harlan, 1990).
We have reported previously that decreased CB1 receptor binding site density in the hippocampus following chronic stress or CORT treatment were accompanied by decreases in CB1 receptor protein (Hill et al., 2005b, 2008a). These findings indicate that the decrease in agonist binding site density is the result of a decrease in CB1 receptor protein expression and suggest that glucocorticoids are negative regulators of CB1 receptor gene expression. This notion is consistent with previous reports that adrenalectomy increases CB1 mRNA in the striatum (Mailleux and Vanderhaeghen, 1993). However, in contrast to those previous findings, chronic CORT treatment via the drinking water did not decrease CB1 receptor mRNA expression. It is possible that increased rates of CB1 receptor degradation, rather than reduced gene transcription, underlie the changes in CB1 receptor binding site density, however, more studies are needed to test this hypothesis.
Chronic CORT significantly reduced AEA content and increased the Vmax. for FAAH within both the hippocampus and amygdala, suggesting that CORT increases FAAH activity, which in turn results in a reduction in AEA content. In support of this finding, the concentrations of two other FAAH substrates, PEA and OEA, are also reduced by chronic CORT. The effects of chronic CORT exposure through the drinking water on AEA content and FAAH activity mirror the effects of chronic stress in both rats and mice (Hill et al., 2010b; Patel et al., 2005b; Rademacher et al., 2008), suggesting that CORT is the likely mediator of changes in FAAH activity and AEA content following chronic stress. The change in the Vmax. of FAAH is not accompanied by an increase in FAAH mRNA. This effect is not surprising given that glucocorticoids have been shown to exert negative regulation over FAAH transcription through activation of a glucocorticoid response element in the FAAH promoter, suggesting that glucocorticoid receptor activation would downregulate FAAH expression (Waleh et al., 2002). It is possible that chronic CORT produces a post-translational modification of FAAH in a manner that increases its hydrolytic activity. Ongoing research will seek to determine the mechanisms underlying the regulation of FAAH activity by glucocorticoids.
Chronic CORT was found to increase 2-AG in the hippocampus, but not amygdala. This finding is in contrast to the effects of CORT injections in the rat, where chronic CORT (by injection) had no effect on 2-AG in the hippocampus but increased it in the amygdala (Hill et al., 2005a, 2008a). Moreover, chronic restraint stress in mice has reliably been found to increase 2-AG in both the amygdala and hippocampus (Patel et al., 2005b, 2009), whereas in the rat it has been found to increase or have no effect on 2-AG in the amygdala (depending on the nature of the stress, e.g. chronic restraint vs. chronic unpredictable stress) and either reduce or have no effect on 2-AG in the hippocampus (Hill et al., 2005b, 2008b, 2010b). Thus, the regulation of 2-AG by glucocorticoids within limbic structures appears to be complex and could be dependent upon the time of tissue collection following stress or glucocorticoid exposure. However, given that 2-AG levels within the amygdala are reliably elevated by repeated exposure to a homotypic stressor (Hill et al., 2010b; Patel et al., 2005b, 2009; Rademacher et al., 2008), it is likely that the difference in these effects from those seen following CORT injections (Hill et al., 2005a) are due to the interactive nature of stress (as would be induced by repeated restraint or chronic injections) and increases in circulating CORT. These data highlight the sensitivity of the ECS to environmental stress, even stressors as mild as daily injections. Accordingly, researchers should be careful in the interpretation of data regarding changes in the ECS when seemingly innocuous manipulations, such as daily injections, are being performed.
These data demonstrate that chronic CORT suppresses limbic CB1 receptor binding and AEA signaling, while having variable effects on 2-AG. Given the role of limbic endocannabinoid signaling in the regulation of emotional behavior (Hill et al., 2010c; Lutz, 2009), these data would suggest that glucocorticoid-induced changes in limbic endocannabinoid signaling could contribute to shifts in emotional behavior, especially increases in anxiety and depression-like behaviors, which occur following sustained glucocorticoid exposure (Sterner and Kalynchuk, 2010; Schulkin, 2006). Protracted glucocorticoid exposure can promote obesity (Karatsoreos et al., 2010), suggesting that alterations in limbic endocannabinoid signaling may be involved in changes in emotional behavior that are related to hormonally mediated obesity phenotypes (including the current paradigm; see Bhagat et al., 2009). This could be particularly relevant for the growing rise of comorbidity of mood and anxiety disorders with the increasing obesity epidemic (Dunbar et al., 2008; Chrousos and Kino, 2009; Takeuchi et al., 2009).
Abbreviations
- AEA
anandamide
- CB1
cannabinoid type 1
- CB2
cannabinoid type 2
- CORT
corticosterone
- Ct
cycle threshold value
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- HPA
hypothalamic pituitary adrenal
- mRNA
messenger RNA
- OEA
oleoylethanolamine
- PEA
palmitoylethanolamine
- 2-AG
2-arachidonoylgycerol
References
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Bhagat SM, Karatsoreos IN, McEwen BS. Sex differences in the effects of chronic corticosterone on physiology and behavior. Soc Neurosci Abstr. 2009;469:23. [Google Scholar]
- Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Ueda N, Yamamoto S. The fatty acid amide hydrolase (FAAH) Prostaglandins Leukot Essent Fatty Acids. 2002;66:201–210. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, Bunker SJ, Best JD, Vartiainen E, Kai Lo S, Janus ED. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31:2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamopituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008a;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008b;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur J Pharmacol. 2005a;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010a;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010b;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010c;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005b;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151:2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Glucocorticoid regulation of cannabinoid receptor messenger RNA levels in the rat caudate-putamen. An in situ hybridization study. Neurosci Lett. 1993;156:51–53. doi: 10.1016/0304-3940(93)90437-p. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Omeir RL, Chin S, Hong Y, Ahern DG, Deutsch DG. Arachidonoyl ethanolamide-[1,2–14C] as a substrate for anandamide amidase. Life Sci. 1995;56:1999–2005. doi: 10.1016/0024-3205(95)00181-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005a;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005b;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, la Fleur SE, Houshyar H, Gomez F, Bhargava A, Akana SF. From Malthus to motive: how the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol. 2006;79:247–340. doi: 10.1016/j.pneurobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J. Angst and the amygdala. Dialogues Clin Neurosci. 2006;8:407–416. doi: 10.31887/DCNS.2006.8.4/jschulkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009;35:32–36. doi: 10.1016/j.diabet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291:203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]