Abstract
The identification of genes influencing sensitivity to stimulants and opioids is important for determining their mechanism of action and may provide fundamental insights into the genetics of drug abuse. We used a panel of C57BL/6J (B6; recipient) x A/J (donor) chromosome substitution strains (CSS) to identify quantitative trait loci (QTL) for both open field activity and sensitivity to the locomotor stimulant response to methamphetamine (MA). Mice were injected with saline (days 1 and 2) and MA (day 3; 2 mg/kg, i.p.). We analyzed the total distance traveled in the open field for thirty minutes following each injection. CSS-8, -11, and -16 showed reduced MA-induced locomotor activity relative to B6 whereas CSS-10 and -12 showed increased MA-induced locomotor activity. Further analysis focused on CSS-11 because it was robustly different from B6 following MA-injection but did not differ in activity following saline injection and because it also showed reduced locomotor activity in response to the mu-opioid receptor agonist fentanyl (0.2 mg/kg, i.p.). Thus, CSS-11 captures a QTL for the response to both psychostimulants and opioids. Using a B6 x CSS-11 F2 intercross, we identified a dominant QTL for the MA response on chromosome 11. We used haplotype association mapping of cis expression QTLs and bioinformatic resources to parse among genes within the 95% confidence interval of the chromosome 11 QTL. Identification of the genes underlying QTLs for psychostimulants and opioid response may provide insights about genetic factors that modulate sensitivity to drugs of abuse.
Keywords: Methamphetamine, fentanyl, psychostimulant, stimulants, opioid, locomotor activity, C57BL/6J, A/J, chromosome substitution stains, CSS, F2, chromosome 11, QTL
INTRODUCTION
Drugs of abuse, including both psychostimulants and opioids increase locomotor activity in rodents (Wise & Bozarth, 1987). This behavior is partially mediated by dopamine release in the nucleus accumbens (NAc) (Di Chiara & Imperato, 1988, Koshikawa et al., 1989), a brain region critical for drug reward. Differences in the sensitivity to the locomotor activating effects of methamphetamine are heritable (Phillips et al., 2008), and we and others hypothesize that some of the genes that mediate differences in methamphetamine-induced locomotor activity may also modulate the rewarding effects of drugs. Consistent with this hypothesis, we previously identified an expression polymorphism in casein kinase 1 epsilon (Csnk1e) that modulates methamphetamine (MA)-induced locomotor activity in mice (Bryant et al., 2009a, Palmer et al., 2005) and translated this finding to humans by showing that a polymorphism in CSNK1E predicted the subjectively euphoric effects of amphetamine in healthy human volunteers (Veenstra-Vanderweele et al., 2006). More recently, a polymorphism in CSNK1E that is associated with heroin dependence has been reported (Levran et al., 2008). These data support the hypothesis that genes that modulate the locomotor stimulant response to methamphetamine in mice may also influence the rewarding effects of drugs and the risk for developing drug abuse in humans.
Several studies have identified chromosomal regions, termed quantitative trait loci (QTL) that are associated with differential sensitivity to the locomotor stimulant effects of psychostimulant drugs in mice (Phillips et al., 2008). Chromosome substitution strains (CSS) provide a rapid means for identifying QTLs for complex traits such as the locomotor stimulant response to methamphetamine (Nadeau et al., 2000, Singer et al., 2004). Each CSS has been bred such that a single chromosome from the donor strain (A/J) has been introgressed onto an otherwise homogenous, recipient background (B6). Phenotypic differences between a CSS and B6 indicate the presence of a QTL on the substituted chromosome. The advantages and disadvantages of using CSS for QTL mapping have been previously discussed (Nadeau et al., 2000); two notable advantages include the elimination of genotyping and the ability to fine map by creating an intercross between B6 and the relevant CSS. Disadvantages include lower initial QTL localization (e.g. compared to an F2), and the inability to detect epistatic interactions.
We surveyed a panel of C57BL/6J (B6) x A/J mouse CSS for open field activity on days 1 and 2 and the locomotor response to MA on day 3. We also used a B6 x CSS-11 F2 population to further map a QTL on chromosome 11. Following localization of this QTL to the distal region of chromosome 11, we used haplotype association mapping to identify cis expression QTLs (eQTLs) for several brain regions. Finally, we used bioinformatic resources to identify non-synonymous coding SNPs between B6 and A/J within the chromosome 11 QTL.
METHODS
Subjects
All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Chicago’s Institutional Animal Care and Use Committee. Breeder pairs for B6, A/J and each CSS were obtained from the Jackson Laboratories (Bar Harbor, ME) and offspring were generated for testing at the University of Chicago. The number following CSS (CSS-#) indicates which B6 chromosome has been substituted with the corresponding A/J chromosome. CSS-13 was not included in the study due to poor breeding which prevented us from obtaining the necessary sample size. F2 mice were generated by crossing B6 females with CSS-11 males to create an F1 generation, and then intercrossing female and male F1 mice to create F2 mice.
Mouse colony rooms were maintained on a 12/12 light/dark cycle with lights on at 0600 h. All mice were provided unlimited access to food and water, except during testing. Two to five same-sex littermates were housed in clear plastic cages with standard corn cob-type bedding; cage mates were always tested on the same days. Testing was conducted between 0900 h and 1600 h. Mice were transported from the vivarium next door into the test room and were allowed to habituate for at least 30 min before testing.
Behavioral testing
Mice were tested over the course of 15 three-day sessions. Approximately 4.5 times more B6 mice were phenotyped compared to any individual CSS, as recommended by (Belknap, 2003). The exact numbers of mice tested are listed in Table 1. B6 mice were included in almost all three-day sessions whereas individual CSS and A/J mice were tested in only a fraction of three-day sessions. Care was taken to randomize and balance the order and sex of strains in each three-day session; an average of 6 strains were included in each three-day session. CSS mice ranged from 7–14 weeks old on the first day of testing; F2 mice ranged from 7–11 weeks old on the first day of testing.
Table 1.
The N, mean age (shown in days), S.E.M. of the mean age, and the age range for B6, A/J and each CSS is listed for each sex.
| Strain | N (females) | Age (females) (mean ± S.E.M. and range) | N (males) | Age (males) (mean ± S.E.M.) |
|---|---|---|---|---|
| B6 | 55 | 67.0 ± 1.2 (50–82) | 53 | 65.9 ± 1.3 (52–79) |
| A/J | 9 | 63.4 ± 1.9 (57–73) | 10 | 71.3 ± 1.2 (66–75) |
| CSS-1 | 4 | 72.5 ± 0.5 (71–73) | 6 | 55.0 ± 1.3 (53–59) |
| CSS-2 | 10 | 66.7 ± 3.6 (52–81) | 10 | 72.6 ± 4.7 (51–83) |
| CSS-3 | 8 | 62.0 ± 1.9 (55–69) | 14 | 60.0 ± 1.5 (54–67) |
| CSS-4 | 10 | 68.1 ± 1.4 (60–72) | 9 | 63.4 ± 2.0 (58–72) |
| CSS-5 | 14 | 64.1 ± 2.2 (54–73) | 10 | 58.9 ± 1.9 (54–73) |
| CSS-6 | 10 | 66.8 ± 1.7 (62–74) | 9 | 71.6 ± 3.6 (60–89) |
| CSS-7 | 10 | 63.2 ± 1.7 (59–71) | 11 | 59.0 ± 0.5 (57–61) |
| CSS-8 | 12 | 64.8 ± 1.4 (60–71) | 8 | 69.1 ± 2.2 (63–75) |
| CSS-9 | 10 | 67.8 ± 2.1 (58–72) | 9 | 66.6 ± 2.1 (60–72) |
| CSS-10 | 13 | 62.8 ± 1.5 (58–70) | 16 | 62.7 ± 1.4 (57–71) |
| CSS-11 | 14 | 60.0 ± 2.7 (48–76) | 23 | 67.7 ± 2.2 (50–78) |
| CSS-12 | 15 | 80.5 ± 2.0 (72–92) | 34 | 78.7 ± 2.0 (62–92) |
| CSS-14 | 9 | 71.4 ± 0.2 (71–72) | 5 | 74.6 ± 5.0 (63–85) |
| CSS-15 | 10 | 62.8 ± 1.5 (54–65) | 13 | 65.7 ± 1.5 (54–70) |
| CSS-16 | 13 | 71.2 ± 1.8 (65–80) | 9 | 71.2 ± 1.6 (65–77) |
| CSS-17 | 3 | 74.3 ± 3.7 (67–78) | 9 | 66.3 ± 0.9 (62–69) |
| CSS-18 | 5 | 62.4 ± 0.6 (60–63) | 9 | 61.4 ± 1.0 (55–64) |
| CSS-19 | 10 | 61.6 ± 2.1 (55–69) | 10 | 66.0 ± 2.2 (56–72) |
| CSS-X | 12 | 75.3 ± 1.2 (72–82) | 6 | 54.0 ± 0.0 |
The procedures for behavioral testing has been described previously (Bryant et al., 2009a, Palmer et al., 2005). Just prior to testing, mice were removed from their home cages and placed in clean holding cages for approximately 5 min after which they received an intraperitoneal injection of saline (10 ml/kg; days 1 and 2) or MA (2 mg/kg, i.p.; day 3) and were immediately placed in the center of the open field; total distance traveled over the subsequent 30 min was recorded. The twelve open fields were cleaned before and after each 30 min recording on each of the three days with 10% isopropanol. Locomotor activity was measured using automated Versamax open field (AccuScan, Columbus, OH). Each open field arena was made of a clear acrylic arena (40 X 40 X 30 cm) placed inside a frame containing evenly spaced photocells and receptors making a grid of infrared photobeams from the front to the back and from the left to the right of the arena. The floor of the open field is white. Beam breaks were recorded on a computer and converted into total distance traveled (cm). Each open field was surrounded by a sound attenuating PVC / lexan environmental chamber (AccuScan). In each open field, overhead lighting provided dim illumination (~80 lux) and a fan provided ventilation and masking of background noise. In a separate series of experiments (described below) we examined the effects of fentanyl (0.2 mg/kg, i.p., day 3; Sigma, St. Louis), a selective mu opioid receptor agonist, on B6 and CSS-11 strains; the protocol was identical except that on day 3, fentanyl was injected instead of MA. This fentanyl dose produces a robust increase in locomotor activity in the open field over 30 min in C57BL/6J mice (Bryant et al., 2009b).
Analysis
The dependent measure for all analyses was total distance traveled over 30 minutes. In some analyses we treated days 1, 2, and 3 as repeated measures, whereas other analyses examined each day separately. In addition, we considered the difference in activity between days 3 and 2 (day 3 – day 2), which we and others have previously used to identify the response to MA treatment (e.g. Palmer et al., 2005).
For the comparisons involving B6 versus A/J and B6 versus CSS-11 mice, we used a three-way repeated measures analysis of variance (ANOVA) to examine the factors day (within group repeated measure), strain, and sex. For analysis of all CSS we used two-way ANOVAs to examine the effect of strain and sex for each day (1, 2 or 3) in separate analyses using age as a covariate. Because there was never a main effect or interaction of sex or age, these variables were collapsed out of the analysis and we followed up with one-way ANOVAs for the factor strain. Main effects and interactions between strain and day were further examined with posthoc tests (unpaired or paired t-tests) as appropriate.
We converted behavioral data into z-scores and used these values to compare each strain to B6, using equation (3) from Belknap (2003). The z-score reflects the number of standard deviations that separate the means of B6 and each CSS. Based on Dunnett’s test, a significance threshold of z > 2.9 (p<0.004) was employed to correct for multiple comparisons against a single B6 background strain whereas z > 1.96 (p<0.05) was considered suggestive (Belknap, 2003). We also used the z-scores to estimate the effect size (proportion of the variance explained, u2CvsB) using equation (4) of Belknap (2003). This value indicates the proportion of total variance explained by fitting the genetic effect. Thus, by generating z-scores we were able to determine which strains captured significant QTLs and the effect of those QTLs on the phenotype.
For the analysis of the F2 cross between B6 x CSS-11, 87 F2 mice (39 females, 48 males) were tested in the same manner as the CSS. DNA was extracted from the tail and used for genotyping. We selected single nucleotide polymorphism (SNP) markers with an average spacing of 7.6 cM as estimated by a previously published genetic map (Shifman et al., 2006). Mb positions are based on build 37 of the mouse genome: rs13480888 (16.9 Mb), rs13480921 (25.7Mb), rs6280308 (32.6 Mb), rs13481015 (48.1 Mb), rs13481044 (57.6 Mb), rs13481117 (79.1 Mb), rs13481170 (95.5 Mb), rs3023315 (99.4 Mb), rs13481220 (108.4 Mb), and rs13481256 (118.0 Mb). All assays were conducted using Applied Biosystems TaqManR SNP Genotyping Assays according to the manufacturer's instructions.
QTL analysis of the F2 mice was conducted using interval mapping (step=1 cM) and the estimation maximization procedure as implemented in R/qtl (Broman et al., 2003). We used a genetic map that was generated from our marker data for mapping; this map showed good agreement with the previously published maps. The significance threshold was set at p<0.05 as determined by 1000 permutations. The 95% confidence interval was estimated by calculating the Bayes credible interval using R/qtl. Effect plots were generated for the marker with the highest LOD score.
Haplotype association mapping of eQTL
An expression QTL (eQTL) is a locus that modulates the expression of a particular gene. QTLs for complex traits can be mediated by gene expression differences due to polymorphisms in regulatory regions near the gene (cis-acting) or elsewhere in the genome (trans-acting). We were interested in identifying cis-eQTL within our chromosome 11 QTL because such eQTL could be causally related to the QTL for differential locomotor response to MA. eQTL can be detected in standard mapping populations including F2 crosses and recombinant inbred strains (Chesler et al., 2004). However, no brain eQTL data are publicly available for crosses between B6 and A/J. Therefore, we conducted haplotype association mapping using a panel of inbred strains (23–29 per brain region) chosen from the Mouse Phenome Project priority strains (Bogue, 2003) for which we had also measured gene expression. This method takes advantage of the fact that laboratory inbred strains were largely derived from the same founders and share regions of the genome that are largely identical by descent (Wiltshire et al., 2003). Therefore, at some locations, B6 and A/J will be identical by descent (IBD) with respect to each other, and thus unlikely to segregate opposite alleles of an eQTL. At other loci, B6 and A/J will not be IBD with respect to one another, but may be IBD with respect to many other inbred strains. By comparing the gene expression of A/J-like strains with B6-like strains at regions where A/J and B6 are not IBD, it can be determined whether the tested location is correlated with a difference in gene expression.
Gene expression in the inbred strains was measured in 5 brain regions (striatum, nucleus accumbens, prefrontal cortex, amygdala, and hippocampus). Adult (10- to 12-week-old) mice were euthanized and brain regions were collected from groups of three experimentally naive male mice. The brain was positioned in a 1mm brain block with the anterior surface abutting a single-edged razor blade placed in the first slot and ventral surface visible. The slot nearest the boundary of medulla was used as the first landmark. Razor blades were placed in slots one and two mm anterior to that boundary. Additional razor blades were placed in remaining anterior slots. Prefrontal cortex was dissected from the section +1.7 to +1.2 mm to Bregma at the anterior surface by taking a triangular section formed by two incisions made 45° from the midsagittal plane using the corpus callosum as a ventral boundary. From the section +1.3 to +0.7 mm to Bregma at anterior surface, nucleus accumbens was dissected by using a 1mm punch to remove tissue ventro-lateral to anterior commissures and striatum was extracted using a 1mm punch between the corpus callosum and anterior commissure. Two 2-mm thick sections -2.0 mm to Bregma at posterior surface were dissected. In the first section, a horizontal cut was made at the ventral boundary of the external capsule. Another cut in line with the external capsule was made to separate the piriform cortex from the amygdala. In the remaining 2 mm thick section, the cortex was peeled apart from the hippocampus. Tissues were quickly frozen on dry ice. Tissues were pulverized while frozen, and total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA), then further processed by using the RNeasy miniprep kit according to manufacturer’s protocols (Qiagen, Chatsworth, CA). The quality of all samples was determined with an Agilent Bioanalyzer (Palo Alto, CA).
5 μg of total RNA was used to synthesize cDNA that was then used as a template to generate biotinylated cRNA. cRNA was fragmented and hybridized to Affymetrix MOE430 gene expression arrays according to the manufacturer’s instructions. The arrays were then washed and scanned with a laser scanner, and images were analyzed by using the MAS5 algorithm. Arrays were normalized by using global median scaling.
10,990 SNPs spaced at ~300 kb intervals were chosen for genotyping the strains and inferring haplotypes. A 3-SNP window was used to assign strains to a haplotype, with a minimum requirement of five strains per haplotype to be considered at a locus (Mcclurg et al., 2006, Pletcher et al., 2004). A marker association algorithm combined with family-wise error rate (gFWER) analysis was used to identify associations and to account for relatedness among strains and thus, decrease the rate of false positive associations (Mcclurg et al., 2006). We report only eQTLs that were located in regions where B6 and A/J were assigned to different haplotypes groups and these eQTLs had – log(p) > 3.5 with a QTL peak within 50 kb of the gene whose expression is being measured.
RESULTS
A/J vs. B6
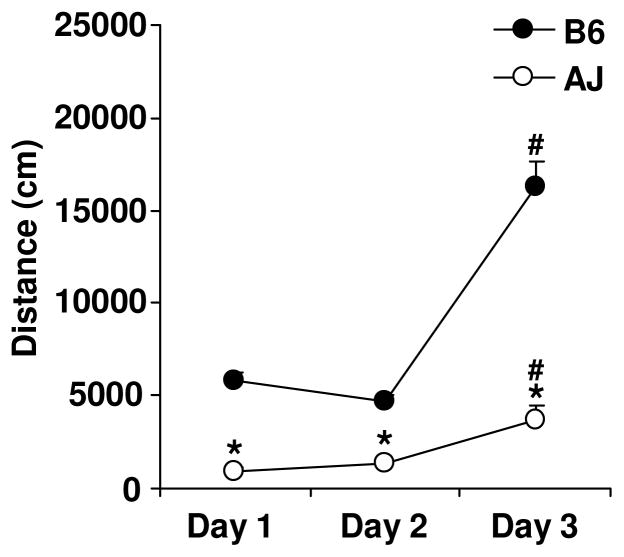
The B6 and A/J strains were significantly different from each other on all three test days (Figure 1) as reflected by main effects of strain (F1,34=112.09; p<0.0001), day (F2,68=93.37; p<0.0001), and an interaction between strain and day (F2,68=37.33; p<0.0001). A/J showed less activity than B6 on all three days (t34=10.86, 8.68 and 8.38, for days 1, 2 and 3, all p<0.0001). Paired t-tests comparing activity on day 1 versus day 2 indicated that B6 mice showed a significant decrease in activity (t16=3.09; p=0.007), whereas A/J mice showed a trend for an increase in activity (t18=1.93; p=0.069). Both B6 and A/J strains showed an increase in activity from day 2 to day 3 (t16=10.21, p<0.0001; t18=3.26, p=0.0044, respectively), reflecting the effect of MA treatment on day 3.
Figure 1. Locomotor response in B6 and A/J.
B6 (N=10 females, 7 males) and A/J mice (N=9 females and 10 males) received saline injections (i.p.) on days 1 and 2 and MA (2 mg/kg, i.p.) on day 3 before placement in the open field. Total distance traveled was recorded over 30 min. Data are presented as the mean ± S.E.M. * = A/J significantly different from B6 for that day. # = significant difference between days 2 and 3.
CSS
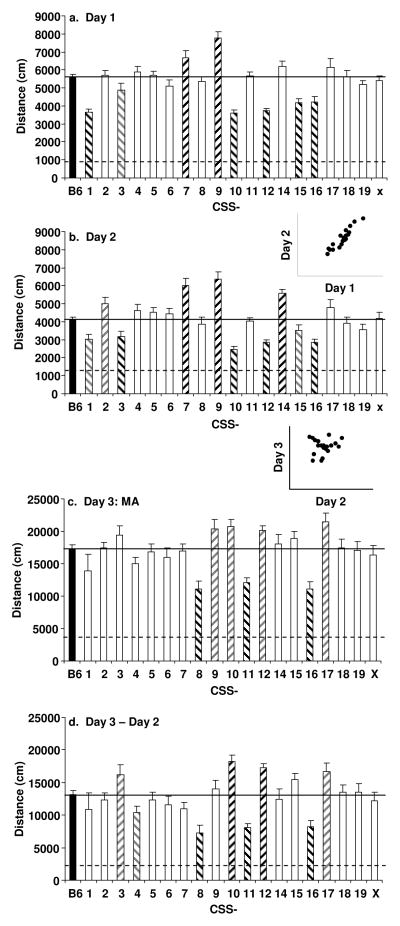
Table 1 lists the N, mean age ± S.E.M., and the age range for B6, A/J and each CSS for each sex; all data are available at the Mouse Phenome Database (www.jax.org/phenome). We found a significant effect of strain on day 1 activity (F19,500=14.20; p<0.0001; Figure 2a). CSS-1, -10, -12, -15, and -16 showed significantly less locomotor activity on day 1 relative to B6 mice whereas CSS-7 and -9 showed significantly more locomotor activity compared to B6. We also found a significant effect of strain on activity on day 2 (F19,500=13.85; p<0.0001; Figure 2b). In general, the mean activity of the strains on day 1 and 2 was highly correlated (r=0.93; p<0.0001) as shown in the inset of Figure 2b. Strains CSS-3, -10, -12, and -16 showed significantly less locomotor activity relative to B6 mice, whereas strains CSS-7, -9, and -14 showed significantly more locomotor activity compared to B6.
Figure 2. Locomotor response in CSS on days 1, 2, 3 and day 3 minus day 2.
Total distance traveled over 30 minutes is shown for a: day 1 (saline), b: day 2 (saline), c: day 3 (MA; 2 mg/kg, i.p.), and d: day 3 minus day 2. The N for each strain and sex and their mean age ± S.E.M are listed in Table 1. The solid, horizontal line indicates the mean value for B6. The dashed, horizontal line indicates the mean value for A/J. The inset of Panel b illustrates the high degree of correlation between day 1 and day 2 activity (r=0.93; p<0.0001). The inset of Panel d illustrates the lack of correlation between day 3 and day 2 activity. Significant differences as compared to B6 are indicated by a black and white hatched pattern. Suggestive differences are indicated by a gray and white hatched pattern. Data are presented as the mean value for each CSS ± S.E.M.
The main focus of our study was to assess activity following treatment with MA, which was measured on day 3. There was a significant main effect of strain on day 3 (F19,500=6.68; p<0.0001; Figure 2c). The strains CSS-8, -11, and -16 showed significantly less locomotor activity following MA administration as compared to B6, none of the CSS showed significantly increased locomotor activity compared to B6.
We also examined the difference between activity on day 3 and day 2 (Figure 2d), which we and others have used in the past as a way to distinguish between differences that are specific to drug treatment versus those that are secondary to differences in basal locomotor activity and occur even in the absence of drug treatment. There was a significant effect of strain (F19,500=8.22; p<0.0001). The results were generally similar to those shown in Figure 2c, however, CSS-10 and -12 showed significantly more locomotor activity compared to B6. Thus, CSS-10 and -12 are greater than B6 only when the difference between day 3 and day 2 is used as the dependent measure. This difference stems from the fact that these strains showed significantly lower activity on days 1 and 2 and a non-significant trend towards higher activity on day 3. Interestingly, there was little correlation between activity on day 2 and day 3, as shown by the inset in Figure 2c (rs=0.16; p>0.05).
To better understand the data shown in Figure 2, we calculated z-scores for each of these phenotypes that are shown in Table 2. Table 2 also lists v2CvsB, which is the proportion of phenotypic variance accounted for by the QTL on a given chromosome (Belknap, 2003). We also examined the correlations between the strain means of the variables shown in Figure 2 and Table 3. In general, there were strong correlations between days 1 and 2, and between day 3 and day 3 - day 2; however, activity on days 1 and 2 was not significantly correlated with the response to MA on day 3.
Table 2.
Significant (bolded) and suggestive (unbolded) Z-scores and ν2CvsB for the phenotypes of CSS relative to the B6 strain.
| CSS- | Day 1 activity (Saline) | Day 2 activity (Saline) | Day 3 activity (MA) | Day 3 Day 2 (MA - Saline) | ||||
|---|---|---|---|---|---|---|---|---|
| Z | v2CvsB | Z | v2CvsB | Z | v2CvsB | Z | v2CvsB | |
| 1 | -4.36 | 0.14 | −2.47 | 0.050 | ||||
| 2 | 2.74 | 0.056 | ||||||
| 3 | −2.32 | 0.041 | −3.12 | 0.071 | 2.37 | 0.042 | ||
| 4 | −2.06 | 0.033 | ||||||
| 5 | ||||||||
| 6 | ||||||||
| 7 | 3.22 | 0.075 | 5.87 | 0.21 | ||||
| 8 | −4.44 | 0.14 | −4.45 | 0.14 | ||||
| 9 | 6.35 | 0.24 | 6.68 | 0.26 | 2.21 | 0.038 | ||
| 10 | −7.15 | 0.27 | −6.03 | 0.21 | 2.90 | 0.058 | 4.50 | 0.13 |
| 11 | −4.80 | 0.14 | −4.93 | 0.15 | ||||
| 12 | −8.05 | 0.30 | −5.55 | 0.17 | 2.85 | 0.050 | 4.34 | 0.11 |
| 14 | 3.74 | 0.10 | ||||||
| 15 | −4.69 | 0.15 | −2.03 | 0.031 | ||||
| 16 | −4.41 | 0.13 | −4.13 | 0.12 | −4.65 | 0.14 | −3.84 | 0.10 |
| 17 | 2.42 | 0.047 | 2.14 | 0.037 | ||||
| 18 | ||||||||
| 19 | ||||||||
| X | ||||||||
Table 3.
Correlation matrix of the average phenotypes for each CSS and B6.
| Variable | day 1 | day 2 | day 3 | day 3–day 2 |
|---|---|---|---|---|
| day 1 | - | - | - | - |
| day 2 | 0.93 | - | - | - |
| day 3 | 0.12 | 0.16 | - | - |
| day 3 – day 2 | −0.21 | −0.19 | 0.94 | - |
B6 vs. CSS-11
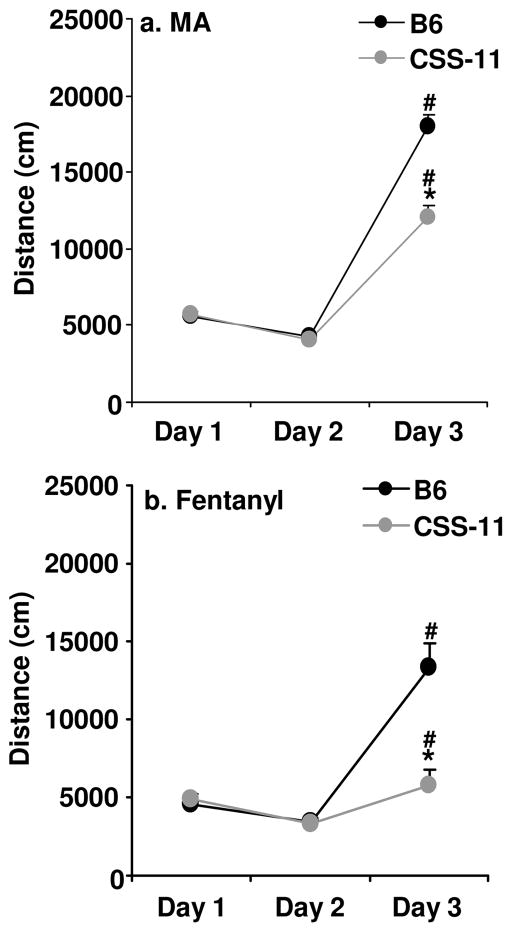
CSS-11 stood out because it had a significantly lower response to MA on day 3 as compared to B6 but was virtually identical to B6 on days 1 and 2 (Figure 2a, b). Additionally, CSS-11 had the highest Z-score for MA-induced activity on Day 3 (−4.80), accounting for 14% of the trait variance. Thus, we chose to examine this strain in greater detail. We compared the response of a subset of B6 mice that were tested on the same days as CSS-11 mice across all three days of treatment, treating day as a repeated measure (Figure 3a). We identified a significant interaction between strain and day (F2,176=20.36; p<0.0001). To investigate the source of the interaction between strain and day, we compared the two strains for each day. As stated above, B6 and CSS-11 were not different on days 1 and 2 but CSS-11 showed a significantly lower response to MA on day 3 (t88=5.29; p<0.0001). Both strains showed a significant increase in activity from day 2 to to day 3 in response to MA (B6: t52=17.57; p<0.0001; CSS-11: t36=12.38; p<0.0001).
Figure 3. Locomotor response in B6 and CSS-11 to MA and Fentanyl.
Two different cohorts of mice received saline injections on days 1 and 2, and received either MA (2 mg/kg, i.p.; Panel a) or fentanyl (0.2 mg/kg, i.p.; Panel b) on day 3. For panel a, N=23 females, 30 males for B6 (average age = 67.8 ± 0.9) and N=14 females, 23 males for CSS-11 (average age = 64.8 ± 1.8). For panel b, N=5 females, 6 males for B6 (average age = 66.2 ± 1.8) and N=6 females, 4 males for CSS-11 (average age = 69.6 ± 0.7). Total distance traveled was recorded over 30 min. Data are presented as the mean ± S.E.M. * = CSS-11 significantly different from B6 for that day. # = significant difference between days 2 and 3.
We were interested to know if these differences would generalize to opioids, which are mechanistically distinct for MA but are also commonly abused by humans. We examined the response to the selective mu opioid receptor agonist fentanyl in a separate cohort of B6 and CSS-11 mice, using a procedure that was identical to that used to examine the response to MA, except that fentanyl (0.2 mg/kg) was given on day 3 in place of MA (Figure 3b). We observed a significant interaction between strain and day (F2,38=14.85, p<0.0001). To investigate the source of this interaction, we examined the difference between strains at each day separately. B6 and CSS-11 were similar on days 1 and 2, but CSS-11 showed a significantly lower response to fentanyl on day 3, as compared to B6 (t19=3.78; p=0.0013). Both strains showed a significant increase in activity from day 2 to day 3 in response to fentanyl (B6: t10=5.94; p=0.0001; CSS-11: t9=2.57; p=0.03). Thus, CSS-11 has a blunted response to both MA and fentanyl as compared to B6 despite similar behavior in the absence of drug administration as measured on days 1 and 2.
B6 x CSS-11 F2 intercross
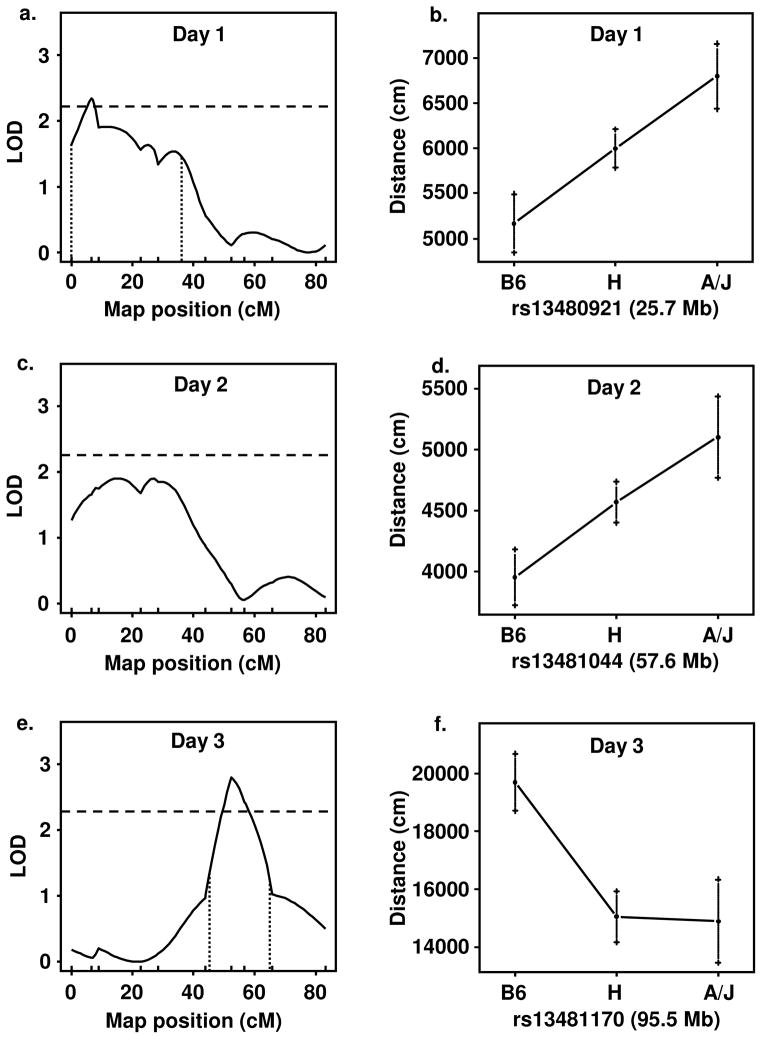
We phenotyped and genotyped 87 B6 x CSS-11 F2 mice in an attempt to better define the QTL for day 3 activity on chromosome 11. Figure 4 illustrates the QTL and effect plots for day 1 (Panels a, b), day 2 (Panels c, d), and day 3 activity (Panels e, f). There was no effect of sex or age, nor an interaction of sex with age for activity on Day 1, 2, or 3 (p>0.05). For day 1 activity, a QTL at 6.5 cM (95% confidence interval = 0–37 cM) with a LOD score of 2.34 (significance threshold = 2.33) was observed; this QTL showed an additive mode of inheritance. The LOD score for day 2 activity was not significant. For day 3 activity, a QTL was observed at 52.4 cM (95% confidence interval = 45–65 cM) with a LOD score of 2.79 (significance threshold = 2.20) and a dominant mode of inheritance. We used the equation 1–10−2 LOD / n to estimate the proportion of trait variance accounted for by this QTL (n = sample size; (Broman et al., 2003). As with CSS-11, the proportion of variance accounted for by the QTL was 14%.
Figure 4. QTL mapping of the locomotor response using a B6 x CSS-11 F2 population.
39 females and 48 males were used for this study. Panels a, c, and e: QTL plots for locomotor Methamphetamine QTL with CSS activity on day 1, day 2, and day 3, respectively. The dashed, horizontal lines indicate significance threshold (1000 permutations). The vertical, dotted lines indicate the 95% confidence interval (Bayes credible interval). Panels b, d, and f: Effect plots of the marker with the highest LOD score showing the mean and standard error for each genotype class: B6 = homozygous for B6. H = heterozygous for B6 and A/J alleles. A/J = homozygous for A/J.
Haplotype association mapping of eQTL
A total of 32 cis-expression QTLs (eQTLs) between A/J and B6 were identified (Table 4) in the 95% confidence interval (79–109 Mb; build 37) derived from our F2 study. We did not explore the possibility that SNPs within a probeset might be responsible for the eQTL that we detected.
Table 4.
Expression QTLs derived from haplotype association mapping for the region between 79 and 109 MB. Genes, expression probes, physical location (build 37), and the –logP values are listed for each of the 5 brain regions. NAc = nucleus accumbens. Str = striatum. PFC = prefrontal cortex. Amyg = amygdala. HC = hippocampus. e-QTLs are only included if B6 and A/J belong to different haplotypes. Darkened horizontal lines encapsulate one or multiple probesets for individual genes. Genes in bold indicate those for which eQTLs were identified in the NAc, Str, or both.
| Gene | Probe | Location (Mb) | NAc | Str | PFC | Amyg | HC |
|---|---|---|---|---|---|---|---|
| AI450353 | 1440345_at | 83.1 | 4.36 | 3.58 | |||
|
| |||||||
| Taf15 | 1438130_at | 83.3 | 5.70 | 4.24 | 4.68 | 6.00 | 3.59 |
|
| |||||||
| Acaca | 1444810_at | 84.0 | 3.57 | ||||
|
| |||||||
| 4632419I22Rik | 1433954_at | 86.0 | 3.80 | 5.40 | 4.42 | ||
|
| |||||||
| Rps6kb1 | 1460705_at | 86.3 | 5.40 | 4.31 | 6.00 | 4.15 | |
|
| |||||||
| Cltc | 1440457_at | 86.5 | 5.40 | 4.60 | 4.89 | ||
|
| |||||||
| Gdpd1 | 1424077_at | 86.8 | 6.00 | 4.19 | 6.00 | 6.00 | 6.00 |
|
| |||||||
| Stxbp4 | 1442267_at | 90.3 | 3.54 | ||||
|
| |||||||
| Utp18 | 1454817_at | 93.7 | 4.74 | 3.53 | 6.00 | 4.48 | 3.53 |
|
| |||||||
| Mbtd1 | 1417241_at | 93.7 | 4.17 | ||||
| Mbtd1 | 1417261_at | 93.7 | 4.03 | 5.52 | 5.10 | ||
| Mbtd1 | 1441100_at | 93.7 | 4.52 | 5.05 | |||
|
| |||||||
| Nme2 | 1448808_a_at | 93.8 | 3.73 | ||||
|
| |||||||
| C77673 | 1444677_at | 94.2 | 4.96 | ||||
|
| |||||||
| Rsad1 | 1437449_at | 94.4 | 4.72 | 3.71 | |||
|
| |||||||
| Mrpl27 | 1415690_at | 94.5 | 3.71 | 4.34 | |||
|
| |||||||
| Pdk2 | 1448825_at | 94.9 | 4.44 | 5.05 | 5.30 | 4.46 | |
|
| |||||||
| Spop | 1458886_at | 95.3 | 4.19 | 4.92 | 4.92 | 3.50 | |
|
| |||||||
| Phb | 1448563_at | 95.5 | 3.52 | 3.78 | |||
|
| |||||||
| Atp5g1 | 1444874_at | 95.9 | 3.60 | ||||
|
| |||||||
| Snx11 | 1424031_at | 96.6 | 4.25 | ||||
|
| |||||||
| Cbx1 | 1436266_x_at | 96.7 | 3.97 | ||||
|
| |||||||
| Pnpo | 1415793_at | 96.8 | 3.91 | 4.57 | |||
|
| |||||||
| Mel13 | 1435017_at | 97.5 | 4.62 | 4.96 | |||
|
| |||||||
| B230217C12Rik | 1428568_at | 97.7 | 6.00 | 6.00 | 6.00 | 6.00 | |
|
| |||||||
| Smarce1 | 1422675_at | 99.1 | 3.88 | 3.98 | |||
|
| |||||||
| Krt12 | 1419230_at | 99.3 | 3.55 | 5.00 | 4.64 | ||
|
| |||||||
| Mpp3 | 1419077_at | 101.9 | 5.40 | ||||
|
| |||||||
| Gfap | 1440142_s_at | 102.7 | 4.18 | 3.54 | |||
|
| |||||||
| Cdc27 | 1426076_at | 104.4 | 4.64 | ||||
|
| |||||||
| Smurf2 | 1429045_at | 106.7 | 3.96 | ||||
| Smurf2 | 1429046_at | 106.7 | 4.77 | 4.10 | |||
|
| |||||||
| Cacng5 | 1434785_at | 107.8 | 4.60 | ||||
|
| |||||||
| Prkca | 1437393_at | 107.8 | 4.40 | ||||
|
| |||||||
| Amz2 | 1417241_at | 109.3 | 3.59 | ||||
Non-synonymous coding SNPs
There are 30,810 reported synonymous (non-coding) and non-synonymous (coding) SNPs between B6 and A/J on chromosome 11 between 79 and 109 Mb (Mouse Phenome Database). Of these, 204 are non-synonymous SNPs. The QTL for MA sensitivity could be due to synonymous and/or non-synonymous polymorphisms that could affect either expression or functionality of a protein. Table S1 lists all 204 non-synonymous coding SNPs that are polymorphic between B6 and A/J on chromosome 11 between 79 and 109 Mb. A total of 117 genes containing coding SNPs were identified, many of which contain multiple coding SNPs. Seven of these genes, Stxbp4 (90.4 Mb), Nme2 (93.8 Mb), Rsad1 (94.4 Mb), Atp5g1 (95.9 Mb), Snx11 (96.6 Mb), Mpp3 (101.9 Mb), and Gfap (102.8) also contain eQTLs (Table 4).
DISCUSSION
B6 and A/J mice show striking differences in locomotor activity both in the presence and absence of drug treatment (Figure 1). Screening B6 x A/J CSS revealed multiple QTLs influencing saline-induced and MA-induced locomotor activity (Figure 2 and Table 2). For CSS-11, we showed a blunted response to both MA and fentanyl (Figure 3), suggesting that a single QTL on chromosome 11 may influence the response to both drugs. We used a B6 x CSS-11 F2 intercross to more accurately map and determine the mode of inheritance of the QTL for MA-induced locomotor activity (Figure 4). Bioinformatic procedures were then used to identify candidate genes within the 95% confidence interval of this QTL. Taken together, these data identify many QTLs for multiple phenotypes that may be relevant for understanding genetic variability in the response to drugs of abuse.
The genetic relationship between locomotor activity following saline versus MA injections can be elucidated using our data. In general, we did not observe any strong relationship between activity on days 1 and 2 compared to activity following MA on day 3. One exception is CSS-16 which showed lower activity on all three days, suggesting that the lower activity following drug treatment may reflect a general tendency toward hypolocomotion. However, both CSS-10 and -12 showed lower activity after saline on days 1 and 2 but significant higher activity following MA on day 3 (using the day 3 minus day 2 measure) demonstrating that in some strains, activity following saline is inversely correlated with MA-induced activity. These different relationships reflect the lack of overall correlation between saline- and MA-induced activity that is shown in Table 3. In some instances, a single QTL may pleiotropically influence activity in both conditions. In other cases, two or more distinct QTLs may be present. We have previously reported MA-induced locomotor stimulation as the difference between day 3 and day 2 (e.g. Palmer et al., 2005). However, Table 3 shows that day 3 and day 3–day 2 are highly correlated with each other. In examining Figure 2c and d, the only minor differences between these two approaches are observed. Overall, we conclude that both measures are similar and that subtraction of day 2 neither significantly adds nor detracts from the utility of the measure.
We conducted follow-up studies on an F2 cross between B6 and CSS-11 because of the observed strain difference was specific to the locomotor response to MA and because CSS-11 was the most divergent strain from B6 when considering the MA response (Figure 2c, d) with Z scores of -4.80 and -4.93 (Table 2). CSS-11 was also much less sensitive to fentanyl-induced hyperlocomotor activity (Figure 3b), suggesting a single QTL on CSS-11 may influence sensitivity to both psychostimulants and opioids (although they could be separate QTL). We have previously reported a QTL on chromosome 11 for decreased MA response using a cross between B6 and DBA/2J (Palmer et al., 2005). This raises the possibility that the same polymorphism causes both QTLs. Interestingly, a previous finding using B6 and A/J progenitor strains localized a QTL for sensitivity to the locomotor stimulant effect of nicotine to the same region of chromosome 11 (95% C.I.: 48–54 cM; Gill & Boyle, 2005) perhaps indicating a QTL with pleiotropic effects on both MA and nicotine induced locomotor activity. Alternatively, there could be separate genes on chromosome 11 regulating sensitivity to MA, fentanyl, and nicotine.
Despite the specificity of CSS-11 for drug-induced locomotor activity, the B6 x CSS-11 F2 study identified a QTL influencing day 1 activity (Figure 4a, b) that we did not observe in the parental CSS-11 (Figures 2a, 3a, b). Nevertheless, the most robust finding of the F2 study was the confirmation and fine mapping of the QTL for response to MA (Figure 4e, f). The QTLs for activity on day 1 (Figure 4a) and day 3 (Figure 4e) are very likely to be independent for several reasons. First, the 95% confidence intervals for these two QTLs do not overlap (Figure 4a, e). Second, the A/J allele for the day 1 QTL increases locomotor activity (Figure 4b) whereas the A/J allele for the day 3 QTL decreases activity (Figure 4f). Third, the day 1 QTL shows an additive mode of inheritance (Figure 4b) whereas the day 3 QTL shows a dominant mode of inheritance (Figure 4f). Thus, we conclude that the QTL on chromosome 11 for day 3 activity is specific for MA-induced locomotor activity and has no effect on activity in the absence of drug.
Both psychostimulants and opioids increase dopamine release, which is associated with both locomotor stimulation and the subjectively rewarding effects of these drugs in humans (Wise and Bozarth, 1987). Thus, if we assume that differences observed in CSS-11 are due to the same QTL, it is possible that genes involved in dopaminergic neurons or their targets underlie this QTL. Darpp-32 (dopamine- and cyclic AMP-regulated phosphoprotein-32) is a potent phosphatase inhibitor that is highly expressed in dopamine-receiving neurons in the NAc (Ouimet et al., 1984, Walaas et al., 1983) and modulates psychostimulant-induced locomotor activity (Fienberg et al., 1998, Greengard, 2001, Lindskog et al., 2002, Snyder et al., 2000, Zachariou et al., 2006) and reward (Zachariou et al., 2002) and opioid-induced locomotor activity (Borgkvist et al., 2007). Ppp1r1b, the gene that encodes Darpp-32, is located on chromosome 11 at 98.2 Mb which is between the two markers that showed the highest LOD scores in our F2 study. Furthermore, we previously found that a mouse line selected for high MA sensitivity exhibited an increase in Darpp-32 transcript abundance in the nucleus accumbens (Palmer et al., 2005). However, when comparing B6 and A/J mice, a previous study found no difference in Darpp-32 protein expression (Brodkin et al., 1998) and we found no e-QTL for Ppp1r1b (Table 4) nor did we find any coding differences in Ppp1r1b between B6 and A/J (Table S1) nor did Mouse Phenome Database contain many polymorphic SNPs between B6 and A/J in the vicinity of Ppp1r1b, suggesting that B6 and A/J are likely to have inherited a functionally equivalent region from a recent common ancestor. Thus, we do not believe that Ppp1r1b underlies this QTL.
The 95% confidence interval for the MA-induced locomotor activity QTL on chromosome 11 is large and contains a correspondingly large number of genes. It is therefore not possible to implicate a specific gene with high confidence. One gene of particular interest that was in a B6-AJ SNP-dense region was Cacna1g, which encodes the alpha 1G subunit of the t-type calcium channel CaV3.1. This gene is located within 1 Mb of the marker with the highest LOD score for MA-induced locomotor activity (rs13481170; 95.5 Mb). Although we did not observe an e-QTL for Cacna1g (Table 4), we identified a single coding mutation rs27076081 (T1078A) in the A/J allele within exon 16 of Cacna1g (Table S1). Calcium channel antagonists reduce psychostimulant locomotor activity (Hori et al., 1998, Mills et al., 1998, Pani et al., 1990, Pierce et al., 1998), cocaine-induced dopamine release in the caudate (Mills et al., 1998) and striatum (Pani et al., 1990) and cocaine reward (Pani et al., 1991). Mice lacking the gene Cacna1e, which encodes the r-type Ca2+ channel Cav2.3, show a complete loss of the locomotor response to cocaine (Han et al., 2002). Interestingly, calcium channel antagonists reduce the subjectively rewarding effects of MA in healthy human volunteers (Johnson et al., 1999). We also identified an e-QTL in the striatum for another calcium channel gene, Cacng5, which encodes the voltage-dependent calcium channel subunit (Table 3).
Last, in considering genes possessing both eQTLs within the nucleus accumbens and/or striatum (both regions arguably being the most relevant for our phenotype) and coding SNPs, we identified two genes, syntaxin binding protein 4 (Stxbp4) and sorting nexin 11 (Snx11). Both of these genes are located in regions that are highly polymorphic between B6 and A/J. However, out of these two genes, Stxbp4 stood out as a particularly interesting candidate. Syntaxins bind to the dopamine transporter (Torres, 2006) and increase dopamine efflux in response to amphetamine (Binda et al., 2008). Syntaxin binding proteins bind syntaxins which results in a decrease in vesicular exocytosis (Zhang et al., 2000) and thus, a decrease in neurotransmitter release. Thus, differential expression of or coding differences in syntaxin binding proteins could affect methamphetamine-induced neurotransmitter release and contribute to differences in the locomotor response.
In summary, we used the B6.A/J CSS panel to dissect the genetic architecture of locomotor behavior after both saline and MA treatment. We mapped a QTL on chromosome 11 to a small interval and utilized bioinformatic resources to further parse among the candidate genes in the identified region. Because this QTL may influence sensitivity to both stimulants and opioids, identification of the underlying gene or genes could significantly enhance our understanding of sensitivity to drugs of abuse.
Supplementary Material
Table S1. Non-synonymous coding SNPs between A/J and B6 from 79 and 109 Mb. Genes, SNP I.D., physical location, A/J and B6 nucleotides, and the amino acid change are listed for all non-synonymous coding SNPs within the region (Mouse Phenome Database; build 37). Darkened horizontal lines encapsulate either one or multiple SNPs for individual genes.
Acknowledgments
We wish to thank Michaelanne Munoz, Tanya Cebollero, Ryan Walters and Michael DeMeyer for their technical assistance and Dr. Greta Sokoloff for her technical assistance and intellectual input. This work was supported by DA021336 and DA007255.
References
- Belknap JK. Chromosome substitution strains: some quantitative considerations for genome scans and fine mapping. Mamm Genome. 2003;14:723–732. doi: 10.1007/s00335-003-2264-1. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Molecular pharmacology. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue M. Mouse Phenome Project: understanding human biology through mouse genetics and genomics. J Appl Physiol. 2003;95:1335–1337. doi: 10.1152/japplphysiol.00562.2003. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Usiello A, Greengard P, Fisone G. Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology. 2007;32:1995–2003. doi: 10.1038/sj.npp.1301321. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Carlezon WA, Jr, Haile CN, Kosten TA, Heninger GR, Nestler EJ. Genetic analysis of behavioral, neuroendocrine, and biochemical parameters in inbred rodents: initial studies in Lewis and Fischer 344 rats and in A/J and C57BL/6J mice. Brain Res. 1998;805:55–68. doi: 10.1016/s0006-8993(98)00663-5. [DOI] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Sokoloff G, Palmer AA. A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology. 2009a;203:703–711. doi: 10.1007/s00213-008-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS. Pavlovian conditioning of multiple opioid-like responses in mice. Drug and alcohol dependence. 2009b;103:74–83. doi: 10.1016/j.drugalcdep.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Wang J, Williams RW, Manly KF. WebQTL: rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat Neurosci. 2004;7:485–486. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Gill KJ, Boyle AE. Genetic basis for the psychostimulant effects of nicotine: a quantitative trait locus analysis in AcB/BcA recombinant congenic mice. Genes, brain, and behavior. 2005;4:401–411. doi: 10.1111/j.1601-183X.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Han W, Saegusa H, Zong S, Tanabe T. Altered cocaine effects in mice lacking Ca(v)2.3 (alpha(1E)) calcium channel. Biochem Biophys Res Commun. 2002;299:299–304. doi: 10.1016/s0006-291x(02)02632-3. [DOI] [PubMed] [Google Scholar]
- Hori Y, Takeda H, Tsuji M, Matsumiya T. Differentiation of the inhibitory effects of calcium antagonists on abnormal behaviors induced by methamphetamine or phencyclidine. Pharmacology. 1998;56:165–174. doi: 10.1159/000028195. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Bordnick PS, Ait-Daoud N. Isradipine, a dihydropyridine-class calcium channel antagonist, attenuates some of d-methamphetamine's positive subjective effects: a preliminary study. Psychopharmacology. 1999;144:295–300. doi: 10.1007/s002130051007. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Mori E, Oka K, Nomura H, Yatsushige N, Maruyama Y. Effects of SCH23390 injection into the dorsal striatum and nucleus accumbens on methamphetamine-induced gnawing and hyperlocomotion in rats. J Nihon Univ Sch Dent. 1989;31:451–457. doi: 10.2334/josnusd1959.31.451. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes, brain, and behavior. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, Fredholm BB, Nairn AC, Greengard P, Fisone G. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature. 2002;418:774–778. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- McClurg P, Pletcher MT, Wiltshire T, Su AI. Comparative analysis of haplotype association mapping algorithms. BMC bioinformatics. 2006;7:61. doi: 10.1186/1471-2105-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Arsah TA, Ali SF, Shockley DC. Calcium channel antagonist isradipine attenuates cocaine-induced motor activity in rats: correlation with brain monoamine levels. Ann N Y Acad Sci. 1998;844:201–207. [PubMed] [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Pani L, Kuzmin A, Diana M, De Montis G, Gessa GL, Rossetti ZL. Calcium receptor antagonists modify cocaine effects in the central nervous system differently. Eur J Pharmacol. 1990;190:217–221. doi: 10.1016/0014-2999(90)94128-k. [DOI] [PubMed] [Google Scholar]
- Pani L, Kuzmin A, Martellotta MC, Gessa GL, Fratta W. The calcium antagonist PN 200–110 inhibits the reinforcing properties of cocaine. Brain Res Bull. 1991;26:445–447. doi: 10.1016/0361-9230(91)90022-c. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32:707–759. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, Lagler E, Korstanje R, Wang X, Nusskern D, Bogue MA, Mural RJ, Paigen B, Wiltshire T. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS biology. 2004;2:e393. doi: 10.1371/journal.pbio.0020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Bell JT, Copley RR, Taylor MS, Williams RW, Mott R, Flint J. A high-resolution single nucleotide polymorphism genetic map of the mouse genome. PLoS biology. 2006;4:e395. doi: 10.1371/journal.pbio.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE. The dopamine transporter proteome. Journal of neurochemistry. 2006;97(Suppl 1):3–10. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, Jr, de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to D-amphetamine. Neuropsychopharmacology. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Pletcher MT, Batalov S, Barnes SW, Tarantino LM, Cooke MP, Wu H, Smylie K, Santrosyan A, Copeland NG, Jenkins NA, Kalush F, Mural RJ, Glynne RJ, Kay SA, Adams MD, Fletcher CF. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3380–3385. doi: 10.1073/pnas.0130101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR. Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or Inhibitor 1. Biol Psychiatry. 2002;51:612–620. doi: 10.1016/s0006-3223(01)01318-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta -cell. The Journal of biological chemistry. 2000;275:41521–41527. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Non-synonymous coding SNPs between A/J and B6 from 79 and 109 Mb. Genes, SNP I.D., physical location, A/J and B6 nucleotides, and the amino acid change are listed for all non-synonymous coding SNPs within the region (Mouse Phenome Database; build 37). Darkened horizontal lines encapsulate either one or multiple SNPs for individual genes.