Abstract
Anti-retroviral therapy (ART) has improved the quality of life for HIV+ individuals but efficacy requires strict adherence and treatment is not curative. Recently, the use of T cells as therapeutic agents have been in the spotlight in the settings of post-transplant opportunistic infections and cancer. Whether T cell therapy can be harnessed for treating HIV remains to be determined but there are a few studies that seek to answer that question. Infusion of ex vivo expanded HIV-specific T cells showed limited efficacy but no adverse events. Genetically modified T cells expressing CD4 chimeric antigen receptors (CAR) have recently been shown to have persistence that outperforms CARs used for cancers. Although the results have not yet been published for many clinical studies using T cells for HIV, preclinical studies and the clinical data that is available highlight the potential for T cell therapy to decrease or eliminate HIV patients’ dependency on ART.
Keywords: Anti-retroviral therapy (ART): lifelong drug regimen necessary for most HIV+ individuals that consists of a combination of drugs that prevent HIV replication; Adoptive T cell transfer/therapy: Autologous T cells are isolated from an individual and may be modified or expanded ex vivo before re-infusing these cells back into the body. Alternatively, 3rd party T cells may be used; Chimeric Antigen Receptors (CAR): an artificial receptor consisting of the variable fragment of an antibody linked to T cell signaling molecules and may also include T cell stimulatory molecules; Zinc finger nucleases: restriction enzymes designed to target specific DNA sequences to ultimately delete protein expression
INTRODUCTION
The adoptive transfer of T cells recognizing multiple viruses in immune-compromised patients (e.g. CMV, EBV) have shown great promise in reconstituting anti-viral immunity in hematopoietic stem cell transplant recipients [1]. Efforts to exploit the same strategy against HIV in patients with AIDS have so far been met with limited success [2, 3]. However, as in other chronic persistent infections like CMV and EBV, T cells appear to play a crucial role in HIV [4]. Ultimately, definitive cures for HIV/AIDS will require both control/elimination of the virus and restoring T cell immunity. In this article we will review the state of the science in this field exploring the potential role of adoptive T cell transfer as a therapeutic strategy for HIV. Specifically we will discuss recent therapeutic strategies using T cell therapy (Table 1) as well as some pre-clinical studies that may improve the clinical efficacy of HIV-specific T cells.
Table 1. Summary of HIV clinical studies using T cells.
| Strategy | Patients | Status | Clinical Trial ID |
|---|---|---|---|
| CD4 zeta-CAR T cell |
ART experienced | 11+ year persistence tissue-specific efficacy in vivo |
NCT01013415[30] |
| Artificial high- affinity gag- specific TCR |
Responsive to ART treatment, HLA-A2+ |
Active and recruiting | NCT00991224 |
| Expanded autologous CD8 T cells |
CD4 between 100-400 cells/mm3 |
No adverse events, no statistically significant efficacy |
NCT00000756[3] |
| Expanded autologous CD8 T cells |
CD4 above 200 cells/mm3 |
Moderate persistence, homing to reservoir sites |
NCT00110578[35] |
| CCR5 deleted CD4 T cells |
ART experienced, responsive and un- responsive to treatment |
Active and recruiting |
NCT01044654, NCT00842634 |
| CCR5 deleted CD4 T cells |
HIV+ with CD4>500 cells/ mm3 and viral load between 1,000 and 1,000,000 copies/mL |
Active and not recruiting | NCT01252641 |
ENDOGENOUS T CELLS POSSESS ANTI-HIV ACTIVITY
HIV-specific CD8+ T cells from patients infected with the virus strongly correlate with transient decreases in viral load. Strong CD8+ T cell responses against HIV are frequently seen in patients[5], and are associated with decreased levels of detectable virus in plasma during acute infections [6]. CD8+ T cells obtained from HIV-positive individuals have also shown specificity and antiviral activity ex vivo, as measured by interferon gamma secretion and tetramer analysis. Cytotoxic T cells are believed to recognize and kill infected cells presenting HIV antigens as well as secrete an antiviral factor, CD8 associated factor (CAF), that inhibits viral replication independent of cytolytic activity[7]. CAFs are believed to block HIV replication following initiation of the reverse transcription step [8].
Patients who are able to keep the virus in check, HIV controllers, have CD8+ T cells that can efficiently recognize and lyse autologous HIV-infected CD4+ T cells ex vivo even without prior stimulation [9]. The HIV controller phenotype does not seem to rely on an inherent resistance to viral infection since CD4+ T cells from these patients can be superinfected with virus. Hence the evidence suggests that the phenotype is the result of potent CD8+ HIV-specific T cell activity [10].
For a majority of other patients, however, prevention of AIDS is not possible without initiating an ART regimen despite the presence of HIV-specific CD8 T cells. The reasons for this are not entirely clear, and are likely to be multifactorial. Regulatory T cells from HIV patients have been observed to inhibit the cytolytic and non-cytolytic activity of anti-HIV specific T cells[11]. Due to the ability of HIV to mutate rapidly, CD8 T cells may be able to elicit an anti-viral response but mutated variants can escape this initial response, accounting for the initial but unsustained decreased in plasma viral loads [12]. Thus it has been observed that CD8+ T cells isolated from acutely infected individuals recognize different epitopes from the chronically infected patients and the breadth of the T cell response during acute infection is narrower. Indeed, while CD8+ T cells recognize a few epitopes during acute infection, CD8+ T cells isolated from chronically infected individuals recognize as many as 11-13 epitopes [13]. Prolonged antigenic stimulation may also lead to exhaustion, as suggested by associations between disease progression and expression of a T cell exhaustion marker, PD-1 on exhausted T cells derived from HIV+ patients [14]. Strong associations between certain HLA alleles and disease progression point to more efficient antigen presentation in some patients versus non-beneficial CD8+ T cell responses in others. For example, the majority of individuals who can control HIV without the use of HAART, termed elite controllers, express HLA-B*5701 or a closely related allele, B*5801. T cells from these individuals are specific for the gag epitope TW10 (240-249) which is relatively conserved amino acid sequence because mutant variants are disadvantaged in replicative capacity [15]. T cells derived from this rare group of individuals were also shown to have a broader response to gag [16]. Finally, the lack of effective help from CD4+ T cells may also be a factor, as CD4+ T cells in patients are obviously defective from HIV infection[17].
POTENTIAL BENEFITS OF T CELL-BASED IMMUNE STRATEGIES
The fact that otherwise potent T-cells with antiviral activity against HIV are limited by the host environment resembles immune responses to other viruses in immunocompromised hosts. The successful use of T cell immunotherapy in these settings has been shown in phase I and phase II clinical trials[1]. Therefore, the infusion of ex vivo manipulated T cells to treat HIV is, at least in theory, a way to utilize “natural immunity” against the virus while overcoming the immunosuppressive environment seen by endogenous cells in vivo.
T cells can home to sites of infection, directly lyse infected cells, secrete cytokines that will recruit other immune cells, and develop into long-lived memory cells that can confer lifelong protection[18]. Moreover, most studies thus far have reported only mild grade 1 and 2 adverse events following infusion[19]. This safety profile increases in the autologous setting, which would apply to the majority of cases in HIV. Furthermore, as shown in the EBV setting, the benefit to cost ratio can be high with the manufacturing, quality testing, and infusion of virus-specific T cells costing approximately $6,000 per patient [20].
The expansion of HIV-specific CD8+ T cells is the current goal of most HIV vaccine trials [4]. However, the direct infusion of these cells into the patient holds two key advantages over vaccines. The precise phenotype and specificity of T cells can be better controlled for when using T cell immunotherapy, and the limitations imposed by eliciting an immune response in an immune deficient state may be circumvented.
T cell therapy may also be advantageous over other existing and alternative HIV therapies. ART, for example, requires long-term administration, which leads to high recurrent costs and toxic side effects [21]. The use of histone deacetylase inhibitors (HDACi) to reactivate viral replication in order to eliminate reservoirs have potential off-target effects, and are also entirely dependent on ART to prevent new infection from propagating and the efficacy of ART in preventing HIV replication is not 100% [22]. Hematopoietic stem cell transplantation with a CCR5delta32 donor (essentially replacing the patient’s own hematopoietic system with one that is resistant to HIV entry by R5 tropic viruses) is controversial. Few patients warrant a stem cell transplant, and both the cost of the procedure and the potential for the development of lethal GVHD limit the palatability of this approach. It is however, currently the only therapeutic strategy shown to sucessfully eradicate HIV.
Adoptively transferred T cells can specifically recognize and kill; HIV-infected cells, offering a targeted therapeutic approach while limiting off-target effects and bystander organ toxicity. Importantly, it is a treatment modality that can be constantly improved upon. Function can be enhanced by altering specificity and persistence of T cells. Increased safety can be achieved by modifying T cells to be resistant to HIV infection as well as the addition of suicide genes that have been incorporated into genetically modified T cells used in the tumor-specific setting[23].
RE-DIRECTING T CELLS TO HIV
The inability of pre-existing HIV-specific T cells to control virus during treatment interruption despite normalized lymphocyte counts after ART and the limited success of infusing mitogenically expanded CD8+ T cells suggests that it is not the quantity but the quality of T cells that should be the focus of therapeutic development. Therefore, one approach is to enhance recognition of the HIV-infected cells.
Currently, the T cell therapy field is split between genetically modified T cells and polyclonal cytotoxic T cells (CTL). The former is further divided into those modified to express a specific T cell receptor and those transduced with a chimeric antigen receptor (CAR), which combines the specificity of an antibody to the signaling of a T cell receptor. Each has its own set of pros and cons. Artificial TCRs have the advantage of controlling the affinity of the T cell receptor but finding the ideal receptor for the ideal epitope may make manufacturing of these products difficult. One advantage of CAR technology is that CAR-transduced T cells are not limited by class I expression, which has been shown to be downregulated during HIV infection by Nef[24, 25]. However, in contrast, the potential for antigen escape is a concern [26]. Meanwhile, polyclonal CTLs that recognize multiple antigens may overcome antigen escape, and concerns regarding safety. They are however, dependent on class I antigen presentation. That said, there are examples from all three groups that have shown efficacy in the viral and tumor-specific setting but efficacy has not yet been shown for HIV.
ARTIFICAL T CELL RECEPTORS
Varela-Rohena et al identified a high-affinity TCR against the HLA-A2 gag epitope, SL9. This epitope has been shown to be well-conserved due to the lower replicative fitness of HIV expressing variants. Preclinical in vitro studies showed that T cells expressing this artificial TCR could bind to antigen longer and have enhanced effector functions compared to untransduced T cells. They could also recognize common escape variants of SL9, suggesting the high potential of these cells to overcome immune escape[27]. A Phase I clinical study testing the in vivo efficacy of these high-affinity gag-specific T cells in ART patients is currently ongoing (NCT00991224). However, because T cell specificity has been re-directed to a HLA-restricted epitope, patient eligibility has been limited to only the HLA-A2 population, severely restricting the number of patients who can enroll on the study. While this approach could be used to generate high-affinity T cell receptors for other epitopes depending on the patient’s HLA-type, it presents an additional obstacle in the manufacturing process. More advanced clinical studies will be needed to show efficacy in addition to safety.
CHIMERIC ANTIGEN RECEPTORS
It has been recently shown that CAR-transduced T cells have the potential for excellent persistence in vivo despite the immunosuppressive environment described in HIV infected patients. A persistence study encompassed three different clinical trials. The Mitsuyasu study was a Phase II placebo controlled trial that tested whether HIV patients with detectable viral load could control virus when infused with CAR transduced T cells with or without IL-2. [28]. The Deeks study was also a Phase II study that administered multiple T cell infusions in ART patients (no detectable viral load) with either CAR T cells or unmodified T cells[29]. The last trial followed was the Aronson study which compared the ability to control viral load with CAR T cells, CAR T cells with IL-2, or IL-2 alone (NCT01013415). All three trials used a CAR expressing a CD4 molecule on its surface that was fused with the CD3zeta signaling domain (CD4z CAR). The CAR was designed to facilitate T cell interaction with HIV infected cells via gp120’s affinity for CD4, leading to T cell activation and theoretically, killing of the now docked target [30].
The significance of these studies is three-fold. First the results showed that retroviral-engineered T cells were safe to infuse into HIV patients. Second, it demonstrated the ability of genetically modified T cells to persist in patients for over 11 years without the need for immunosuppression, thereby lowering risk further. Lastly, these T cells were shown to be at least compartmentally functional. CAR T cells were able to home to infected rectal tissue sites and lower HIV RNA in some patients[28].
HIV-SPECIFIC CYTOTOXIC T CELLS
While genetically modified T cells may cause adverse events such as insertional mutagenesis or cytokine storms resulting from over-stimulated T cells,[31] polyclonal CTL therapy or the transfer of T cell clones is relatively low risk because it simply infuses an enriched and expanded population of endogenous, naturally occurring T cells. On-going or completed clinical trials have isolated CD8 T cells from patients, screened against HIV peptides for high IFNg release and cytotoxicity ex vivo to select the most promising clones, and then expanded these clones for re-infusion. This process enriches for functional T cells, and is usually augmented with cytokines such as IL-2. Viral load was shown to be decreased following CTL infusion in patients with detectable viremia prior to therapy but this effect was short-lived (~2 weeks) and did not reach statistical significance [3].
There could be a multitude of reasons for the limited persistence and efficacy of these T cells in vivo. One possibility is that the initial burst of CTL activity stimulates antigen escape variants or the epitope recognized by the infused T-cell clone is not abundantly expressed in vivo. This problem may be overcome by infusing a polyclonal T cell product. In contrast to expanding T cells using a single epitope, methods to confer polyclonal specificity are being developed pre-clinically. One way is to electroporate mRNA encoding HIV antigens into dendritic cells (DCs), which are the most potent antigen presenting cell in the immune system. These DCs are then used to stimulate and expand T cells ex vivo[32]. This approach can be supplemented with the use of immunomodulatory drugs such as thalidomide, lenalidomide, and pomalidomide, which have been shown to enhance T cell function in vitro [33]. The advantage of this approach is that T cells may be primed against epitopes that are not immunodominant in vivo, thus expanding the breadth of the T cell response.
INCREASING PERSISTENCE
Other reasons for the limited success of previous CTL trials include the lack of a true central memory population in the T cell product, the immunosuppressive environment found in HIV+ patients, and the lack of CD4 T cells. Infused T cell products containing a central memory population have been shown to exhibit improved persistence in vivo in the cancer setting[34]. These T cells have self-renewing properties unlike effector memory T cells, thus potentially providing a long-term source of antigen-specific T cells. Chapuis et al expanded CD8 T cell clones from ART patients, and confirmed the cytotoxic ability and presence of central memory markers (CD28, CD62L) prior to re-infusion. Infused Tcells were able to persist for up to 84 days in vivo and an increase of T cells expressing these memory markers was observed over time. While the investigators did not report reduction in viral load in vivo, they were able to visualize homing of T cells to the gut-associated lymphoid tissue, which serves as a major anatomical reservoir for HIV [35]. The importance of central memory T cells in controlling HIV infection is also strongly insinuated by the fact that elite controllers maintain levels of highly functional HIV-specific central memory T cells[16]. Therefore, enrichment for central memory T cells, especially in ART experienced patients where T cells may not receive constant antigen stimulation, may be an important step for the generation of effective HIV-specific T cells.
A significant obstacle for the persistence of highly functional T cells is the immunosuppressive environment resulting from HIV infection. HIV+ individuals also have higher percentages of regulatory T cells in vivo[36] and effector T cells are found to express exhaustion markers such as PD-1 in the chronically infected. A potential strategy to decrease T cell susceptibility to negative regulation by PD-1/PD-L1 signaling is to use blocking antibodies. Indeed it has been shown in mice that treatment with PD-L1 blocking antibodies enhanced gag-specific CD8 T cell responses after vaccination with a DC vaccine [37].Although systemic PD-1/PDL-1 blockade has been used clinically and has been tolerated advanced cancer patients, some adverse events were reported [38, 39]. Therefore, we speculate that identifying a strategy to specifically block PD-1/PD-L1 signaling on HIV-specific T cells would improve in vivo function and persistence of these cells in vivo thereby avoiding the potential for adverse off-target side effects when the antibody is administered systemically.
PROTECTING CD4 HELP
While CD8+ T cells play the main role in targeting and killing HIV-infected cells, cytotoxic HIV-specific CD4+ T cells are associated with viral suppression in elite controllers. CD4 T cells are also known to improve persistence and function of CD8 T cells as well as aid in memory formation[40]. However, because CD4 is the main entry receptor used by HIV, CD4+ T cells are immediately susceptible to infection and subsequent dysfunction if they were to be infused into HIV+ patients. Therefore, many have studied ways to protect this important subset of HIV-specific T cells from infection. The CD4 molecule is responsible for the recruitment of enzymes necessary for proper T cell activation as well as interaction with MHC Class II molecules[41]. Deletion of CD4 may render T cells resistant to HIV infection but at the cost of their function. Fortunately HIV utilizes additional co-receptors for infection. CCR5 is a chemokine receptor known to be the major co-receptor utilized by HIV in conjunction with CD4. The success story of the Berlin patient (a now “cured” HIV+ man who received a stem cell transplant for a co-existing acute myeloid leukemia from a donor who was naturally deficient in the CCR5 receptor [42]) demonstrated that the administration of HIV-resistant stem cells may be an efficacious strategy. However, the question of whether stem cell transplantation outside of the cancer context can be justified, coupled with the difficulty of finding an HLA-matched CCR5 deficient donor, provided rationale for determining whether autologous cells could be artificially manipulated to be CCR5 deficient.
Clinical studies (NCT00842634, NCT01252641,NCT01044654) are currently being conducted which evaluate the safety and efficacy of infusing expanded, autologous CD4+ T cells that have the co-receptor CCR5 deleted using zinc finger nucleases. In pre-clinical studies, these T cells were shown to be resistant to HIV infection in culture as well as in vivo in humanized mouse models [43]. Although CCR5 knockdown has been successfully achieved through other methods such as lenti-viral encoded shRNA[44] and nanoparticle delivered siRNA[45], the zinc finger nucleases offer permanent CCR5 gene knockdown.
While the Berlin patient seems to remain seronegative after engraftment of CCR5 deficient cells, some strains of HIV can utilize CXCR4, or both CXCR4 and CCR5 for entry. It has been reported that up to 50% of patients who have been on ART therapy, have persisting CXCR4 or dual-tropic viruses[46]. To address this, these knockdown strategies have expanded to target CXCR4, including a recent publication reporting the development of a CXCR4 zinc finger nuclease and its efficacy in protecting CD4 T cells in a NSG mouse model[47]. Another strategy is to not only block CCR5 but also other proteins that are necessary for HIV replication. Anderson et al developed a triple lenti-viral vector that not only deletes CCR5 expression but also theoretically prevents integration using viral uncoating protein TRIM5α and prevents transcription by using a TAR (transactivation response element) decoy molecule [48]. Although these studies have not yet been translated into humans, broader inhibition may prove to be necessary to prevent HIV escape mutants and enlargement of the viral reservoir.
FUTURE PERSPECTIVE
While the Berlin patient has inspired the resurgence of hope for the definitive cure, the field still does not have a robust therapeutic modality to reliably achieve a cure for every HIV+ individual, especially those without a hematologic malignancy. T cell therapy, which has recently been in the public spotlight for cancer, may also be promising for the HIV setting. To date, T cell therapies have not achieved durable statistically significant clinical benefits for HIV infected individuals, in spite of the many T cell therapy clinical trials for HIV which have been conducted. However, while these early studies using unmodified T cells may not have been successful, the lack of adverse events enabled these clinical studies to be a springboard for methods to enhance recognition of HIV-infected cells and improve the persistence and function of HIV-specific T cells.
Unlike the cancer setting, many HIV+ individuals in the Western world who can benefit from T cell therapy are generally healthy if they are on a rigorous ART regimen. Therefore, T cell therapy could arguably have more promise for HIV patients than for patients with cancer in terms of their safety and persistence. This is highlighted by the ability of CD4zeta CAR T cells to persist longer than many cancer-specific CAR-transduced T cells in vivo [30].
The potential for high specificity, proliferative and migratory abilities, and the feasibility of manufacturing a safe, clinical grade product are universal advantages of T cell therapy. Given the advances in the T cell therapy field for cancer, these approaches may be also applied to the HIV setting. For example, the incorporation of suicide genes on gene modified T cells can further increase the safety of adoptive T cell therapy [23]. Additionally, exogenous cytokines can be administered to improve in vivo persistence. T cells are also being developed as an off-the-shelf or 3rd party product targeting other latent viruses such as CMV and EBV (NCT00711035) and if successful, could potentially also utilize HIV-specific T cells as an “off the shelf” therapy. This enabling broadening of the technology for less developed countries which may lack facilities to manufacture autologous T cell products on site or those countries without continuous access to ART.
In conclusion, there are still several major obstacles to achieve a durable cure versus simply lowering dependency on ART. Whether T-cell therapies could eliminate the need for antivirals altogether is dependent on the ability of T cells to target the latent reservoirs. Although Chapuis et al demonstrated that ex vivo expanded T cells can home to the gut-associated lymphoid tissue, it remains unknown whether these cells can eliminate latent HIV. Furthermore the brain serves as a second major anatomical reservoir with the presence of HIV-infected microglial cells and it is uncertain whether T cells can safely migrate to this part of the body and kill the infected cells without brain damage. Nevertheless, it is likely that similar to the cancer setting, curing HIV patients that do not otherwise need a stem cell transplant will lie in the successful development of a combination of different therapeutic modalities from antiretroviral agents to immune based therapies.
EXECUTIVE SUMMARY.
ENDOGENOUS T CELLS POSSESS ANTI-HIV ACTIVITY
HIV+ individuals do have HIV-specific T cells.
T cells can initially inhibit HIV but this response is not sustained in the majority of HIV patients.
There are functional and phenotypic differences found in T cells isolated from patients after acute infection versus chronic infection.
POTENTIAL BENEFITS OF T CELL-BASED IMMUNE STRATEGIES
T cells can proliferate, migrate, and specifically kill targets in response to antigen stimulation.
T cell therapy is already being used in clinical trials for the treatment of cancer and opportunistic viral infections post-transplant.
It has shown to be safe in the HIV setting.
RE-DIRECTING T CELLS TO HIV
Artificial T cell receptors with specificity for an HLA-A2 restricted epitope of Gag has been developed and are now being tested in Phase I clinical trials.
CD4zeta CAR T cells have been infused into HIV+ patients and have shown long-term safety and persistence.
Adoptive transfer of HIV-specific cytotoxic T cells have shown safety but no clear efficacy.
INCREASING PERSISTENCE
HIV-specific T cells with central memory phenotypes may have increased persistence in vivo.
Inhibiting T cell exhaustion by blocking PD-1/PDL-1 has shown benefit in HIV murine studies but has not been tested clinically.
PROTECTING CD4 HELP
CD4 T cells are essential for optimal CD8 T cell function and memory formation.
Various strategies aiming to delete HIV entry receptors such as CCR5 on CD4 T cells are being tested in clinical trials and may be able to imitate the effect of a hematopoetic stem cell transplant with a CCR5delta donor.
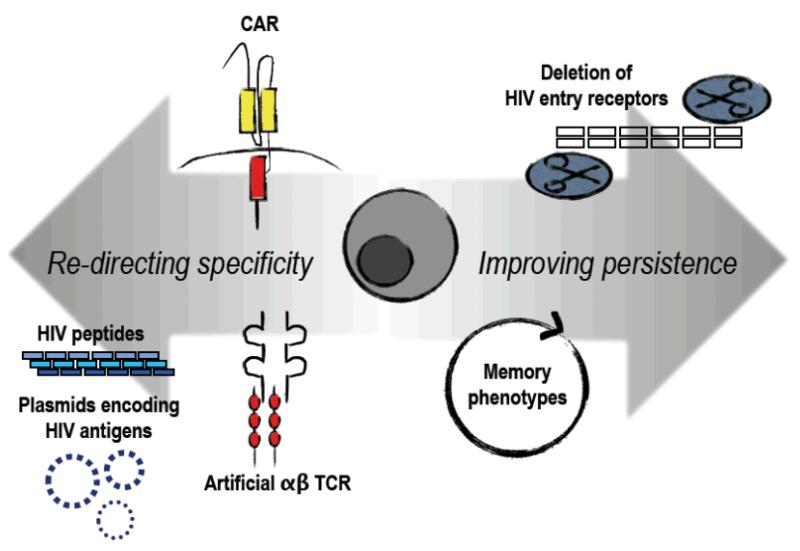
Figure 1. Strategies to improve anti-HIV T cells.
T cells can be modified to more effectively target HIV infected cells by increasing the specificity to HIV antigens. This can be achieved by genetically modifying T cells with a chimeric antigen receptor (CAR) or an artificial T cell receptor that may have extraordinarily high affinity to HIV epitopes. T cells can also be stimulated using peptides or mRNA for HIV antigens. HIV-specific T cells can be enriched for memory phenotypes prior to infusion which have more potential for persistence versus effector T cells. HIV entry can also be inhibited to protect the CD4 pool, allowing immune reconstitution as well as supporting CD8 T cell activity.
Acknowledgments
This work was supported in part by the Baylor-UTHouston Center for AIDS Research Core Support Grant number AI36211 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature medicine. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 2.Koenig S, Conley AJ, Brewah YA, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nature medicine. 1995;1(4):330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman J, Skolnik PR, Parkerson GR, 3rd, et al. Safety of autologous, ex vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte infusion in HIV-infected patients. Blood. 1997;90(6):2196–2206. [PubMed] [Google Scholar]
- 4.Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harbor perspectives in medicine. 2011;1(1):a007252. doi: 10.1101/cshperspect.a007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker BD, Chakrabarti S, Moss B, et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 6.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of virology. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saksena NK, Wu JQ, Potter SJ, Wilkinson J, Wang B. Human immunodeficiency virus interactions with CD8+ T lymphocytes. Current HIV research. 2008;6(1):1–9. doi: 10.2174/157016208783572008. [DOI] [PubMed] [Google Scholar]
- 8.Le Borgne S, Fevrier M, Callebaut C, Lee SP, Riviere Y. CD8(+)-Cell antiviral factor activity is not restricted to human immunodeficiency virus (HIV)-specific T cells and can block HIV replication after initiation of reverse transcription. Journal of virology. 2000;74(10):4456–4464. doi: 10.1128/jvi.74.10.4456-4464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saez-Cirion A, Pancino G, Sinet M, Venet A, Lambotte O. HIV controllers: how do they tame the virus? Trends in immunology. 2007;28(12):532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kinter A, Mcnally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. Journal of virology. 2003;77(3):2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 15.Miura T, Brockman MA, Schneidewind A, et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. Journal of virology. 2009;83(6):2743–2755. doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndhlovu ZM, Proudfoot J, Cesa K, et al. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. Journal of virology. 2012;86(12):6959–6969. doi: 10.1128/JVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nature medicine. 1999;5(5):518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 18.Mackay CR. Dual personality of memory T cells. Nature. 1999;401(6754):659–660. doi: 10.1038/44309. [DOI] [PubMed] [Google Scholar]
- 19.Cruz CR, Hanley PJ, Liu H, et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy. 2010;12(6):743–749. doi: 10.3109/14653241003709686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard J. The Side Effects of HAART. 2010;2012(December 26) [Google Scholar]
- 22.Crowe SM, Sonza S. HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. Journal of leukocyte biology. 2000;68(3):345–350. [PubMed] [Google Scholar]
- 23.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen GB, Gandhi RT, Davis DM, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10(6):661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 25.Lundquist CA, Tobiume M, Zhou J, Unutmaz D, Aiken C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. Journal of virology. 2002;76(9):4625–4633. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganusov VV, Goonetilleke N, Liu MK, et al. Fitness costs and diversity of the cytotoxic T lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. Journal of virology. 2011;85(20):10518–10528. doi: 10.1128/JVI.00655-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature medicine. 2008;14(12):1390–1395. doi: 10.1038/nm.1779. This publication demonstrated the development and in vitro efficacy of a HIV-specific artificial T cell receptor.
- 28.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–793. [PubMed] [Google Scholar]
- 29.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2002;5(6):788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 30*.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Science translational medicine. 2012;4(132) doi: 10.1126/scitranslmed.3003761. 132ra153. This publication highlights the potential for CAR-modified T cells to persist long term in vivo in HIV+ individuals without the need for lymphodepletion.
- 31.Heslop HE. Safer CARS. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18(4):661–662. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gulck ER, Vanham G, Heyndrickx L, et al. Efficient in vitro expansion of human immunodeficiency virus (HIV)-specific T-cell responses by gag mRNA-electroporated dendritic cells from treated and untreated HIV type 1-infected individuals. Journal of virology. 2008;82(7):3561–3573. doi: 10.1128/JVI.02080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Keersmaecker B, Allard SD, Lacor P, Schots R, Thielemans K, Aerts JL. Expansion of polyfunctional HIV-specific T cells upon stimulation with mRNA electroporated dendritic cells in the presence of immunomodulatory drugs. Journal of virology. 2012;86(17):9351–9360. doi: 10.1128/JVI.00472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. The Journal of clinical investigation. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapuis AG, Casper C, Kuntz S, et al. HIV-specific CD8+ T cells from HIV+ individuals receiving HAART can be expanded ex vivo to augment systemic and mucosal immunity in vivo. Blood. 2011;117(20):5391–5402. doi: 10.1182/blood-2010-11-320226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clinical and experimental immunology. 2009;155(1):44–52. doi: 10.1111/j.1365-2249.2008.03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai B, Xiao L, Bryson PD, Fang J, Wang P. PD-1/PD-L1 blockade can enhance HIV-1 Gag-specific T cell immunity elicited by dendritic cell-directed lentiviral vaccines. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(9):1800–1809. doi: 10.1038/mt.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiesel M, Oxenius A. From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. European journal of immunology. 2012;42(5):1080–1088. doi: 10.1002/eji.201142205. [DOI] [PubMed] [Google Scholar]
- 41.Koretzky GA. Multiple roles of CD4 and CD8 in T cell activation. Journal of immunology (Baltimore, Md.: 1950) 2010;185(5):2643–2644. doi: 10.4049/jimmunol.1090076. [DOI] [PubMed] [Google Scholar]
- 42*.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. This study is the case of the Berlin patient and showed that hematopoetic stem cell transplant using a CCR5 donor has cured this patient of HIV and to date is the only strategy to achieve this.
- 43.Cannon P, June C. Chemokine receptor 5 knockout strategies. Current opinion in HIV and AIDS. 2011;6(1):74–79. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson JS, Walker J, Nolta JA, Bauer G. Specific transduction of HIV-susceptible cells for CCR5 knockdown and resistance to HIV infection: a novel method for targeted gene therapy and intracellular immunization. Journal of acquired immune deficiency syndromes (1999) 2009;52(2):152–161. doi: 10.1097/QAI.0b013e3181b010a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SS, Peer D, Kumar P, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. American Society of Gene Therapy. 2010;18(2):370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medco Health Solutions I CCR5 Tropism testing for Maraviroc. 2004-2012;2012(December 26) [Google Scholar]
- 47.Yuan J, Wang J, Crain K, et al. Zinc-finger nuclease editing of human cxcr4 promotes HIV-1 CD4(+) T cell resistance and enrichment. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(4):849–859. doi: 10.1038/mt.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson JS, Javien J, Nolta JA, Bauer G. Preintegration HIV-1 Inhibition by a Combination Lentiviral Vector Containing a Chimeric TRIM5α Protein, a CCR5 shRNA, and a TAR Decoy. Molecular Therapy: the jounral of the American Society of Gene Therapy. 2009;17(12):2103–2114. doi: 10.1038/mt.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]