Abstract
The inflammasome has emerged as an important molecular protein complex which initiates proteolytic processing of pro-IL-1β and IL-18 into mature inflammatory cytokines. In addition, inflammasomes initiate pyroptotic cell death that may be independent of those cytokines. Inflammasomes are central to elicit innate immune responses against many pathogens, and are key components in the induction of host defenses following bacterial infection. Here, we review recent discoveries related to NLRP1, NLRP3, NLRC4, NLRP6, NLRP7, NLRP12 and AIM2-mediated recognition of bacteria. Mechanisms for inflammasome activation and regulation are now suggested to involve kinases such as PKR and PKCδ, ligand binding proteins such as the NAIPs, and caspase-11 and caspase-8 in addition to caspase-1. Future research will determine how specific inflammasome components pair up in optimal responses to specific bacteria.
Introduction
The innate immune system is the first line of defense against pathogens and is initiated by genome-encoded pattern recognition receptors (PRRs) which respond to invading microbes. Upon infection, PRRs recognize microbial pathogen-associated molecular patterns (PAMPs) and endogenous danger-associated molecular patterns (DAMPs), leading to the activation of host defense pathways that result in the clearance of the infection. Toll-like receptors (TLRs) are a well-defined group of membrane-bound extracellular and endosomal receptors that play an important role in pathogen detection. A relatively new and interesting PRR-containing complex in innate immunity is the inflammasome, a multi-protein complex that acts as a platform for the activation of the pro-inflammatory caspase-1; the active form of which then proteolytically cleaves the cytosolic-sequestering leader sequence from pro-IL-1β, pro-IL-18, and pro-IL-33 [1,2] to generate mature cytokines which are released from the cell to mediate downstream inflammatory effects.
Typical inflammasomes are constructed of pro-caspase-1 and proteins in the cytosolic NLR (nucleotide-binding domain and leucine-rich repeat containing) family, or AIM2. Some require the adapter protein ASC that mediates interaction between the NLR or AIM2 and caspase-1. NLRs are comprised of a pyrin-domain (or an amino-terminal caspase-activation and recruitment domain (CARD)), a nucleotide-binding and oligomerization domain (NOD), and leucine-rich repeats (LRRs) [2] that are responsible for the recognition of PAMPs or other signals (Figures 1 and 2). Inflammasome-mediated cytokine release follows a multi-step activation pathway: first an NF-κB-dependent upregulation of the inactive pro-forms of IL-1β and IL-18 and also of some NLRs like NLRP3 [3], and second, activation of the NLR or AIM2 and inflammasome formation (Figure 1). Recently, a 3-step activation pathway has been described for some Gram-negative bacteria that involves caspase-11 and TLR4/TRIF [4,5]. It should be noted that some cells may have a simpler activation process due to higher basal levels of the pro-forms of caspase-1 and/or pro-cytokines [6]. Some inflammasomes have been well characterized for their role in bacterial recognition (NLRC4, NLRP3, AIM2), whereas details are emerging for others (NLRP1b, NLRP6, NLRP7, NLRP12). Furthermore, negative regulators of inflammasomes have also been proposed, although their relation to bacterial infection has yet to be defined [7]. Inflammasome activation has also been linked to cell death pathways (e.g., pyroptosis) [8]. This review centers on recent observations that have led to better understanding of inflammasome-mediated host defenses against invading bacterial pathogens.
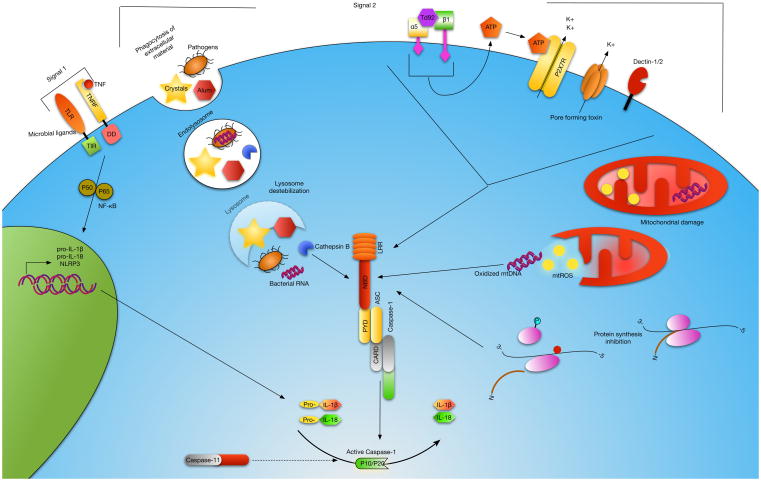
Figure 1. Model of NLRP3 activation.
Activation of caspase-1 by the NLRP3 inflammasome is a multi-signal process. Signal 1 occurs when TNF or a TLR ligand binds its cognate receptor resulting in the translocation of NF-κB into the nucleus where expression of NLRP3 and the immature (pro-) forms of IL-1β and IL-18 are induced. Signal 2 is the activation of NLRP3 resulting in recruitment and cleavage of pro-caspase-1 to its active form leading to cleavage of the immature inflammatory cytokines. At least three distinct NLRP3 activation pathways have been identified. Phagocytosis of extracellular particulates and pathogens results in lysosomal destabilization and release of cathepsin B and bacterial mRNA which trigger NLRP3 activation. A decrease in intracellular K+ has been shown to result in activation of NLRP3. K+ efflux occurs by engagement of extracellular ATP with the P2X7R or directly through bacterial pore-forming toxins. ROS generated during mitochondrial damage and oxidized mitochondrial DNA (mtDNA) produced during apoptosis lead to activation of NLRP3. Td92, a surface protein of Treponema denticola, can interact with the α5β1 integrin resulting in ATP release and K+ efflux. Inhibition of ribosomal function and protein synthesis can also direct NLRP3 activation, and this mechanism may involve lysosomal destabilization, K+ efflux and ROS. Caspase-11 has been defined upstream of caspase-1 during NLRP3 inflammasome activation.
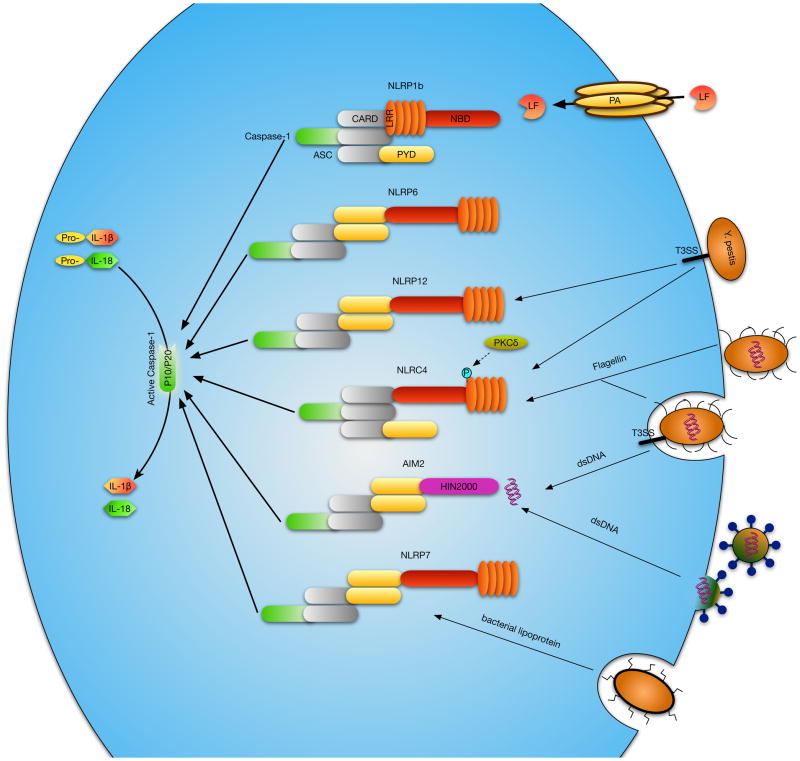
Figure 2. Inflammasome design and activators.
Inflammasome-forming NLRs share the same general features, although mechanisms for ligand recognition may differ. NLRP3, 6, 7 and 12 all share a C-terminal leucine-rich-repeat (LRR) region, an internal nucleotide-binding-domain (NBD), and a N-terminal Pyrin domain (PYD), which recruits the adapter protein ASC - a caspase recruitment and activation domain (CARD) and PYD containing protein which links the NLR or AIM2 to caspase-1. The ASC adapter is believed to be an integral part of NLRP3, 6, 7 and 12 inflammasomes. NLRC4 contains an N-terminal CARD domain which can recruit caspase-1 directly, though ASC involvement may increase caspase-1 processing activity. NLRP1b, like NLRC4, has a N-terminal CARD domain, but has an internal LRR and a C-terminal NBD domain. Lastly, AIM2 has a HIN200 DNA binding domain and a PYD for ASC recruitment. Different types of stimuli signal inflammasome activation via the various NLRs or AIM2, some of which are depicted.
The NLRP3 inflammasome
The NLRP3 inflammasome is described to be involved in host responses to a wide variety of pathogenic microorganisms (Table I). It is activated by a number of PAMPs and DAMPs, and is upregulated in cells after TLR stimulation (Figure 1) [3]. NLRP3 activation and subsequent inflammatory damage has also been linked to the pathogenesis of diseases characterized by crystal-mediated sterile inflammation, e.g., atherosclerosis caused by the deposition of cholesterol crystals [9]. Other examples of exogenous NLRP3 activators include silica and asbestos, leading to silicosis and asbestosis, respectively [2].
Table 1.
Bacterial inflammasome activators
Bacterial pathogens-ligands and their known signaling inflammasomes. The table contains upstream, and in some cases, downstream inflammasome effects after pathogen activation.
| Pathogen | Putative Bacterial Activator | Inflammasome | Proposed Mechanism | Role in host defense in vivo | References |
|---|---|---|---|---|---|
| Bacillus anthracis | Lethal toxin | NALP1b/NLRP1b | phospho-PKR, ATP leakage/K+ efflux, pyroptosis | Yes | 19, 40, 58, 59 |
| Burkholderia pseudomallei | BsaK | NLRP3, NLRC4 | nd | Yes | 23, 60 |
| Burkholderia thailandensis | BsaK | NLRC4/NAIP2 | Ligand binding to NAIP | nd | 22 |
| Chlamydia pneumoniae | nd | NLRP3 | oxidized mtDNA, K+ efflux, lysosomal acidification, cathepsin B release | nd | 16, 61 |
| Chromobacterium violaceum | CprI | NLRC4/human NAIP | Ligand binding to NAIP | nd | 22 |
| Citrobacter rodentium | mRNA | NLRP3 | TLR4/TRIF, Caspase-11 | nd | 5 |
| Escherichia coli | mRNA | NLRP3 | TLR4/TRIF, Caspase-11, lysosomal rupture | Yes | 5, 13 |
| nd | NLRP3 | phospo-PKR | Yes | 19 | |
| EprJ, Escl | NLRC4/NAIP5, NLRC4, | nd | Yes | 23, 30 | |
| Franciscella tularensis | DNA | AIM2 | K+ efflux, lysosomal acidification | yes | 35, 36 |
| Group B Streptococcus | β-hemolysin | NLRP3 | K+ efflux | Yes | 12 |
| Legionella pneumophila | Flagellin | NLRC4/NAIP5 | Ligand binding to NAIP, cPLA2, eicosanoid release, caspase-7 activation | Yes | 22, 27, 31 |
| Listeria monocytogenes | nd | NLRP3 | GBP5 | Yes | 18 |
| LLO, DNA? | NLRP3, AIM2 | nd | nd | 57 | |
| Mycobacterium tuberculosis | DNA | AIM2 | nd | Yes | 62 |
| ESX-1, ESAT-6 | NLRP3 | pore formation | nd | 63 | |
| ASC/Caspase-1 | dependent in vitro, not in vivo | yes | 66 | ||
| Pseudomonas aeruginosa | PscL | NLRC4 | nd | nd | 23 |
| Flagellin | NLRC4/NAIP5 | Ligand binding to NAIP | nd | 22 | |
| Salmonella enterica serovar Typhimurium | nd | NLRP3, NLRC4 | GBP5, phospo-PKR | nd | 18, 19 |
| PrgJ | NLRC4, NLRC4/NAIP2 | Ligand binding to NAIP | Yes | 23, 22, 26 | |
| T3SS, Flagellin | NLRP3, NLRC4 | phospho-Ser533, PKCδ, TLR4/TRIF, Caspase-11 | Yes | 28, 30, 33, 4 | |
| Shigella flexneri | Mxil | NLRC4 | nd | nd | 23 |
| Staphylococcus aureus | α-hemolysin | NLRP3 | K+ efflux | Yes | 64 |
| Streptococcus pneumoniae | pneumolysin | NLRP3 | K+ efflux | Yes | 65 |
| Treponema denticola | Td92 | NLRP3 | ATP leakage/K+ efflux | nd | 67 |
| Vibrio cholera | cholera toxin B | NLRP3 | Caspase-11 dependent | nd | 53 |
| Yersinia pestis | T3SS | NLRP3, NLRC4 | ND | Yes | 24 |
| YopJ | NLRP3 | K+ efflux | nd | 68 | |
| T3SS, YopJ | NLRP12, NLRP3 | nd | Yes | 46 |
nd, not determined
Models for NLRP3 activation
Three models of NLRP3 activation in response to microbial ligands have been proposed [2]. The channel model proposes that extracellular ATP from microbial pathogens activates the P2X7 receptor and allows the efflux of intracellular potassium ions (K+) resulting in NLRP3 activation [10,11]. A number of bacterial pore-forming toxins (e.g. Group B Streptococcus β-hemolysin [12]) can also cause cellular ion dysregulation and subsequent NLRP3 activation (Table I). However, cellular activation induced by a number of bacteria is independent of P2X7R, emphasizing that multiple pathways can lead to NLRP3 activation [11].
Escape from the lysosome after phagocytosis is an important step during the movement of many pathogens, toxins, and cholesterol-dependent cytolysins. The lysosomal rupture model for NLRP3 activation posits that the release of lysosomal enzymes, such as cathepsin B, into the cell cytoplasm during lysosomal destabilization leads to NLRP3 activation [2]. Recent studies have shown that prokaryotic mRNA released from the lysosome into the cytosol during degradation of phagocytosed live bacteria, can activate NLRP3 [13], suggesting that bacterial RNA may be a key trigger of the NLRP3 inflammasome during many infections.
Reactive oxygen species (ROS) released from the mitochondria is considered to be a cellular stress-induced alarm and may trigger NLRP3 inflammasomes [14]. The ROS model is based on observations that NLRP3 is activated upon mitochondrial damage and release of ROS [15]. This activity is dependent on the mitochondrial voltage-dependent ion channels which facilitate the exchange of ions between the intermembrane space and the cell cytosol. Oxidized mitochondrial DNA (mtDNA) from mitochondria damaged by bacterial infection or other means was suggested to bind and activate NLRP3 [16]. This phenomenon was negatively regulated by the anti-apoptotic protein Bcl2, suggesting a link between apoptosis and inflammasome activation. The idea that the NLRP3 inflammasome senses mitochondrial dysfunction could potentially help in understanding the prior observations suggesting an association of mitochondrial damage with inflammatory diseases [17].
Newly identified factors controlling NLRP3 activation
Recently, GBP5 (guanylate-binding protein 5) and PKR (double-stranded RNA-dependent protein kinase) have been proposed to play integral roles in NLRP3 activation. GBP5 has been demonstrated to interact with the pyrin domain of NLRP3 and aids in the oligomerization of the inflammasome complex [18]. Importantly, GBP5 promotes a role in NLRP3 activation by live bacteria, but not during sterile inflammation in vitro. Infected GBP5-deficient mice had higher bacterial burdens and faster disease progression in comparison to wild-type mice. PKR has also been shown to autophosphorylate upon macrophage stimulation with NLRP3 ligands, and active PKR can bind NLRP3 (as well as NLRP1b, NLRC4 and AIM2) [19]. This study proposes that PKR can directly activate the NLRP3 inflammasome and promote release of the pro-inflammatory cytokine HMGB1 (high-mobility group protein B1) when stimulated with a cohort of ligands including bacterial pathogens. Since many of the NLRP3 stimuli lead to inhibition of protein synthesis, it has also been found that direct blocking of ribosomal function, independent of K+ efflux, leads to inflammasome activation [20]. It is however unclear how the PKR phosphorylation leading to NLRP3 inflammasome activation is triggered during infection. Although multiple pathways lead to NLRP3 activation, the array of ligands which trigger the NLRP3 inflammasome indicates that common denominators exist (Figure 1).
The NLRC4 inflammasome and pathogen detection
A number of bacteria including Pseudomonas, Legionella, Salmonella, Yersinia, and Listeria are thought to induce caspase-1 activation and IL-1β/IL-18 maturation via NLRC4 activation [21–25]. NLRC4 is specifically activated by a functional bacterial type III or IV secretion system (T3SS/T4SS) or flagellin [22,23]. Salmonella typhimurium was one of the first bacteria shown to activate caspase-1 via the NLRC4-inflammasome; and mutants of S. typhimurium lacking the fliC and flipB genes that encode flagellin monomers are unable to activate caspase-1 via NLRC4 [23]. Ligands are believed to directly bind distinct members of the NAIP (neuronal apoptosis inhibitor protein) NLR subfamily to subsequently activate the NLRC4 inflammasome [26]. NAIP5/NAIP6 interact with flagellin, whereas NAIP2 interacts with the T3SS rod components Salmonella PrgJ and Burkholderia BsaK [22,26]. Although this model of ligand binding and differentiation by NAIPs is appealing, it should be pointed out that there is only one human NAIP protein and this recognizes the Chromobacterium violaceum T3SS needle protein CprI [22]. Interestingly, NLRC4/NAIP5 inflammasome activation has been directly tied to eicosanoid release from resident peritoneal macrophages [27]. NLRC4 has been implicated in a pro-inflammatory defense mechanism in the intestine against foreign, but not commensal, bacteria by intestinal mononuclear phagocytes [28]. Recently, Ayres et al proposed that NLRC4 activation can be detrimental to the host during infection, because the growth of pathogenic E. coli emerges in the intestine after antibiotic treatment which severely alters the composition of microbiota [29]. Perhaps analogous to NLRP3 activation by PKR, new convincing evidence show that NLRC4 is activated upon phosphorylation at Ser533 by Protein Kinase C δ (PKCδ) [30]. NLRC4 is also tied to other processes which do not involve IL-1β and IL-18 release; such as the degradation and restriction of intercellular bacterial growth of Legionella pneumophila downstream of caspase-7 activation [31] and NLRC4-dependent cell death [32]. Activation of caspase-1 by the inflammasome has been linked to pyroptosis, a proinflammatory form of caspase-1-dependent cell death [8]. NLRC4-dependent pyroptosis occurs in response to a number of bacterial infections including Salmonella, L. pneumophila, and Francisella tularensis [8]. This cell death pathway exerts a protective role in host defense. Activation of caspase-1 via NLRC4 by S. typhimurium persistently expressing flagellin led to antibacterial defenses independently of IL-1β and IL-18 [32], as the inflammatory cell death drove clearance of the pathogen from the surrounding tissue via neutrophil recruitment. It has also been shown that NLRP3 and NLRC4 work together for an optimal response to Salmonella [33]. It is likely that many bacteria will trigger multiple inflammasomes upon infection, as they contain ligands for multiple NLRs, or NLRs and AIM2. Future studies will undoubtedly cast more light on how inflammasome components cooperate for optimal host responses to pathogens and clarify the involvement of subsets of components for specific downstream effects.
The AIM2 Inflammasome and intracellular bacteria
AIM2 (Absent in Melanoma 2) is a cytosolic binding receptor for double-stranded DNA, known to form an inflammasome and activate caspase-1 in the presence of bacteria and viruses [34–36]. It contains an N-terminal pyrin domain and a C-terminal DNA-binding HIN200 domain; and is the only known HIN200 domain-containing protein with the ability to mature IL-1β and IL-18 via interactions with ASC and caspase-1 [34]. Bone marrow-derived macrophages (BMDMs) from AIM2−/− mice are deficient in pro-caspase-1, pro-IL1β, and pro-IL18 processing after infection with Listeria monocytogenes and Francisella [35–37]; thus emphasizing the importance of inflammasome activation against bacteria that replicate intracellularly. An anti-bacterial phenotype was verified in vivo as AIM2-deficient mice were more susceptible to subcutaneous infection with F. tularensis, correlating to reduced serum IL-18 levels [35].
NLRP1b, NLRP6, NLRP7, NLRP12 inflammasomes
The original description of the inflammasome complex involved human NLRP1 (Figure 2) [38]. Studies in THP1 cells showed that NLRP1 forms a complex with CARD8, ASC, caspase-5, and caspase-1 to subsequently process IL-1β [38]. NLRP1 has been linked to IL-1β production by muramyl-dipeptide in a Bcl-2- and Bcl-XL-regulated fashion [39]. Moreover, mouse NLRP1b has been described as a receptor for lethal toxin from Bacillus anthracis in the host cytosol and participates in caspase-1-mediated IL-1β production and pyroptosis, in vivo and in vitro [40].
NLRP6 has been reported to be involved in obesity, intestinal inflammation and tumorigenesis, the regulation of commensal microflora, and most recently, bacterial recognition [41–44]. The NLRP6 inflammasome function has been proposed as NLRP6-deficient mice showed altered gut microbiota and a predisposition for colitis as a result of decreased levels of IL-18 secretion by intestinal epithelial cells [41]. However, Anand et al. [42] presented a novel function for NLRP6 during certain bacterial infections as a negative regulator of innate immunity, since mice deficient for NLRP6 were resistant to infection with L. monocytogenes, Salmonella, and E. coli.
An early study of NLRP12 (Monarch-1/PYPAF7) showed that the protein could function as an inflammasome component [45]. Consistent with this, the NLRP12 inflammasome has been described to have a pro-inflammatory role during bacterial infection as an important regulator of IL-1β and IL-18 release [46]. In this study, NLRP12-deficient mice were unable to control infection with a modified Y. pestis strain, and had reduced circulating IL-1β and IL-18 and increased spleen bacterial loads [46]. Other studies have suggested NLRP12 as a negative regulator of colon inflammation and tumorigenesis in a DSS colitis model, and to dendritic cell recruitment [47–49]. It is possible that NLRP6 and NLRP12, and also other NLRs, can play multiple roles in immune function, perhaps dependent upon expression levels in tissues and cells central for specific pathology in various diseases and cooperation with other signaling molecules. NLRP7 is not expressed in mice, but hNLRP7 has been linked to inflammasome function in response to bacterial lipopeptides (TLR2 ligands) using a siRNA knockdown system [50]. The main function of another family member, NLRC5, appears to be in regulation of MHC class I genes [51], although knock-down data in human cells suggest NLRC5 may participate in inflammasome activation during infection [52].
Caspase-1 is not alone – roles of caspase-8 and caspase-11
Recent studies have shown that mouse caspase-11, an orthologue of human caspase-4 and caspase-5, contributes to caspase-1-independent cell death in response to a number of bacterial pathogens [4,5,53]. To the great surprise of the field, it was revealed that that widely used caspase-1-deficient mice, generated on a 129 background, also lack a functional allele of caspase-11, and are therefore functionally caspase-1/caspase-11 double knockouts [53]. Importantly, caspase-11 was found to be a key molecule in inflammasome activation by cholera toxin, E. coli, Vibrio cholerae and C. rodentium, and a central mediator of LPS-induced lethal shock [53], although caspase-11 works upstream of caspase-1 for IL-1β processing and independently of caspase-1 for the induction of cell death. Rathinam et al. subsequently found that TLR4/TRIF-dependent type I IFN production is crucial for caspase-11 activation, and this licenses NLRP3-inflammasome-induced caspase-1 processing, thus providing another link between TLR and NLR signaling. Broz et al. [4] supported this role for TRIF, and it appears that in the absence of caspase-1 [4] or neutrophil-mediated phagocytosis [32], lysis of macrophages and the release of intracellular Salmonella can be detrimental to the host in a caspase-11-dependent manner.
Other developments have showed that caspase-1-independent IL-1β processing can be dependent on the caspase-8 inflammasome [54]. This was triggered by antagonism of IAP (inhibitor of apoptosis) proteins and involved the RIP3 kinase and ROS production. The findings demonstrated that activation of the cell death-inducing ripoptosome platform generates IL-1β and IL-1β-driven inflammation, although another study suggested that IAP deficiencies reduced caspase-1 activation [55]. Dectin-1 and caspase-8 have also been implicated in IL-1β release in response to Mycobacterium bovis BCG and M. leprae [56]. Taken together, these studies suggest there may be more paths to IL-1β and IL-18 processing than via caspase-1.
Conclusions and further directions
Inflammasome function and pyroptotic cell death are key events in the host response to bacterial pathogens. However, this is a double-edged sword as dysfunction and dysregulation can drive human inflammatory diseases, and there is a need for balance between resolution of infection and excessive inflammation. Thus, we will likely see further studies of regulators of inflammasome activity and infections [7]. A number of pathogens activate multiple inflammasomes, such as Y. pestis, Salmonella, and L. monocytogenes, [33,46,57] and increasing the knowledge of how multiple NLRs or AIM2 and specific caspases cooperate during infections is likely to be the focus of several future studies. Continuing investigation into inflammasome activation mechanisms, including proposed upstream activators such as cathepsins, ROS, GBP5, PKR and PKC will drive our understanding of inflammation and hopefully elucidate novel drug targets for both antimicrobial and anti-inflammatory uses.
Highlights.
The inflammasomes are central for many anti-bacterial host defenses.
In addition to caspase-1, caspases-11 and -8 are emerging as important players in pro- inflammatory signaling.
NLRP1, NLRP3, NLRC4, NLRP6, NLRP7, NLRP12, AIM2, ASC and NAIPs are inflammasome components
Kinases may control both NLRP3 and NLRC4 inflammasomes
Cooperation between different inflammasomes may be necessary for optimal responses
Acknowledgments
The authors apologize to those whose citations were excluded due to space limitations. This work was supported by the NIH (grants AI057588-American Recovery and Reinvestment Act and AI075318 to E.L., AI095213 to G.I.V., the Research Council of Norway, and the Norwegian Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franchi L, Muñoz -Planillo R, Nuñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis BK, Wen H, Ting JP-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012 doi: 10.1038/nature11419. This study describes the function of caspase-11 during NLRP3 activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Rathinam VAK, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. This study describes the function of caspase-11, downstream of TRIF, in NLRP3 activation by bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Netea MG, Nold-Petry CA, Nold MF, Joosten LAB, Opitz B, van der Meer JHM, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathinam VAK, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 11.Franchi L, Kanneganti T-D, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 12.Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, Midiri A, Galbo R, Trieu-Cuot P, Papasergi S, et al. Activation of the NLRP3 inflammasome by group B streptococci. The Journal of Immunology. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. This paper described the role for mRNA from live bacteria in eliciting immune responses as detected by the NLRP3 inflammasome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2009;11:136–140. doi: 10.1038/ni.1831. This paper links oxidative stress and inflammasome activation. [DOI] [PubMed] [Google Scholar]
- 15••.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. This paper proposes a role for mitochondria during NLRP3 activation. [DOI] [PubMed] [Google Scholar]
- 16••.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. The study linked NLRP3 immune responses directly to cell damage by describing inflammasome activation by oxidized mitochondrial DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 19••.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes -Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. The activity of PKR was suggested to be necessary for the mechanism of action of NLRP3 downstream from many activators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyleta ML, Wong J, Magun BE. Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome. PLoS ONE. 2012;7:e36044. doi: 10.1371/journal.pone.0036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proceedings of the National Academy of Sciences. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. The authors propose ligand binding by mouse and human NAIP proteins in NLRC4 dependent responses to the type III secretion system of bacteria. [DOI] [PubMed] [Google Scholar]
- 23.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 2010;7:376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. This study suggests that ligand binding by mouse NAIP proteins in NLRC4 dependent responses to the type III secretion system of bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Moltke von J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012 doi: 10.1038/nature11351. This report describes a novel role for the NAIP5-NLRC4-caspase-1 inflammasome inducing eicosanoids independently of IL-1β and IL-18 storm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim Y-G, Nuñez G. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13:449–456. doi: 10.1038/ni.2263. Intestinal phagocytes are proposed to be central in theNLRC4-dependent sensing of pathogens vs commensals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012 doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Qu Y, Misaghi S, Izrael -Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Kömüves L, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012 doi: 10.1038/nature11429. The paper shows that the mechanism of NLRC4 activation may be direct phosphorylation by PKCδ. [DOI] [PubMed] [Google Scholar]
- 31.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. This study describes the link of caspase-1 and pro-inflammatory cell death independently of IL-1β and IL-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. Journal of Experimental Medicine. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Hornung V, Ablasser A, Charrel -Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. This paper describes a DNA sensing AIM2-inflammasome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Fernandes -Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. One of the papers describing the importance of cytosolic DNA sensing and inflammasome activation in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. This paper also describes the importance of cytosolic DNA sensing and inflammasome activation in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proceedings of the National Academy of Sciences. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 39.Bruey J-M, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 40.Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. Cutting Edge: Resistance to Bacillus anthracis Infection Mediated by a Lethal Toxin Sensitive Allele of Nalp1b/Nlrp1b. The Journal of Immunology. 2009;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elinav E, Strowig T, Kau AL, Henao -Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Anand PK, Malireddi RKS, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti T-D. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. These data suggested that NLRP6, which had previously been described in pro-inflammatory roles, could also have an anti-inflammatory immune effect, and mice deficient in this NLR are protected during bacterial challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen GY, Liu M, Wang F, Bertin J, Nuñez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. The Journal of Immunology. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He nao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Manji GA, Grenier JM, Al -Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 46••.Vladimer G, Weng D, Paquette SWM, Vanaja SK, Rathinam VAK, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, et al. The NLRP12 Inflammasome Recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. This study showed that NLRP12 could have a pro-inflammatory role during bacterial infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford R-MT, Davis BK, Uronis JM, Herfarth HH, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaki MH, Vogel P, Malireddi RKS, Body -Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti T-D. The NOD-Like Receptor NLRP12 Attenuates Colon Inflammation and Tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, Schneider M, Roney KE, O’Connor BP, Moore CB, et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. The Journal of Immunology. 2010;185:4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. This is the first study to describe a role for human NLRP7 in a pro-inflammatory fashion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meissner TB, Li A, Biswas A, Lee K-H, Liu Y-J, Bayir E, Iliopoulos D, van den Elsen PJ, Kobayashi KS. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proceedings of the National Academy of Sciences. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper M-A, et al. Cutting edge: NLRC5-dependent activation of the inflammasome. The Journal of Immunology. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. This story described a role for mouse caspase-11 during inflammasome activation and revealed that 129 mice express a non-functional caspase-1. [DOI] [PubMed] [Google Scholar]
- 54•.Vince JE, Wong WW-L, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. This study makes an important connection between IAP/RIP kinase pathways and IL-1β activation. [DOI] [PubMed] [Google Scholar]
- 55.Labbé K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 56•.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vli st M, Boekhout T, Geijtenbeek TBH. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. This report proposed that caspase-8 could participate in IL-1β release. [DOI] [PubMed] [Google Scholar]
- 57.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Niz et V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP-Y, Koller BH. NLRP1-Dependent Pyroptosis Leads to Acute Lung Injury and Morbidity in Mice. The Journal of Immunology. 2012;189:2006–2016. doi: 10.4049/jimmunol.1201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ceballos -Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. The Journal of Immunology. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. Critical role of AIM2 in Mycobacterium tuberculosis infection. International immunology. 2012 doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 63.Mishra BB, Moura -Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. Mycobacterium tuberculosis Protein ESAT-6 is a Potent Activator of the NLRP3/ASC Inflammasome. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 64.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus -Hemolysin Mediates Virulence in a Murine Model of Severe Pneumonia Through Activation of the NLRP3 Inflammasome. Journal of Infectious Diseases. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayer -Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nuñez G, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. The Journal of Immunology. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jun H-K, Lee S-H, Lee H-R, Choi B-K. Integrin α5β1 activates the NLRP3 inflammasome by direct interaction with a bacterial surface protein. Immunity. 2012;36:755–768. doi: 10.1016/j.immuni.2012.05.002. [DOI] [PubMed] [Google Scholar]