Abstract
Methylmercury (MeHg) is a potent neurotoxin that enters mammalian cells as a conjugate with L-cysteine through L-type large neutral amino acid transporter, LAT1, by a molecular mimicry mechanism by structurally resembling L-methionine. Caenorhabditis elegans (C. elegans) has been increasingly used to study the neurotoxic effects of MeHg, but little is known about uptake and transport of MeHg in the worm. This study examined whether MeHg uptake through LAT1 is evolutionarily conserved in nematodes. MeHg toxicity in C. elegans was blocked by pre-treatment of worms with L-methionine, suggesting a role for amino acid transporters in MeHg transport. Knockdown of aat-1, aat-2, and aat-3, worm homologues to LAT1, increased the survival of C. elegans following MeHg treatment and significantly attenuated MeHg content following exposure. These results indicate that MeHg is transported in the worm by a conserved mechanism dependent on functioning amino acid transporters.
Keywords: methylmercury, L-type large neutral amino acid transporter, molecular mimicry
Introduction
Methylmercury (MeHg) is a toxic heavy metal that poses a considerable risk to human health. Major sources of exposure to MeHg occur through manufacturing and consumption of seafood (Clarkson and Magos, 2006; Fitzgerald and Clarkson, 1991). Mass poisonings in Japan and Iraq, as well as the examination of seafood-rich diets of the Seychelles and Faroe Islands, have illustrated the effects of MeHg on human populations (Ekino et al., 2007; Grandjean et al., 2010). In adults, MeHg causes focal lesions, such as loss of cerebellar granular cells and occipital lobe damage, and during extreme poisonings can lead to ataxia, numbness of extremities, muscle weakness, vision and hearing problems, and paralysis (Clarkson and Magos, 2006). As MeHg can cross the placenta, the developing fetus is also at risk; where MeHg exposure leads to microcephaly and inhibition of neuronal migration, distortion of cortical layers, cerebellar abnormalities, alterations in glial cells, and alterations in neurotransmitter systems (Clarkson and Magos, 2006). MeHg has also been implicated in neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (Landrigan et al., 2005).
Recently the Caenorhabditis elegans (C. elegans) model has been used to characterize in vivo MeHg toxicity. C. elegans is a powerful model organism for exploring toxicity of metals due to their high homology to humans, short lifespan, ease of genetic manipulability, low cost, and transparency for imaging. Many of the toxic effects of MeHg in mammals occur in C. elegans, including lethality, growth and developmental delays, and altered behavior (Helmcke and Aschner, 2010; Helmcke et al., 2009). MeHg increases oxidative stress and depletes cellular glutathione levels in C. elegans, as well as induces stress response genes, such as glutathione S-transferases, heat shock proteins, and γ-glutamylcysteine synthetase (Helmcke and Aschner, 2010; McElwee and Freedman, 2011). These effects are directly related to the ability of MeHg to enter the worm. C. elegans readily accumulate Hg following MeHg exposure (Helmcke et al., 2009), however it is unclear how worms uptake and transport MeHg.
The molecular mechanisms responsible for the absorption and transport of MeHg are not fully characterized. While MeHg is lipid soluble and may distribute throughout the body by diffusion, it also has a high affinity for -SH (thiol) groups, forming conjugates with L-cysteine and glutathione (Hughes, 1957). MeHg-L-cysteine conjugates are structurally similar to L-methionine, and thus by molecular mimicry enter cells through the L-type large neutral amino acid transporter 1 (LAT1) (Aschner and Clarkson, 1988; Kajiwara et al., 1996; Simmons-Willis et al., 2002; Yin et al., 2008). LAT1 is a member of the L-type Na+-independent heteromeric amino acid transporter system family, which is composed of a catalytic multi-transmembrane spanning light chain and a type II glycoprotein heavy chain complexed together by a disulfide bond (Kanai and Endou, 2003; Mastroberardino et al., 1998). Substrates for transport by LAT1 include branched and aromatic amino acids, such as leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, histidine and methionine. C. elegans have nine genes encoding amino acid transporters homologous to the light chain, aat-1-9, and two genes encoding glycoprotein heavy chains, atg-1 and atg-2 (Veljkovic et al., 2004b). AAT-1 through AAT-3 have the highest homology to LAT1 and contain the cysteine responsible for the disulfide bond between heavy and light chains (Veljkovic et al., 2004b). AAT-1 and AAT-3, but not AAT-2, have been shown to associate with ATG-2 in Xenopus oocytes and transport L-alanine (Veljkovic et al., 2004b). However the function of these transporters has not been characterized in the worm in vivo. AAT-4 through AAT-9 have the least homology to human LAT1 and do not contain the cysteine residue required for bonding with the heavy chain (Veljkovic et al., 2004a). Recently it has been reported that AAT-6 interacts with the scaffold protein Na+/H+ exchanger regulatory factor, NRFL-1, to localize to the membrane (Hagiwara et al., 2012), but it has not been reported whether NRFL-1 is required for other AAT proteins as well. Herein we examined the hypothesis that MeHg is transported in C. elegans by a mechanism similar to mammals: entering cells through AAT transporters as a cysteine conjugate, which may be competitively blocked by excess L-methionine. Additionally we examined whether NRFL-1 was involved in MeHg-induced toxicity.
Materials and Methods
Reagents
Unless otherwise stated all reagents were obtained from Sigma-Aldrich (St. Louis, MO).
C. elegans strains and handling of the worms
C. elegans strains were handled and maintained at 20°C on Nematode Growth Medium (NGM) plates seeded with OP-50 strain of Escherichia coli, as previously described (Brenner, 1974). The following strains were used in this study: N2 and NL2099 (rrf-3(pk1426)). All strains were provided by the Caenorhabditis Genetic Center (CGC; University of Minnesota). Synchronous L1 populations were obtained by isolating embryos from gravid worms using a bleaching solution (1% NaOCl and 0.25 M NaOH), and segregating eggs from worm and bacterial debris by flotation on a sucrose gradient, as previously described (Stiernagle, 1999).
RNAi feeding and assessment of gene knock down
Synchronized L1 NL2099 worms were fed bacteria expressing RNAi constructs for aat-1 (F27C8.1), aat-2 (F07C3.7), aat-3 (F52H2.2), nrfl-1 (C01F6.6) (Thermo scientific, Waltham, MA) or empty L4440 RNAi vector (Addgene, Cambridge, MA) as previously described (Timmons et al., 2001). Adults were harvested, and either treated with MeHg or immediately used for RNA extraction. RNA from 20,000 worms per RNAi feeding was isolated using Trizol followed by chloroform extraction, as previously described (Chomczynski and Mackey, 1995). 1 ug of input RNA was reverse transcribed to cDNA by Applied Biosystems’ high capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Expression analysis was performed by Custom TaqMan® Array Analysis utilizing the corresponding TaqMan® Gene Expression Assays for aat-1 (Ce02458013_g1), aat-2 (Ce02479487_g1), and aat-3 (Ce02492836_g1) (Applied Biosystems). Target gene expression was normalized to the expression values of gpd-3 (Ce02616909_gH), and data was expressed as fold change compared to L4440 control fed worms.
Acute MeHg treatments and dose-response curves
The lethal dose 50% (LD50) of MeHg in C. elegans was determined by treating 5,000 adult RNAi-fed NL2099 worms were treated with doses of methylmercuric chloride (CH3HgCl) ranging from 10 µM to 1 mM for 30 min at 25°C, washed 3 times with M9 buffer, and transferred to RNAi-expressing bacteria spread plates until scored for lethality or extracted for inductively coupled plasma mass spectrometry (ICP-MS) analysis. Synchronized L1 N2 worms were also pre-treated with 1 mM L-methionine for 1 h prior to MeHg exposure washed 3 times with M9 buffer, and transferred to NGM plates containing 1 mM L-methionine until worms were scored for lethality. All treatments were carried out in duplicate and repeated 4–7 times. Worms were also treated with water as a vehicle control. Worms were manually counted for lethality 24 h post treatment.
Quantification of Hg by inductively coupled plasma-mass spectrometry (ICP-MS)
4×105 RNAi-fed adult C. elegans were treated with MeHg as described above. After 24 h of culture on RNAi-expressing bacteria spread plates, samples were collected and washed twice with ultrapure H2O (Millipore, Billerica, MA). Pellets were dried at room temperature and subjected to acid digestion with concentrated nitric acid (HNO3) and hydrochloric acid (HCl) (optima grade, Fisher Chemical, Pittsburg, PA) at 100°C in Teflon jars (Savillex, Eden Prairie, MN). The acid digested samples were then diluted with ultrapure water and gold (Au) solution to the level of 2% HNO3 + 2% HCl + 200 ppb Au. High Resolution-Inductively Coupled Plasma-Mass Spectrometry (HR-ICP-MS, Element 2, Thermo Fisher Scientific, Bremen, Germany) was utilized to perform quantitative analysis. The parameters of operation are listed in table 1.
Table 1.
| ICP-MS conditions | |
|---|---|
| RF power | 1250 W |
| Cool gas | 16.00 L min−1 |
| Auxiliary gas | 0.8 L min−1 |
| Sample gas | 1.0 L min−1 |
| Resolution mode | Medium resolution (4400) |
| Isotopes measured | 202Hg |
| Samples per peak | 20 |
| Runs | 10 |
| Passes | 1 |
Statistics
All statistical analyses were performed using Prism 5 (Graphpad software). Dose-response lethality curves and LD50 determination were generated using a sigmoidal dose-response model with a top constraint at 100%. Statistical analysis of significance was carried out by a two-tailed t-test for comparison of LD50 concentrations and a one-way ANOVA for the gene expression analysis, and a two-way ANOVA for the ICP-MS quantification of MeHg content. Values of P < 0.05 were considered statistically significant.
Results
L-methionine protects worms against MeHg-induced lethality
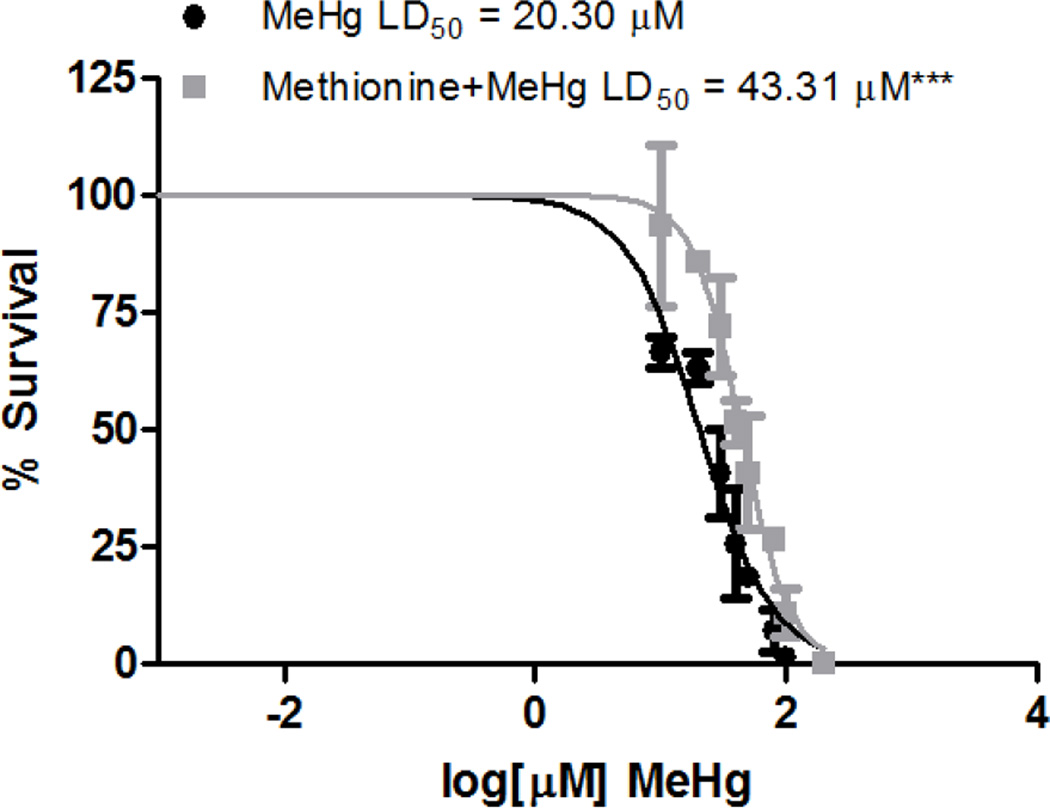
In mammals, MeHg is transported as a L-cysteine conjugate by amino acid transporters, however it is unknown whether there are additional transport mechanisms or how MeHg is transported in invertebrates. To address whether a shared mechanism for MeHg transport via molecular mimicry occurs in worms, C. elegans were pre-treated with 1 mM L-methionine, to compete as a substrate for amino acid transporters. Pretreated and untreated worms were then treated with increasing concentrations of MeHg (10 µM to 1 mM) and lethality was assessed after 24 h. MeHg was toxic to C. elegans, with an LD50 of 20.30 µM, while pre-treatment with methionine significantly shifted the dose-response curve to the right (LD50 = 43.31 µM) (Figure 1). This protection by L-methionine treatment suggests that MeHg toxicity may result from the MeHg-L-cysteine conjugate in nematodes and that L-methionine reduces the MeHg burden in worms (see below).
Figure 1.
Pre-treatment with L-methionine, a substrate for LAT1, protects against MeHg-induced lethality. N2 worms were treated for 30 min with increasing concentrations of MeHg in the presence or absence of a 1 h pre-treatment with L-methionine (1 mM). Data are expressed as means ± SEM from 3 independent experiments. *** p < 0.001.
Knockdown of AAT-1 through AAT-3 transporters protect against MeHg-induced lethality
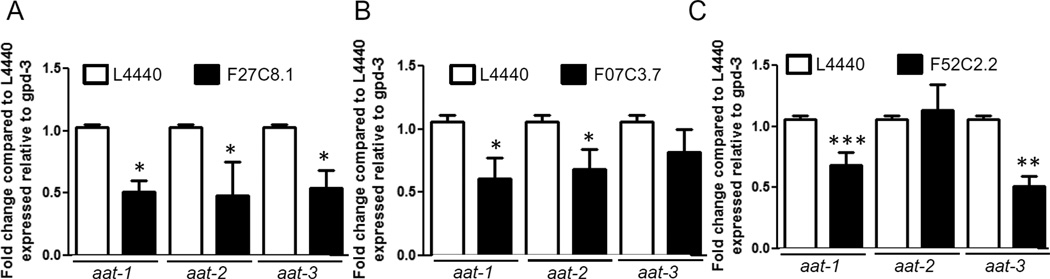
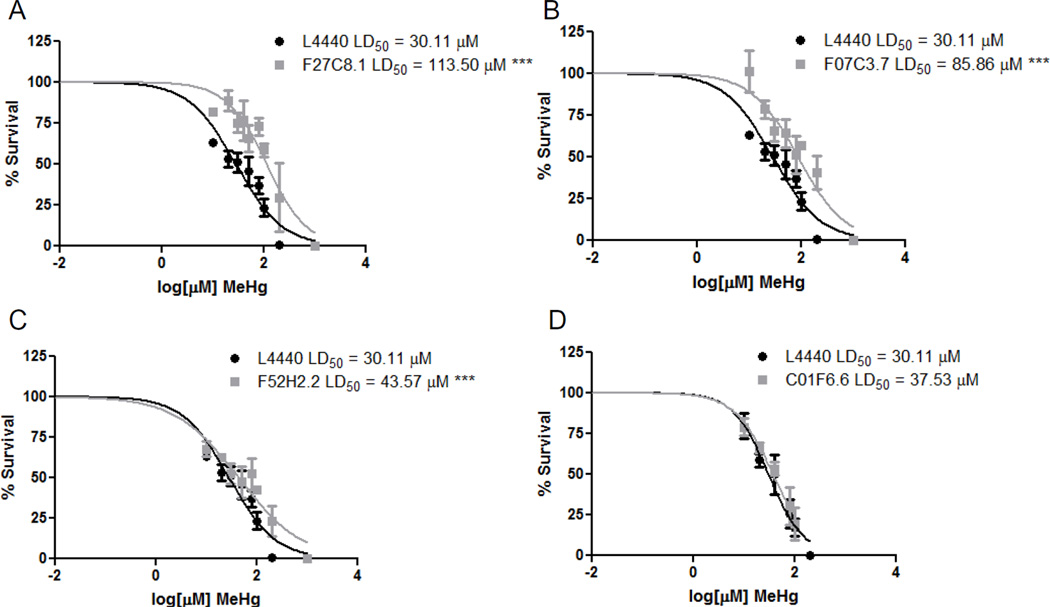
As C. elegans have 9 homologs (aat-1-9) of the mammalian LAT1 with varying degrees of homology, we investigated whether the three most homologous AATs to human LAT1 were involved in MeHg transport. dsRNA for aat-1 (F27C8.1), aat-2 (F07C3.7), and aat-3 (F52C2.2) were fed to the RNAi sensitive strain NL2099. Feeding worms with the construct for aat-1 (F27C8.1) resulted in decreased levels of aat-1, aat-2 and aat-3 (Figure 2A). While feeding for aat-2 (F07C3.7) knocked down aat-1 and aat-2 and feeding for aat-3 (F52C2.2) resulted in decreased levels of aat-1 and aat-3 (Figure 2B and C). Multiple AAT knockdowns resulting from the RNAi feeding may be due to the high homology between these proteins; comparison of AAT-1 through AAT-3 proteins sequences reveals that the three transporters share 43–70% identity (Figure S1). Feeding worms with either of these dsRNA constructs provided protection from MeHg toxicity. MeHg was toxic to adult NL2099 worms fed the control vector construct (L4440), with a LD50 of 30.11 µM (Figure 3). Worms fed the F27C2.2 construct showed the most protection from MeHg treatment, with an LD50 of 113.5 µM (Figure 3A). Feeding worms with the F07C3.7 or the F52C2.2 constructs were also significantly protective against MeHg, with LD50 of 85.86 µM and 43.57 µM, respectively (Figure 3B and C). C. elegans were also fed with dsRNA against nrfl-1 (C01F6.6), a scaffold protein required for AAT-6 localization and function, however there was no alleviation of MeHg toxicity (Figure 3D). This suggests that the protection from knocking down aat-1 through aat-3 was specific and did not involve aat-6 or nrfl-1.
Figure 2.
RNA levels of knocked down of AAT transporters. NL2099 worms were fed dsRNA against (A) F27C8.1 (aat-1), (B) F07C3.7 (aat-2), and (C) F52H2.2 (aat-3). mRNA levels of aat-1, aat-2 and aat-3 for each feeding were quantified by qPCR and normalized to gpd-3. Data are expressed as fold change in mRNA levels in dsRNA-fed worms as compared to levels in L4440-fed worms from 10–15 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 3.
Knockdown of AAT transporters protect against MeHg-induced lethality. NL2099 dsRNA-fed worms (A) F27C8.1 (aat-1), (B) F07C3.7 (aat-2), (C) F52H2.2 (aat-3), and (D) C01F6.6 (nrfl-1) were treated for 30 min with increasing concentrations of MeHg. Data are expressed as means ± SEM from 7 independent experiments. *** p < 0.001.
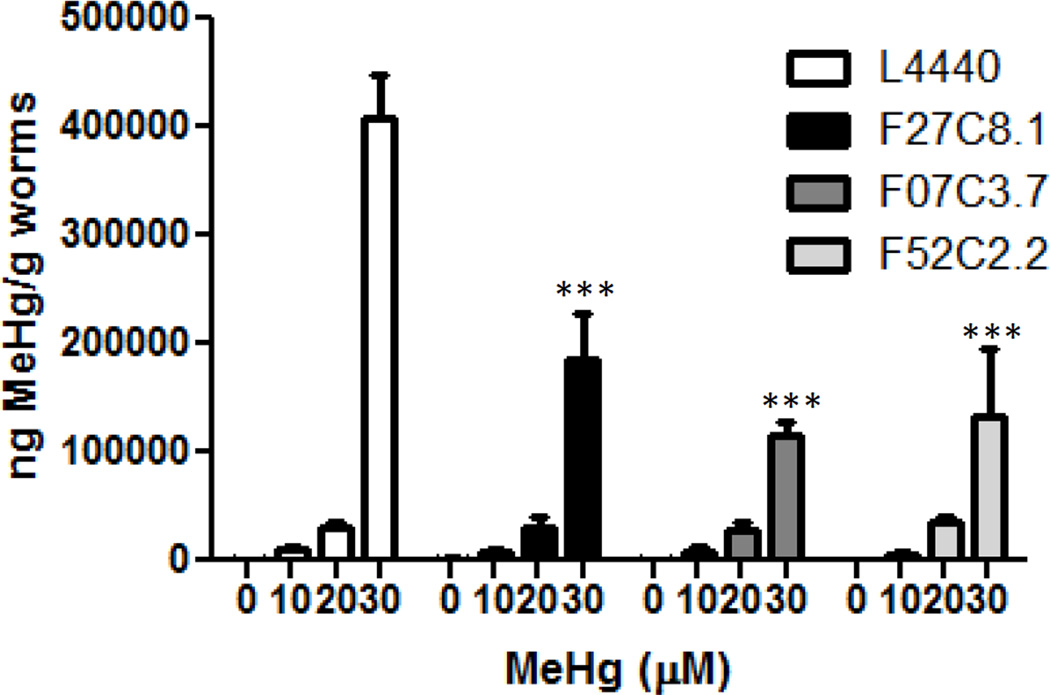
AAT transporters mediate MeHg accumulation
To determine whether the AAT transporters aat-1, aat-2 and aat-3 were involved in the transport of MeHg, Hg content was measured 24 post exposure in dsRNA fed worms treated for 30 min with 10, 20, or 30 µM MeHg. These treatments were all at or below the LD50 for MeHg in control L4440-fed worms. Hg content was dose-dependently increased in control L4440-fed worms (Figure 4). Worms fed with either dsRNA against F27C8.1, F07C3.7, or F52H2.2 contained significantly less Hg following 30 µM treatment with MeHg (Figure 4), suggesting that these transporters are involved in the uptake of MeHg in C. elegans.
Figure 4.
Hg accumulation is attenuated by knockdown of aat-1 through aat-3. Hg content was measured in NL2099 worms fed RNAi F27C8.1, F07C3.7, and F52H2.2 treated with 10, 20 or 30 µM MeHg. *** p < 0.001.
Discussion
C. elegans is an emerging model organism for MeHg toxicity research (Helmcke and Aschner, 2010; Helmcke et al., 2009; McElwee and Freedman, 2011; Vanduyn et al., 2010), however little is known about how worms uptake or transport MeHg. As C. elegans contain 60–80% gene homology with mammals (Kaletta and Hengartner, 2006; McDonald et al., 2006), examining MeHg transport in nematodes can provide further insight on both known and unknown transport mechanisms. The present study provides direct evidence for an evolutionarily conserved transport mechanism for MeHg via LAT1 homologues in C. elegans.
Amino acid transporters play a crucial role in the transport of the MeHg. While MeHg is highly lipophilic, it is mostly complexed with water soluble sulfhydryl-containing molecules, such as glutathione and L-cysteine (Hughes, 1957). Studies in rodents have demonstrated that administration of L-cysteine (enantiomorph specific) can increase MeHg uptake by the blood-brain-barrier endothelial cells and that uptake of the MeHg-cysteine conjugate can be inhibited by administration of L-methionine (Aschner and Clarkson, 1988; Hirayama, 1980). Pre-treatment of C. elegans with L-methionine significantly increased the survival rate following MeHg exposure, suggesting that transport in nematodes may occur through conserved mechanisms to mammals. The L-type Na+-independent large neutral amino acid transporters, LAT1 and LAT2, have been shown to transport MeHg-cysteine conjugates in CHO-k1 cells and in Xenopus laevis oocytes expressing LAT1 or LAT2 (Simmons-Willis et al., 2002; Yin et al., 2008). Of the 9 AAT amino acid transporters in C. elegans, AAT-1 through AAT-3 are the most homologous to LAT1. AAT-1 through AAT-3 are also homologous to each other, which resulted in multiple gene knock downs in RNAi fed worms. Knock down of aat-1 and aat-3 and aat-1 and aat-2 were both protective against MeHg induced lethality, while knockdown of all three aat-1 through aat-3 was the most protective. Furthermore, knockdown of aat1-3 attenuated MeHg accumulation in the worms. Interestingly there was no significant difference in the intracellular Hg levels in the worms with knockdown of all three aat-1 through aat-3, or the combinations of aat-1 and aat-2, and aat-1 and aat-3. AAT-1 is the most homologous to LAT1, and was knocked down in all three RNAi feedings, which may explain the lack of differential uptake across the three RANi fed worms. However the contributions of AAT-2 and AAT-3 to MeHg transport should not be overlooked as there was differential survival following MeHg exposure. In mammals, LAT1 is localized to multiple cell types and is present on both sides of polarized cells, such as endothelial cells, implicating that the transporter is involved in transcellular movement and distribution of MeHg (Boado et al., 1999; Dave et al., 2004; Duelli et al., 2000; Manjarin et al., 2011; Ritchie and Taylor, 2001; Yin et al., 2008). Localization of AAT1 through AAT-3 remains to be determined.
While RNAi was not directly performed for AAT-4 through AAT-9, it is unlikely that these transporters are involved in MeHg transport. AAT-4 through AAT-9 are the least homologous to LAT1 and show 23–29% homology to AAT-1 through AAT-3. Additionally, AAT-4 through AAT-9 do not form complexes with the glycoprotein heavy chain subunit, which is required for AAT-1, AAT-3, and LAT1 (Kanai and Endou, 2003; Veljkovic et al., 2004a; Veljkovic et al., 2004b). Our studies indirectly examined whether AAT-6 is involved in MeHg toxicity by examining the knockdown of nrfl-1. NRFL-1 has been shown to be required for AAT-6 localization to the plasma membrane in the intestines of adult worms (Hagiwara et al., 2012). Knockdown for nrfl-1 did not alter survival after MeHg exposure, suggesting that this scaffolding protein may not be involved in AAT-1 through AAT-3 localization and that AAT-6 may not be involved in MeHg transport.
Collectively, our studies support a conserved role for amino acid transporters in MeHg transport in C. elegans. Furthermore we found that MeHg toxicity was dependent specifically on AAT-1 -3. Using RNAi against these transporters or generation of mutant strains will be beneficial for genetic screens for modifiers of MeHg uptake and analyzing regulation of cell signaling pathways through the AATs.
Supplementary Material
Figure 1S: Sequence alignment of AAT-1, AAT-2, and AAT-3. Sequence alignment of C. elegans AAT-1 [NP_501707.1], AAT-2 [NP_505394.2], and AAT-3 [NP_508461.1] was performed using Clustal Omega multiple sequence alignment algorithm (Larkin et al., 2007). Conserved identical amino acid residues are in green and homologous residues are in yellow.
Highlights.
Methylmercury toxicity in worms is blocked by L-methionine.
Amino acid transporters transport methylmercury in the worm.
Knockdown of aat, increase worm the survival following methylmercury treatment
Knockdown of aat also attenuate worm methylmercury content.
Acknowledgements
This work was funded by NIEHS R01 ES007331 and S10 RR026742.
Abbreviations
- ICP-MS
Inductively coupled mass spectrometry
- LAT1
L-type neutral amino acid transporter
- MeHg
methylmercury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462:31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558:597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli R, Enerson BE, Gerhart DZ, Drewes LR. Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab. 2000;20:1557–1562. doi: 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Clarkson TW. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Nagamori S, Umemura YM, Ohgaki R, Tanaka H, Murata D, Nakagomi S, Nomura KH, Kage-Nakadai E, Mitani S, et al. NRFL-1, the C. elegans NHERF orthologue, interacts with amino acid transporter 6 (AAT-6) for age-dependent maintenance of AAT-6 on the membrane. PLoS One. 2012;7:e43050. doi: 10.1371/journal.pone.0043050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmcke KJ, Aschner M. Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2010;248:156–164. doi: 10.1016/j.taap.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmcke KJ, Syversen T, Miller DM, 3rd, Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2009;240:265–272. doi: 10.1016/j.taap.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K. Effect of amino acids on brain uptake of methyl mercury. Toxicol Appl Pharmacol. 1980;55:318–323. doi: 10.1016/0041-008x(80)90093-9. [DOI] [PubMed] [Google Scholar]
- Hughes WL. A physicochemical rationale for the biological activity of mercury and its compounds. Ann N Y Acad Sci. 1957;65:454–460. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70:310–314. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci. 2003;28:1–17. doi: 10.2131/jts.28.1. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Manjarin R, Steibel JP, Zamora V, Am-In N, Kirkwood RN, Ernst CW, Weber PS, Taylor NP, Trottier NL. Transcript abundance of amino acid transporters, beta-casein, and alpha-lactalbumin in mammary tissue of periparturient, lactating, and postweaned sows. J Dairy Sci. 2011;94:3467–3476. doi: 10.3168/jds.2011-4163. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;26:593–618. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee MK, Freedman JH. Comparative toxicology of mercurials in Caenorhabditis elegans. Environ Toxicol Chem. 2011;30:2135–2141. doi: 10.1002/etc.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JW, Taylor PM. Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J. 2001;356:719–725. doi: 10.1042/0264-6021:3560719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367:239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C elegans: A Practical Approach. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Vanduyn N, Settivari R, Wong G, Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci. 2010;118:613–624. doi: 10.1093/toxsci/kfq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljkovic E, Bacconi A, Stetak A, Hajnal A, Stasiuk S, Skelly PJ, Forster I, Shoemaker CB, Verrey F. Aromatic amino acid transporter AAT-9 of Caenorhabditis elegans localizes to neurons and muscle cells. J Biol Chem. 2004a;279:49268–49273. doi: 10.1074/jbc.M404470200. [DOI] [PubMed] [Google Scholar]
- Veljkovic E, Stasiuk S, Skelly PJ, Shoemaker CB, Verrey F. Functional characterization of Caenorhabditis elegans heteromeric amino acid transporters. J Biol Chem. 2004b;279:7655–7662. doi: 10.1074/jbc.M309528200. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107:1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S: Sequence alignment of AAT-1, AAT-2, and AAT-3. Sequence alignment of C. elegans AAT-1 [NP_501707.1], AAT-2 [NP_505394.2], and AAT-3 [NP_508461.1] was performed using Clustal Omega multiple sequence alignment algorithm (Larkin et al., 2007). Conserved identical amino acid residues are in green and homologous residues are in yellow.