Abstract
Rationale
Craving is a primary feature of opiate addiction and is clinically significant because of its potential to trigger opiate use and relapse. Opiate use can also produce abnormal pain perception. We predicted that for opiate addicts (OAs), there may be an association between these two major features of addiction (drug craving and abnormal pain responses).
Objectives
To examine pain responses in abstinent opiate addicts in comparison with healthy controls using a cold-pressor test (CPT) and investigate the correlations of cue-induced drug craving with pain responses.
Material and methods
Fifty-four abstinent OAs and 46 healthy subjects participated in the CPT, and the OAs were also exposed to heroin-related cues the day before the pain test. Outcome measures included pain-tolerance time, VAS ratings of pain intensity and distress, and (in the cue-exposure procedure) VAS ratings of heroin craving and anxiety.
Results
In the CPT, abstinent addicts showed shorter pain-tolerance time (85.1±14.1 s vs. 133.7±16.7 s, p<0.05) and higher ratings of pain distress (61±3.2 vs. 45.6±3.2, p< 0.01) compared to healthy controls. When we divided the addicts and controls into pain-sensitive (PS) and pain-tolerant (PT) groups by dichotomizing each group in terms of pain-tolerance time, we again found differences between the two PS groups (37.3±3.5 s vs. 57.4±5.1 s, p<0.01 for pain-tolerance time; 66.7±3.2 vs. 52.4±3.3, p<0.01 for distress ratings). For all participants, pain-tolerance time was negatively correlated with VAS ratings for pain intensity and distress. More importantly, the PS addicts reported greater cue-induced craving than the PT addicts (17.8±2.2 vs. 4.5±4.2, p<0.05). For the addict group as a whole, pain distress (the affective aspect of pain) was positively correlated with intensity of cue-induced craving measured on a different day (r=0.33, p=0.01).
Conclusions
A hyperalgesic state persists for at least 5 months in abstinent OAs and is predictive of cue-induced craving. Longitudinal research is needed to clarify the direction of causation between hyperalgesia and opiate addiction.
Keywords: Craving, Heroin, Addiction, Pain
Introduction
Craving for a drug is a primary feature of addictive disorders and is clinically significant because of its potential to trigger drug use and relapse (Childress et al. 1999). Relapse to drug addiction is frequently associated with subjective reports of craving, a state that precedes and accompanies drug-seeking behaviors (Grimm et al. 2001). Craving for drugs can be induced in a laboratory setting using the cue-reactivity paradigm. In this paradigm, drug-dependent patients show specific physical and psychological responses to drug-related stimuli. These responses are assessed before and after exposure to a wide range of drug-related cues, including the sight of drug paraphernalia (Yu et al. 2007), scripted imagery of drug use (Weinstein et al. 1997), and drug- related pictures (Waters et al. 2003), words, or videos (Ooteman et al. 2006; Ren et al. 2009; Shi et al. 2007; Upadhyaya et al. 2004). The most commonly collected psychological measures are craving, urge to use drug, drug-induced arousal, mood changes, and anxiety (Fox et al. 2007; Fox et al. 2005). Physiological measures often include heart rate, blood pressure, salivation, body temperature, skin conductance, and withdrawal signs (Carter and Tiffany 1999; LaRowe et al. 2007).
Pain is a complex experience encompassing sensory, affective, and cognitive elements, which are mediated by different parts of the central nervous system (Brooks et al. 2005; Davis 2000; Singer et al. 2004; Talbot et al. 1991). Opiate administration can produce abnormal pain perception, either hyperalgesia, or hypoalgesia, depending on the schedule (Celerier et al. 2001; Laulin et al. 1999; White 2004). Opiate-induced hyperalgesia is most broadly defined as a state of nociceptive sensitization caused by exposure to opiates (Chu et al. 2008; Mao et al. 1994). Abnormal pain perceptions have been reported in different stages of opiate addiction, including the development, maintenance, and withdrawal periods and periods of abstinence during treatment (Compton 1994; Compton et al. 2000; Doverty et al. 2001; Lehofer et al. 1997). Among OAs, preexisting pain problems are especially likely among those who used prescription opiates only or initially (Brands et al. 2004); among substance-misusing physicians, one of the most common precipitating factors is physical pain (Akvardar et al. 2002). On the other hand, pain can also be a result of chronic opiate use. OAs exhibit hyperalgesia after long-term opiate use, as shown by significantly shorter durations of ability to withstand immersion of the hand in ice-cold water compared to healthy individuals (Compton 1994; Compton et al. 2001; Martin and Inglis 1965; Pud et al. 2006). Patients on methadone maintenance are relatively intolerant of pain, a finding hypothesized to reflect a hyperalgesic state induced by chronic opiate administration (Compton et al. 2001). Also, OAs undergoing partial-agonist maintenance may have difficulty achieving analgesia when in acute pain (Alford et al. 2006), and pain is also among the causes of discontinuation of methadone maintenance and triggers of relapse to addiction (Calsyn et al. 2006; Cruciani et al. 2008).
We hypothesized that for opiate addicts, there may be an association between two major features of addiction–drug craving and abnormal pain responses, both of which can trigger relapse to drug use. We know of no published reports testing this hypothesis. Therefore, we compared pain responses (pain-tolerance time and VAS ratings for pain intensity and distress), during a CPT in abstinent OAs and healthy controls, and investigated the correlations between pain responses and cue-induced opiate craving using a cue-reactivity paradigm.
Materials and methods
Participants
Fifty-five individuals who met DSM-IV criteria for heroin dependence upon admission into a long-term rehabilitation program were recruited. The participants had been abstinent from heroin for an average of around 4 months upon recruitment. Those who met DSM-IV criteria for dependence on another psychoactive substance (except nicotine) were excluded. Also excluded were those who were currently on medications for physiological or psychological disorders, those who had chronic pain conditions and ongoing acute pain, and those needing to use prescribed drugs. The control group consisted of 47 healthy volunteers recruited via word of mouth and advertisement. Control-group participants were required to meet the following criteria: (1) no history of alcohol or substance abuse, (2) chronic pain conditions and ongoing acute pain, (3) no current or prior serious physiological and psychological disorders, (4) no current use of medication, and (5) ability to understand the purpose and instructions of the study. A urine sample was required for the detection of opiates and methamphetamine.
The study was approved by Human Investigation Committee of the Peking University School of Medicine, and written informed consent was obtained from all participants before they started the study. Three of the enrolled OAs were not included in the analyses; two were dropped from the study for hypertension and one completed the study session but, due to experimenter error, did not have his pain-tolerance time recorded.
The participants in this study were all male. The demographic characteristics of the participants are summarized in Table 1; no significant differences were found between the OAs and healthy controls.
Table 1.
Characteristics of opiate addicts vs. the control group (mean ± SEM)
| Opiate addicts (n=52) |
Controls (n=47) |
|
|---|---|---|
| Age (years) | 31.6±0.8 | 30.3±0.9 |
| Weight (kg) | 68.5±1.5 | 64.7±2.2 |
| Years of education | 9.7±0.5 | 10.8±0.4 |
| Years of regular heroin use | 4.3±0.5 | N/A |
| Amount of heroin use per day (g) | 0.6±0.05 | N/A |
| Duration of abstinence (months) | 4.9±0.2 | N/A |
No significant differences were found between the opiate addicts and controls.
Experimental procedure
Pain response was determined with a CPT, which is believed to be the best method for simulating the quality, duration, and urgency of clinical pain in the laboratory (Turk et al. 1983). The test was conducted as described before (Chen et al. 1989; Liebmann et al. 1997). Briefly, all participants underwent a CPT; tolerance time and subjective reports of pain intensity and distress were recorded. OAs also underwent a drug-cue-reactivity paradigm, in which changes in craving were recorded; this paradigm was always performed between 8:00 and 10:00 A.M. OAs completed the two tests on two consecutive days. Cue exposure always preceded the CPT. This fixed order was chosen to prevent a “carry-over” effect of stress from the cold-presssor test; cues and stress in combination could increase drug craving (Carter and Tiffany 1999; Sinha et al. 2003).
Cold-pressor test
The apparatus for the CPT was a container of ice and water stirred to maintain a constant temperature of 2.0± 2°C throughout the session. At the onset of the test, participants were instructed to submerge one of their hands into the container such that the ice water covered 4 in. of the arm above the wrist. Participants were instructed to indicate to say “stop” when they were no longer willing or able to tolerate the pain, at which point they withdrew their submerged hand. A maximum time limit of 5 min was imposed.
Subjective pain ratings
Participants were instructed that at the point when they withdrew their submerged hand, they would be asked to rate the pain in terms of its intensity (a sensory aspect of pain) and its unpleasantness (an affective aspect of pain) using separate visual analogue scale (VAS, 0–100). The patient was instructed to distinguish between the concepts of pain intensity and distress as follows: “To understand the difference between pain intensity and distress, think of listening to music on a radio. As I turn the volume up, I can ask you how loud the music is or I can ask you how pleasant or unpleasant the music is to listen to. The intensity of pain is like the loudness of music. How pleasant or unpleasant the music is depends on how much you like or dislike the music, and the distress of pain depends on how much you dislike that sensation” (Price et al. 1983). The experimenter held up one VAS for intensity rating, with the 100 mm line anchored by the words “not at all intense” and “the most intense pain imaginable” and another VAS for distress rating, with the 100 mm line anchored by the words “not at all unpleasant” and “the most unpleasant pain imaginable”.
Drug-cue-induced craving and anxiety
OAs were exposed for 5 min to a film of drug users injecting and smoking heroin. Heroin craving and anxiety were measured at baseline and immediately following cue exposure using two separate visual analog scales starting at 0 (none at all) to 100 (more than ever; Sinha et al. 2003).
Data analysis
Independent t tests were used to compare demographic characteristics and pain measurements in OAs vs. controls. Participants were empirically dichotomized into PS and PT groups as reflected by pain-tolerance time. Independent t tests were used to compare the differences between PS and PT groups. Spearman correlations were used to assess associations between pain responses and craving.
Results
Hyperalgesia in opiate addicts after prolonged abstinence
Table 2 shows mean pain-tolerance times and VAS ratings of pain intensity and pain distress in OAs and healthy controls. There were obvious dichotomies in pain tolerance within both the OA group and the control group. The PT subjects endured almost the entire CPT (pain-tolerance time=295.4±4.6 s for PT OAs, 291.3±5.4 s for PT controls); the PS subjects had mean pain-tolerance times of only 37.3±3.5 s for OAs and 57.4±5.1 s for controls. Within PS subjects, pain-tolerance time was shorter in OAs than in controls (t=−3.36, p<0.01); within PT subjects, there was no difference between OAs and controls (p>0.05).
Table 2.
Abnormal pain responses in opiate addicts (mean ± SEM)
| Control | Opiate addicts | t value | p value | ||
|---|---|---|---|---|---|
| Tolerance time (s) | Total | 133.7±16.7 | 85.1±14.1 | −2.13 | <0.05* |
| Pain-sensitive | 57.4±5.1 | 37.3±3.5 | −3.36 | <0.01** | |
| Pain-tolerant | 291.3±5.4 | 295.4±4.6 | 0.53 | 0.60 | |
| Pain intensity (VAS) | Total | 54.7±2.6 | 53.5±3.1 | −0.35 | 0.73 |
| Pain-sensitive | 59.6±2.8 | 55.0±3.2 | −0.93 | 0.44 | |
| Pain-tolerant | 46.0±4.9 | 47.3±8.6 | 0.14 | 0.90 | |
| Pain distress (VAS) | Total | 45.6±3.2 | 60.9±3.2 | 3.38 | <0.01** |
| Pain-sensitive | 52.4±3.4 | 66.7±3.1 | 3.07 | <0.01** | |
| Pain-tolerant | 30.8±5.5 | 37.1±6.4 | 0.74 | 0.47 |
Tolerance time and VAS ratings of pain intensity and pain distress were presented in total, pain-sensitive, and pain-tolerant opiate addicts and healthy controls, respectively
p<0.05,
p<0.01 compared with healthy controls
The mean VAS rating of distress in the total sample of OAs was 61±3.2, higher than the mean of 45.6±3.2 in the controls (t=3.38, p<0.01). For VAS rating of pain intensity, there was no difference between OAs and controls (t=−0.351, p=0.73). However, within the PS subjects, distress was higher in OAs (66.7±3.2) than in controls (52.4±3.3; t=3.07, p<0.01). No such difference was found for pain intensity between the two PS groups (t=−0.93, p=0.36). Furthermore, no differences were found between the two PT groups in VAS ratings for distress or pain intensity (t=0.74, p=0.47 for distress; t=−0.14, p=0.90 for pain intensity).
Correlations between tolerance time and VAS ratings for pain intensity and distress
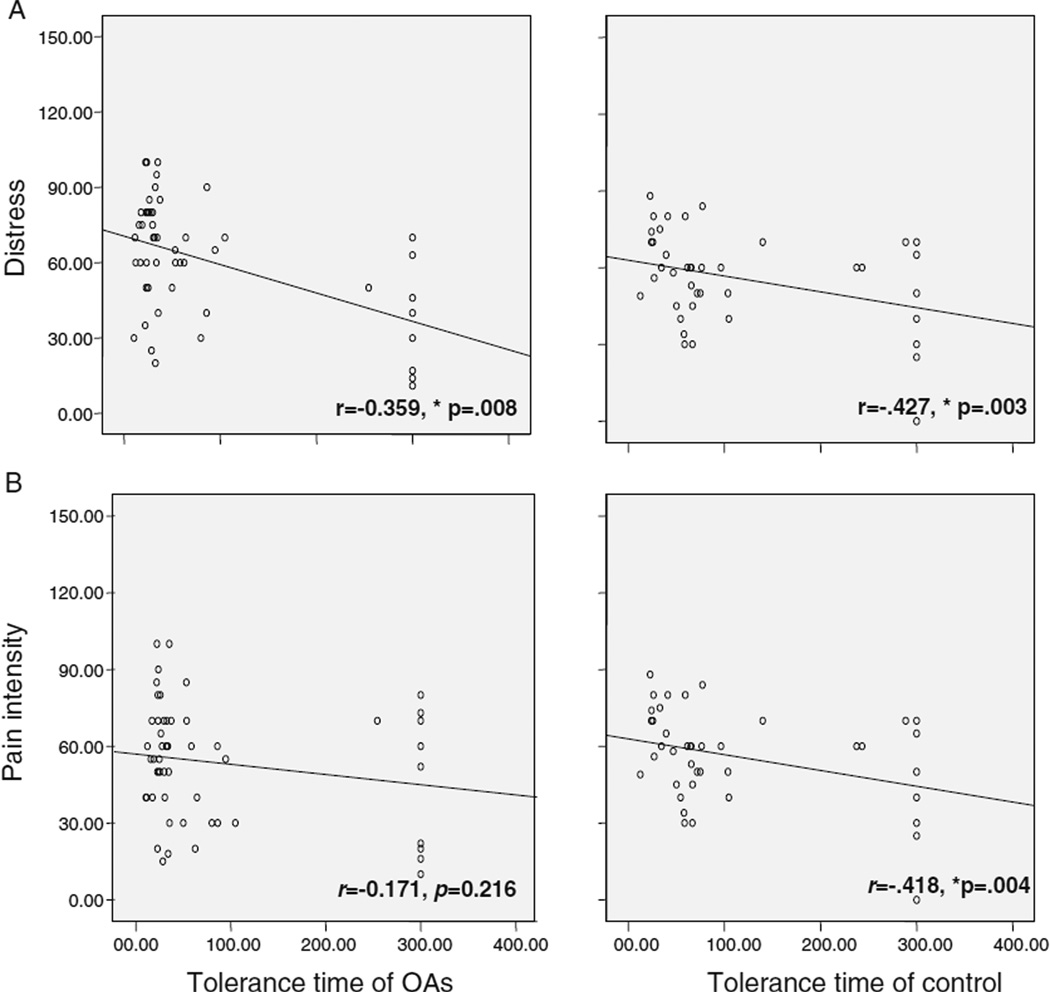
Both the OAs and the controls showed significant negative correlations between pain-tolerance times and distress ratings (r=−0.359, p=0.008 for OAs and r= −0.427, p=0.003 for controls, Fig. 1a). For pain intensity, only the controls showed a significant negative correlation with pain-tolerance time (r=−0.171, p=0.216 for OAs and r=−0.418, p=0.004 for controls, Fig. 1b).
Fig. 1.
Correlations between pain-tolerance time and distress and pain intensity. a Negative correlations between pain-tolerance time and distress in OAs and controls (r=−0.359, p=0.008 for OAs and r=−0.427, p=0.003 for the controls). b Correlations between pain-tolerance time and pain intensity in OAs and controls (r=−0.171, p=0.216 for OAs and r=−0.418, p=0.004 for the controls). Only controls showed significant negative correlations between pain-tolerance time and pain intensity. *p<0.01
Increased cue-induced drug craving in pain-sensitive opiate addicts after prolonged abstinence
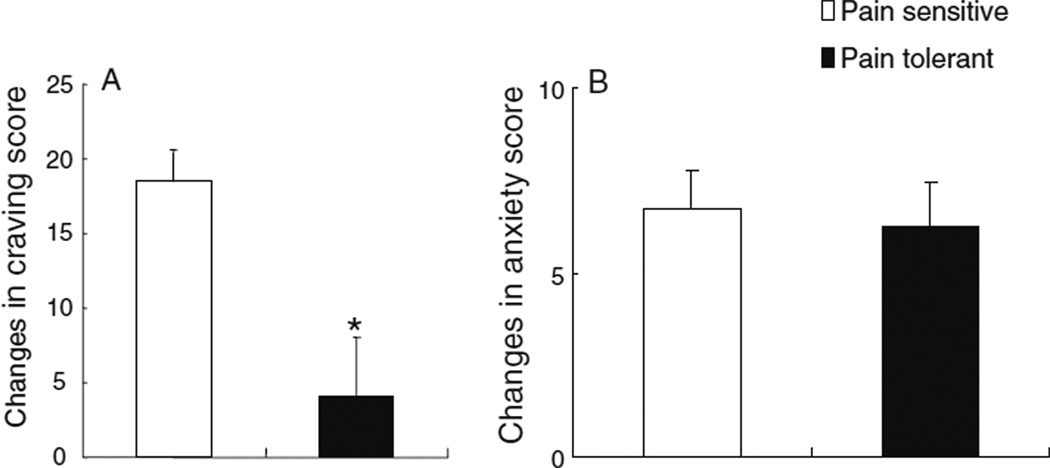
Figure 2 shows changes in heroin-cue-induced drug craving and anxiety in the total sample of OAs. No differences were found in baseline craving and anxiety ratings between PS and PT OAs (data not shown). However, PS individuals reported greater changes in cue-induced craving than the PT individuals (17.8±2.2 vs. 4.5±4.2, t=2.76, df=50, p<0.01). The anxiety changes were similar in PS and PT OAs (6.7±1.0 vs. 6.3±1.2, t= 0.21, df=50, p=0.8).
Fig. 2.
Comparison of cue-induced drug craving and anxiety between pain-sensitive and pain-tolerant opiate addicts. a Pain-sensitive opiate addicts reported greater craving increases than pain-tolerant opiate addicts when exposed to heroin-related cues. b Pain-sensitive opiate addicts did not show greater anxiety increases than pain-tolerant opiate addicts when exposed to heroin-related cues. *p<0.01 between PS and PT
Correlations between cue-induced craving changes and pain responses
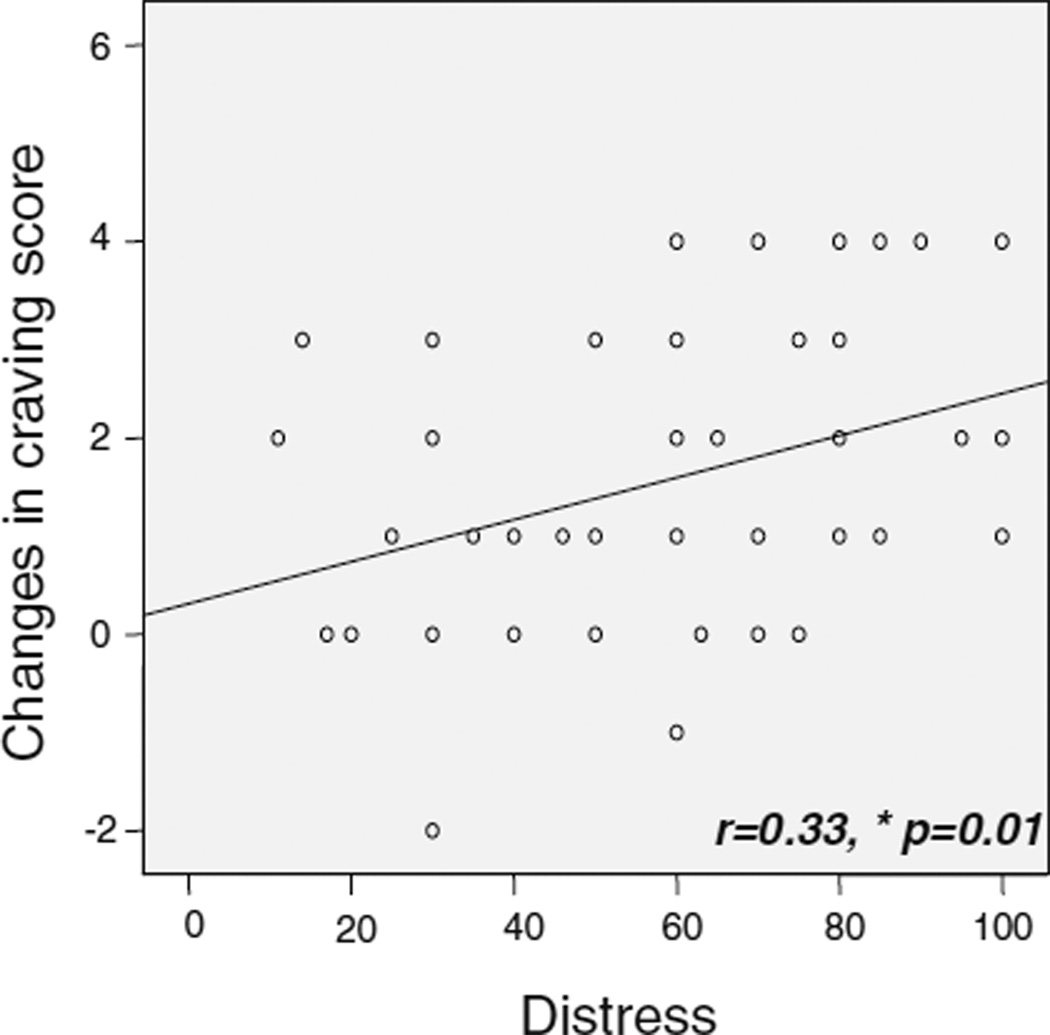
Among OAs, there was a significant positive correlation between cue-induced craving changes and VAS rating for pain distress (r=0.33, p=0.01, Fig. 3). Craving changes did not correlate with tolerance time or pain-intensity ratings.
Fig. 3.
Correlations between cue-induced craving changes and pain-induced distress in OAs. OAs showed a positive correlation between craving changes and ratings of pain distress (r=0.33, p=0.01). *p<0.05
Discussion
The current study demonstrated that 4-month-abstinent OAs, compared with healthy controls, showed shorter pain-tolerance times, and higher ratings of pain-related distress in the cold-pressor test, indicating that formerly drug-dependent individuals were in a hyperalgesic state, at least by those measures. This is consistent with previous reports (Compton et al. 2000; Pud et al. 2006). When we empirically divided participants into PS and PT groups, the same differences were observed between the two PS groups, but not between the two PT groups. This division by pain-tolerance time was supported by the negative correlations between pain-tolerance time and distress. Most importantly, among the OAs, cue-induced heroin craving in a separate task was greater in PS individuals than in PT individuals, and the degree of craving was positively correlated with ratings of pain distress.
Our findings suggest that the difference between the OAs and the controls was mainly driven by participants at the higher end of a continuum of pain sensitivity (PS rather than PT individuals). As previously suggested (Compton et al. 2000), there are at least two possible explanations: (1) Chronic opiate misuse may cause hyperalgesia (Doverty et al. 2001; Ho and Dole 1979; Martin and Inglis 1965), and for heroin-dependent patients entering treatment with established hyperalgesia, subsequent maintenance on methadone or buprenorphine neither exacerbates nor reduces that hyperalgesia (White 2004); or (2) persons at risk for opiate addiction may be inherently intolerant of pain (Compton et al. 2000), a possibility supported by animal and human studies of genetic polymorphisms (Bond et al. 1998; Mogil 1999; Mogil et al. 1996).
Unexpected findings in the present study were that cue-induced craving was positively correlated with ratings of pain distress, and that craving was greater among pain-sensitive OAs than among pain-tolerant OAs. Still, the absence of significant correlations between anxiety and pain responses supports the specificity of cue-induced drug craving in this cue-reactivity paradigm. These findings suggest that hyperalgesia after long-term opiate use may contribute to drug craving during prolonged opiate abstinence.
The dissociation of pain intensity from pain distress has been reported in patients with frontal lobotomies or cingulotomies (Foltz and White 1968; 1962; Hurt and Ballantine 1974). The endogenous opioid system, through the activation of µ-opioid receptors in specific brain regions, is involved in the attenuation of sensory and affective responses to painful stimuli. In our participants, similar ratings of pain intensity between OAs and healthy controls suggest that the brain regions involved in sensory aspects of pain were functionally normal. But, the increased distress in the PS OAs suggests a functional abnormality in brain regions involved in affective aspects of pain.
Several limitations of the present study might be addressed in a replication. First, it remains undetermined how other psychological variables may affect pain responses in OAs. Second, sex differences (Yu et al. 2007) could not be addressed in our study due to difficulty in recruiting female OAs. We will address these issues in future studies.
Our data suggest that the difference in pain tolerance between OAs and healthy controls may persist through at least 4 months of abstinence. This abnormal hyperalgesic state in OAs could predispose them to increased opiate craving. Numerous studies have shown that the neuro-adaptations resulting from long-term opiate misuse do not normalize even long after abstinence (Liebman et al. 1994; Liebmann et al. 1997; Pud et al. 2006). Therefore, the persistent hyperalgesia seen in our OAs may reflect a neuroadaptation to opiates, although longitudinal studies are needed to rule out the equally plausible possibility of a preexisting difference. In either case, it is possible that treatment aimed at ameliorating the hyperalgesia could help relieve opiate craving and prevent relapse to addiction.
Acknowledgments
This work was supported in part by the grant from the National Basic Research Program of China (973 Program, 2007CB512302 and 2009CB522004), the National High Technology Research and Development Program of China (863 Program, 2006AA02Z4D1), and the China–Canada Joint Health Research Program (No: 30611120528). We thank Xiaoli Zhang, Jingjie Yu, and Tangying Lu for technical assistant and Yu Liu for helpful comments on an early version of the manuscript.
Contributor Information
Zhen-Yu Ren, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, HaiDian District, Beijing 100083, China.
Jie Shi, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, HaiDian District, Beijing 100083, China.
David H. Epstein, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD 21224, USA
Jun Wang, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, HaiDian District, Beijing 100083, China.
Lin Lu, Email: linlu@bjmu.edu.cn, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, HaiDian District, Beijing 100083, China.
References
- Akvardar Y, Turkcan A, Cakmak D. Is substance abuse among physicians a problem? Turk Psikiyatri Derg. 2002;13:238–244. [PubMed] [Google Scholar]
- Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Malcy JA, Saxon AJ. Slow tapering from methadone maintenance in a program encouraging indefinite maintenance. J Subst Abuse Treat. 2006;30:159–163. doi: 10.1016/j.jsat.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Dworkin SF, Haug J, Gehrig J. Human pain responsivity in a tonic pain model: psychological determinants. Pain. 1989;37:143–160. doi: 10.1016/0304-3959(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. J Pain Symptom Manage. 1994;9:462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Cruciani RA, Esteban S, Seewald RM, Altilio T, Bookbinder M, Sheu R, Portenoy RK. MMTP patients with chronic pain switching to pain management clinics. A problem or an acceptable practice? Pain Med. 2008;9:359–364. doi: 10.1111/j.1526-4637.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Davis KD. The neural circuitry of pain as explored with functional MRI. Neurol Res. 2000;22:313–317. doi: 10.1080/01616412.2000.11740676. [DOI] [PubMed] [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE., Jr Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE. The role of rostral cingulumotomy in “pain” relief. Int J Neurol. 1968;6:353–373. [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuro-adaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Dole VP. Pain perception in drug-free and in methadone-maintained human ex-addicts. Proc Soc Exp Biol Med. 1979;162:392–395. doi: 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- Hurt RW, Ballantine HT., Jr Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. Clin Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Lehofer M, Liebmann PM, Moser M, Legl T, Pernhaupt G, Schauenstein K, Zapotoczky HG. Decreased nociceptive sensitivity: a biological risk marker for opiate dependence? Addiction. 1997;92:163–166. [PubMed] [Google Scholar]
- Liebman PM, Lehofer M, Schonauer-Cejpek M, Legl T, Pernhaupt G, Moser M, Schauenstein K. Pain sensitivity in former opioid addicts. Lancet. 1994;344:1031–1032. doi: 10.1016/s0140-6736(94)91697-7. [DOI] [PubMed] [Google Scholar]
- Liebmann PM, Lehofer M, Moser M, Hoehn-Saric R, Legl T, Pernhaupt G, Schauenstein K. Persistent analgesia in former opiate addicts is resistant to blockade of endogenous opioids. Biol Psychiatry. 1997;42:962–964. doi: 10.1016/S0006-3223(97)00337-5. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Inglis J. Pain tolerance and narcotic addiction. Br J Soc Clin Psychol. 1965;4:224–229. doi: 10.1111/j.2044-8260.1965.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proc Natl Acad Sci U S A. 1996;93:3048–3055. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006;30:57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ren ZY, Zhang XL, Liu Y, Zhao LY, Shi J, Bao Y, Zhang XY, Kosten TR, Lu L. Diurnal variation in cue-induced responses among protracted abstinent heroin users. Pharmacol Biochem Behav. 2009;91:468–472. doi: 10.1016/j.pbb.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Epstein DH, Zhang XL, Lu L. Long-term methadone maintenance reduces protracted symptoms of heroin abstinence and cue-induced craving in Chinese heroin abusers. Pharmacol Biochem Behav. 2007;87:141–145. doi: 10.1016/j.pbb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic–pituitary–adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Turk D, Meichenbaum D, Genest M. Pain and behavioral medicine: theory, research, and a clinical guide. New York, USA: Guildford Press; 1983. [Google Scholar]
- Upadhyaya HP, Drobes DJ, Thomas SE. Reactivity to smoking cues in adolescent cigarette smokers. Addict Behav. 2004;29:849–856. doi: 10.1016/j.addbeh.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Wilson S, Bailey J, Myles J, Nutt D. Imagery of craving in opiate addicts undergoing detoxification. Drug Alcohol Depend. 1997;48:25–31. doi: 10.1016/s0376-8716(97)00098-7. [DOI] [PubMed] [Google Scholar]
- White JM. Pleasure into pain: the consequences of long-term opioid use. Addict Behav. 2004;29:1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007;86:485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]