Abstract
Objective
To examine the association of fast-adapting receptor-mediated vibrotactile sensitivity and slow-adapting receptor-mediated pressure sensitivity with self-selected usual gait speed and gait speed over a challenging narrow (20 cm wide) course.
Design
Participants from the population-based older cohort of the Health ABC study were included (n = 1721; age: 76.4 ± 2.8 yrs). Usual gait speed over 6 m and gait speed over a 6-m narrow course were measured. Vibration perception threshold (100 Hz) was measured on the plantar surface, and monofilament testing (1.4 and 10 g) was performed on the dorsum of the great toe. Covariates including knee extensor torque, standing balance, visual acuity and contrast sensitivity, knee pain, depressive symptoms, high fasting glucose levels, and peripheral arterial disease were evaluated.
Results
Vibrotactile and monofilament sensitivity were significantly worse in slower gait speed groups in both walking conditions (P < 0.001 to P = 0.015). Adjusting for covariates, vibrotactile (P < 0.001) but not monofilament sensitivity (P = 0.655) was independently associated with self-selected normal gait speed. Neither sensory function was associated with narrow-base gait speed.
Conclusions
In the elderly, poor lower limb vibrotactile sensitivity measured on the plantar surface of the great toe, but not the pressure sensitivity as measured by monofilament testing on the dorsum of the great toe, is independently associated with slower self-selected normal gait speed. Narrow-based walking seems to depend on other neuromuscular mechanisms.
Keywords: Aging, Gait Speed, Cutaneous Vibration Sensitivity, Monofilament Sensitivity
Cutaneous sensory information from the lower limbs plays a significant role in maintaining stability during walking by activating appropriate re-flexes that are specific to the phase of the step cycle and the context in which the locomotor task is performed.1,2 It is proposed that the contribution of cutaneous information is particularly important under more challenging conditions, for example when walking on uneven or compliant surfaces3,4 or for regaining stability following a strong destabilization that induces a stepping recovery response.5 Deterioration in lower limb cutaneous sensitivity induced by experimental manipulation (for example by applying local anesthetics) or because of a pathologic process such as peripheral neuropathy is associated with reduced gait speed,3,6,7 possibly in an attempt to increase stability. However, none of these previous studies examined which lower limb cutaneous sensory function was in fact associated with the observed responses.
Researchers have reported a significant age-related decline in lower limb cutaneous sensitivity to detect a vibratory and pressure stimulus.8,9 The physiologic properties of the neural circuitry involved in vibration and pressure sensation are different.10 Compared with activation of slow-adapting receptors that mediate pressure sensation, activation of fast-adapting cutaneous receptors responsible for vibrotactile sensation has a better capacity to modulate reflex responses in lower limb muscles.11 Moreover, age-related decline in cutaneous vibration and pressure sensory functions do not occur in parallel.9 However, it is not known whether vibration sensitivity and pressure sensitivity are differentially associated with walking performance in older adults or whether this relationship is further affected by the challenges induced during walking.
The primary objective of this study was to examine the association of vibrotactile sensitivity and pressure sensitivity with gait speed at a self-selected normal pace which is a global indicator of walking performance and a strong predictor of incident walking disability in the elderly.12 The second objective was to gain an insight whether the relationship between the cutaneous sensitivity parameters and gait speed is modified under a challenging condition that constrains foot-placement in the mediolateral direction by narrowing the walking path. Monitoring quick changes in the center of foot-pressure is imperative for maintaining dynamic stability during walking. Therefore, we hypothesized that compared with pressure sensitivity, fast-adapting receptor-based vibrotactile sensitivity would be more strongly associated with gait speed under normal and in a challenging condition.
METHODS
Participants
The Health, Aging and Body Composition (Health ABC) study is a prospective cohort study on the relationship of changes in body composition and health conditions on physiologic and functional status in older adults initially aged 70 –79 yrs. Briefly, between April 1997 and June 1998, 3075 well-functioning nondisabled participants were recruited from a list of Medicare beneficiaries living in the areas surrounding Pittsburgh, Penn-sylvania and Memphis, Tennessee in the United States. Inclusion criteria for initial recruitment were no reported difficulty walking a quarter mile, climbing 10 steps, or performing basic activities of daily living; no life-threatening illness and no plans to leave the area for 3 yrs. Clinic-based follow-ups were conducted annually with 6-mo interval; and telephone follow-up was done for recording vital status, health events and hospitalizations, and physical function. The study protocol was approved by the institutional review boards at both the study sites. All participants received a detailed description of the study and all signed an informed consent.
The present study uses data collected in year 4 of the study during which sensory function assessment was performed. A total of 2165 participants completed the test for self-selected walk speed over 6 m and participated in the in-clinic examination. Of this group, 1721 participants had complete data available for all the variables of interest and were included in the analysis.
Outcome Measures
Walking Performance
Participants were asked to stand with their feet behind and just touching the tape line marking the start of the 6 m walking course and following the command “go,” to walk at their usual pace along the course and to stop a few steps beyond the finish line. Timing started with the first foot fall and ended with the first foot fall after the 6 m finish line. Speed of the faster of the two walking trials constitutes self-selected normal gait speed (normal speed) used in the analysis.13 For increasing challenge/demands to walking performance, a narrow base walk test was administered.14,15 On the same walking course, participants were instructed to walk at their usual pace, but to stay between lines of colored tape placed 20 cm apart. Failure to complete this test was recorded if the participant stepped on or outside the tape lines three or more times. Three attempts were allowed to obtain two valid trials. Speed of the faster of the two valid trials constitutes narrow-base gait speed (narrow-base speed). For those who successfully completed only one trial, the speed of this trial was used. If the participant could not successfully complete even one narrow-base trial, a zero value was assigned for his/her narrow-base gait speed.
Cutaneous Sensory Function
Vibration Perception Threshold (VPT)
Testing was performed in a quiet room using a commercial device (Medoc VSA 3000 Advance Medical Systems, MN). The diameter of the stimulating probe was 1.25 cm. If the initial limb temperature was below 30°C, the foot was heated for 5 min using a heating pad. With the participant seated, the examiner placed his/her bare testing foot on the foot plate with the probe surface under the distal phalanx of the big toe. The vibrating rod on the Medoc device is designed to provide a standard 50 g force on the plantar aspect of the big toe. The vibratory stimulus was delivered at 100 Hz and the method of limits was used. The machine delivered a stimulus that increased in amplitude by 0.8 μm/sec. The participant was instructed to concentrate on the foot and to press the button on the hand-held clicker as soon as s/he perceived the vibration. The test was repeated five times. The highest and the lowest scores were eliminated and the average of the remaining three trials was recorded as the VPT (μm). Testing was performed on the right foot unless contraindicated. Participants were excluded from the testing if both big toes were amputated.
Monofilament Testing
A two step monofilament protocol was used on the dorsum of the right great toe 1 cm proximal to the nail bed using monofilaments of 1.4 and 10.0 g (North Coast Medical Inc., CA). With the participant seated and eyes closed the examiner applied pressure first using a 1.4 g monofilament until the monofilament was bent to half its length. If the participant was unable to detect three out of four applications, the same test was repeated using the 10.0 g monofilament. The measurement was graded as follows: 0 = unable to detect either 1.4 or 10.0 g monofilament; 1 = able to detect 10.0 g monofilament 3 out of 4 times; and 2 = able to detect 1.4 g monofilament 3 out of 4 times. Testing was performed on the right foot unless contraindicated. Participants were excluded from the testing if both big toes were amputated.
Covariates
Demographic factors included age, sex, race, and body mass index (BMI, weight in kilograms/height in meters squared). Concentric knee extensor torque was measured on the right side at 60 degrees per second (Kin-Com Isokinetic Dynamometer, TN). Maximum torque was normalized to the participant’s body weight. Participants were excluded from the torque testing if they had a history of cerebral aneurysm, cerebral bleeding within the past 6 mos, blood pressure >199/109 mm Hg, or bilateral severe knee pain or joint replacement. In case of severe knee pain or joint replacement on the right side, the left knee was tested. Standing balance was evaluated using three progressively more difficult stands, semitandem, full tandem, and single leg, all to be held for maximum 30 sec. For both the full tandem and single stands two attempts were permitted. The total time was calculated by adding the time spent in each stand before losing balance. A ratio of total time to the total time possible (90 sec) was computed for each participant (range 0 –1).15 Participants did not undergo balance assessment if they refused the test or if the testing was deemed unsafe by the tester.
Participants were considered having high fasting glucose levels if their fasting blood glucose level was ≥126 mg/dL.16 Peripheral arterial disease was delineated as ankle brachial index <0.9.17 Persons with open wounds including venous stasis ulcers or rashes were excluded. Visual acuity was tested using the standard Bailey-Lovie chart,18 and contrast sensitivity was measured using the standard Pelli-Robson chart.19 Total number of correctly identified letters was the unit of analysis. Since vision assessment was not performed in year 4, data from year 3 was used. Knee joint pain was evaluated by the Western Ontario MacMaster Questionnaire.20 The Center for Epidemiologic Studies Depression scale,21 a 20-item self-report questionnaire, was used to assess severity of depressive symptoms.
Statistical Analysis
Distributions of continuous variables were tested for normality. Log transformations of skewed variables (VPT, Western Ontario MacMaster Questionnaire and Center for Epidemiologic Studies Depression scale scores, and standing balance ratios) were used in subsequent analyses and data were back-transformed for presentation. Initially, participants were stratified according to four standard gait speed cut-points of self-selected normal gait speed (<0.8, <1.0, <1.2, and ≥1.2 m/sec).22–24 Because participants were asked to walk at their normal speed even in the narrow-base condition, similar cut-points were used for this walking condition as well. Analysis of covariance was performed using a General Linear Model to test for differences in VPT between gait speed groups while adjusting for age, sex, BMI, and height. The fifth group in the narrow-base condition comprised participants who were unable to successfully complete the task. A multinomial logistic regression analysis (χ2 test) was performed to identify differences in the monofilament sensitivity grades of participants between the gait speed groups while adjusting for age, sex, BMI, and height.
First, the relationship of each cutaneous sensory function with the two gait speeds was assessed by a correlation analysis partialling out age, sex, race, BMI, and height. Cutaneous sensory functions with correlational significance of P < 0.20 were included in the multiple linear regression models to identify their independent association with gait speed when adjusted for age, sex, BMI, and height. For this and the above-mentioned correlation analysis, monofilament sensitivity was treated as a continuous measure and was log transformed. If a significant independent association (P < 0.05) was found with either vibrotactile or pressure sensitivity in this initial model, further analysis was done by adding additional covariates to test the robustness of this association (knee extensor torque, standing balance, high fasting glucose levels, peripheral vascular disease, visual acuity, visual contrast sensitivity, knee pain, and Center for Epidemiologic Studies Depression scale severity of depressive symptoms). Self-selected gait speed was included as a covariate in the fully adjusted model for the analysis with narrow base gait speed.
All statistical analyses were conducted in SPSS (version 13.0, SPSS Inc, Chicago, IL). P < 0.05 was considered statistically significant.
RESULTS
Table 1 describes the overall characteristics of study participants included in this analysis (n = 1721). Compared with those seen in the clinic but not included because of incomplete data set (n = 444), included participants were slightly younger (age, 76.4 ± 2.8 vs. 76.7 ± 2.9 yrs, P = 0.001), were more likely to be men (49% men vs. 41% women, P = 0.011), had a faster average gait speed (1.14 ± 0.23 vs. 1.01 ± 0.25 m/sec, P < 0.001), and lower VPT (49.7 ± 35.6 vs. 54.3 ±38.5 μm, P = 0.001). Also, 7.9% of the included participants did not perceive either of the monofilaments compared with 9.5% of those who were not included (P = 0.113).
TABLE 1.
Characteristics of Health ABC study participants included in this analysis (n = 1721)
| Variable | Summary Statistics |
|---|---|
| Age (yrs) | 76.4 (2.8) |
| Height (m) | 1.65 (0.09) |
| Gender (men%) | 46.1% |
| Race (white/black) | 64.5%/35.5% |
| BMI (kg/m2) | 27.10 (4.5) |
| Vibration perception threshold (μm) | 49.4 (35.3) |
| Monofilament grade (none/only 10.0 g/1.4 g) | 7.6%/34.0%/58.4% |
| Normalized knee torque (Nm/kg) | 1.28 (0.4) |
| Standing balance ratioa | 0.74 (0.25) |
| High fasting glucoseb | 9.5% |
| Peripheral arterial diseasec | 13.9% |
| Visual acuityd | 54.9 (7.0) |
| Visual contrast sensitivitye | 34.4 (3.5) |
| Total knee painf | 2.4 (4.9) |
| Depressive symptomsg | 6.0 (5.9) |
| Walk speed (normal) (m/sec) | 1.1 (0.2) |
| Walk speed (narrow-base) (m/sec) | 0.9 (0.5) |
Mean (SD) if not otherwise specified.
Standardized balance ratio: Standing balance was evaluated using three progressively more difficult stands, semi-tandem, full tandem, and single leg, all to be held for maximum 30 sec. For both the full tandem and single stands two attempts were permitted. The total time was calculated by adding the time spent in each stand before loosing balance. A ratio of total time to the total time possible (90 sec) was computed for each participant (range 0 –1).
High fasting glucose: fasting glucose level ≥126 mg/dL.
Peripheral arterial disease: ankle brachial index <0.9.
Visual acuity: total number of correctly identified letters on the standard Bailey-Lovie chart.
Visual contrast sensitivity: total number of correctly identified letters on the standard Pelli-Robson chart.
Total knee pain: Western Ontario MacMaster Questionnaire scores.
Depressive symptoms: The Center for Epidemiological Studies Depression scale scores.
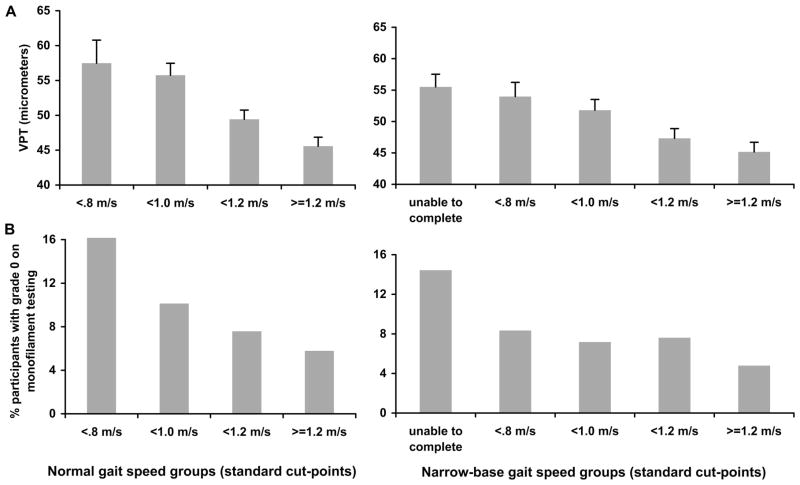
Participants walked significantly slower in narrow-base walking condition (normal gait speed: 1.14 ± 0.23 m/sec; narrow-base gait speed: 0.92 ± 0.49 m/sec, F(1,1720) = 431.41; P < 0.001). After adjusting for age, sex, race, BMI, and height, VPT was significantly higher in the slower gait speed groups (normal gait speed groups: F(3,1712) = 8.724, P < 0.001; narrow-base gait speed groups: F(4,1711) = 5.195, P < 0.001) (Fig. 1A). Significantly more participants in the slower gait speed groups had poorer grade of monofilament sensitivity (normal gait speed groups: χ2(6) = 15.839, P < 0.015; narrow-base gait speed groups: χ2(8) = 19.247, P = 0.014) (Fig. 1B).
FIGURE 1.
Mean vibration perception thresholds (SEM) according to normal gait speed groups and narrow-base gait speed groups using standard cut-points (A). Percent of participants who did not perceive either of the monofilaments (grade 0) according to normal gait speed groups and narrow-base gait speed groups using standard cut-points (B).
Independent of age, sex, race, BMI, and height, both logVPT and log monofilament sensitivity were significantly correlated with self-selected gait speed and narrow-base gait speed (all four P values <0.01).
In multiple regression analyses that included the demographic factors, both logVPT and log monofilament sensitivity were independently associated with self-selected normal gait speed (Table 2, model 1). After adjusting for confounders, the co-efficient for VPT was only moderately reduced and was still significantly different from zero. In contrast, the size of coefficient for monofilament sensitivity was reduced by more than 75% and was no longer significantly different from zero (Table 2, model 2).
TABLE 2.
Multiple linear regression models relating vibrotactile sensitivity and monofilament sensitivity to normal gait speed and narrow-base gait speed
| Normal Gait Speed
|
Narrow-base Gait Speed
|
|||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t | P | β | SE | t | P | |
| Model 1 | ||||||||
| Vibration threshold (μm) | −0.081 | 0.014 | −5.656 | 0.000 | −0.096 | 0.032 | −3.019 | 0.003 |
| Monofilament grade | 0.086 | 0.038 | 2.252 | 0.024 | 0.324 | 0.085 | 3.818 | 0.000 |
| Age (yrs) | −0.012 | 0.002 | −6.683 | 0.000 | −0.028 | 0.004 | −6.866 | 0.000 |
| Height (m) | 0.182 | 0.084 | 2.156 | 0.031 | 0.334 | 0.187 | 1.785 | 0.074 |
| Sex | 0.069 | 0.015 | 4.574 | 0.000 | 0.090 | 0.034 | 2.672 | 0.008 |
| Race | −0.132 | 0.011 | −12.067 | 0.000 | −0.163 | 0.024 | −6.710 | 0.000 |
| BMI (kg/m2) | −0.005 | 0.001 | −4.614 | 0.000 | −0.015 | 0.003 | −5.856 | 0.000 |
| Constant | 2.042 | 0.213 | 9.575 | 0.000 | 2.983 | 0.474 | 6.296 | 0.000 |
| Model 2 | ||||||||
| Vibration threshold (μm) | −0.057 | 0.014 | −4.141 | 0.000 | −0.004 | 0.028 | −0.135 | 0.893 |
| Monofilament grade | 0.016 | 0.037 | 0.446 | 0.655 | 0.122 | 0.076 | 1.608 | 0.108 |
| Age (yrs) | −0.007 | 0.002 | −3.651 | 0.000 | −0.009 | 0.004 | −2.394 | 0.017 |
| Height (m) | 0.185 | 0.081 | 2.287 | 0.022 | 0.198 | 0.167 | 1.188 | 0.235 |
| Sex | −0.001 | 0.016 | −0.051 | 0.960 | −0.051 | 0.032 | −1.574 | 0.116 |
| Race | −0.122 | 0.011 | −11.324 | 0.000 | −0.058 | 0.023 | −2.532 | 0.011 |
| BMI (kg/m2) | −0.001 | 0.001 | −0.625 | 0.532 | −0.006 | 0.002 | −2.475 | 0.013 |
| Nor max torque (Nm/kg) | 0.120 | 0.015 | 7.870 | 0.000 | 0.093 | 0.032 | 2.904 | 0.004 |
| Standing balance ratio | 0.497 | 0.075 | 6.663 | 0.000 | 1.607 | 0.156 | 10.316 | 0.000 |
| Visual acuity | 0.001 | 0.001 | 0.856 | 0.392 | 0.004 | 0.002 | 2.276 | 0.023 |
| Contrast sensitivity | 0.004 | 0.002 | 2.184 | 0.029 | 0.004 | 0.004 | 0.987 | 0.324 |
| Total knee pain | −0.021 | 0.012 | −1.739 | 0.082 | 0.015 | 0.025 | 0.606 | 0.544 |
| Depressive symptoms | −0.030 | 0.013 | −2.400 | 0.017 | −0.047 | 0.026 | −1.807 | 0.071 |
| High fasting glucose | −0.037 | 0.016 | −2.249 | 0.025 | −0.015 | 0.034 | −0.455 | 0.650 |
| Peripheral arterial disease | −0.024 | 0.014 | −1.717 | 0.086 | 0.007 | 0.029 | 0.229 | 0.819 |
| Normal gait speed (m/sec) | 0.711 | 0.050 | 14.249 | 0.000 | ||||
| Constant | 1.078 | 0.222 | 4.852 | 0.000 | −0.178 | 0.461 | −0.386 | 0.700 |
Model 1 adjusted for demographic factors and model 2 adjusted for other possible confounding factors in addition to demographics. Normal gait speed was included as a covariate for the analysis of narrow-base gait speed.
Results of the multiple regression analysis showed that VPT and monofilament sensitivity were also independently associated with narrow-base gait speed, when adjusted for demographics (Table 2, model 1). However, in the fully adjusted model (Table 2, model 2) neither vibrotactile nor pressure sensitivity was independently associated with narrow-base gait speed.
DISCUSSION
Using data collected on a large population-based sample of older adults, we demonstrated that older persons with slower gait speeds have higher VPT and poorer pressure sensitivity. After adjusting for demographics and other confounding factors, VPT, but not monofilament sensitivity, was associated with self-selected normal gait speed.
Substantial evidence from neurophysiological studies demonstrates that cutaneous sensory information from the foot plays an important role during locomotion in modulating lower limb muscle activity through spinal and supraspinal reflex pathways.25 Fallon et al.11 recently found that activation of the fast-adapting receptors in the foot sole is a more potent stimulus for modulating lower limb muscle activity than activation of slow-adapting receptors. Although Fallon et al.11 tested their participants in a non–weight-bearing recumbent position, it is possible that a similar relationship would hold during walking as well, because the fast-adapting receptors respond to the beginning of the mechanical deformation and the end of the stimulus and are therefore, better suited to detect quick changes in the center of foot-pressure location during a dynamic task such as walking. Results from this study clearly indicate that unlike mono-filament sensitivity, the association between vibrotactile sensitivity and self-selected normal gait speed is robust even when adjusted for multiple possible confounding factors. Further, even when interactions between the vision parameters and the peripheral sensory parameters were included in the analysis, these results were not influenced considerably. Although the exact mechanism underlying this relationship is not clear, it is possible that in older persons reduced ability to detect quick changes in pressure (vibration) under the foot results in a global strategic adaptation of reduced gait speed, possibly to improve stability. Our speculation is supported by studies that have reported similar strategic changes in peripheral neuropathy patients3,7 and when foot cutaneous sensations are experimentally reduced in healthy young adults.6 Our study extends the findings of these previous reports and also suggests that in addition to decline in lower limb muscle strength, balance deficits, and impaired vision,26 decline in vibrotactile sensitivity could be a key physiologic factor that independently contributes to age-related decline in gait speed.
Contrary to our initial hypothesis, when adjusted for confounding factors, the association between vibrotactile and pressure sensitivity and gait speed in the narrow-based walk challenge disappeared. There could be two reasons for this. First, we increased the challenge by restricting the allowable walking area using visible marking tapes rather than manipulating surface conditions. A high level of attention is involved in this task, particularly for planning/selecting the foot placement. However, considering the high level of challenge that is induced on this population (age 76.4 ± 2.8 yrs) for postural control while performing this task, it would demand the overall sensory-motor integration for maintaining stability. It is possible that a more reactive walking task would result in a stronger independent association. The regression analysis results suggested that for maintaining stability under the biomechanical challenge thus induced, BMI, knee extensor torque, balance, and visual acuity were possibly more relevant to performance. Also, the induced challenge was specifically in the mediolateral direction. It is possible that ankle inversion and eversion detection threshold and/or vestibular information, which were not available in this study, would be more relevant for this particular task than lower limb vibrotactile and cutaneous sensory feedback.
The overall protocol of the Health ABC study was not specifically designed to assess the association between loss of peripheral sensory function and gait and therefore, our measures of sensory function were not chosen solely for this reason. Nonetheless, great toe is a standard evaluation site for vibration testing that has been previously validated in large epidemiologic studies (e.g., The Women’s Health and Aging Study). The results from The Women’s Health and Aging Study have also shown that VPT at the plantar surface of the great toe is associated with walking speed in their population.7 Different studies have used different test sites (such as various sites on plantar surface of the foot, medial or lateral malleolus, or tibial tuberosity) for assessing VPT. VPT at all these sites show age-related or disease-related deterioration in vibrotactile sensitivity. The results of the studies that used multiple sites suggest that there is global reduction in foot vibration sensitivity. Surprisingly, however, there is no study that directly reports the between-site correlation of this deterioration. Because sensitivity of different areas of the footsole may influence different phases of the gait cycle, it would be absolutely interesting to examine whether there is between-site differences in the strength of this independent association. It is noteworthy, however, that vibration sensitivity even at the tibial tuberosity was found to be associated with walking speed.3
As for the testing site for monofilament test, in addition to other sites on the foot plantar surface and malleoli, dorsum of the great toe proximal to the nail bed is recommended in clinical studies. This site was selected in the Health ABC study primarily to achieve a distal, but more sensitive surface area where callus formation may not forbid the testing. Our results indicated that cutaneous sensitivity as assessed by monofilament testing in this study was not independently associated with the gait speed in either condition in fully adjusted regression models. We conducted further analysis to test whether this was because the testing was performed on the dorsal nonweight bearing aspect of the foot or because lower limb pressure sensitivity is less relevant for functional mobility. Because monofilament testing assesses sensitivity to maintained pressure, we repeated the multiple regression analyses using balance performance as a dependent variable instead of gait speed. We found that in the fully adjusted model monofilament sensitivity and not vibrotactile sensitivity was independently associated with balance performance (vibrotactile sensitivity: β = −0.005, SE = 0.004, t = −1.044, P = 0.297; monofilament sensitivity: β = 0.038, SE = 0.012, t = 3.188, P = 0.001). These results lead us to speculate that the foot cutaneous sensitivity as measured by monofilament testing could be more relevant for postural control when there is a maintained foot contact (for example during quiet standing, while standing on unstable surfaces, while performing a sit-to stand maneuver, etc.), rather than when there is a necessity to monitor a continuous change in touch/pressure, such as during walking. Thus, the difference in the independent association of the two sensory functions may not be due to the difference in sites but could be because their relevance in postural control is task-dependent. Nonetheless, considering the multiple sites of testing used in various studies for peripheral sensory assessment, further research is warranted to identify if age-related or disease-related deterioration in pressure sensitivity and vibration sensitivity at different locations on the foot are correlated.
In patients with diabetic peripheral neuropathy, a lack of protective sensation and the risk for unrecognized foot injury is associated with lack of sensitivity to 10 g monofilament.27 However, these patients also demonstrate poor mobility and increased risk of falls.4,7,28 Significant differences have been reported in global and spatiotemporal gait parameters between healthy elderly and elderly with diabetic neuropathy.29 Further, Thies et al.30 have demonstrated that a composite score derived from sensory parameters, strength of distal muscles, and quality of stretch reflexes is significantly correlated with the step width variability of patients with diabetic neuropathy. Future studies should investigate whether impaired vibrotactile sensitivity in patients with peripheral neuropathy is specifically associated with an accelerated decline in dynamic postural control observed in these patients and whether there is a specific cut-off point for decline in vibrotactile sensitivity when the risk of deficits in functional mobility and falls increases substantially.
The main limitation of this study is the cross-sectional nature of the analysis that suggests but does not prove a direct causal relationship between the age-related decline in vibrotactile sensitivity and gait speed. Therefore, the findings should be confirmed by using a longitudinal approach. The second limitation is that the monofilament sensitivity was tested using three grades. It is possible that finer gradation could have provided a more sensitive measure of cutaneous pressure sensitivity.
In conclusion, among various factors that can contribute to age-related decline in gait speed in the elderly, poor lower limb vibrotactile sensitivity is independently associated with slower self-selected normal gait speed. Vibrotactile sensitivity may significantly contribute to stability during walking by directly monitoring the change in the location of center of pressure on the plantar surface during a dynamic task such as walking. The results of this study clearly indicate that decline in vibrotactile sensitivity may have a direct bearing on age-related decline in self-selected gait speed. The role of the lower limb cutaneous information during a challenging walking task may be specific to the dynamics of the induced challenge.
Footnotes
Disclosures:
This research was supported in part by the intramural research program of the NIH, Institute on Aging (Contracts N01-AG-6-2101, NO1-AG-2103, and NO1-AG-2106). The findings were presented at The Gerontological Society of America’s 60th Annual Scientific Meeting, San Francisco, CA, USA, 2007.
References
- 1.Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol. 2003;90:2850– 61. doi: 10.1152/jn.00531.2003. [DOI] [PubMed] [Google Scholar]
- 2.Duysens J, Trippel M, Horstmann GA, et al. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res. 1990;82:351– 8. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- 3.Menz HB, Lord SR, St George R, et al. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85:245–52. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40:1008–12. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 5.Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877:401–6. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell M, Warden-Flood A. Effect of partial foot anaesthesia on normal gait. Aust J Physiother. 2000;46:115–20. doi: 10.1016/s0004-9514(14)60319-6. [DOI] [PubMed] [Google Scholar]
- 7.Resnick HE, Stansberry KB, Harris TB, et al. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25:43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 8.Perry SD. Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci Lett. 2006;392:62–7. doi: 10.1016/j.neulet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 9.Sands ML, Schwartz AV, Brown BW, et al. Relationship of neurological function and age in older women. The study of osteoporotic fractures. Neuroepidemiology. 1998;17:318–29. doi: 10.1159/000026186. [DOI] [PubMed] [Google Scholar]
- 10.Gardner EP, Martin JH, Jessell TM. The bodily senses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Sciences. 4. New York: Mc Graw-Hill; 2000. pp. 430–50. [Google Scholar]
- 11.Fallon JB, Bent LR, McNulty PA, et al. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol. 2005;94:3795– 804. doi: 10.1152/jn.00359.2005. [DOI] [PubMed] [Google Scholar]
- 12.Brach JS, Studenski SA, Perera S, et al. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:983– 8. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 14.Bandinelli S, Pozzi M, Lauretani F, et al. Adding challenge to performance-based tests of walking: The Walking InCHIANTI Toolkit (WIT) Am J Phys Med Rehabil. 2006;85:986–91. doi: 10.1097/01.phm.0000233210.69400.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonsick EM, Newman AB, Nevitt MC, et al. Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–9. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 16.Expert committee on the diagnosis and classification of diabetes mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837– 45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 18.Bailey IL, Lovie JE. The design and use of a new near-vision chart. Am J Optom Physiol Opt. 1980;57:378–87. doi: 10.1097/00006324-198006000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Pelli DG, Robson PG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1998;2:187–99. [Google Scholar]
- 20.Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7:216–21. doi: 10.1053/joca.1998.0153. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385– 401. [Google Scholar]
- 22.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 23.Shumway-Cook A, Patla AE, Stewart A, et al. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther. 2002;82:670– 81. [PubMed] [Google Scholar]
- 24.Langlois JA, Keyl PM, Guralnik JM, et al. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87:393–7. doi: 10.2105/ajph.87.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aniss AM, Gandevia SC, Burke D. Reflex responses in active muscles elicited by stimulation of low-threshold afferents from the human foot. J Neurophysiol. 1992;67:1375– 84. doi: 10.1152/jn.1992.67.5.1375. [DOI] [PubMed] [Google Scholar]
- 26.Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51:390–5. doi: 10.1159/000088703. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong DG, Lavery LA, Vela SA, et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289–92. doi: 10.1001/archinte.158.3.289. [DOI] [PubMed] [Google Scholar]
- 28.Volpato S, Leveille SG, Blaum C, et al. Risk factors for falls in older disabled women with diabetes: The women’s health and aging study. J Gerontol A Biol Sci Med Sci. 2005;60:1539– 45. doi: 10.1093/gerona/60.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson JK, Thies SB, DeMott TK, et al. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004;52:1532–7. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- 30.Thies SB, Richardson JK, Demott T, et al. Influence of an irregular surface and low light on the step variability of patients with peripheral neuropathy during level gait. Gait Posture. 2005;22:40–5. doi: 10.1016/j.gaitpost.2004.06.006. [DOI] [PubMed] [Google Scholar]