Abstract
Objective
Previous studies addressing racial disparities in treatment for early-stage prostate cancer have focused on the etiology of undertreatment of black men. Our objective was to determine whether racial disparities are attributable to undertreatment, overtreatment, or both.
Methods
Using the SEER-Medicare dataset, we identified men 67–84 years-old diagnosed with localized prostate cancer from 1998 to 2007. We stratified men into clinical benefit groups using tumor aggressiveness and life expectancy. Low-benefit was defined as low-risk tumors and life expectancy <10 years; high-benefit as moderate-risk tumors and life expectancy ≥10 years; all others were intermediate-benefit. Logistic regression modeled the association between race and treatment (radical prostatectomy or radiotherapy) across benefit groups.
Results
Of 68,817 men (9.8% black and 90.2% white), 56.2% of black and 66.3% of white men received treatment (adjusted odds ratio (OR)=0.65; 95% CI, 0.62–0.69). The percent of low-, intermediate-, and high-benefit men who received treatment was 56.7%, 68.4%, and 79.6%, respectively (P=<0.001). In the low-benefit group, 51.9% of black vs. 57.2% of white patients received treatment (OR=0.74; 95% CI, 0.67–0.81) compared to 57.2% vs. 69.6% in the intermediate-benefit group (OR=0.64; 95% CI, 0.59–0.70). Racial disparity was largest in the high-benefit group (64.2% of black vs. 81.4% of white patients received treatment; OR=0.57; 95% CI, 0.48–0.68). The interaction between race and clinical benefit was significant (P<0.001).
Conclusion
Racial disparities were largest among men most likely to benefit from treatment. However, a substantial proportion of both black and white men with a low clinical benefit received treatment, indicating a high level of overtreatment.
Keywords: Prostatic neoplasms, Healthcare disparities, Minority health, Standard-of-care, Population, Geriatrics
1. Introduction
In the United States, racial and ethnic disparities in cancer treatment and overall cancer survival are well-documented.1–3 Black patients continue to have the highest mortality and shortest survival for all major cancer sites, and disparities persist despite an improved understanding of early detection, genetics, and socioeconomic risk factors.1,3–7 Prostate cancer imposes a disproportionate burden on black men as they have a higher incidence, more unfavorable tumor characteristics, and greater mortality than white men.8–12 Several studies have called for increasing treatment of black men as the key approach to resolving cancer disparities.11–14 It is unclear whether overtreatment of white men is an exacerbating factor to these observed racial disparities. This is important to determine, as disparities related to overtreatment of white men would require a markedly different solution than those directed at addressing undertreatment of black men.
Prior analyses of prostate cancer disparities have not incorporated the marked clinical heterogeneity in prognosis. Many men diagnosed with early-stage disease will die from other causes, and treatment may cause significant adverse side-effects.15,16 Both tumor and patient characteristics strongly influence how a prostate cancer diagnosis will affect health outcomes and the benefits of treatment. Older patients with shorter life expectancies and less aggressive tumors are less likely to benefit from surgical treatment, and expectant management may be more appropriate.17–19 Prior studies have not shown a significant mortality benefit with radical prostatectomy compared to expectant management in men >65 years of age with low-risk disease.17,20 And to our knowledge, there are no randomized-control trials comparing different radiation modalities vs. expectant management among older men. In order to understand the contribution of undertreatment and overtreatment to racial disparities in prostate cancer treatment, amore clinically relevant framework is needed: both life expectancy and tumor aggressiveness are accepted as determinants of treatment benefit at the bedside and are included in recommendations by the National Comprehensive Cancer Network (NCCN).9 Treatment within a low-benefit group may be considered overuse, while no treatment in a high-benefit group may be considered underuse.21 The potential remedies to decreasing overuse of treatment drastically differ from approaches to improving underuse of treatment. Only by viewing racial disparities through a lens of clinical appropriateness can clinicians and policy-makers rectify disparities. We therefore created a framework of clinically appropriate care based on clinical evidence and national guidelines, and then applied this framework to empirically assess racial disparities in prostate cancer treatment.9,17
To understand appropriate care in terms of overtreatment and undertreatment in localized prostate cancer, we stratified men into clinical benefit groups based on life expectancy and tumor characteristics based on NCCN guidelines. We analyzed the patterns of treatment in each benefit group and the degree to which specific treatment modalities contributed to disparities. We hypothesized that racial disparities in prostate cancer treatment would vary across clinical benefit groups.
2. Methods
2.1. Data Source and Study Sample
We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database.22 This database includes demographics and tumor characteristics for 20 different registries across the United States and covered 26% of the U.S. population during 2000–2007.22 Compared to the general population ≥65 years of age, SEER is comparable in terms of age and sex distribution; however, SEER has a higher proportion of non-white persons and urban-dwellers and a lower proportion of persons living below poverty level.23 The linked database also includes Medicare claims for all beneficiaries in the SEER registries and a 5% random sample of Medicare beneficiaries with and without cancer in the SEER regions. We included the 13 SEER registries available before the 2000 SEER expansion.24 The Yale Human Investigation Committee does not classify this study as human subject research.
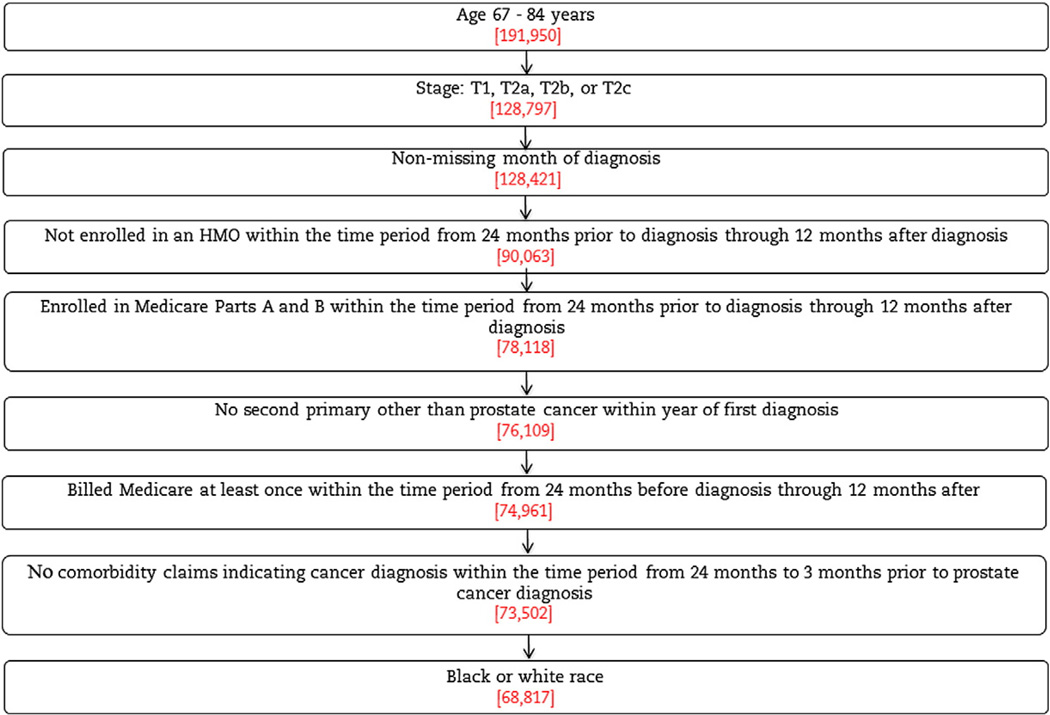
We selected all black and white men aged 67–84 years of age diagnosed with stages T1–T2 prostate cancer from 1998 to 2007. We excluded patients whose reporting source of cancer was a death certificate or autopsy, those with missing grade or stage, those with prior malignancy or a second primary tumor diagnosed within a year of their prostate cancer diagnosis, and those who did not have fee-for-service Medicare Parts A and B coverage during the study period. Patients must have had at least one Medicare claim billed during the study period (Fig. 1).
Fig. 1.
Sample selection.
2.2. Construction of Variables
Comorbid conditions were assessed by Medicare claims in the 24 through 3 months prior to diagnosis. We searched International Classification of Diseases, 9th revision (ICD-9) diagnosis codes for the conditions recommended by Elixhauser et al. that were significantly associated with survival in a sample of non-cancer patients.25 We selected ICD-9 codes that appeared on an inpatient claim or at least two outpatient claims billed ≥30 days apart. Median household income at the zip code level reported by the 2000 Census was used as a proxy for socioeconomic status.
2.3. Clinical Benefit Groups
The sample was stratified into low- and moderate-risk tumor categories. Low-risk disease included SEER histologic grade 1 or 2 and clinical stage T1 or T2a, while moderate-risk disease included SEER grade 3 or 4 or T2b–T2c disease.9,22 Though T1a is potentially curable by transurethral resection of the prostate (TURP), we included T1a patients in low-risk disease to include those classified as T1 without further detail. The SEER Program Coding and Staging Manual (Appendix C) indicates that SEER grade 1 = Gleason scores 2–4, grade 2 = scores 5–6, and grades 3–4 = scores 7–10. These are consistent with the NCCN and American Urological Association guidelines. Since not all men in the SEER database had Gleason scores and the scoring system changed in the 1990s, we felt that SEER grading was a more consistent tool to use in our analysis.26
To estimate life expectancy, we used a sample of non-cancer patients from the Medicare 5%randomsample. We constructed age- and comorbidity-specific life tables using the annual mortality rates. To validate our life expectancy method, we applied age- and comorbidity-specific life expectancy estimates to men diagnosed in 1998–99 (for whom 10-year follow up data was available). Ten-year survival among men with a life expectancy ≥10 years was 76.1%, compared to 48.0% among men with a life expectancy of <10 years.
NCCN guidelines recommend that patients with moderate-risk disease and life expectancy ≥10 years receive treatment. Conversely, for patients with low-risk tumors and life expectancy <10 years, treatment risks may outweigh the benefits with little mortality benefit; therefore, expectant management is an acceptable treatment option.9 Based on NCCN guidelines, low-benefit patients were defined as having a low-risk tumor and life expectancy <10 years, intermediate-benefit as having a low-risk tumor and life expectancy ≥10 years or a moderate-risk tumor and life expectancy <10 years, and high-benefit as having a moderate-risk tumor and life expectancy ≥10 years.
Because PSA and Gleason scores were only available in SEER starting in 2004, we performed a sensitivity analysis including only men diagnosed between 2004 and 2007. Low-risk was defined as stages T1–T2a, Gleason scores 2–6, and PSA<10, and with SEER staging indicators verifying no lymph node involvement or metastases. Moderate-risk was defined as stages T2b–T2c, Gleason score 7, and PSA 10–20.Men with high-risk tumors were excluded from the study sample (consistent with our main analysis).
2.4. Treatment
We searched Medicare claims for Healthcare Common Procedure Coding System and ICD-9 procedure codes for radiotherapy and radical prostatectomy (Table 1). We defined treatment as receipt of radiotherapy or prostatectomy within 9 months of diagnosis. Patients were considered to be under expectant management if there were no claims billed with the listed codes or if they received primary androgen deprivation therapy. Radiotherapy included external beam radiotherapy (including standard external beam, intensity modulated radiotherapy, stereotactic radiosurgery, and proton beam) and brachytherapy. Men who received external beam or intensity modulated radiotherapy had to receive at least four treatments to be categorized as treated.
Table 1.
Procedure codes used to identify treatment.
| Treatment | Codes | |
|---|---|---|
| External beam radiation | HCPCS | 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77301, 77418, 77371–77373, 77520, 77522, 77523, 77525, 0082T, 0073T, G0174, G0173, G0243, G0251, G0339, G0340 |
| Brachytherapy | HCPCS | 77776, 77777, 77778, 77781, 77782, 77783, 77784, 77799, G0256, G0261 |
| Prostate surgery | HCPCS | 55810, 55812, 55815, 55840, 55842, 55845, 55866, 55801, 55821, 55831 |
| ICD-9 | 60.3, 60.4, 60.5, 60.6, 60.62, 60.69 |
2.5. Statistical Analysis
Chi-squared tests were used to test the bivariate associations between receipt of curative therapy and each covariate and to determine whether the receipt of curative therapy differed between black and white patients within all strata of the covariates. Logistic regression modeled the association between race and receipt of treatment. The multivariable model included covariates independently associated with receipt of treatment, and we tested the interaction between race and clinical benefit category. The predicted probability of receiving treatment was calculated for each individual patient based on his actual values for each covariate except for race. Using the parameter estimates output from the logistic regression, two predicted probabilities were calculated for each patient—one in which we set the patient's race to white and the other to black, regardless of the patient's actual race. The mean of these probabilities was taken within each clinical benefit group to compare the average predicted probability of treatment for black and white patients. A 95% confidence interval for each average predicted probability was derived using a bootstrapping technique. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
3. Results
Among 68,817 eligible men, 6723 (9.8%) were black and 62,094 (90.2%) were white (Table 2). Approximately 13.9% of black men were in the high-benefit group vs. 12.6% of white men (P=0.002). Overall, 56.7%of low-benefit men received treatment compared to 68.4% of intermediate-benefit men and 79.6% of high-benefit men (P=<0.001).
Table 2.
Patient characteristics and receipt of curative therapy.
| Patient characteristic | Percent receiving curative therapy |
Black vs. White | ||||||
|---|---|---|---|---|---|---|---|---|
| Full sample a |
Black a |
White a |
||||||
| N | % | N | % | N | % | P-value b | ||
| Age | 67–69 | 15,493 | 77.2 | 1822 | 64.6 | 13,671 | 78.9 | <0.001 |
| 70–74 | 25,166 | 73.3 | 2510 | 63.9 | 22,656 | 74.4 | <0.001 | |
| 75–79 | 19,141 | 59.5 | 1692 | 48.5 | 17,449 | 60.5 | <0.001 | |
| 80–84 | 9017 | 34.8 | 699 | 25.5 | 8318 | 35.6 | <0.001 | |
| Tumor risk | Low | 38,783 | 62.0 | 3464 | 54.7 | 35,329 | 62.7 | <0.001 |
| Moderate | 30,024 | 69.6 | 3259 | 57.9 | 26,765 | 71.0 | <0.001 | |
| Marital status | Married | 49,445 | 69.2 | 3725 | 61.2 | 45,720 | 69.9 | <0.001 |
| Not married | 13,984 | 57.6 | 2479 | 51.4 | 11,505 | 58.9 | <0.001 | |
| Unknown | 5388 | 49.1 | 519 | 43.9 | 4869 | 49.6 | 0.01 | |
| Comorbidity | 0 | 37,081 | 67.6 | 3232 | 56.0 | 33,849 | 68.7 | <0.001 |
| 1,2 | 24,091 | 65.6 | 2390 | 59.6 | 21,701 | 66.2 | <0.001 | |
| ≥3 | 7645 | 53.4 | 1101 | 49.7 | 6544 | 54.0 | 0.008 | |
| Income | Q1: <$35,528 | 17,458 | 57.7 | 3807 | 51.4 | 13,651 | 59.4 | <0.001 |
| Q2: $35,528–<47,930 | 17,344 | 65.1 | 1520 | 59.9 | 15,824 | 65.6 | <0.001 | |
| Q3: $47,930–<65,852 | 17,104 | 67.9 | 942 | 64.4 | 16,162 | 68.1 | 0.02 | |
| Q4: >$65,852 | 16,911 | 70.7 | 454 | 66.3 | 16,457 | 70.9 | 0.04 | |
| Life expectancy | <10 years | 47,708 | 60.6 | 4771 | 53.6 | 42,937 | 61.4 | <0.001 |
| ≥10 years | 21,109 | 75.9 | 1952 | 62.6 | 19,157 | 77.2 | <0.001 | |
| Clinical benefit | Low c | 26,410 | 56.7 | 2445 | 51.9 | 23,965 | 57.2 | <0.001 |
| Intermediate d | 33,681 | 68.4 | 3345 | 57.2 | 30,336 | 69.6 | <0.001 | |
| High e | 8726 | 79.6 | 933 | 64.2 | 7793 | 81.4 | <0.001 | |
| Overall | 68,817 | 65.3 | 6723 | 56.2 | 62,094 | 66.3 | <0.001 | |
Chi-square test for receipt of curative therapy and each covariate: P<0.001 for all comparisons.
P-value is for chi-square test of whether receipt of curative therapy differs between black and white patients.
Low-benefit: low-risk tumors and life expectancy <10 years.
Intermediate-benefit: low-risk tumors and life expectancy ≥10 years or moderate-risk tumors and life expectancy <10 years.
High-benefit: moderate-risk tumors and life expectancy ≥10 years.
Similar to prior studies, decreasing age, fewer comorbidities, and being married were positively associated with receipt of treatment regardless of race.27,28 Only a slightly higher percentage of men with moderate-risk disease were treated (69.6%) compared to those with low-risk disease (62.0%). Men in the highest income quartile were significantly more likely to receive treatment (70.7% vs. 57.7% of patients in the lowest income quartile (P<0.001)).
Approximately 56.2% of black men versus 66.3% of white men received treatment. Black men were less likely to receive treatment regardless of their age, marital status, tumor risk, or comorbidity status (P<0.001). Among men with low-risk tumors, 54.7% of black men vs. 62.7% of white men received treatment (P<0.001). Similarly, 57.9% of black vs. 71.0% of white men with moderate-risk tumors received treatment (P<0.001).
As the potential for clinical benefit increased, the likelihood of receiving treatment also increased. In the full sample, 56.7% of low-benefit men received treatment compared to 68.4% of intermediate- and 79.6% of high-benefit men (P<0.001). Within the low-benefit group, 51.9% of black vs. 57.2% of white men received treatment compared to 57.2% vs. 69.6% in the intermediate-benefit group, and 64.2% vs. 81.4% in the high-benefit group, respectively (P<0.001 for each black-white comparison).
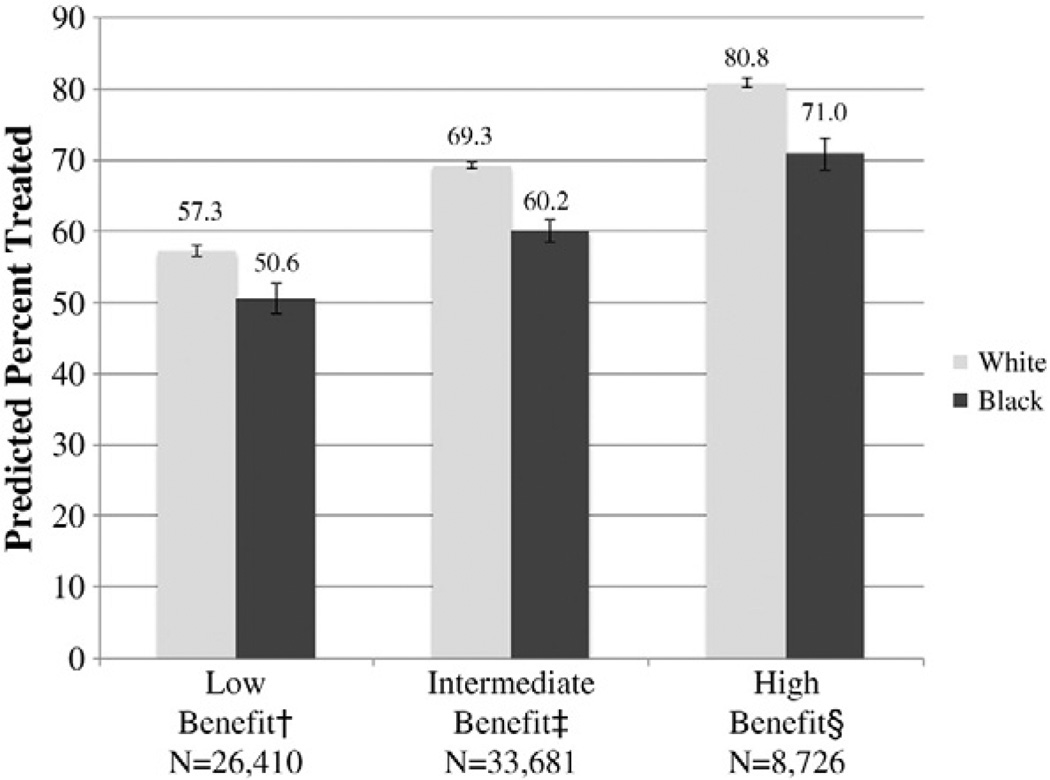
In the multivariable model, age, marital status, and income were significantly associated with receipt of treatment (Table 3). The largest magnitude of racial disparity was in the high-benefit group (adjusted odds ratio (OR) for treatment for black vs. white men 0.57; 95%CI, 0.48–0.68). This disparity was smaller among the intermediate- and low-benefit groups (OR=0.64; 95% CI, 0.59–0.70 and OR=0.74; 95%CI, 0.67–0.81, respectively). In the full model, the interaction between race and clinical benefit group was significant (P<0.001), indicating that the association between race and receipt of treatment varied across benefit groups. The average predicted probability of receiving treatment increased from low- to high-benefit groups, as did the absolute magnitude of racial disparity (Fig. 2).
Table 3.
Receipt of curative therapy according to clinical benefit group.
| Patient characteristic | Adjusted odds ratio for receipt of curative therapy a |
||||
|---|---|---|---|---|---|
| Full sample |
Low benefit b |
Intermediate benefit c |
High benefit d |
||
| N=68,817 | N=26,410 | N=33,681 | N=8726 | ||
| Race | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.65 (0.62–0.69) | 0.74 (0.67–0.81) | 0.64 (0.59–0.70) | 0.57 (0.48–0.68) | |
| Agee | 67–69 | 1.00 | 1.00 | 1.00 | 1.00 |
| 70–74 | 0.80 (0.77–0.84) | 0.79 (0.72–0.86) | 0.83 (0.77–0.88) | 0.86 (0.78–0.96) | |
| 75–79 | 0.42 (0.40–0.44) | 0.40 (0.73–0.44) | 0.54 (0.50–0.58) | – | |
| 80–84 | 0.15 (0.14–0.16) | 0.13 (0.12–0.14) | 0.20 (0.19–0.22) | – | |
| Tumor risk f | Low | 1.00 | – | – | – |
| Moderate | 1.62 (1.57–1.68) | – | – | – | |
| Marital status | Married | 1.00 | 1.00 | 1.00 | 1.00 |
| Not married | 0.68 (0.66–0.71) | 0.73 (0.68–0.78) | 0.68 (0.64–0.72) | 0.57 (0.50–0.65) | |
| Unknown | 0.44 (0.42–0.47) | 0.48 (0.43–0.55) | 0.41 (0.38–0.45) | 0.45 (0.37–0.55) | |
| Comorbidity e | 0 | 1.00 | 1.00 | 1.00 | – |
| 1,2 | 1.02 (0.99–1.06) | 0.95 (0.89–1.01) | 1.32 (1.25–1.40) | – | |
| ≥3 | 0.67 (0.63–0.71) | 0.62 (0.57–0.67) | 0.84 (0.78–0.91) | – | |
| Income | Q1: <$35,528 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: $35,528–<47,930 | 1.28 (1.22–1.35) | 1.21 (1.12–1.31) | 1.34 (1.25–1.43) | 1.27 (1.10–1.48) | |
| Q3: $47,930–<65,852 | 1.42 (1.35–1.50) | 1.30 (1.20–1.41) | 1.50 (1.39–1.61) | 1.55 (1.32–1.83) | |
| Q4: >$65,852 | 1.60 (1.51–1.69) | 1.37 (1.26–1.49) | 1.72 (1.59–1.87) | 1.98 (1.66–2.37) | |
Model adjusted for all variables in table including SEER registry and year of diagnosis. P-value<0.001 for the interaction between clinical benefit and each covariate.
Low-benefit: patients with low-risk tumors and life expectancy <10 years.
Intermediate-benefit: low-risk tumors and life expectancy ≥10 years or moderate-risk tumors and life expectancy <10 years.
High-benefit: moderate-risk tumors and life expectancy ≥10 years.
Odds ratios for the high benefit category not calculated for certain categories because all high-benefit patients had age <75 years and no comorbidities.
Odds ratios for tumor risk not calculated with benefit groups because tumor risk was used to create benefit categories.
Fig. 2.
Average predicted probability of receipt of treatment. The P-value for the race × benefit group interaction was significant (P<0.001) and the average predicted probability was adjusted for marital status, age, comorbidity, median household income, year of diagnosis, and SEER registry. †Low-benefit: low-risk tumors and life expectancy <10 years. ‡Intermediate-benefit: low-risk tumors and life expectancy ≥10 years or moderate-risk tumors and life expectancy <10 years. §High-benefit: moderate-risk tumors and life expectancy ≥10 years.
In the sensitivity analysis, 34,607 black or white men were diagnosed from 2004 to 2007, of whom 9644 (27.9%) were low- and 18,645 (53.9%) were moderate-risk. We then incorporated the new risk categories into the clinical benefit categories. Using the new subgroup categories and rerunning our multivariable model, the adjusted odds ratio for receipt of treatment for blacks versus whites did not change significantly.
In the full study sample, radiotherapy was more common than surgical treatment, although prostatectomy tended to be relatively more common among high-benefit men (Table 4). Radical prostatectomy was the major contributor to observed racial differences. For instance, black men in the high-benefit group were less likely to receive radical prostatectomy than white men (22.4% vs. 38.5%, P<0.001), which was not due to underlying factors such as age or comorbidity. Among low-benefit men, the difference in prostatectomy rate was smaller (7.0% vs. 8.8%; P=0.002). Unlike with prostatectomy, the magnitude of the racial disparity in brachytherapy use varied little across clinical benefit groups, holding steady at an absolute difference of approximately 4%–6%. There was no significant racial disparity between black and white men receiving external beam radiotherapy in any of the clinical benefit groups.
Table 4.
Curative therapy according to race and treatment modality.
| Full sample N (%) |
Low benefit a N (%) |
Intermediate benefit b N (%) |
High benefit c N (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | P-value d | Black | White | P-value d | Black | White | P-value d | Black | White | P-value d | |
| Any curative therapy | 3781 | 41,152 | <0.001 | 1270 | 13,698 | <0.001 | 1912 | 21,108 | <0.001 | 599 | 6346 | <0.001 |
| (56.2) | (66.3) | (51.9) | (57.2) | (57.2) | (69.6) | (64.2) | (81.4) | |||||
| Treatment modality | ||||||||||||
| Radical prostatectomy | 752 | 10,587 | <0.001 | 170 | 2113 | 0.002 | 373 | 5473 | <0.001 | 209 | 3001 | <0.001 |
| (11.2) | (17.1) | (7.0) | (8.8) | (11.2) | (18.0) | (22.4) | (38.5) | |||||
| External beam radiation | 2104 | 18,810 | 0.09 | 728 | 6917 | 0.34 | 1103 | 9824 | 0.49 | 273 | 2069 | 0.08 |
| (31.3) | (30.3) | (29.8) | (28.9) | (33.0) | (32.4) | (29.3) | (26.6) | |||||
| Brachytherapy | 925 | 11,755 | <0.001 | 372 | 4668 | <0.001 | 436 | 5811 | <0.001 | 117 | 1276 | 0.003 |
| (13.8) | (18.9) | (15.2) | (19.5) | (13.0) | (19.2) | (12.5) | (16.4) | |||||
Low-benefit: low-risk tumors and life expectancy <10 years.
Intermediate-benefit: low-risk tumors and life expectancy ≥10 years or moderate-risk tumors and life expectancy <10 years.
High-benefit: moderate-risk tumors and life expectancy ≥10 years.
P-value is for bivariate test of receipt of curative therapy between black and white patients within each row.
4. Discussion
We found that the presence and magnitude of cancer treatment disparities varied according to a patient's likelihood for clinical benefit. By incorporating the NCCN framework into our analysis, we view this as a novel approach to furthering our understanding of how to address racial disparities in the treatment of prostate cancer. Racial disparities were the largest among prostate cancer patients with the highest likelihood of clinical benefit (moderate-risk tumors and a life expectancy ≥10 years). However, the highest absolute numbers of men receiving treatment were in the low- and intermediate-benefit groups. These results suggest that efforts to improve both quality and equity of prostate cancer care in older men should address two domains: improving equitable access to treatment for high-benefit patients, but more importantly, decreasing overtreatment of all men with a low potential for clinical benefit.
The undertreatment of black men in the high-benefit group is of particular concern, as black men have a higher incidence of prostate cancer and present with aggressive tumors.3,5,22 Recent studies suggest that underuse of treatment is a growing concern for high-risk disease and our study confirms that this is particularly true among black men.29 In our sample, black men were more likely to have moderate-risk tumors and a high-benefit as compared to white men. This results in a two-fold hazard for black men: they are more likely to develop tumors with poor prognostic characteristics, and when they are diagnosed with such tumors, the racial disparities in receipt of treatment are substantial.
Treatment of over ten-thousand low-benefit black and white men reflects overuse of treatment among all men regardless of race. Older men with low-risk disease and shorter life expectancies are unlikely to die from their prostate cancer; therefore, the treatment of early-stage disease may not outweigh the adverse side-effects of treatment.10,16,18 Reduction of overtreatment among both white and black men with low-benefit would diminish racial disparities while decreasing the adverse side-effects of aggressive treatment for all men.9,21
Surgery is a potential contributor to observed disparities in high-benefit patients. Increasing access and availability of radical prostatectomy to high-benefit black patients may ameliorate current racial disparities in treatment practices. However, some older men with low-risk prostate cancer do not experience a mortality benefit from radical prostatectomy.17,30 Interestingly, there was little difference in receipt of radiation treatment between black and white men. Perhaps this suggests that providers are improving communication and decreasing mistrust surrounding the use of radiation therapy.31 Although prostate cancer treatment recommendations are controversial among older men, the NCCN guidelines and recent randomized control trials state that men with higher risk tumors and life expectancy >10 years are more likely to benefit from aggressive management. This does not imply that the solution to observed racial disparities includes surgical treatment of all high-benefit men. The most recent surgical trial was not able to determine a significant difference in mortality between radical prostatectomy and expectant management for men >65 years-old, although it is unclear whether that study was adequately powered to detect important differences in prostate cancer-specific outcomes in this age group.17 Future studies are needed to determine which factors are associated with receipt of surgical treatment for moderate and high-risk prostate cancer and whether there is a mortality benefit from surgical intervention in the treatment of higher risk tumors in men >65 years-old.
Our findings build upon prior work regarding racial disparities in the treatment of non-cancerous diseases. In cardiovascular care, white patients are more likely to undergo percutaneous transluminal coronary angiography for an inappropriate indication than similar black patients.32,33 However, overuse did not account for the entirety of treatment disparity, suggesting that underuse may have played a role. Similarly, studies of renal transplantation demonstrate that black patients with end-stage renal disease were less likely to be referred, have a completed work-up, and undergo transplantation than white patients, regardless of the clinical indication.34 This racial disparity was also attributed to both underuse among black patients who were appropriate transplant candidates and overuse among white patients who were inappropriate transplant candidates.34 More research is needed to optimize patient care and outcomes for minority populations.35
There are several limitations to our study. Medicare claims are for administrative purposes and may not include all treatment and comorbidity data. While age and comorbidity are frequently used to gage life expectancy at the bedside, some patients may live longer or shorter than their life expectancy might suggest. Patients with T1a and T1b diseases, curable by TURP, were included, though they did not strictly meet the definition of “low-risk” by NCCN criteria. Tumor grading and staging were also subject to error of interpretation and are different if a patient has surgery versus a biopsy. We used SEER grade to be consistent throughout our analysis because PSA values were not available prior to 2004, and the Gleason scoring system changed during our study period.9,22,26 Other important factors such as geriatric assessment and frailty scoring were not included in this study as it was limited to Medicare data.36 Despite these limitations, our study included a large, diverse sample of men with Medicare.
In conclusion, the greatest racial disparity present in the receipt of treatment among black and white prostate cancer patients was among those most likely to benefit from treatment. Because it is uncertain whether patients with a lower clinical benefit will derive any mortality benefit, addressing racial disparities should also focus on developing evidence to determine whether treatment is more likely to provide benefit than harm, and ensuring informed consent regarding the uncertain benefits of treatment. Sweeping approaches to increase the use of treatment for all patients, regardless of the likelihood of clinical benefit, have the potential to do more harm than good.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Funding/support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA149045. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures and Conflict of Interest Statements
Employment or leadership position: None
Consultant or advisory role: Cary P. Gross, FAIR Health, Inc.
Scientific Advisory Board member
Stock ownership: None
Honoraria: None
Research funding: None
Expert testimony: None
Other remuneration: Cary P. Gross, Medtronic grant for work on sharing of clinical trial data
Author Contributions
Conception and design: All authors.
Collection and assembly of data: Pamela R. Soulos, Laura D. Cramer, Jessica B. Long, Cary P. Gross
Data analysis and interpretation: All authors.
Manuscript writing: All authors
Final approval: All authors
REFERENCES
- 1.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. J Am Med Assoc. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 2.Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: early-versus late-stage cancer diagnosis. Health Aff. 2009;28:160–168. doi: 10.1377/hlthaff.28.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Underwood W, DeMonner S, Ubel P, Fagerlin A, Sanda MG, Wei JT. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol. 2004;171:1504–1507. doi: 10.1097/01.ju.0000118907.64125.e0. [DOI] [PubMed] [Google Scholar]
- 8.Zeliadt SB, Potosky AL, Etzioni R, Ramsey SD, Penson DF. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-medicare trends 1991 to 1999. Urology. 2004;64:1171–1176. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Scardino P. Update: NCCN prostate cancer clinical practice guidelines. J Natl Compr Canc Netw. 2005;3 [PubMed] [Google Scholar]
- 10.Roberts CB, Albertsen PC, Shao Y, Moore DF, Mehta AR, Stein MN, et al. Patterns and correlates of prostate cancer treatment in older men. Am J Med. 2011;124:235–243. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman RM, Harlan LC, Klabunde CN, Gilliland FD, Stephenson RA, Hunt WC, et al. Racial differences in initial treatment for clinically localized prostate cancer. J Gen Intern Med. 2003;18:845–853. doi: 10.1046/j.1525-1497.2003.21105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barocas DA, Penson DF. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106:322–328. doi: 10.1111/j.1464-410X.2010.09467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13:93–100. doi: 10.1200/JCO.1995.13.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Underwood W, Jackson J, Wei JT, Dunn R, Baker E, DeMonner S, et al. Racial treatment trends in localized/regional prostate carcinoma: 1992–1999. Cancer. 2005;103:538–545. doi: 10.1002/cncr.20796. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the united states. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 17.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Konety BR. Management of localized prostate cancer in men over 65 years. Curr Opin Urol. 2009;19:309–314. doi: 10.1097/MOU.0b013e328329a303. [DOI] [PubMed] [Google Scholar]
- 19.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kattan MW, Cowen ME, Miles BJ. A decision analysis for treatment of clinically localized prostate cancer. J Gen Intern Med May. 1997;12(5):299–305. doi: 10.1046/j.1525-1497.1997.012005299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll PR. Early stage prostate cancer—do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173:1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 22.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975. Available from URL: http://seer.cancer.gov/csr/1975_2007/. [Google Scholar]
- 23.Warren JL, Klabunde C, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [ http://www.ncbi.nlm.nih.gov/pubmed/12187163]. [DOI] [PubMed] [Google Scholar]
- 24.SEER Registry Groupings for Analyses. Surveillance Epidemiology and End Results. National Cancer Institute; http://seer.cancer.gov/registries/terms.html. [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PDH, Sanders M, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005 Sep 7;97:1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125–3130. [PubMed] [Google Scholar]
- 28.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 29.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farjah F, Wood DE, Yanez ND, III, Vaughan TL, Gaston-Symons R, Krishnadasan B, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–18. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halbert CH, Weathers B, Delmoor E, Mahler B, Coyne J, Thompson HS, et al. Racial differences in medical mistrust among men diagnosed with prostate cancer. Cancer. 2009;115:2553–2561. doi: 10.1002/cncr.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein AM, Weissman JS, Schneider EC, Gatsonis C, Leape LL, Piana RN. Race and gender disparities in rates of cardiac revascularization do they reflect appropriate use of procedures or problems in quality of care? Med Care. 2003;41:1240–1255. doi: 10.1097/01.MLR.0000093423.38746.8C. [DOI] [PubMed] [Google Scholar]
- 33.Schneider EC, Leape LL, Weissman JS, Piana RN, Gatsonis C, Epstein AM. Racial differences in cardiac revascularization rates: does “overuse” explain higher rates among white patients? Ann Intern Med. 2001;135:328–337. doi: 10.7326/0003-4819-135-5-200109040-00009. [DOI] [PubMed] [Google Scholar]
- 34.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, et al. Racial disparities in access to renal transplantation: clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss T, Weinberger MI, Holland J, Nelson C, Moadel A. Falling through the cracks: a review of psychological distress and psychosocial service needs in older Black and Hispanic patients with cancer. J Geriatr Oncol. 2012 Apr 1;3(2):163–173. [Google Scholar]
- 36.Gironés R, Torregrosa D, Maestu I, Gómez-Codina J, Tenias JM, Costa RR. Comprehensive Geriatric Assessment (CGA) of elderly lung cancer patients: a single-center experience. J Geriatr Oncol. 2012 Apr 1;3(2):98–103. [Google Scholar]