Summary
We have investigated the role of CD40 signaling in islet-reactive, diabetogenic CD4 Th1 T cell clones. Using multispectral flow cytometry, we showed that CD40 and CD154 are co-expressed and form complexes on the surface of activated T cells. We also demonstrate that activated T cells can transactivate CD4+CD40+ T cells through the CD40-CD154 pathway. To investigate the role of CD40 signaling on Th1 cells, we used the diabetogenic clone BDC-5.2.9 retrovirally transduced with a truncated form of the CD40 molecule to produce a CD40 dominant-negative T cell clone. Upon challenge with antigen in vitro, the production of IFN-γ by BDC-5.2.9 CD40DN was greatly reduced and in vivo, the dominant-negative variant was unable to induce diabetes. Transduction with the CD40DN vector was also effective in preventing transfer of disease by primary NOD CD4 T cells. Ex vivo analysis of pancreatic infiltrates after transfer of BDC-5.2.9 CD40DN revealed an overall reduction of cell numbers and cytokine production by both T cells and macrophages. These data indicate that CD40 is an important signaling molecule on autoreactive CD4 T cells and contributes to their pathogenic effector function.
Keywords: CD40, CD4 T cells, NOD, Type 1 Diabetes
Introduction
CD40 is a widely distributed cell surface receptor belonging to the tumor necrosis factor-receptor (TNF-R) superfamily (1, 2) and its ligand, CD154, is expressed on the surface of activated T cells. While interactions between CD40 on APC and CD154 on T cells are necessary for efficient immune responses, increased signaling through CD40 is linked to inflammation and autoimmunity (3, 4). CD40 was first identified and functionally characterized on B cells, but in recent years, it has been well established that CD40 is also expressed on T cells (5) and more specifically, that it can be a marker of autoreactive T cells in mouse models of rheumatoid arthritis and type 1 diabetes (T1D) (6-8).
We have previously reported that in addition to producing pro-inflammatory Th1 cytokines in response to pancreatic islet β-cell antigens (18), diabetogenic CD4 T cell clones derived from the non-obese diabetic (NOD) mouse express CD40 (6, 8). Because CD40 appears on pathogenic T cells and can be upregulated via antigen/MHC stimulation, we hypothesized that it plays a role in promoting the diabetogenicity of autoreactive T cells. To explore the mechanism by which pathogenicity of CD4 T cells may be enhanced by expression of CD40, we pursued two lines of experimentation using an islet-reactive T cell clone, BDC-5.2.9, that expresses high levels of CD40 and is highly diabetogenic in vivo. The first was to demonstrate that CD40 and CD154 are co-expressed on the surface of activated T cell clones and that signaling through CD40 on T cells actually takes place via interaction of CD40 and CD154, both expressed on T cells. The other experimental approach was to generate through retroviral transduction CD40 dominant-negative T cells, first with the diabetogenic T cell clone BDC-5.2.9, and then in CD4 T cells isolated from diabetic mice. Using this system, we could investigate directly whether inhibition of signaling through CD40 on islet-reactive T cells would change their functional properties. Our results show that T cells can interact with other T cells through CD40 and CD154 and, if signaling is blocked through expression of a dominant negative form of the CD40 molecule, T cell effector function is impaired, both in vitro and in vivo.
Results
CD40 and CD154 can be expressed simultaneously on diabetogenic T cell clones
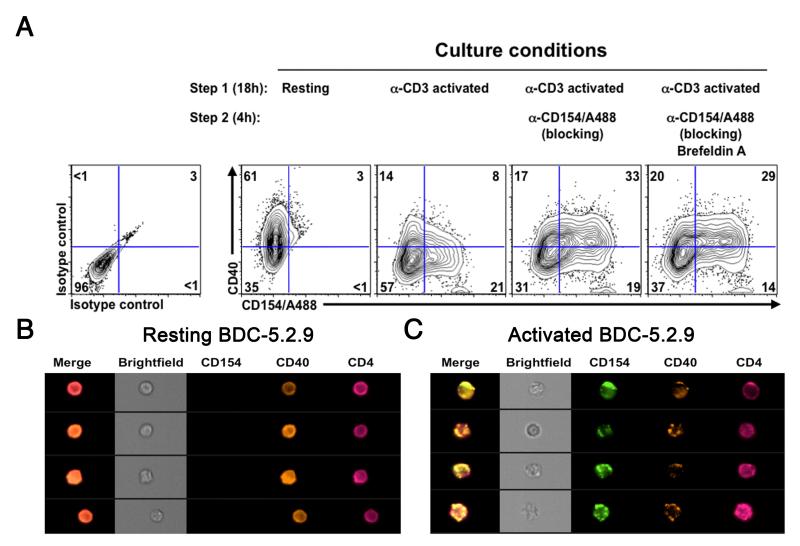
We previously reported that diabetogenic CD4+ T cell clones from the BDC panel constitutively express CD40 on their surface (6). CD154, the ligand for CD40 is also expressed on the T cell clones but only upon activation (8). Based on these observations we investigated whether these two molecules could be expressed at the same time on a T cell. The BDC-5.2.9 T cell clone was activated and expression of CD40 and its ligand was analyzed by flow cytometry (Fig. 1A). Resting T cell clones expressed CD40 and no CD154. Upon activation, expression of CD154 increased and was present on up to 30% of the cells after 20 hours, while at the same time, CD40 levels appeared to go down. To determine whether expression of CD154 leads to downregulation of CD40 or whether CD40 expression is in some way masked through interaction with CD154, we used a neutralizing antibody to CD154 to disrupt CD40-CD154 interaction. For this experiment, the T cell clone BDC-5.2.9 was activated overnight with plate-bound anti-CD3 and then incubated with a fluorescently labeled anti-CD154 antibody (anti-CD154/A488). Under these conditions, the conjugated anti-CD154 antibody can serve to both block CD40-CD154 interaction and label CD154+ cells (21). When T cells were activated overnight and anti-CD154/A488 was added for the last 4 hours of culture, CD40 levels were comparable to those on resting T cells, indicating that upon expression of CD154, there was no alteration of CD40 expression. Moreover, the number of cells expressing both CD40 and CD154 was increased to >30% compared to 5-8% in the absence of the antibody. T cells were also incubated under the same conditions in the presence of Brefeldin A, and as shown in the 4th panel, CD40 expression was unaffected by the addition of this reagent, indicating that CD40 was not synthesized de novo. These results suggest that observation of simultaneous expression of CD40 and CD154 on T cells is facilitated when CD40-CD154 interaction is disrupted and further, that CD40 and CD154 may form complexes on the surface of activated T cells.
Figure 1.
CD40 and CD154 are co-expressed on T cells upon activation. (A) The diabetogenic T cell clone BDC-5.2.9 was analyzed at resting state (first panel) or was activated with immobilized anti-CD3 antibody (2 μg/ml). Cells were harvested and resuspended in original media (second panel) supplemented with anti-CD154/A488 (10 μg/ml) (third panel) or anti-CD154/A488 and Brefeldin A (GolgiPlug) (far right panel) and cultured at 37°C for an additional 4 hr. Cells were then harvested and stained with anti-CD154/A488, anti-CD40 PE and anti-CD4 PerCP-Cy5.5 antibodies or appropriate isotype control antibodies. Expression of CD40, CD154 and CD4 was then monitored by flow cytometry. Data is representative of a least 3 independent experiments. Gates were set on live/CD4 cells and quadrants were set according to isotype control staining. (B) Resting BDC-5.2.9 T cell clones were analyzed on an ImageStream Flow cytometer and representative images of CD4+CD40+ T cells are represented. (C) ImageStream analysis of the BDC-5.2.9 T cell clone after activation in the presence of anti-CD154/A488. Representative images of T cells expressing both CD40 and CD154 are shown.
To investigate how CD40 and CD154 were localized on the surface of the T cell clone, we analyzed expression of these two molecules by multispectral imaging flow cytometry, a method that combines the advantages of a high-throughput flow cytometer and fluorescence microscopy (22). As shown in Fig. 1B, CD40 staining on resting clones was homogeneous. In contrast, CD40 and CD154 staining on T cells activated in the presence of anti-CD154/A488 was punctate (Fig. 1C) and both molecules co-localized in more than 70% of the cells (data not shown). These data clearly demonstrate that CD40 and CD154 can be expressed at the same time on the surface of activated autoreactive T cells.
Interactions between T cells occur through CD40 and CD154 on T cells
To more fully investigate the co-expression of CD40 and CD154 on T cells and how signaling might take place through this pathway, we generated three GFP-positive variant forms of the diabetogenic T cell clone BDC-5.2.9 by retroviral transduction: a control clone transduced with the empty MIGR vector, a clone over-expressing the full-length signaling CD40 receptor (CD40hi), and a dominant negative (CD40DN) variant containing the CD40 isoform in which the intracellular domain is truncated (23). CD40 levels were assessed by flow cytometry on the GFP-sorted BDC-5.2.9 T cell clones and as shown in Fig. S1, there was good correlation between CD40 expression and the GFP reporter in the CD40hi and CD40DN variants. CD40 expression was not affected by transduction of BDC-5.2.9 with the empty vector.
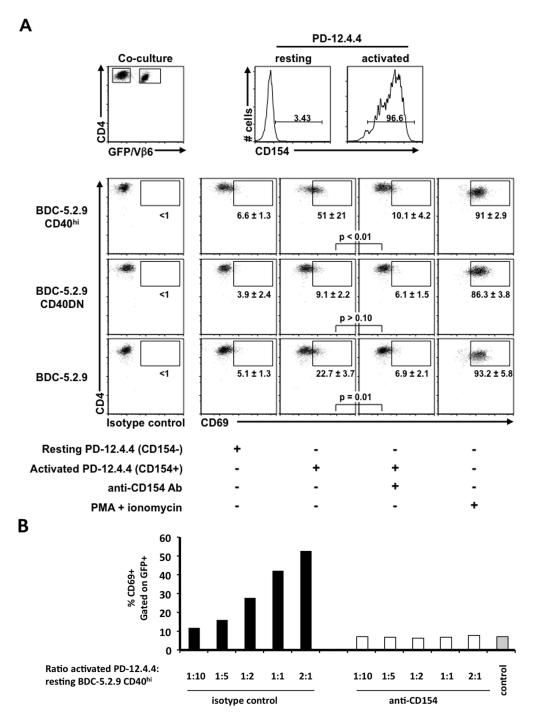
The BDC-5.2.9 variants transduced with different forms of CD40 allowed us to test for different levels of signaling through CD40 on T cells and at the same time, track the T cells through expression of the GFP reporter molecule. Since the TCR of BDC-5.2.9 is Vβ6, the parent clone can be detected through Vβ6 staining. To test the functionality of CD40 on BDC-5.2.9 CD40hi or CD40DN T cells, we co-cultured these clones with an insulin-reactive (Vβ6-negative) T cell clone, PD-12.4.4 (24). PD-12.4.4 served as an interacting partner, providing the ligand for CD40 and expressing high levels of CD154 upon activation through TCR. As a measure of transactivation, the clones were co-cultured in the presence or absence of an anti-CD154 antibody (MR1) and were monitored for expression of the early activation marker CD69 on the GFP+ T cells. As predicted, CD69 expression was low when the BDC-5.2.9 CD40hi or CD40DN clones were co-cultured with PD-12.4.4 in the resting state (Fig. 2A). In contrast, when the activated form of PD-12.4.4 expressing high levels of CD154 was used, CD69 expression was markedly increased on the BDC-5.2.9 CD40hi variant (Fig. 2B). Furthermore, the degree of CD69 expression correlated with the number of activated PD-12.4.4 cells in the cultures (Fig. 2B). In the presence of anti-CD154, however, the upregulation of CD69 expression was completely prevented (Fig. 2B), indicating that interaction between CD40 and CD154 was required for transactivation of the CD40hi clone. Also, when the BDC-5.2.9 CD40DN variant was used instead of the BDC-5.2.9 CD40hi clone, no transactivation of the CD40DN T cell clone was observed, indicating that signaling through CD40 was necessary for transactivation of a CD40+ T cell clone by an activated CD154+ T cell clone (Fig. 2A). The transactivation between clones resulting from CD40-CD154 interaction is clearly illustrated by use of the BDC-5.2.9 CD40hi T cell clone, but could also be demonstrated using the parent BDC-5.2.9 clone (Fig. 2A).
Figure 2.
CD154-positive T cells can activate CD40-positive T cells. The insulin-reactive T cell clone PD-12.4.4 (Vβ6-negative) was activated with immobilized anti-CD3 (2 μg/ml). After 18 hr, the PD-12.4.4 T clone was extensively washed in CM and co-cultured 1:1 (or at the ratios indicated) with 1 × 105 resting T cell clones: BDC-5.2.9 (Vβ6+), BDC-5.2.9 CD40hi (GFP+) or BDC-5.2.9 CD40DN (GFP+), in the presence or absence of anti-CD154 Ab (10 μg/ml) or isotype control Ham IgG. Four hours after co-culture, cells were harvested and stained with anti-Vβ6 FITC, anti-CD4 APC and anti-CD69 PE or anti-CD154 PE or appropriate isotype control. (A) Expression of GFP, Vβ6, CD154, CD69 and CD4 was monitored by flow cytometry and gates were set on live CD4 cells. CD69 expression was monitored on GFP+ cells and was comparable on all the clones when stimulated with PMA/ionomycin. (B) Gates were set on live CD4+ GFP+ cells and the percentage of CD69+ cells is represented for each condition. Black bars: co-cultures in the presence of isotype control; white bars: co-cultures in the presence of anti-CD154 blocking antibody; gray bar: co-culture of BDC-5.2.9 CD40hi with resting PD-12.4.4 (CD154-negative). Data are representative of at least three independent experiments.
Prevention of signaling through CD40 on autoreactive T cells inhibits effector function in vitro
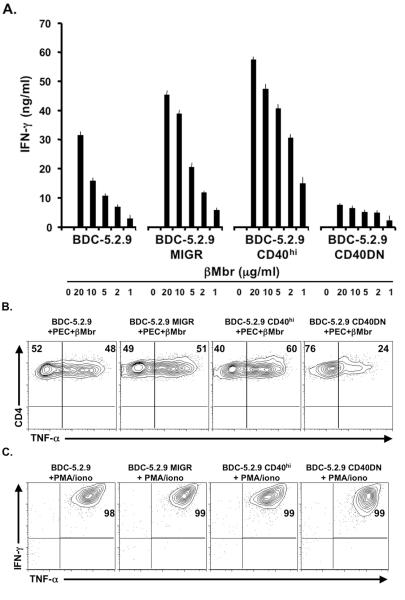
In order to investigate whether blocking signaling between CD40 and CD154 on T cells could inhibit their effector function, we investigated the effects of a non-functional form of the CD40 molecule in the diabetogenic T cell clone, BDC-5.2.9. We first tested in vitro whether T cell effector function could be altered by measuring the responses of BDC-5.2.9 to antigen with the CD40 dominant-negative variant of the clone. This assay allowed us to investigate the role of T-T cell interaction though the CD40-CD154 axis, leaving intact the interactions between CD40 expressed on APCs and CD154 expressed on T cells. Functional activity of the three variant forms of BDC-5.2.9 was measured through production of the inflammatory cytokines IFN-γ and TNF-α in response to APC and varying concentrations of β-cell antigen (β-Mbr). As shown in Fig. 3A, the BDC-5.2.9 MIGR empty vector variant and BDC-5.2.9 parent clone made comparable levels of IFN-γ whereas over-expression of CD40 on the surface of the clone resulted in higher IFN-γ production. In contrast, the BDC-5.2.9 T cell clone transduced with CD40DN produces little IFN-γ, even at high antigen concentrations. These data suggest that signaling through CD40 is essential for effector function of autoreactive Th1 clones. We also investigated the effect of blocking CD40 signaling on TNF-α production by autoreactive T cells. Since TNF-α is produced by both PEC and T cells, TNF-α production was measured by intracellular cytokine staining in CD4 T cells. Fig. 3B shows that in comparison to the parent clone, TNF-α production by BDC-5.2.9 CD40DN was reduced by half after 3 hours of antigen stimulation. No differences in IFN-γ or TNF-α production between T cell clone variants were observed upon activation with PMA/ionomycin (Fig. 3C).
Figure 3.
Transduction of a diabetogenic T cell clone with a dominant negative CD40 impairs effector function. (A) T cell clones (2 × 104) were challenged with PEC (2.5 × 104) in the presence or absence of β-Mbr as antigen (20-1.2 μg/ml). After 48 hr of culture, IFN-γ was measured from supernatants from duplicate wells by ELISA. Results are representative of three independent experiments and normalized average IFN-γ production for clones stimulated with 20 μg/ml of β-Mbr was 81 ± 15.6 ng/ml for BDC-5.2.9 and 23.3 ± 11.9 ng/ml for BDC-5.2.9 CD40DN (p=0.002), 117.8 ± 7.1 ng/ml for BDC-5.2.9 MIGR and 146.3 ± 20.2 ng/ml for BDC-5.2.9 CD40hi. (B) T cell clones were tested with PEC, in the presence or absence of antigen and TNF-α production was measured by intracellular staining (ICS). (C) T cell clones were cultured with Brefeldin A in the presence of PMA + ionomycin. Cells were then harvested and IFN-γ and TNF-α production was assessed by intracellular cytokine staining (ICS). No difference in IFN-γ or TNF-α production was observed between the parent clone and variant clones upon PMA/ionomycin activation. Data are representative of three independent experiments.
Blocking CD40 signaling on autoreactive T cell clones prevents transfer of disease
Our previous work showed that the diabetogenicity of T cell clones from the BDC panel is prevented when T cells are treated with anti-CD40 antibodies (8). Since antibody-mediated inhibition can occur through various mechanisms, including complement fixation or antibody-dependent cell-mediated cytotoxicity (ADCC), the interpretation of experiments involving treatment of T cells with anti-CD40 is not entirely straight-forward. In order to demonstrate in an unequivocal manner that blocking signaling between CD40 and CD154 on T cells could prevent transfer of disease, we investigated the CD40DN form of the T cell clone BDC-5.2.9 in vivo.
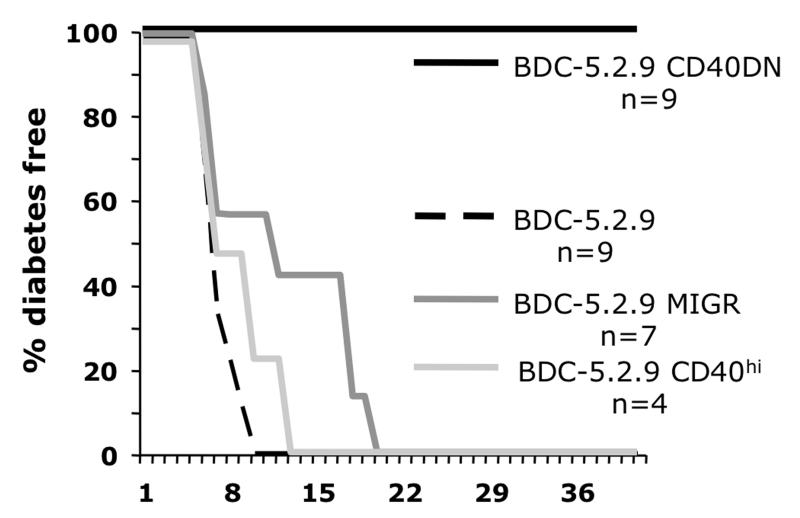
Adoptive transfer of the diabetogenic T cell clone BDC-5.2.9 induces diabetes only in young NOD or NOD.scid mice (<2 weeks old) and typically recipients become diabetic within 2-3 weeks of transfer (18). To determine whether signaling through CD40 on the T cell clone could affect the capacity of BDC-5.2.9 to transfer disease, we carried out adoptive transfer experiments into NOD.scid mice. The parent BDC-5.2.9 T cell clone, the BDC-5.2.9 variant containing the empty MIGR vector, the BDC-5.2.9 CD40hi, or the BDC-5.2.9 CD40DN clone were transferred into young NOD.scid recipient mice. As expected, the parent BDC-5.2.9 clone and the BDC-5.2.9 CD40hi variant transferred disease rapidly, with all mice becoming diabetic 10-14 days after transfer (Fig. 4A). Transfer with the empty vector variant was slower in some recipients, but all of the mice became diabetic within three weeks after injection. In contrast, the BDC-5.2.9 CD40DN variant did not transfer diabetes in any of the mice, indicating that altering CD40 signaling impairs the diabetogenic properties of the BDC-5.2.9 T cell clone. Histological analysis of pancreatic sections of mice receiving BDC-5.2.9 shows complete degranulation of β-cells in the islets with massive infiltration of leukocytes and loss of pancreatic architectural structure, whereas pancreatic histology from mice that received the BDC-5.2.9 CD40DN showed well-granulated islets and little or no infiltrate (Fig. S2).
Figure 4.
Blocking CD40 signaling on diabetogenic clone BDC-5.2.9 abrogates disease transfer capacity. BDC-5.2.9 , BDC-5.2.9 MIGR, BDC-5.2.9 CD40hi, or BDC-5.2.9 CD40DN T cells (1 × 107) were injected i.p. into 6-14 day-old NOD.scid recipients and mice were monitored for hyperglycemia. Results from disease transfer: all mice receiving the parent clone BDC-5.2.9, BDC-5.2.9 CD40hi, or the BDC-5.2.9 MIGR (empty vector) became diabetic within 3 weeks of being injected, whereas none of the mice receiving the CD40DN variant became diabetic (p < 0.001). Data summarizes at least three independent experiments for the CD40DN and BDC-5.2.9 and two experiments for BDC-5.2.9 MIGR.
Effector function of autoreactive T cells in the pancreas is reduced after transfer of T cells in which CD40 signaling is blocked
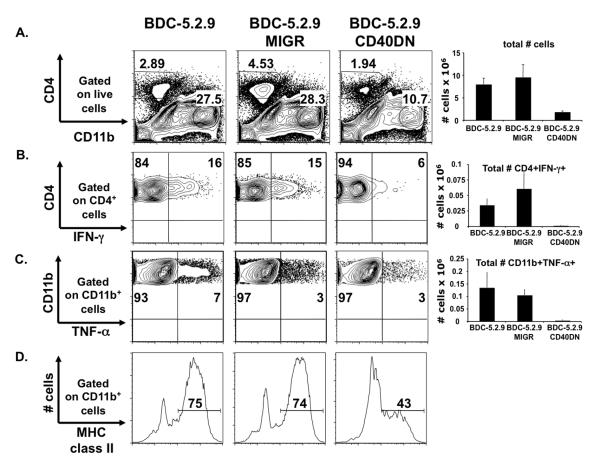
To more closely investigate the reasons for why diabetogenicity of the BDC-5.2.9 clone was inhibited by blocking signaling through CD40, we carried out ex vivo analysis of the pancreatic infiltrate following adoptive transfer. As previously reported, diabetogenic T cell clones infiltrate the pancreas of recipient mice upon transfer and secrete cytokines and chemokines, resulting in recruitment and activation of macrophages (20, 25). Since BDC-5.2.9 CD40DN did not induce disease when transferred into NOD.scid recipients, we asked whether these cells failed to migrate to the pancreas or whether the T cells infiltrated the pancreas but failed to induce inflammation in the target organ.
The parent BDC-5.2.9 clone, or BDC-5.2.9 MIGR or CD40DN variant clones were transferred into NOD.scid recipients and, pancreatic tissue was removed 6 days after transfer for ex vivo analysis by flow cytometry. In recipient mice receiving BDC-5.2.9 CD40DN, we found a four-fold decrease in the total number of infiltrating cells in the pancreas, compared to when mice received either BDC-5.2.9 or BDC-5.2.9 MIGR (Fig. 5A). When we analyzed the composition of the pancreatic infiltrate, there was a 10-fold reduction in the number of CD4+ and CD11b+ cells in mice that received BDC-5.2.9 CD40DN compared to mice transferred with the parent clone or BDC-5.2.9 MIGR. These data demonstrate that CD40 signaling in diabetogenic CD4 Th1 T cell clones is important for both T cell accumulation in the pancreatic tissue and recruitment of other cell types such as CD11b+ cells. Pro-inflammatory cytokines such as IFN-γ and TNF-α play a central role in the pathogenesis of T1D, as inflammatory mediators and through activation of other cell types (20, 25). When we assessed cytokine production of the pancreatic infiltrate, we found that after transfer of BDC-5.2.9 or BDC-5.2.9 MIGR, 14-17% of the T cells produced IFN-γ. In contrast, when BDC-5.2.9 CD40DN was transferred into young NOD.scid recipients, there was a 10 to 20-fold reduction in the total number of CD4+ IFN-γ+ cells (Fig. 5B). Lastly, when we analyzed activation of macrophages in recipient mice, levels of MHC class II and the total number of CD11b+TNF-α+ cells were reduced in pancreata of mice receiving the BDC-5.2.9 CD40DN clone (Fig. 5C and D). These studies were performed in immunodeficient NOD.scid recipients, but the same experiments in immunocompetent NOD mice yielded similar results (data not shown). Taken together, our data indicate that when signaling through CD40 on diabetogenic CD4 Th1 T cell clones is altered, their effector function is impaired.
Figure 5.
Effect of CD40 signaling blockade on pancreatic infiltrate through ex vivo analysis. BDC-5.2.9, BDC-5.2.9 MIGR, and BDC-5.2.9 CD40DN (1 × 107) were expanded in subcultures and were injected i.p. into 10-day-old NOD.scid recipients. Six days after transfer, pancreatic single cell suspensions were prepared, counted, and then incubated at 37°C in the presence of Brefeldin A for 3 h. Cells were analyzed by flow cytometry for CD4, CD11b, TNF-α, IFN-γ and MHC class II expression. (A) The percentage of CD4 and CD11b cells were monitored on live gates. Absolute numbers of infiltrating T cells are represented. (B) IFN-γ production was monitored by ICS on CD4+ cells. Absolute number of CD4+IFN-γ + cells are represented. (C) TNF-α production was monitored by ICS on infiltrating CD11b+ cells. Absolute number of CD11b+TNF-α+ cells are represented. (D) Expression of MHC class II was assessed on CD11b+ cells. Gates were set on live cells and quadrants were set according to isotype control staining. Results are from one experiment with 2 NOD.scid recipient mice for each clone. The data (not shown) are similar to those obtained in the same experiments performed in immunocompetent NOD mice.
Prevention of CD40 signaling in primary CD4 T cells can also inhibit diabetogenicity
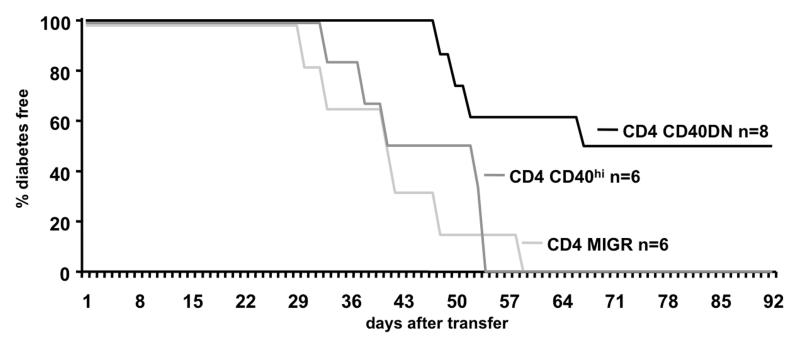
We have previously reported that CD40-positive CD4 T cells isolated from diabetic NOD mice transfer disease whereas CD40-negative CD4 T cells do not (6). Using the CD40DN approach, we examined whether preventing CD40 signaling on primary CD4 T cells from diabetic mice would decrease their capacity to transfer disease. Figure 6 shows that mice that received CD4 T cells transduced with either GFP only (MIGR) or over-expressing CD40 (CD40hi) became diabetic within 8 weeks following injection. In contrast, disease was prevented in 50% of the mice that received CD40DN-transduced CD4 T cells. These data indicate that primary CD4 T cells from diabetic NOD mice use CD40 signaling and that this pathway contributes to their pathogenicity.
Figure 6.
Prevention of CD40 signaling in polyclonal NOD CD4 T cells impairs their diabetogenicity. CD4 T cells were isolated from spleen and lymph nodes of diabetic NOD mice and transduced either with GFP alone, CD40 full-length or CD40DN. After in vitro expansion in IL-2, 12.5 × 106 T cells were transferred i.v. into adult NOD.scid recipients and mice were monitored for hyperglycemia. All mice receiving cells transduced with GFP or CD40 full-length (CD40hi) became diabetic whereas in mice transferred with CD40DN cells, diabetes was prevented in 4 of 8 recipients for at least 90 days after transfer (p < 0.01). Data are representative of at least 2 independent experiments.
Discussion
Expression of CD40 on T cells has been a highly controversial topic (5) and this is due in part to the difficulties encountered in its detection. In this study we have demonstrated that CD40 and CD154 can be co-expressed on T cell clones upon activation. The expression of both molecules at the same time on activated T cells can be revealed in the presence of anti-CD154 antibody, which prevents CD40-CD154 interactions. By disrupting this interaction, visualization of both molecules is possible and this observation, in conjunction with the punctate staining pattern, suggests that CD40 and CD154 form a complex on the surface of activated T cells. When the two molecules are in complex, their detection is hindered. Further, we demonstrated that CD154+ T cells can also activate CD40+ resting T cells in a CD40-CD154 dependent manner as specific blocking of this interaction with anti-CD154 prevented transactivation. Extrapolating to the inflammatory site in the pancreas, activation of diabetogenic T cells expressing CD40 may be prolonged through interaction of CD40 with CD154 expressed by activated bystander T cells. Engagement of CD40 on T cells could also increase costimulation and lead to increased T cell effector function, thereby promoting inflammatory reactions in the autoimmune environment.
To further investigate whether T-T cell interaction plays a role in disease pathogenesis through the CD40-CD154 pathway, we used a dominant-negative version of the CD40-expressing T cell clone, BDC-5.2.9. Upon antigen stimulation in vitro, the BDC-5.2.9 CD40DN T cell clone produced decreased amounts of IFN-γ and TNF-α compared to controls. In vivo, the T cell clone transduced with the CD40DN molecule failed to transfer disease into NOD.scid mice, indicating that CD40 signaling contributes to T cell pathogenicity. Ex vivo analysis of pancreata from mice transferred with the CD40DN T cell clone showed that the variant clone failed to induce a pancreatic infiltrate and that secretion of cytokines by both T cells and macrophages was impaired. Finally, experiments with polyclonal CD4 T cells isolated from diabetic mice show that these findings extend to primary T cells. We conclude from these studies that upon activation, autoreactive T cells express both CD40 and its ligand, CD154. CD154 can subsequently engage CD40 expressed on other autoreactive T cells, resulting in transactivation of those T cells. If signaling through CD40 on autoreactive T cells is blocked, transactivation can not occur and effector function is impaired, resulting in decreased production of proinflammatory cytokines and inability to transfer disease.
CD40 has been well characterized and its engagement triggers a powerful signaling cascade. In NOD T cells, CD40 acts as a costimulatory molecule (8), and signaling through CD40 on T cells activates NFκB pathways (6) and leads to production of IFN-γ and TNF-α (7). These Th1 T cell cytokines play a major role in diabetes pathogenesis via direct cytotoxicity to pancreatic beta-cells (26), through recruitment of other cell types such as macrophages (20), and by generally contributing to tissue inflammation (27). T cells interacting with other T cells via the CD40-CD154 axis could provide a mechanism by which the inflammatory reaction mediated by pathogenic T cells is amplified and extended. Our hypothesis in this study was that interaction between CD40 and CD154 expressed on autoreactive T cells promotes their diabetogenic properties. Interaction between T cells through CD40-CD154 has also been observed in other contexts, such as provision of CD4 help for the differentiation of CD8 T cells to memory cells (28). Phenotypically, the diabetogenic CD4 T cell clones from NOD mice are memory effector T cells and it is likely that CD40-CD154 interaction applies to memory effector T cells in immune response situations other than autoimmune diabetes.
CD40 is being intensively investigated as a therapeutic target. For example, treatment with an anti-CD40 antibody has been the focus of a recent phase I clinical trial in patients affected with non-Hodgkin’s lymphoma and the results showed that a humanized anti-CD40 monoclonal antibody (Dacetuzumab) was safe and well tolerated (29). This study opens new avenues for immunotherapies directed toward CD40-bearing cells in humans (30), and may be applicable to a variety of disease states including autoimmune diabetes. It has been shown that the CD40-CD154 costimulatory pathway plays a critical role in development of diabetes in the NOD mouse (12, 13). In humans, islet-reactive T cells also express CD40 (19) and it is possible that anti-CD40 antibodies could bind to CD40 expressed on autoreactive T cells in T1D patients. T-T cell interaction through CD40 and CD154 would be blocked and T cell costimulation through CD40 would be impaired, resulting in altered T cell effector function. New technology such as engineering of bi-specific antibodies could provide more specific targeting of pathogenic T cells and perhaps avoid some of the negative effects of broader based immunosuppression.
Materials and methods
Mice
NOD and NOD.scid breeding mice were acquired from The Jackson Laboratory and were bred and housed in specific pathogen-free conditions at National Jewish Health. NOD.scid litters (<2 wks old) were used as recipients in adoptive transfer experiments of T cell clones (transfer of disease with T cell clones takes place only in very young mice); adult NOD.scid mice were used in adoptive transfer of polyclonal CD4 T cells. Breeding mice and experimental animals were monitored for development of disease by urine glucose (Diastix, Bayer) and hyperglycemia was confirmed by OneTouch Ultra glucometer (LifeScan). Mice were considered diabetic when blood glucose levels were >15 mmol/l (270 mg/dl). All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Culture, expansion, and adoptive transfer of T cell clones
The BDC panel of diabetogenic T cell clones was established from spleen and lymph nodes of diabetic NOD mice as previously described (31, 32). T cell clones were restimulated every 2 weeks with a granule-enriched membrane fraction obtained from β-cell tumors as a source of Ag, referred to as β-Membrane (β-Mbr) (33), irradiated NOD spleen cells as APCs, and EL-4 supernatant as a source of IL-2 in complete medium (CM). CM is DMEM (Gibco) supplemented with 44 mM sodium bicarbonate, 0.55 mM L-arginine, 0.27 mM L-asparagine, 1.5 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-ME, 10 mM HEPES, and 10% FCS. Cell numbers were expanded for transfer experiments by subculturing 3–6 × 106 T cells 4 days after restimulation in a 5-fold volume of CM and additional IL-2. T cells were harvested, washed three times, and resuspended in HBSS for injection into young (<2 wk-old) NOD.scid recipients. Transfer of disease with the BDC T cell clones only occurs in mice less than 3 weeks of age and therefore, we typically use recipients under 2 weeks old.
CD40 constructs
The cloned CD40 cDNA was kindly provided by Dr R.Torres (34) (NJH, Denver, Colorado). cDNA for full-length CD40 and dominant negative CD40 (CD40DN) with engineered restriction sites were generated by PCR with forward primer 5′-AGC CTC GAG CCA CCA TGG TGT CTT TGC CTC GGC TGT-3′ and reverse primers (Invitrogen) 5′-GCT GCT CAA TTG TCA GAC CAG GGG CCT CAA GGC TAT GCT-3′ for full-length CD40 and 5′-GCT GCT CAA TTG TTA TCC TTT GGT TTC TTG ACC ACT GAT ATA GAG AAA CAC-3′ for CD40DN. cDNA for full-length CD40 and CD40DN were then subsequently cloned into a mouse stem cell virus vector-based retroviral plasmid with an internal ribosomal entry site plus sequence encoding green fluorescent protein as a reporter (MSCV-IRES-GFP or MIGR). Sequence was identical to those published by Tone et al (Accession number NM_011611.2 (full-length CD40) and NM_170704.2 (CD40DN)). The full-length CD40 or CD40DN or empty vector (MIGR) retroviral plasmid were then transfected into Phoenix packaging cells together with the pCLEco accessory plasmid with Fugene6 (Roche), according to manufacturer’s protocol. Retrovirus-containing supernatants were collected 48 hr after transfection, centrifuged and filtered for removal of debris.
Retroviral transduction of T cell clones
Three days after activation with antigen/MHC, the BDC-5.2.9 T cells were spin-infected at 3300 × g for 90 min at 37°C in retrovirus-containing supernatants supplemented with polybrene (8 μg/ml). GFP-expressing cells were isolated 72 hr later on a MoFlo cell sorter (Dakocytomation).
Retroviral transduction and adoptive transfer of primary CD4 T cells
CD4 T cells were isolated from spleen and lymph nodes of diabetic NOD mice using magnetic bead separation (Miltenyi Biotech, USA) and were then activated with soluble anti-CD3 and anti-CD28 antibodies (1 μg/ml) in the presence of IL-2 (40 U/ml). Cells were harvested 24 hr post stimulation and divided into 2 aliquots. One aliquot was spin-infected with the CD40DN construct and the other with control vector (CD40 full-length or MIGR) followed by a 2-day culture in IL-2 (100 U/ml). After an additional 3-day expansion in IL-2 (100 U/ml), cells were injected i.v. into adult NOD.scid recipients and mice were monitored for onset of diabetes. A small aliquot of cells was used to monitor efficiency of transduction and this analysis confirmed that >30% of the cells were GFP-positive.
Surface and cytokine analysis by intracellular staining
Single cell suspensions were surface-stained in 100 μl of staining buffer (PBS, 0.5% BSA) containing antibodies at optimized concentrations. Antibodies used for surface staining were: anti-CD154/A488 (MR1; a generous gift from Dr. Ross Kedl, NJH, Denver), anti-Vβ6 FITC (RR4-7; PharMingen), anti-CD40 PE (3/23; PharMingen), anti-CD69 PE (H1.2F3; Biolegend), anti-CD154 PE (MR1; BD), anti-CD4 PE (GK1.5; BD), anti-CD11b PerCP-Cy5 (M1/70; BD), anti-CD4 PerCP-Cy5.5 (GK1.5; eBioscience), anti-CD4 APC (GK1.5; eBioscience) and corresponding isotype controls. After incubation on ice for 30-45 min, followed by three washes, surface markers were monitored by flow cytometry or cells were fixed in 2% paraformaldehyde for 10 min in the dark. Cells were washed once more before resuspending in permeabilization buffer (staining buffer, 0.5% saponin), containing an isotype control or specific antibody mix for intracellular cytokines (anti-IFN-γ APC (XMG1.2; BD), anti-TNF-α PE (MP6-XT22; BD), anti-TNF-α APC (MP6-XT22; eBioscience)). After 30 min of incubation, cells were washed three times in permeabilization buffer and resuspended into staining buffer. Stained cells were analyzed on a FACScalibur or FACScan flow cytometer (BD). For each experiment, gates were set to exclude dead cells based on FSC/SSC (Fig. S3) and quadrants were set according to isotype control staining. Data were analyzed using FlowJo software (Tree Star, Inc, OR).
ImageStream analysis
The T cell clone BDC-5.2.9 was activated on plate-bound anti-CD3 or used in a resting state. After 18 hr, cells were harvested and cultured for an additional 4 hr in media only, or media supplemented with anti-CD154/Alexa488 (10 μg/ml), in the presence or absence of Brefeldin A. Cells were harvested and stained with anti-CD154 Alexa488, anti-CD40 PE and anti-CD4 PerCP-Cy5.5 at 4°C. Cells were then washed with cold staining buffer and analyzed on an ImageStream Flow Cytometer (Amnis corporation). Classifiers were used to eliminate collection of debris (based on low area in the brightfield imagery) while camera-saturating events (based on the presence of peak intensities greater than 1022) and doublets were also excluded. 10,000 events were collected for each condition and each cell was imaged with brightfield, side scatter, and three channels of fluorescence. Images were analyzed using IDEAS software. Spectral compensation was digitally performed on a pixel-by-pixel basis before data analysis. Co-localization was determined using the similarity parameter and punctate fluorescence was determined using the maximum pixel parameter.
Transactivation assay
The PD-12.4.4 T cell clone (24) was activated with plate-bound anti-CD3 (2 μg/ml) overnight. After extensive washing, activated T cells were co-cultured at different ratios with resting BDC-5.2.9, BDC-5.2.9 CD40hi or CD40DN for 4 hours at 37°C. CD40-CD154 interactions were blocked using a neutralizing anti-CD154 (MR1) antibody (10 μg/ml) or a Ham IgG isotype control. Cells were stained with anti-Vβ6 FITC, anti-CD4 APC, anti-CD154 PE or anti-CD69 PE or appropriate isotype control. Expression of CD69 on CD4+GFP+/Vβ6+ cells and expression of CD154 on CD4+GFP-/Vβ6- was assessed by flow cytometry.
Antigen stimulation assay
For assay of cytokine production by intracellular staining and/or ELISA, BDC T cell clones (2 × 104) were assayed 2 weeks after restimulation in duplicate wells with β-Mbr (20-1.2 μg/ml) and freshly isolated PEC (2.5 × 104) as APCs at 37°C. Supernatants were collected after 48 hr of culture and IFN-γ concentrations were determined by specific ELISA. For intracellular cytokine staining, T cell clones (4 × 104) were stimulated with PEC (5 × 104) in the presence or absence of islet Ag for 3 hr and Brefeldin A was added for the last 4 hr of culture. As a positive control, brefeldin A (1X), PMA (100 ng/ml) and ionomycin (1 μg/ml) were added for the last 4 hr of culture.
Adoptive transfer of T cell clones and histological analysis
For disease transfer experiments, cell cultures were expanded and after harvest, T cells (1 × 107) were injected i.p. into age-matched 6-14 day-old NOD.scid recipient mice. Pancreata were harvested at the time of disease onset or 45-60 days after adoptive transfer of T cells; tissue was fixed in formalin, embedded in paraffin, sectioned and stained with aldehyde/fuchsin. Histology scores were generated from multiple sections throughout each pancreas to allow for more thorough examination of the entire pancreas.
Ex vivo analysis of T cells recovered from the pancreas
Six days after adoptive transfer with T cell clones, recipient NOD.scid mice were sacrificed and single cell suspensions from pancreata were obtained as previously described (20). After washing and counting, cells were suspended in 1 ml of CM/GolgiPlug and incubated at 37°C for 3 hr, with or without PMA (100 ng/ml) and ionomycin (1 μg/ml) in 24-well plates. After culture ex vivo, cells were harvested using Cell Dissociation Buffer (Invitrogen) and washed with PBS/GolgiPlug before proceeding to surface and intracellular staining.
Statistics
Statistical significance was determined by a two-tailed Student’s test. For disease transfer experiments, a Wilcoxon rank sum test was used. A p value ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Brenda Bradley, Gene Barbour, and Shirley Scobus for technical assistance, Dr. Gregory Veltri for analysis of the ImageStream experiment and Dr Ross Kedl for reagents.
This work was supported by grants to K. Haskins from the National Institutes of Health (RO1 DK50561) and the American Diabetes Association (Research award 1-10-BS-69 and Mentor award MN 7-06-MN-27). R. Baker received support from NIH T32 AI074491 and NIH T32 AI007405.
Abbreviations
- T1D
type 1 diabetes
- PEC
peritoneal elicited cells
- APC
antigen-presenting cells
- DN
dominant-negative
- ICS
Intracellular cytokine staining
Footnotes
Conflict of interest: The authors declare no financial conflict of interest.
References
- 1.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 3.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 4.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munroe ME. Functional roles for T cell CD40 in infection and autoimmune disease: the role of CD40 in lymphocyte homeostasis. Semin Immunol. 2009;21:283–288. doi: 10.1016/j.smim.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DH, Jr., Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci U S A. 2002;99:3782–3787. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munroe ME, Bishop GA. A Costimulatory Function for T Cell CD40. J Immunol. 2007;178:671–682. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- 8.Baker RL, Wagner DH, Jr., Haskins K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J Autoimmun. 2008;31:385–392. doi: 10.1016/j.jaut.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Kirk AD, Blair PJ, Tadaki DK, Xu H, Harlan DM. The role of CD154 in organ transplant rejection and acceptance. Philos Trans R Soc Lond B Biol Sci. 2001;356:691–702. doi: 10.1098/rstb.2001.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liossis SN, Sfikakis PP. Costimulation blockade in the treatment of rheumatic diseases. BioDrugs. 2004;18:95–102. doi: 10.2165/00063030-200418020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Davidson A, Wang X, Mihara M, Ramanujam M, Huang W, Schiffer L, Sinha J. Co-stimulatory blockade in the treatment of murine systemic lupus erythematosus (SLE) Ann N Y Acad Sci. 2003;987:188–198. doi: 10.1111/j.1749-6632.2003.tb06048.x. [DOI] [PubMed] [Google Scholar]
- 12.Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt HO, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–4627. [PubMed] [Google Scholar]
- 13.Eshima K, Mora C, Wong FS, Green EA, Grewal IS, Flavell RA. A crucial role of CD4 T cells as a functional source of CD154 in the initiation of insulin-dependent diabetes mellitus in the non-obese diabetic mouse. Int Immunol. 2003;15:351–357. doi: 10.1093/intimm/dxg035. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 15.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, Shimamura T, N., et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9:1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Zheng X, Zhang X, Ichim TE, Sun H, Kubo N, Beduhn M, et al. Inhibition of allergic responses by CD40 gene silencing. Allergy. 2009;64:387–397. doi: 10.1111/j.1398-9995.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 17.Margolles-Clark E, Umland O, Kenyon NS, Ricordi C, Buchwald P. Small-molecule costimulatory blockade: organic dye inhibitors of the CD40-CD154 interaction. J Mol Med. 2009;87:1133–1143. doi: 10.1007/s00109-009-0519-3. [DOI] [PubMed] [Google Scholar]
- 18.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 19.Waid DM, Wagner RJ, Putnam A, Vaitaitis GM, Pennock ND, Calverley DC, Gottlieb P, et al. A unique T cell subset described as CD4loCD40+ T cells (TCD40) in human type 1 diabetes. Clin Immunol. 2007;124:138–148. doi: 10.1016/j.clim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol. 2005;175:7738–7745. doi: 10.4049/jimmunol.175.11.7738. [DOI] [PubMed] [Google Scholar]
- 21.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 22.McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods. 2008;336:91–97. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci U S A. 2001;98:1751–1756. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 25.Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760–5767. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 29.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, Harrop K, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 30.Khubchandani S, Czuczman MS, Hernandez-Ilizaliturri FJ. Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies. Curr Opin Investig Drugs. 2009;10:579–587. [PubMed] [Google Scholar]
- 31.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 32.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989;86:8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergman B, Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994;43:197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- 34.Torres RM, Clark EA. Differential increase of an alternatively polyadenylated mRNA species of murine CD40 upon B lymphocyte activation. J Immunol. 1992;148:620–626. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.