Abstract
Because of their important roles in mediating the stabilization and expression of p53, we hypothesized that high-risk genotypes of polymorphisms in p53-related genes, including p53, p73, p14ARF, MDM2 and MDM4, may be associated with an increased risk of second primary malignancy (SPM) after index squamous cell carcinoma of the head and neck (SCCHN). We analyzed data from a cohort of 1283 patients with index SCCHN who were recruited between 1995 and 2007 at MD Anderson Cancer Center and followed for SPM development. Patients were genotyped for nine polymorphisms of p53-related genes. A log-rank test and Cox models were used to compare SPM-free survival and risk. Our results demonstrated that each p53-related polymorphism had a moderate effect on increased SPM risk, but when we combined risk genotypes of these nine polymorphisms together, we found that SPM-free survival was significantly shorter among risk groups with a greater number of combined risk genotypes. SPM risk increased with increasing number of risk genotypes (P < 0.0001 for trend). Compared with the low-risk group (0–3 combined risk genotypes), both the medium-risk (4–5 combined risk genotypes) and high-risk (6–9 combined risk genotypes) groups had significantly increased SPM risk [hazard ratio (HR): 1.6; 95% confidence interval (CI): 1.0–2.6 and HR: 3.0; 95% CI: 1.8–5.0, respectively]. Moreover, such significant associations were even higher in several subgroups. Our findings suggest that combined risk genotypes of p53-related genes may jointly modify SPM risk, especially in patients who are smokers and those with index non-oropharyngeal cancers. However, larger studies are needed to validate our findings.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is characterized by highly aggressive local–regional tumor growth and results in significant morbidity. Despite the advances in loco-regional control achieved with modern treatment, the survival of patients with SCCHN has not essentially improved, partly because about 20% of these patients develop a second primary malignancy (SPM) (1–4). Although drinking alcohol and smoking cigarettes have been linked to SPM risk, patients with SCCHN do not always develop a SPM, even if they continue to consume alcohol and cigarettes (5–7). This suggests that genetic susceptibility may also contribute to SPM etiology. Identifying genetic markers associated with SPM in patients with SCCHN would allow for the identification of a subpopulation of SCCHN survivors with a high risk of SPM.
Tobacco and alcohol consumption prior to the diagnosis of the primary SCCHN significantly increases the risk of SPM (8). Tobacco smoke can cause DNA damage that deregulates cell cycle control and apoptosis, which results in carcinoma if the DNA damage is left unrepaired (9,10), and genetically inherited DNA repair capacity can modulate individual susceptibility to tobacco-induced carcinogenesis (11,12). The p53 molecular pathway plays a central role in maintaining genomic integrity and protecting cells against such damage (13).
p53, a tumor suppressor known as ‘the guardian of the genome’, plays an important role in the prevention of carcinogenesis induced by DNA damage from various agents (14). p53-Related genes, such as p73, p14 and murine double minute proteins 2 and 4 (MDM2 and MDM4), respond to a variety of stress signals to affect cellular homeostatic mechanisms (15,16). Functionally, p73, located on the 1p36 locus, activates transcription of p53-responsive genes (17,18). p14ARF, which represents the product of exons 1b, 2 and 3 of the CDKN2A locus on 9p21, interacts directly with MDM2, thereby indirectly regulating the level of p53 (19). MDM2, described as the first p53 E3-ubiquitin ligase, induces polyubiquitylation and degradation of p53 when overexpressed (20). MDM4 is a negative regulator of p53 and cooperates with MDM2 to inhibit p53 activity in the cellular response to DNA damage (21).
The putatively functional polymorphisms in these p53-related genes (p53, p73, p14ARF, MDM2 and MDM4), which regulate the cell cycle and apoptosis, may collectively modify genetic susceptibility to primary SCCHN or SPM after index SCCHN (17–21). The p53 codon 72 at exon 4 encodes either a proline (Pro) or arginine (Arg) that appears to influence individual susceptibility to cancer by functionally affecting the p53 protein. The non-coding exon 2 polymorphism of p73 G>A rs2273953 may functionally affect the p73 protein by affecting the efficiency of p73 translation initiation. Since the two p14ARF polymorphisms are within the functional regions of the gene’s promoter (p14ARF-rs3731217) and 3′-untranslated region (UTR) (p14ARF-rs3088440), we speculated that they may potentially affect p14ARF expression levels by altering the efficiency of translational initiation. Among the polymorphisms of MDM2, two polymorphisms in promoter, MDM2-rs2279744 and MDM2-rs937283, may lead to change of MDM2 transcription levels, resulting in altered p53-MDM2 binding affinity and regulation of cell cycle control. Unlike p53, p73 and MDM2, few studies have investigated the role of MDM4 variants in the risk of human cancers. We identified three common tagging SNPs, rs11801299 G>A and rs1380576 C>G in 3′-UTR and rs10900598 G>T in 5′-UTR, which may alter or influence MDM4 expression and subsequently increase susceptibility to cancer.
We have reported previously that SPM risk among SCCHN patients was associated with individual genetic variants of these p53-related genes. In these studies, we found that the variant genotypes of p53 codon 72, p14ARF-rs3731217 and p14ARF-rs3088440 polymorphisms were associated with a significantly increased SPM risk compared with their corresponding homozygous wild-type genotypes, respectively [hazard ratio (HR): 1.58, 95% confidence interval (CI): 1.07–2.34 for p53 codon 72; HR: 1.48, 95% CI: 1.00–2.19 for p14ARF-rs3731217 and HR: 1.61, 95% CI: 1.07–2.43 for p14ARF-rs3088440, respectively], but a significantly reduced SPM risk for p73 G>A rs2273953 polymorphism (HR: 0.59; 95% CI: 0.39–0.89) (17–19). However, the similar associations have not been assessed for the two promoter polymorphisms of MDM2: MDM2-rs2279744 and MDM2-rs937283 and the three common tagging SNPs of MDM4: MDM4-rs11801299 G>A and MDM4-rs1380576 C>G in 3′-UTR and MDM4-rs10900598 G>T in 5′-UTR. Given the role of each of these variants in regulation of cell cycle and apoptosis and genetic susceptibility to primary SCCHN or SPM after index SCCHN (17–21), we hypothesize that these nine polymorphisms collectively modify the risk of SPM and that these combined risk genotypes could serve as susceptibility markers for identifying high-risk subgroups of patients who might benefit from personalized prevention and treatment, and we evaluated the combined effects of these polymorphisms on risk of SPM in a well-established cohort of SCCHN patients.
Materials and methods
Study subjects
We analyzed data from a cohort of 1283 patients with index SCCHN who were consecutively recruited between May 1995 and January 2007 as part of an ongoing prospective molecular epidemiological study at The University of Texas MD Anderson Cancer Center, as described previously (18). All subjects provided Institutional Review Board-approved informed consent and were recruited regardless of age, sex, ethnicity or cancer stage. The exclusion criteria included any previous cancer (except non-melanoma skin cancer), distant metastases at presentation, primary sinonasal cancers, salivary gland cancers, cervical metastases of unknown origin and cancers outside the upper aerodigestive tract. Patients were monitored throughout their treatment and post-treatment course with regularly scheduled clinical and radiographic examinations.
All patients were interviewed at presentation for completion of an epidemiological questionnaire that included data on alcohol and smoking status. Alcohol status was categorized as ‘ever drinkers’ (those who had drunk at least one alcoholic beverage/week for at least 1 year during their lifetimes) or ‘never drinkers’ (those who never had such a pattern of drinking). Smoking status was categorized as ‘ever smokers’ (those who had smoked at least 100 cigarettes in their lifetimes) or ‘never smokers’ (those who had smoked fewer than 100 cigarettes in their lifetimes).
Additional clinical data were obtained from review of the patients’ medical records at initial presentation and follow-up, including overall stage, site and treatment at presentation of the index tumor. A SPM was carefully defined according to the modified criteria of Warren et al. (22). Briefly, SPMs were considered if the second lesions had different histopathologic types or if they developed over 5 years after treatment for the index tumor and/or clearly separated by normal epithelium according to clinical and radiographic assessment. Pulmonary lesions were included as a SPM if they had a non-squamous histology or if they were isolated squamous lesions over 5 years from index SCCHN and considered by both thoracic oncologist and thoracic surgeon as a SPM. If there was discrepancy or difference in opinions regarding recurrence or SPM, the second lesion was not considered a SPM but a local recurrence. SPMs were categorized as tobacco-associated (SCCHN or cancers of the esophagus, lung or bladder) or non-tobacco-associated SPM (prostate cancer, papillary thyroid carcinoma, colon adenocarcinoma, etc.).
Genotyping
Genomic DNA was extracted from patient blood samples and genotyped for the following polymorphisms: p53 codon 72, p73 G4C14-to-A4T14, p14ARF-rs3731217, p14ARF-rs3088440, MDM2-rs2279744, MDM2-rs937283, MDM4-rs11801299, MDM4-rs1380576 and MDM4-rs10900598. The details of genotyping for these polymorphisms have been described previously (17–21). There was 100% concordance when 10% of the genotyping assays were repeated.
Statistical analysis
The primary endpoint of the study was SPM occurrence. Time-to-event was calculated from the date of diagnosis of the index SCCHN to the date of diagnosis of the SPM. Student’s t-test was used to compare the mean age and follow-up time between the patients who developed an SPM and those who did not. The chi-square test was used to assess the differences in ethnicity, sex, smoking and alcohol status, primary tumor site and stage, treatment, and genotype distributions between the two groups. Kaplan–Meier curves were used to estimate SPM-free survival, and the log-rank test was used to evaluate significant differences (α = 0.05) in SPM-free survival between the different genotyping groups. In light of the crossed over Kaplan–Meier curves, we also applied alternative tests to the log-rank test for testing homogeneity of the survival functions. Specifically, we applied the Wilcoxon (Gehan’s) test to investigate the survival difference at the early stage of follow-up, whereas we used Fleming-Harrington’s test (two parameters p = q = 0.5) to investigate the survival difference at the late stage of follow-up (23). In the univariate logistic regression analysis, we estimated the association between risk of SPM and selected demographic variables, risk factors and clinical variables by computing the HRs and their 95% CIs. The associations between individual epidemiological factors, clinical characteristics, and treatment variables, and time-to-event (SPM), were initially assessed using univariate Cox proportional hazards regression models. The data were consistent with the assumptions of the Cox proportional hazards regression model from the examination of Kaplan–Meier survival curves and log-minus-log survival plots (24,25). In the multivariable logistic regression models, adjusted for age, sex, ethnicity, smoking and alcohol consumption, we evaluated the effects of single polymorphism or combined polymorphisms of p53-related genes on the risk of SPM. The joint effects were further stratified by smoking status, index tumor site and SPM site. Statistical analyses were performed using Statistical Analysis System (SAS) software (version 9.1.3; SAS Institute, Cary, NC). For all analyses, statistical significance was set at P < 0.05, and all tests were two sided.
Results
Patient characteristics
Demographic, risk and clinical characteristics of the 1283 patients (overall and according to SPM occurrence) are shown in Table I. With a median follow-up time of 34.0 months (range, 2.4–142.4 months), 1163 patients remained SPM free, whereas 120 patients developed SPMs. Of these 120 patients, 85 developed SPMs at tobacco-associated sites and 35 patients developed SPMs at other sites. Although this patient cohort included predominantly men (76.0%), sex was not associated with SPM development (P = 0.5285). We did not observe significant differences between patients with and without SPMs with regard to smoking history (P = 0.1204), alcohol consumption (P = 0.3442), index cancer site (P = 0.3184), index cancer stage (P = 0.6926) or treatment (P = 0.8832). However, compared with SPM-free patients, patients who developed SPMs were more likely to be older (P < 0.0001) and non-Hispanic whites (P = 0.0440).
Table I.
Distribution of selected participant characteristics
| Characteristic | Total | SPM free | SPM | P-valuea | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Total patients | 1283 | 100 | 1163 | 90.7 | 120 | 9.3 | |
| Age | |||||||
| <Median (57 years) | 662 | 51.6 | 623 | 53.6 | 39 | 32.5 | <0.0001 |
| >Median (57 years) | 621 | 48.4 | 540 | 46.4 | 81 | 67.5 | |
| Sex | |||||||
| Male | 975 | 76.0 | 881 | 75.7 | 94 | 78.3 | 0.5285 |
| Female | 308 | 24.0 | 282 | 24.3 | 26 | 21.7 | |
| Ethnicity | |||||||
| Non-Hispanic white | 1086 | 84.6 | 992 | 85.3 | 94 | 78.3 | 0.0440 |
| Other | 197 | 15.4 | 171 | 14.7 | 26 | 21.7 | |
| Smoking | |||||||
| Never | 344 | 26.8 | 319 | 27.4 | 25 | 20.8 | 0.1204 |
| Ever | 939 | 73.2 | 844 | 72.6 | 95 | 79.2 | |
| Alcohol | |||||||
| Never | 335 | 26.1 | 308 | 26.5 | 27 | 22.5 | 0.3442 |
| Ever | 948 | 73.9 | 855 | 73.5 | 93 | 77.5 | |
| Index cancer site | |||||||
| Oral cavity | 416 | 32.4 | 378 | 32.5 | 38 | 31.7 | 0.3184 |
| Oropharynx | 572 | 44.6 | 524 | 45.0 | 48 | 40.0 | |
| Larynx/hypopharynx | 295 | 23.0 | 261 | 22.5 | 34 | 28.3 | |
| Index cancer stage | |||||||
| 1 or 2 | 323 | 25.2 | 291 | 25.0 | 32 | 26.7 | 0.6926 |
| 3 or 4 | 960 | 74.8 | 872 | 75.0 | 88 | 73.3 | |
| Treatment | |||||||
| Surgery only | 229 | 17.8 | 208 | 17.9 | 21 | 17.5 | 0.8832 |
| Surgery plus adjuvant treatment | 318 | 24.8 | 285 | 24.5 | 33 | 27.5 | |
| XRTb | 328 | 25.6 | 300 | 25.8 | 28 | 23.3 | |
| XRT plus chemotherapy | 408 | 31.8 | 370 | 31.8 | 38 | 31.7 | |
aP-values were calculated from chi-square tests.
bXRT: radiotherapy.
Combined effects of the p53-related genetic variants on risk of SPM
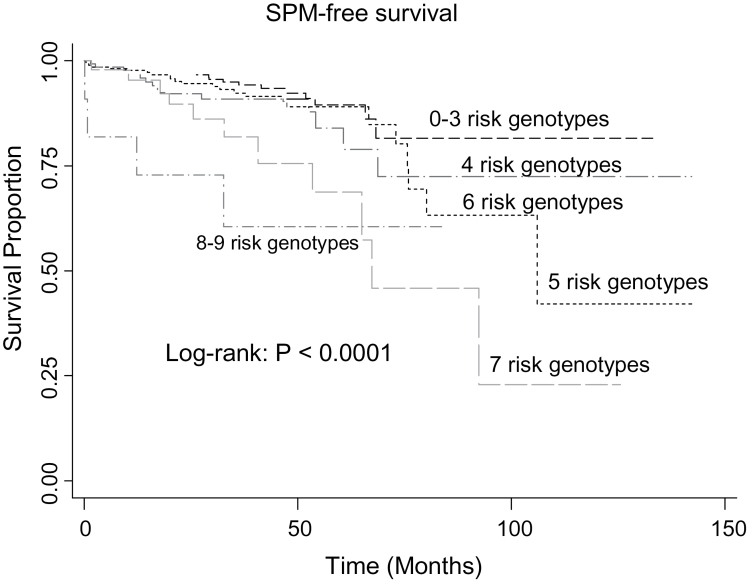
Because each of these polymorphisms appeared to have a minor or moderate effect on SPM risk (Table II) and no polymorphisms were in linkage disequilibrium (LD) to each other between variants belonging to the same genes (data not shown), we categorized the nine polymorphisms under investigation into a new variable. Specifically, in the study subjects who had data available on all nine polymorphisms, we categorized all putative risk (HRs > 1.0) genotypes of each polymorphism into a new variable according to the number of risk genotypes carried by an individual for each of the nine polymorphisms in a dominant model (for the p73 G>A rs2273953 and MDM4-rs10900598 G>T genotypes, we reversed the reference group to reflect the protective effects of the variant genotypes: p73 GA/AA and MDM4-rs10900598 GT/TT). Therefore, according to the number of risk genotypes carried by each individual and the level of SPM risk linked to the risk genotypes of each individual polymorphism, we categorized the individuals into different risk groups with different combined risk genotypes to evaluate the collective effects of the p53, p73, p14, MDM2 and MDM4 polymorphisms on the risk of SPM as shown in Table III. When we combined the risk genotypes of the nine polymorphisms together, we found that SPM-free survival decreased significantly as the number of combined risk genotypes increased (log-rank test; P < 0.0001) (Figure 1). In light of the crossed over Kaplan–Meier curves, we used other alternative tests, such as Wilcoxon (Gehan’s) and Fleming-Harrington’s test, to the log-rank test; and all were highly significant (all P-values < 0.0001), indicating that there was survival difference between different groups. As shown in Table III, there was a significant trend in SPM risk (P < 0.0001 for trend) between increased SPM risk and the increasing number of combined risk genotypes; in particular, patients with eight or nine risk genotypes had an ~7-fold (HR: 7.1; 95% CI: 2.4–21.0) higher risk of SPM compared with patients carrying 0–3 risk genotypes.
Table II.
Association of individual polymorphisms of p53-related genes with SPM risk after index SCCHN
| Polymorphisms | Total (n = 1283) | SPM free (n = 1163) | SPM (n = 120) | Pa | HR (95% CI)b | P | |||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| p53 | |||||||||
| WW | 661 | 51.5 | 615 | 52.9 | 46 | 38.3 | 0.0024 | 1.0 | 0.009 |
| WP/PP | 622 | 48.5 | 548 | 47.1 | 74 | 61.7 | 1.6 (1.1–2.4) | ||
| p73 rs2273953 | |||||||||
| GA/AA | 746 | 58.1 | 665 | 57.2 | 81 | 67.5 | 0.0291 | 1.0 | 0.035 |
| GG | 537 | 41.9 | 498 | 48.2 | 39 | 32.5 | 1.5 (1.0–2.2) | ||
| p14ARF-rs3731217 | |||||||||
| TT | 963 | 75.1 | 880 | 75.7 | 83 | 69.2 | 0.1172 | 1.0 | 0.032 |
| TG/GG | 320 | 24.9 | 283 | 24.3 | 37 | 30.8 | 1.5 (1.0–2.3) | ||
| p14ARF-rs3088440 | |||||||||
| GG | 1034 | 80.6 | 951 | 81.8 | 83 | 69.2 | 0.0009 | 1.0 | 0.026 |
| GA/AA | 249 | 19.4 | 212 | 18.2 | 37 | 30.8 | 1.6 (1.1–2.4) | ||
| MDM2-rs2279744 | |||||||||
| TT | 741 | 57.7 | 656 | 56.4 | 85 | 70.8 | 0.0023 | 1.0 | 0.002 |
| GT/GG | 542 | 42.3 | 507 | 43.6 | 35 | 29.2 | 1.9 (1.2–2.7) | ||
| MDM2-rs937283 | |||||||||
| AA | 343 | 26.7 | 316 | 27.2 | 27 | 22.5 | 0.2710 | 1.0 | 0.461 |
| AG/GG | 940 | 73.7 | 847 | 72.8 | 93 | 77.5 | 1.2 (0.8–1.8) | ||
| MDM4-rs11801299 | |||||||||
| GG | 835 | 65.1 | 757 | 65.1 | 78 | 65.0 | 0.9842 | 1.0 | 0.956 |
| AG/AA | 448 | 34.9 | 406 | 34.9 | 42 | 35.0 | 1.1 (0.7–1.5) | ||
| MDM4-rs1380576 | |||||||||
| CC | 547 | 42.6 | 500 | 43.0 | 47 | 39.2 | 0.4198 | 1.0 | 0.492 |
| CG/GG | 736 | 57.4 | 663 | 57.0 | 73 | 60.8 | 1.1 (0.8–1.6) | ||
| MDM4-rs10900598 | |||||||||
| GT/TT | 883 | 68.8 | 810 | 69.7 | 73 | 60.8 | 0.0472 | 1.0 | 0.048 |
| GG | 400 | 31.2 | 353 | 30.3 | 47 | 39.2 | 1.4 (1.0–2.1) | ||
aChi-square test for differences in the distribution of genotypes in p53-related genes between the patients who developed SPM and the patients who did not.
bAdjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
Table III.
Association of combined risk genotypes of p53-related genes with SPM risk after index SCCHN
| Total (n = 1283) | SPM free (n = 1163) | SPM (n = 120) | P a | HR (95% CI)b | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| No. of risk genotypes | |||||||||
| 0–3 risk genotypes | 463 | 36.1 | 436 | 37.5 | 27 | 22.5 | <0.001 | 1.0 | |
| 4 risk genotypes | 353 | 27.5 | 325 | 27.9 | 28 | 23.3 | 1.5 (0.9–2.5) | 0.155 | |
| 5 risk genotypes | 266 | 20.7 | 238 | 20.5 | 28 | 23.3 | 1.8 (1.1–3.1) | 0.025 | |
| 6 risk genotypes | 141 | 11.0 | 122 | 10.5 | 19 | 15.9 | 2.1 (1.2–3.8) | 0.015 | |
| 7 risk genotypes | 49 | 3.8 | 35 | 3.0 | 14 | 11.7 | 5.5 (2.8–10.9) | <0.0001 | |
| 8–9 risk genotypes | 11 | 0.9 | 7 | 0.6 | 4 | 3.3 | 7.1 (2.4–21.0) | 0.0004 | |
| Trend | <0.001 | ||||||||
| Combined risk genotypesc | |||||||||
| Low-risk group | 463 | 36.1 | 436 | 37.5 | 27 | 22.5 | <0.001 | 1.0 | |
| Medium-risk group | 619 | 48.2 | 563 | 48.4 | 56 | 46.7 | 1.6 (1.0–2.6) | 0.039 | |
| High-risk group | 201 | 15.7 | 164 | 14.1 | 37 | 30.8 | 3.0 (1.8–5.0) | <0.0001 | |
| Trend | <0.001 | ||||||||
aChi-square test for differences in the distribution of genotypes in p53-related genes between the patients who developed SPM and the patients who did not.
bAdjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
cLow-risk group, individuals carrying 0–3 risk genotypes; medium-risk group, individuals carrying 4–5 risk genotypes; high-risk group, individuals carrying 6–9 risk genotypes.
Fig. 1.
SPM-free survival stratified by number of risk genotypes of p53-related genes.
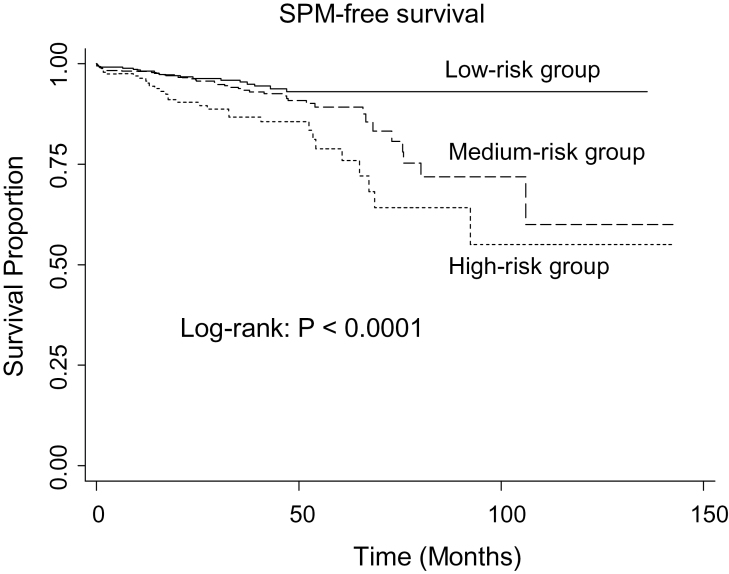
We further categorized the patients into three main groups: (i) the low-risk group (0–3 combined risk genotypes); (ii) the medium-risk group (4–5 combined risk genotypes) and (iii) the high-risk group (6–9 combined risk genotypes). The SPM-free survival differed significantly among the three risk groups (log-rank test; P < 0.0001) (Figure 2). Furthermore, the patients in the medium-risk and high-risk groups had a 1.6-fold (HR: 1.6; 95% CI: 1.0–2.6) and 3.0-fold (HR: 3.0; 95% CI: 1.8–5.0) increased risk of SPM compared with those in the low-risk group (Table III). When the similar analysis was performed among non-Hispanic whites only, we found that the results were similar when SPM risk was limited to non-Hispanic whites (data not shown).
Fig. 2.
SPM-free survival by the combined risk genotypes of p53-related genes in three risk groups.
Stratification analysis of the combined p53-related genetic variants with risk of SPM
We further evaluated the associations between the combined risk genotypes and risk of SPM stratified by age, smoking status, index cancer site and SPM type, as summarized in Table IV. When we used the low-risk group as comparison group, a significant higher HR was observed among young patients (HR: 4.2; 95% CI: 1.7–10.4) than among older patients (HR: 2.3; 95% CI: 1.3–4.4) in the high-risk group, whereas the interaction between age and combined risk genotypes on risk of SPM was not significant (P int. = 0.100 for the six risk groups and P int. = 0.190 for the three risk groups). Compared with the low-risk group, the increased risk of SPM was statistically significant for ever smokers in both the medium-risk group (HR: 1.7; 95% CI: 1.0–2.9) and high-risk group (HR: 3.0; 95% CI: 1.7–5.4). The increase in risk was also higher for patients who had index non-oropharynx tumors in both the medium-risk (HR: 2.4; 95% CI: 1.2–4.8) and high-risk (HR: 5.0; 95% CI: 2.4–10.5) groups as well as for SPMs at either tobacco-associated sites or non-tobacco-associated sites (HR: 3.0; 95% CI: 1.6–5.5 for SPM at tobacco-associated sites and HR: 3.3; 95% CI: 1.3–8.3 for SPM at non-tobacco-associated sites).
Table IV.
Stratified analysis of combined risk genotypes of p53-related genes by smoking, index tumor site and SPM type
| Variables | Low-risk group (reference) | Medium-risk group | P | High-risk group | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPM free | SPM | HR (95% CI)a | SPM free | SPM | HR (95% CI)a | SPM free | SPM | HR (95% CI)a | |||
| Age (median = 57 years) | |||||||||||
| ≤Median | 228 (36.6) | 8 (20.5) | 1.0 | 313 (50.2) | 19 (48.7) | 1.9 (0.8–4.4) | 0.122 | 82 (13.2) | 12 (30.8) | 4.2 (1.7–10.4) | 0.002 |
| >Median | 208 (38.5) | 19 (23.5) | 1.0 | 250 (46.3) | 37 (45.7) | 1.5 (0.9–2.7) | 0.143 | 82 (15.2) | 25 (30.9) | 2.3 (1.3–4.4) | 0.007 |
| Smoking | |||||||||||
| Never | 114 (35.7) | 6 (24.0) | 1.0 | 158 (49.6) | 10 (40.0) | 1.2 (0.4–3.5) | 0.691 | 47 (14.7) | 9 (36.0) | 2.3 (0.8–6.8) | 0.142 |
| Ever | 322 (38.2) | 21 (22.1) | 1.0 | 405 (48.0) | 46 (48.4) | 1.7 (1.0–2.9) | 0.045 | 117 (13.8) | 28 (29.5) | 3.0 (1.7–5.4) | 0.0002 |
| Index tumor site | |||||||||||
| Oro. | 203 (38.7) | 16 (33.3) | 1.0 | 249 (47.6) | 20 (41.7) | 1.1 (0.6–2.1) | 0.723 | 72 (13.7) | 12 (25.0) | 1.7 (0.8–3.6) | 0.180 |
| Non-oro. | 233 (36.5) | 11 (15.3) | 1.0 | 314 (49.1) | 36 (50.0) | 2.4 (1.2–4.8) | 0.010 | 92 (14.4) | 25 (34.7) | 5.0 (2.4–10.5) | <0.0001 |
| SPM type | |||||||||||
| TAS | 436 (37.5) | 19 (22.4) | 1.0 | 563 (48.4) | 41 (48.2) | 1.7 (0.9–3.0) | 0.052 | 164 (14.1) | 25 (29.4) | 3.0 (1.6–5.5) | 0.0005 |
| Non-TAS | 436 (37.5) | 8 (22.9) | 1.0 | 563 (48.4) | 15 (42.9) | 1.7 (0.7–3.9) | 0.250 | 164 (14.1) | 12 (34.2) | 3.3 (1.3–8.3) | 0.010 |
Oro.: oropharyngeal cancer; TAS: tobacco-associated SPM.
aAdjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
Discussion
It is well established that genes in the p53 pathway play a critical role in DNA repair, apoptosis and cell cycle regulation to conserve genomic stability and prevent mutations induced by tobacco and other carcinogens (13,15). Single nucleotide polymorphisms of these genes may affect their corresponding protein expression or function and, thus, potentially affect cancer risk or risk of SPMs (17–21,26,27). In the present study, we found a significant association between the combined risk genotypes of p53-related genes and the risk of developing SPM following index SCCHN.
Interactions among these genes in the p53 pathway are involved in the regulation of p53 activity, which likely provides biological plausibility for the observed associations between these polymorphisms and SPM risk. For example, the polymorphism p73 G>A rs2273953 is completely linked with another nearby variant, p73 C>T rs1801173, which form a stem-loop structure and may influence p73 expression. It was reported that the enhanced binding of p53 codon 72 to p73 can neutralize p73-induced apoptosis (28), suggesting a possible interaction between p73 and p53 polymorphisms in the development of human cancers. Our group also reported that an increased risk of SPM after index SCCHN was associated with p53 and p73 polymorphisms both individually and in combination (29). p14ARF, which plays an important role in the ARF-MDM2-p53 pathway, releases p53 by binding to and inactivating the MDM2 protein, resulting in p53-mediated growth arrest or apoptosis in the oncogene-expressing cells (30). An increased risk of SPM after index SCCHN was associated with each of p14ARF polymorphisms investigated in our previous study (19). The MDM2-rs2279744 T>G polymorphism creates a binding site for the common transcription factor Sp1, leading to low expression of p53 (31). Like MDM2 that includes the p53-binding domain, a zinc finger motif and a C-terminal RING finger domain, MDM4, a structural homolog of MDM2, can also bind to p53 and inhibit its ability to transactivate gene expression (32). Since most of these variants are low-penetrance polymorphisms that confer a minor risk, the combination of p53-related gene polymorphisms could result in more complete and accurate estimates of risk of SPM than that from a single variant (33).
Although the functional relevance of these variants except p53 R72P and MDM2-rs2279744 (also called MDM2 SNP309) has not yet been clarified, some biologically plausible information from public data may provide additional evidence to support our observed associations. We performed LD analysis from the recently released public information on 1000 genome data (March 2012) and used the SNPinfo tool (FuncPred, NIEHS, NIH, 2012) to predict relevant functionality of other seven variants. Our results indicated that all of these variants are potentially functional or highly in LD (r2 > 0.8) with other nearby functional variants. p73 G>A (rs2273953) is completely in LD (r2 = 1.0) with another SNP rs1801173, which is located at the exonic splicing enhancer and might affect the splicing process. p14ARF-rs3731217 is highly in LD with another variant, p14ARF rs2811711, which may influence the binding activity of transcription factors, whereas p14ARF-rs3088440 in the 3′-UTR of p14ARF is within the putative binding sites of several micro-RNAs (e.g. hsa-miR-328, hsa-miR-1291 and has-miR-663b). All three MDM4 variants in this study were found to be highly in LD with other functional variants. For example, MDM4-rs1380576 is highly in LD with several other variants in the vicinity of this candidate variant to influence the binding activity of transcription factors (e.g. rs11240754, rs11240755, rs7367519, rs2926533, rs12032733, rs12738124 and rs4951077), the micro-RNA binding (e.g. rs4245739) and exonic splicing process (e.g. rs4245738). MDM4-rs11801299 is highly in LD with variant rs12028476, which is located at the 5′ flanking of MDM4 and may have the potential to influence the binding of transcription factors. Finally, MDM4-rs10900598 is highly in LD with two variants, rs12034564 and rs4252745, and these two variants may, respectively, influence the binding activities of transcription factors or micro-RNAs (hsa-miR-425 and hsa-miR-542-3p). Taken together, such information may additionally support our observed associations, which to some extent may be driven by limited study power due to small sample sizes in SPM events.
In our analysis of 1283 SCCHN patients, we analyzed the combined effects of the nine well-studied polymorphisms in p53-related genes on genetic susceptibility to SPM. Results showed that these variants jointly and significantly increased the risk of SPM with index SCCHN, and an increasing number of putative risk genotypes was associated with increasing risk of SPM. These results support the notion that the development of SPM following index SCCHN may be a polygenic process, and risk evaluation in a pathway-based approach may yield higher predictive estimates of association (34).
We further performed a stratified analysis of the effects of combined low-risk, medium-risk and high-risk genotypes of these nine polymorphisms on risk of SPM among several subgroups. Although we found that young patients had a higher risk of SPM than the older patients in the high-risk group, the interaction of age with the combined risk genotypes was not statistically significant. Therefore, the residue confounding effect or bias caused by age in estimates of association in this study might not be severe. We found that significantly increased risk of SPM associated with the combined risk genotypes was confined to smokers and patients with index non-oropharyngeal cancers. Epidemiological studies have consistently demonstrated that most non-oropharyngeal cancers are associated with tobacco and alcohol use, whereas most oropharyngeal cancers are etiologically associated with human papillomavirus infection (35,36). The risk of developing SPMs in the aerodigestive tract is clearly associated with tobacco use before the diagnosis of the index tumor (5–7). Hence, it is biologically plausible that the risk of SPM in patients with index non-oropharyngeal cancers is likely attributable to tobacco use before and after diagnosis of the primary tumor.
The etiological concept of field cancerization might help to explain why patients with SCCHN may have a high possibility of developing SPM (37). Field cancerization, in which environmental carcinogens, such as tobacco, induce a field of the mucosa afflicted with premalignant lesions, may elevate risk of developing epithelial cancer throughout the upper aerodigestive tract (38). It was shown that these unresected fields contributed to SPM occurrence in patients treated for SCCHN (39). Our findings in index non-oropharyngeal cancers suggest that genetic variants in p53-related genes could modulate tobacco-induced development of SPM, possibly through gene–environment interactions.
Another finding in the present study was that the pronounced association between the combined risk genotypes and SPM risk was similar for all anatomical sites of SPM. Our results also showed that the high-risk group was significantly associated with the development of SPMs after index SCCHN in both tobacco-associated sites and non-tobacco-associated sites, suggesting that these polymorphisms of p53-related genes may play roles in both smoking-driven and non-smoking-induced SPMs. Although our results were significant in several subgroups, the insufficient numbers of SPMs in each subgroup, especially when combining the nine polymorphisms, may have limited the study power to detect a weak association. Therefore, larger sample sizes and well-characterized studies are needed to validate these results.
The present study has the following limitations. (i) Possible selection bias could not be ruled out because of the hospital-based study design. (ii) Approximately 85% of the patients were non-Hispanic whites, despite a large cohort of SCCHN patients. The results may, therefore, not be applicable to all ethnic populations. (iii) Clinical outcomes such as SPM were collected retrospectively, but the demographic, carcinogen exposure and clinical data for the cohort were collected prospectively. (iv) We did not have information on human papillomavirus infection or on continued smoking behavior after the index SCCHN diagnosis because of the retrospective study design. (v) The patients may not have had enough time to develop SPM or could have been lost to follow-up because the high proportion of never smokers and late stage index cancer patients as well as our strict criteria for determining SPM resulted in a SPM rate that was lower than expected. Therefore, the low rate of SPM and short follow-up time of study patients limited statistical power for the analysis, particularly for the stratified analysis. It is highly likely that most of these significant associations could be false positive results. Thus, our results could be chance findings and should be confirmed in larger studies.
Thus, our future studies will incorporate information such as human papillomavirus tumor status and continued smoking behavior. Finally, the small sample sizes of SPM events should be noted when interpreting the results as the small sample size increases the possibility that the statistically significant results could be due to chance. SPM rate (9.3%) in this study was lower than expected, and the SPM sample size limits statistical power in the subgroup analyses. Such low SPM rate may have been limited by short duration of follow-up for SPM development during the study period as the large proportion of study patients had a stage III or IV index cancer (75%) who were lost to follow-up, with only 120 patients having developed SPM, thus limiting statistical power to detect modest associations. Furthermore, the high prevalence (~27%) of never smokers and our strict definition of SPM may have also limited the observed incidence of SPM in this patient cohort. All of these limitations may have biased the observed association. Therefore, our findings are preliminary, and future prospective studies with larger sample sizes of SPM events and longer follow-up time in different populations are warranted to validate the results in multi-institutional groups such as INHANCE, the International Head and Neck Cancer Epidemiology Consortium.
In conclusion, the present study provides epidemiologic support for the combined effects of genetic variants of p53-related genes on risk of SPM in patients after index SCCHN. We also noted that the combined genetic effects were higher in ever smokers than in never smokers and in patients with index non-oropharyngeal cancers than with oropharyngeal cancers. The value of examining multiple polymorphisms simultaneously in genes involved in the same pathway is highlighted by our approach, which may improve the precision of risk estimates.
Funding
National Institute of Environmental Health Sciences (R01 ES-11740 to Q.W.); National Institutes of Health (P-30 CA-16672 to The University of Texas MD Anderson Cancer Center); National Institutes of Health (CA135679 and CA133099 to G.L.)
Acknowledgements
The authors wish to thank Ms Margaret Lung, Ms Angeli Fairley, Ms Liliana Mugartegui and Ms Kathryn Tipton for their assistance with patient recruitment. The authors gratefully thank Dawn Chalaire for editing the article.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CI
confidence interval
- HR
hazard ratio
- LD
linkage disequilibrium
- MDM
murine double minute protein
- SCCHN
squamous cell carcinoma of the head and neck
- SPM
second primary malignancy
- UTR
untranslated region.
References
- 1. Curado M.P., et al. (2009). Recent changes in the epidemiology of head and neck cancer. Curr. Opin. Oncol., 21, 194–200 [DOI] [PubMed] [Google Scholar]
- 2. Di Martino E., et al. (2002). Survival in second primary malignancies of patients with head and neck cancer. J. Laryngol. Otol., 116, 831–838 [DOI] [PubMed] [Google Scholar]
- 3. Manikantan K., et al. (2011). Current concepts of surveillance and its significance in head and neck cancer. Ann. R. Coll. Surg. Engl., 93, 576–582 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Sturgis E.M., et al. (1995). Second primary malignancies in the head and neck cancer patient. Ann. Otol. Rhinol. Laryngol., 104, 946–954 [DOI] [PubMed] [Google Scholar]
- 5. Dahlstrom K.R., et al. (2008). Squamous cell carcinoma of the head and neck in never smoker-never drinkers: a descriptive epidemiologic study. Head Neck., 30, 75–84 [DOI] [PubMed] [Google Scholar]
- 6. Hymowitz N. (2011). Smoking and cancer: a review of public health and clinical implications. J. Natl Med. Assoc., 103, 695–700 [DOI] [PubMed] [Google Scholar]
- 7. Leemans C.R., et al. (2011). The molecular biology of head and neck cancer. Nat. Rev. Cancer, 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 8. Leon X., et al. (2009). Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with a head and neck cancer. A case-control study. Cancer Causes Control, 20, 645–652 [DOI] [PubMed] [Google Scholar]
- 9. Hang B. (2010). Formation and repair of tobacco carcinogen-derived bulky DNA adducts. J. Nucleic Acids, 2010, 709521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moktar A., et al. (2011). Cigarette smoke condensate-induced oxidative DNA damage and its removal in human cervical cancer cells. Int. J. Oncol, 39, 941–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sturgis E.M., et al. (2000). XPD/ERCC2 polymorphisms and risk of head and neck cancer: a case-control analysis. Carcinogenesis, 21, 2219–2223 [DOI] [PubMed] [Google Scholar]
- 12. Taioli E. (2008). Gene-environment interaction in tobacco-related cancers. Carcinogenesis., 29, 1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfeifer G.P., et al. (2002). Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene, 21, 7435–7451 [DOI] [PubMed] [Google Scholar]
- 14. Lane D.P. (1992). Cancer. p53, guardian of the genome. Nature, 358, 15–16 [DOI] [PubMed] [Google Scholar]
- 15. Harris S.L., et al. (2005). The p53 pathway: positive and negative feedback loops. Oncogene, 24, 2899–2908 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann T.K., et al. (2008). Alterations in the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol., 44, 1100–1109 [DOI] [PubMed] [Google Scholar]
- 17. Li F., et al. (2010). Association of p53 codon 72 polymorphism with risk of second primary malignancy in patients with squamous cell carcinoma of the head and neck. Cancer, 116, 2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li F., et al. (2009). p73 G4C14-to-A4T14 polymorphism and risk of second primary malignancy after index squamous cell carcinoma of head and neck. Int. J. Cancer., 125, 2660–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y., et al. (2011). p14ARF genetic polymorphisms and susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Cancer, 117, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu H., et al. (2011). Effects of MDM2 promoter polymorphisms and p53 codon 72 polymorphism on risk and age at onset of squamous cell carcinoma of the head and neck. Mol. Carcinog., 50, 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu H., et al. (2011). Polymorphisms of MDM4 and risk of squamous cell carcinoma of the head and neck. Pharmacogenet. Genomics, 21, 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warren S., et al. (1932). Multiple primary malignant tumors: a survey of the literature and statistical study. Am. J. Cancer, 51, 1358 [Google Scholar]
- 23. Klein J.P., et al. (2003). Survival Analysis: Techniques for Censored and Truncated Data. 2nd edn. Springer, New York: [Google Scholar]
- 24. Collett D. (2003). Modelling Survival Data in Medical Research. 2nd edn. Chapman & Hall, Bristol, UK: [Google Scholar]
- 25. Cox DR. (1972). Regression models and life tables. J. R. Stat. Soc. B., 34, 187–220 [Google Scholar]
- 26. Liu F., et al. (2011). p73 G4C14-A4T14 polymorphism and cancer risk: a meta-analysis based on 27 case-control studies. Mutagenesis, 26, 573–581 [DOI] [PubMed] [Google Scholar]
- 27. Sun T., et al. (2010). Single-nucleotide polymorphisms in p53 pathway and aggressiveness of prostate cancer in a Caucasian population. Clin. Cancer Res., 16, 5244–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L., et al. (2012). Combined analysis of the association between p73 G4C14-to-A4T14 polymorphisms and cancer risk. Mol. Biol. Rep., 39, 1731–1738 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y., et al. (2012). Genetic variants of the p53 and p73 genes jointly increase risk of second primary malignancies in patients after index squamous cell carcinoma of the head and neck. Cancer, 118, 485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber A., et al. (2002). INK4a-ARF alterations and p53 mutations in primary and consecutive squamous cell carcinoma of the head and neck. Virchows Arch., 441, 133–142 [DOI] [PubMed] [Google Scholar]
- 31. Xia L., et al. (2004). p53 activation in chronic radiation-treated breast cancer cells: regulation of MDM2/p14ARF. Cancer Res., 64, 221–228 [DOI] [PubMed] [Google Scholar]
- 32. Marine J.C. (2011). MDM2 and MDMX in cancer and development. Curr. Top. Dev. Biol., 94, 45–75 [DOI] [PubMed] [Google Scholar]
- 33. Kotnis A., et al. (2012). Multiple pathway-based genetic variations associated with tobacco related multiple primary neoplasms. PLoS ONE, 7, e30013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu X., et al. (2009). Novel susceptibility loci for second primary tumors/recurrence in head and neck cancer patients: large-scale evaluation of genetic variants. Cancer Prev. Res. (Phila.)., 2, 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris L.G., et al. (2011). Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control., 22, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris L.G., et al. (2011). Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol., 29, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almadori G., et al. (2004). Multistep laryngeal carcinogenesis helps our understanding of the field cancerisation phenomenon: a review. Eur. J. Cancer., 40, 2383–2388 [DOI] [PubMed] [Google Scholar]
- 38. Slaughter D.P., et al. (1953). Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer, 6, 963–968 [DOI] [PubMed] [Google Scholar]
- 39. Muto M., et al. (2002). Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis., 23, 1759–1765 [DOI] [PubMed] [Google Scholar]