Abstract
Background:
There is increasing evidence of significant and dynamic systemic activation and upregulation of complement in multiple sclerosis (MS), which may contribute to disease pathogenesis.
Objective:
We aimed to investigate the pathological role of complement in MS and the potential role for complement profiling as a biomarker of MS disease state.
Methods:
Key components of the classical, alternative and terminal pathways of complement were measured in plasma and cerebrospinal fluid (CSF) of patients with MS in different clinical phases of disease and in matched controls.
Results:
Increased plasma levels of C3 (p<0.003), C4 (p<0.001), C4a (p<0.001), C1 inhibitor (p<0.001), and factor H (p<0.001), and reduced levels of C9 (p<0.001) were observed in MS patients compared with controls. Combined profiling of these analytes produced a statistical model with a predictive value of 97% for MS and 73% for clinical relapse when combined with selected demographic data. CSF-plasma correlations suggested that source of synthesis of these components was both systemic and central.
Conclusion:
These data provide further evidence of alterations in both local and systemic expression and activation of complement in MS and suggest that complement profiling may be informative as a biomarker of MS disease, although further work is needed to determine its use in distinguishing MS from its differential.
Keywords: multiple sclerosis, demyelination, biomarkers, complement, immunology
Introduction
Multiple Sclerosis (MS) is a complex disease of the central nervous system (CNS) in which a diverse interplay of immunological factors contribute to a characteristically variable pathology, phenotypic presentation, disease course and prognosis.1,2 With the possible exception of magnetic resonance imaging (MRI), where use in prediction of progression is not established with any degree of certainty,3 there are currently no biomarkers for MS which reliably predict prognosis at disease onset or enable monitoring of disease progression. With the recent introduction of therapies which can significantly alter the natural history of MS, but which may have significant risks associated with their use,4-6 the importance of accurate diagnosis and prediction of disease severity and outcome at an early stage of disease is becoming increasingly apparent. Identification of effective, accessible biomarkers would have significant translational benefits and an immediate and important role in the clinical management and treatment of patients with MS.
The exact characterisation of the immunopathology of MS remains an emerging field, and recent advances have implicated innate immune mechanisms as playing an important role.7,8 In addition, although no complement genes were among the top hits in the recent genomewide association study in MS, multiple immunologically relevant genes, several of which interact with and influence propagation of the complement system (e.g. IL2 and its receptor; IL7 and its receptor) were identified. Complement, a key component of the innate immune system, has an established role in the pathogenesis of MS (for review see Ingram et al.9); however, despite evidence from pathological,10,11 functional12,13 and animal14 models, the extent and precise nature of complement activation in MS, and its contribution to disease phenotype and long-term outcome, remain unclear.
Recently, we have evaluated complement regulator factor H (fH)15 and an activation fragment of C4, C4a,16 in MS as potential biomarkers for MS disease course. Building on evidence from these initial studies, we set out to examine the role and extent of activation and regulation of complement in a comprehensively phenotyped population of patients with MS. Representative proteins from all complement activitation pathways (classical, alternative and terminal) were strategically chosen to provide a comprehensive picture of complement components, activation products and regulators. Plasma complement profiles were established for different disease courses and evaluated as predictive models of disease state.
Methods
Subjects
Plasma samples were obtained prospectively between 2006 and 2010, from 176 patients with clinically definite MS,17 including 37 patients with stable relapsing–remitting MS (S-RRMS) with no clinically evident relapse for at least 12 months, 68 with acute relapsing–remitting MS (A-RRMS) sampled in relapse, 34 with secondary progressive MS (SPMS), and 37 with primary progressive MS (PPMS). Assays were initially performed in a pilot study with 20 patients in each group. For components showing some variation between groups (including all components except Bb and clusterin), additional analysis was performed to increase subgroup size to between 30 and 40 patients, which from the pilot study gave a calculated power of 99% with confidence levels of 95%. Further analysis of 44 patients serially sampled in acute relapse and at intervals thereafter was performed on assays showing variation in acute relapse (including all components except Bb, C1 inhibitor (C1inh), clusterin and FI). The total number of patients analysed in each subgroup is shown in the results. Detailed clinical information was available on all patients including age, age at onset, disease duration and disability assessed by the Expanded Disability Status Scale (EDSS).18 No patients were on disease-modifying treatments. Of the 68 patients with A-RRMS, 44 remained relapse free over a 5–7-month period and underwent serial plasma sampling at intervals of between 2–3 months and 5–7 months post relapse. No patients seen in relapse had intercurrent infections; 71.8% were treated with oral or intravenous steroids after plasma samples had been collected, and the reminder were untreated. Relapses were classified according to detailed anatomical and clinical characteristics; 10 patients had experienced brainstem or cerebellar relapses, 40 pyrimidal or long tract motor relapses, four optic neuritis and six pure long tract sensory relapses. In patients who were serially sampled, mean EDSS at relapse was 4.72 (SD 1.64); this improved by a mean of 1.08 points (SD 1.07) at 2–3 months and by 1.09 points (SD 1.15) at 5–7 months following relapse. The control group comprised 35 non-related subjects with no personal or family history of neurological disease. Demographic details of patients and controls are displayed in Table 1. Patients selected for this study included the RRMS and SPMS patients and controls used in the replication phase of prior work, and therefore some of the fH data shown for these patients have been published previously.15
Table 1.
Demographic details of total multiple sclerosis population and controls.
| No. | Gender | Age |
DD |
EDSS |
MSSS |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % f | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Control | 35 | 60.00 | 50.57 | 19.54 | N/A | N/A | N/A | N/A | N/A | N/A |
| Total MS | 176 | 68.18 | 44.39 | 12.44 | 11.63 | 10.44 | 4.90 | 2.09 | 6.52 | 2.60 |
| S-RRMS | 37 | 83.78 | 41.76 | 9.65 | 8.27 | 9.71 | 2.59 | 1.41 | 4.41 | 2.65 |
| A-RRMS | 68 | 77.94 | 36.28 | 9.05 | 8.47 | 8.09 | 4.56 | 1.53 | 6.64 | 2.53 |
| SPMS | 34 | 50.00 | 54.03 | 9.07 | 22.88 | 10.31 | 6.97 | 1.21 | 7.29 | 2.06 |
| PPMS | 37 | 51.35 | 53.05 | 11.62 | 10.43 | 8.02 | 5.92 | 1.66 | 7.71 | 1.86 |
DD, disease duration; EDSS, Expanded Disability Status Scale; MSSS, MS Severity Score; f, female; SD, standard deviation; S-RRMS, stable relapsing–remitting MS with no relapses reported for at least 12 months; A-RRMS, acute relapsing–remitting MS sampled in relapse; SPMS, secondary progressive MS; PPMS, primary progressive MS.
Cerebrospinal fluid (CSF) was obtained with paired plasma samples from 66 patients who had been admitted for investigation of suspected or known MS. Of these, five had a clinically isolated syndrome (CIS) and 24 patients were subsequently found to have symptoms not related to demyelinating disease; these samples were used as controls. Subsequent neurological diagnosis within the control group included structural cervical myelopathy, cerebrovascular disease, trigeminal neuralgia, fibromyalgia, vestibular neuronitis and Sjogren’s syndrome. Of the 37 patients with MS, disease subgroups consisted of 28 patients with S-RRMS, five patients with A-RRMS in acute relapse and four patients with progressive disease.
Informed consent was obtained from all patients and ethical approval was gained from South East Wales Ethics committee (ref no.05/WSE03/112 and ref no. MRCE 04/9/025).
Samples
Plasma samples were separated (2000 g/10 mins) within 3 h of collection and stored in aliquots at -80°C until analysis. CSF was taken in line with published guidelines,19 atraumatically from vertebral body L3–5; bloody samples were discarded. Samples were centrifuged (2000 g/10 mins) to remove cells and debris within 30 min of collection before being aliquoted and frozen at −80°C until use.
Measurement of factor B (fB)
A sandwich enzyme-linked immunosorbent assay (ELISA) for quantification of fB in plasma, serum or CSF samples was developed in-house. Maxisorp (Nunc Life Technologies) plates were coated with in-house monoclonal mouse anti-human fB (JC1, 100 µl, 5 mg/l) overnight at 4°C, washed in 0.1% Tween 20 in PBS (washing buffer) and blocked with 200 µl 1% BSA/10 mM EDTA (blocking buffer) in PBS. After a single wash in washing buffer, 100 µl of standards, plasma (diluted 1:3000 in blocking buffer) or CSF (diluted 1:10 in blocking buffer) samples were added in duplicate and incubated. Wells were washed three times in washing buffer and incubated with 100 µl of HRP-labelled affinity-purified rabbit polyclonal anti-human fB (in-house; 1 mg/l). All incubations were stationary, 1 h, 37°C, unless stated otherwise. Wells were washed three times in washing buffer and bound antibody was visualised with orthophenylenediamine (SIGMAFAST™ OPD). Development was stopped by the addition of 10% sulphuric acid, and absorbance at 492 nm was measured. Purified human fB was used as a standard for estimation of plasma fB. Control standards were included on each plate. Concentration of fB in plasma or CSF was calculated by reference to the appropriate calibration curve prepared from the standards and expressed as mg/l of plasma or CSF. A nonlinear regression model was used to fit standard curves generated by ELISA. The calculated detection limit of the assay was 0.006 mg/l and the working range 0.01–0.2 mg/l. The assay performance was assessed by taking multiple measures from independently diluted aliquots of the same plasma samples, either within the same assay or in separate assays. The within-assay precision (coefficient of variation (CV) %), ranged from 6–8% with an average of 7%. Between-assay precision ranged from 5–11% with an average of 9%.
Measurement of C9
A sandwich ELISA for quantification of C9 in plasma, serum or CSF samples was developed in-house with methods as described above. Coating antibody for C9 detection was used at 1 mg/l, 100 μl/well (B7 monoclonal anti-C9, in-house). Plasma samples were diluted 1:2000 in blocking buffer and CSF was diluted 1:10 in blocking buffer. HRP-labelled monoclonal mouse anti-human C9 (MAC68, in-house) was added to wells for detection of C9 (100 μl; 1 mg/l). The assay was standardised using pure C9. The calculated detection limit was 0.003 mg/l with a working range of 0.010–0.16 mg/l. The within-assay precision (CV%) ranged from 0–4% with an average of 3% and between-assay precision ranged from 0–11% with an average of 6%.
Measurement of C1s
A sandwich ELISA for quantification of C1s in plasma, serum or CSF samples was developed in-house with methods as described above. Coating antibody for C1s (F33, in-house) was used at 5 mg/l, 100 μl/well. Plasma samples were diluted 1:2000 in blocking buffer and CSF was diluted 1:10 in blocking buffer for both assays. For detection of C1s, goat polyclonal antiserum to human C1s (A302, Quidel, www.quidel.com) was added to each well (100 μl; 1:2000), followed by HRP-labelled polyclonal rabbit anti-goat immunoglobulins (P0449, Dako UK Ltd, www.dako.com). The assay was standardised using pure C1s. The calculated detection limit of the assay was 0.003 mg/l with a working range of 0.015–0.25 mg/l. The within-assay precision (CV%) ranged from 0–5% with an average of 4% and between-assay precision ranged from 0–12% with an average of 6%.
Measurement of fH
The assay for the detection of fH has previously been described in detail.15,20 Of note, many papers quote higher levels of serum fH; the extinction coefficients for fH standards on which our normal range is based have been described previously.20 The calculated detection limit of the assay was 0.007 mg/l and the working range 0.01–0.2 mg/l. The within-assay precision ranged from 4–7% with an average of 5% and between-assay precision ranged from 5–10% with an average of 8%.
Measurement of factor I
A sandwich ELISA for quantification of fI in plasma, serum or CSF samples was developed in-house with methods as described above. Coating antibody for fI detection was used at 5 mg/l, 100 μl/well (MBI-1 monoclonal anti-fI, in-house). Plasma samples were diluted 1:100 in blocking buffer and CSF was diluted 1:5 in blocking buffer. Commercial polyclonal goat anti-human fI (A238, Complement Technology Inc, www.complementtech.com) was added to wells (100 μl at 1:1000 dilution), followed by HRP-labelled polyclonal rabbit anti-goat immunoglobulins (100 μl at 1:4000 dilution). The assay was standardised using pure fI. The calculated detection limit of the fI assay was 0.015 mg/l with a working range of 0.025–0.6 mg/l. The within-assay precision ranged from 6–8% with an average of 7% and between-assay precision was 10%.
Measurement of clusterin, TCC, Bb and C4a
Clusterin was quantified using a commercial assay from ALPCO diagnostics (44-CLUHU-E05, www.alpco.com). TCC was quantified using a commercial assay from Hycult Biotech, working range 8.2–2000 mAU (activating units)/ml (HK328, www.hycultbiotech.com). Activation product Bb was quantified using a commercial assay from Quidel (A027, www.quidel.com). Activation product C4a was quantified using a commercial assay from BD Biosciences (550947, www.bdbiosciences.com) as described elsewhere.16
Measurement of C3, C4, C1 inhibitor and C-reactive protein
C3, C4 and C1inh concentrations were measured by nephelometry on a Beckman BN11 nephlometer in the University Hospital of Wales Clinical Immunology laboratory using commercial standards. The assay working range for C3 was 0.02–4.1 g/l, for C4 was 0.01–1.9 g/l and for C1inh was 0.02–0.6 g/l. C-reactive protein (CRP) was measured by laser nephelometry in the University Hospital of Wales Clinical Immunology laboratory, with a lower detection limit of 0.04mg/l.
Routine CSF analysis
CSF and paired serum samples were analysed in the routine laboratory for IgG, albumin and oligoclonal bands (OCB). IgG and albumin were measured on the BN 11 nephlometer, and OCBs on the Sebia Hydrasys system (400-1705 Corporate Drive, Norcross, GA 30093; www.sebia-usa.com) using Hydrogel 9 CSF Iso-electric focussing gels. Reference ranges were: serum IgG 5.2–15.5 g/l, CSF IgG <58 mg/l, serum albumin 35–50 g/l, CSF albumin 160–360 mg/l, albumin/IgG index 0.3–0.8.
Statistical analyses
Data analysis was performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA) statistical package. All data were normally distributed. Quantitative concentrations were compared between disease subgroups using either Student’s t-test for 2-way analysis (MS versus controls), or analysis of variance (ANOVA) for multiple comparisons between subgroups. If the ANOVA showed a statistical difference between subgroups a post-hoc analysis with Bonferonni correction for multiple comparisons was performed. Variables showing statistical significant difference at t-test were included in a logisitic regression model and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Objective clinical characteristics such as age, gender and disease duration were added to the model and concordance, or the C statistic was calculated based on the area under Receiver operated characteristics (ROC) curve, to assess whether individual components or combined models contributed to predicting binary outcomes (a perfect score would be 1.0 or 100% predictability). Correlations were performed using Pearson’s correlation coefficient and then modelled using multivariate regression analysis to assess any dominant effect. Subgroup analysis of CSF was limited by number of samples and is therefore presented as median values and analysed using the non-parametric Mann–Whitney test to compare groups. Sample sizes for 2-way analysis of CSF, comparing subgroups with normal and raised IgG and albumin ratios, were larger and normally distributed and therefore are presented as means and compared using the Student’s t-test.
Results
Plasma complement levels predict MS compared with a control population
Compared with healthy controls, the total MS population analysed showed increased mean levels of plasma C3 and C4, reduced levels of plasma C9, and no difference in mean levels of plasma proteins C1s and fB (Table 2, Figure 1). There was a significant increase in plasma levels of activation product C4a; however, as shown previously, this elevation was seen only in the A-RRMS group.16 No changes were seen in activation product Bb, and levels of TCC were undetectable in the plasma with a lower assay detection limit of 8.2 mAU/ml (Table 3). Complement regulators C1inh and fH were significantly raised in patients with MS compared with controls, with no difference seen in plasma levels of clusterin or factor I (Table 4, Figure 1). A logistic regression model examining patients with MS and normal healthy controls was constructed using variables showing a significant difference on t-test and limited to analysis of C9 (OR 0.87; 95% CI 0.80–0.93, p<0.001), C3 (OR 1.00; 95%CI 1.00–1.01, p=0.035), C1inh (OR 1.03; 95% CI 1.01–1.05, p=0.002) and fH (OR 1.01; 95% CI 1.00–1.02, p=0.077). C4 (OR 1.01; 95% CI 0.99–1.02, p=0.520) was excluded from the model (the significance of change if removed p=0.518). A ROC curve was established based on the probabilities of the predicted values in the model and the C statistic based on this model was 0.97 (95% CI 0.93–1.00, p<0.001) and superior to any individual component (C9 0.14, 95% CI 0.07–0.22; C3 0.67, 95% CI 0.56–0.77; C1inh 0.83, 95% CI 0.76–0.90; fH 0.76, 95% CI 0.67–0.85) (Figure 2).
Table 2.
Complement components in multiple sclerosis disease subgroups and controls.
| No. | FB mg/l |
C3 mg/l |
C1s mg/l |
C4 mg/l |
C9 mg/l |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | Mean | SD | p | p* | ||
| Control | 35 | 194.02 | 68.28 | 1263.14 | 268.38 | N/A | 97.91 | 44.75 | 187.43 | 57.09 | N/A | 98.22 | 23.26 | N/A | <0.001 |
| Total MS | 176 | 183.98 | 53.52 | 1454.40 | 350.18 | 0.003 | 104.66 | 46.65 | 236.56 | 84.63 | 0.001 | 73.31 | 18.82 | <0.001 | N/A |
| S-RRMS | 37 | 171.97 | 35.25 | 1440.41 | 363.56 | 0.266 | 99.89 | 50.19 | 223.83 | 68.43 | 0.571 | 68.49 | 13.74 | <0.001 | 0.102 |
| A-RRMS | 68 | 199.10 | 69.27 | 1397.68 | 326.42 | 0.561 | 102.72 | 48.09 | 228.03 | 66.61 | 0.153 | 78.73 | 22.51 | <0.001 | N/A |
| SPMS | 34 | 173.31 | 33.74 | 1539.21 | 304.96 | 0.008 | 119.01 | 48.99 | 263.06 | 103.16 | 0.001 | 70.45 | 14.47 | <0.001 | 0.426 |
| PPMS | 37 | 177.99 | 44.54 | 1499.58 | 413.23 | 0.042 | 99.79 | 36.15 | 249.00 | 145.97 | 0.263 | 70.81 | 17.44 | <0.001 | 0.463 |
SD, standard deviation. p-values are measured using students t-test to compare MS and controls and ANOVA for multiple comparisons. p-values are shown for the subgroup analysis if a significant difference was seen at ANOVA. p = comparison with control group. p* = comparison with A-RRMS group. S-RRMS, stable relapsing–remitting MS with no relapses reported for at least 12 months; A-RRMS, acute relapsing–remitting MS sampled in relapse; SPMS, secondary progressive MS; PPMS, primary progressive MS.
Figure 1.
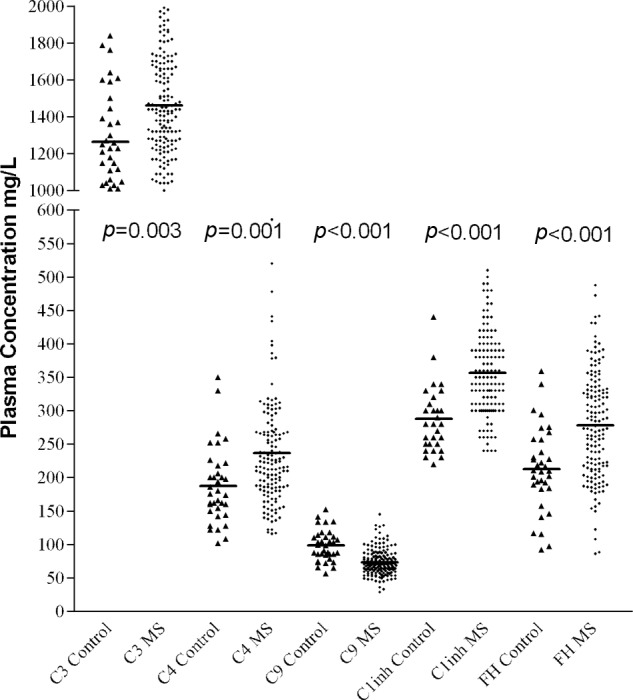
Plasma levels of C3, C4, fH and C1 inhibitor were increased and plasma levels of C9 were decreased in MS patients compared with normal controls.
Table 3.
Complement activation products in multiple sclerosis disease subgroups and controls.
| Bb mg/l |
C4a mg/l |
p | p* | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | |||
| Control | 35 | 1.04 | 0.21 | 35 | 819.65 | 570.94 | N/A | <0.001 |
| Total MS | 82 | 1.07 | 0.30 | 176 | 1477.27 | 941.15 | <0.001 | N/A |
| S-RRMS | 20 | 1.03 | 0.32 | 37 | 1180.66 | 784.56 | 1.000 | 0.011 |
| A-RRMS | 20 | 0.98 | 0.19 | 68 | 1692.26 | 1080.47 | <0.001 | N/A |
| SPMS | 22 | 1.14 | 0.31 | 34 | 1288.56 | 736.92 | 0.316 | 0.190 |
| PPMS | 20 | 1.31 | 0.42 | 37 | 1579.80 | 646.69 | 0.154 | 1.000 |
SD, standard deviation p-values are measured using students t-test to compare MS and controls and ANOVA for multiple comparisons. p-values are shown for the subgroup analysis if a significant difference was seen at ANOVA. p = comparison with control group. p* = comparison with A-RRMS group. S-RRMS, stable relapsing–remitting MS with no relapses reported for at least 12 months; A-RRMS, acute relapsing–remitting MS sampled in relapse; SPMS, secondary progressive MS; PPMS, primary progressive MS.
Table 4.
Complement regulators in multiple sclerosis disease subgroups and controls.
| C1 inhibitor mg/l |
p | p* | Clusterin mg/l |
Factor H mg/l |
p | p* | Factor I mg/l |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | |||||
| Control | 35 | 288.06 | 48.26 | N/A | <0.001 | 35 | 52.74 | 23.09 | 35 | 212.94 | 63.08 | N/A | 0.007 | 35 | 29.42 | 7.95 |
| Total MS | 140 | 356.79 | 68.48 | <0.001 | N/A | 82 | 57.69 | 21.01 | 176 | 278.05 | 79.08 | <0.001 | N/A | 140 | 29.97 | 6.86 |
| S-RRMS | 37 | 350.94 | 64.53 | 0.002 | 1.000 | 20 | 55.48 | 14.90 | 37 | 238.90 | 78.52 | 1.000 | 0.719 | 37 | 28.23 | 8.34 |
| A-RRMS | 32 | 368.44 | 87.55 | <0.001 | N/A | 20 | 67.22 | 23.09 | 68 | 267.04 | 60.17 | 0.007 | N/A | 32 | 29.30 | 6.08 |
| SPMS | 34 | 369.06 | 63.62 | <0.001 | 1.000 | 22 | 56.99 | 23.72 | 34 | 341.94 | 71.60 | <0.001 | <0.001 | 34 | 29.60 | 5.99 |
| PPMS | 37 | 340.29 | 53.60 | 0.013 | 0.777 | 20 | 51.13 | 18.91 | 37 | 274.11 | 77.26 | 0.003 | 1.000 | 37 | 32.66 | 6.21 |
SD, standard deviation. p-values are measured using students t-test to compare MS and controls and ANOVA for multiple comparisons. p-values are shown for the subgroup analysis if a significant difference was seen at ANOVA. p = comparison with control group. p* = comparison with A-RRMS group. S-RRMS, stable relapsing–remitting MS with no relapses reported for at least 12 months; A-RRMS, acute relapsing–remitting MS sampled in relapse; SPMS, secondary progressive MS; PPMS, primary progressive MS.
Figure 2.

Receiver operated characteristic (ROC) curve to predict the probability of MS compared to control subjects.
Combined model C statistic 0.97. For the individual components of the model; C3 C statistic 0.67, C9 C statistic 0.14, C1inh C statistic 0.83 and fH C statistic 0.76.
Dynamic changes in plasma complement levels in acute relapse
Analysis of disease course subgroups demonstrated elevated mean levels of complement components C9 (p=0.102) and fB (p=0.174) (Table 2), activation product C4a (p=0.011) (Table 3) and regulator fH (p=0.719) (Table 4) in A-RRMS compared with S-RRMS. A logistic regression model was developed to assess A-RRMS compared with S-RRMS; an initial model using fB, C9, C4a and fH along with objective clinical features age, gender and disease duration; revealed no contribution to the model from fB (the significance of change if fB was removed from the model = 0.446), gender (0.280), C4a (0.414) or fH (0.191). The optimal model used C9 (OR 1.04, 95% CI 1.01–1.07, p=0.023), age (OR 0.88, 95% CI 0.82–0.95, p=0.001) and disease duration (OR 1.12, 95% CI 1.03–1.22, p=0.009). The C statistic based on the area under the ROC curve for this model to predict A-RRMS from S-RRMS was 0.73, 95% CI 0.63–0.83 (Figure 3).
Figure 3.
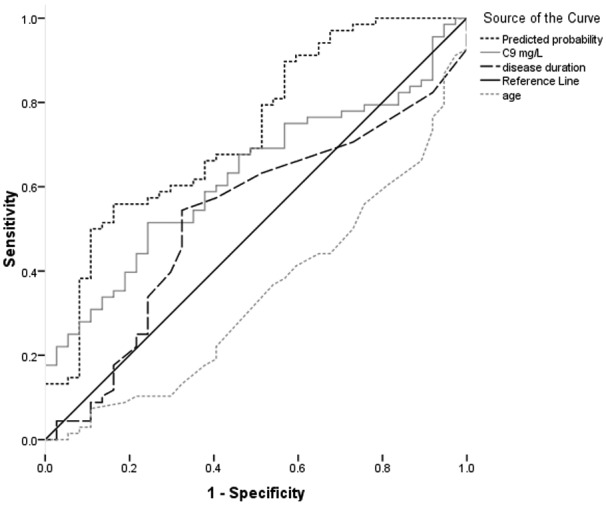
ROC curve to predict the probability of A-RRMS from S-RRMS.
Combined model C statistic 0.73. For the individual components of the model; C9 C statistic 0.63, disease duration C statistic 0.53 and age C statistic 0.34.
Where higher mean plasma analyte levels were seen in A-RRMS, an extended analysis was conducted with measurement of convalescent samples post relapse at 2–3 and 5–7 months. There were no differences in mean plasma levels between acute relapse and convalescent samples for either C9 or fB (Table 5). Mean plasma fH levels were significantly higher in A-RRMS compared with both 2–3 (p=0.013) and 5–7 (p=0.007) month convalescent samples (Table 5, Figure 4); levels were reduced in 26 of 44 subjects at 2 months and 30 of 44 subjects at 6 months, suggesting inter-individual variability in the convalescent response. Use of steroids post relapse did not alter the plasma complement concentrations (Supplemental Table 1). Changes in C4a post relapse have been reported previously.16
Table 5.
Serum factor H, factor B and C9 post relapse.
| Months post relapse | No. | Factor H mg/l |
Factor B mg/l |
C9 mg/l |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p | Mean | SD | p | Mean | SD | p | ||
| 0 | 44 | 205.72 | 63.80 | N/A | 247.50 | 86.18 | N/A | 96.95 | 31.47 | N/A |
| 2-3 | 44 | 178.81 | 42.93 | 0.013 | 270.69 | 70.05 | 0.158 | 99.79 | 29.54 | 0.678 |
| 5-7 | 44 | 176.39 | 39.28 | 0.007 | 243.62 | 71.52 | 0.813 | 97.88 | 32.14 | 0.891 |
FH, Factor H; FB, Factor B; SD, standard deviation. p = p-value for comparison with acute relapse (month 0).
Figure 4.

Plasma factor H levels post relapse.
A reduction in plasma factor H is seen post relapse in 26 of 44 patients at 2-3 months and 30 of 44 patients at 5-7 months. Mean levels are significantly reduced at both time points (p=0.013 at 2–3 months and p=0.007 at 5–7 months).
Analysis of phenotypic parameters showed no correlation of any measured components with gender, disease duration, EDSS, Multiple Sclerosis Severity Score or time to secondary progressive disease from onset. There was weak correlation of both plasma C9 (r=0.16) and C1s (r=0.12) with age; however, using age as a covariate in our previous analysis did not alter results (data not shown). There was no difference in levels of CRP between the total MS population (mean CRP 2.82, SD 3.98, n=82) and the control group (mean 2.76, SD 6.48, n=35), or between MS disease subgroups (S-RRMS mean 3.14, SD 4.37, n=20; A-RRMS mean 2.65, SD 3.82, n=20; SPMS mean 2.48, SD 3.87, n=22; PPMS mean 3.04, SD 4.11, n=20), demonstrating the lack of acute phase response at times of complement upregulation.
Changes in complement levels in the CSF
It was possible to measure complement components fB, C9, C1s, clusterin, fI and TCC in CSF using the assays described (levels of fH and C4a in CSF have been described previously);15,16demographic details of patients and controls are shown in Table 6. Levels of fB and C9 in both CSF and plasma were reduced in patients with MS and CIS compared with the control population; however, this only reached significance in plasma C9 levels (Table 7). C9 CSF/plasma ratio and C9 index were also non-significantly reduced in MS patients compared with controls (Table 7). Levels of CSF and plasma C1s, clusterin and TCC were increased in patients with MS and CIS compared with controls; this only reached significance for plasma clusterin in CIS cases; given the sample size, this finding may be the result of a type 1 error. There were no changes seen in CSF or plasma fI.
Table 6.
Demographic details and routine cerebrospinal fluid results.
| No. | Age |
Gender | Disease duration |
EDSS |
Albumin ratio |
Ig index |
OCB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | %f | Mean | SD | Mean | SD | Mean | SD | Mean | SD | % +ve | ||
| Control | 24 | 49.79 | 13.52 | 79.17 | N/A | N/A | N/A | N/A | 5.24 | 2.79 | 0.50 | 0.05 | 0.00 |
| MS | 37 | 38.81 | 10.76 | 75.68 | 7.11 | 8.57 | 2.55 | 2.10 | 5.33 | 1.93 | 1.00 | 0.48 | 91.89 |
| CIS | 5 | 40.00 | 9.35 | 100.00 | 0.80 | 0.45 | 2.00 | 1.58 | 6.14 | 2.31 | 0.79 | 0.44 | 40.00 |
EDSS, Expanded Disability Status Scale; Albumin ratio, CSF albumin mg/l / serum albumin g/l; Ig Index, derived from cerebrospinal fluid /plasma ratios of IgG and albumin concentrations; OCB, oligoclonal bands; SD, standard deviation; CIS, clinically isolated syndrome.
Table 7.
Cerebrospinal fluid complement in patients with MS, CIS and controls.
| CSF |
Plasma |
CSF:plasma ratio |
Calculated Index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Med | IQR | Med | IQR | Med | IQR | Med | IQR | ||
| FB | Control | 0.71 | 0.56–1.33 | 185.11 | 145.50–197.70 | 3.78 | 2.70–4.78 | 0.82 | 0.72–0.86 |
| MS | 0.74 | 0.47–0.10 | 179.74 | 147.97–218.12 | 3.92 | 3.00–5.74 | 0.79 | 0.58–1.00 | |
| CIS | 0.78 | 0.68–0.82 | 178.66 | 122.03–226.12 | 4.92 | 2.92–6.68 | 0.75 | 0.58–0.88 | |
| C9 | Control | 0.27 | 0.18–0.36 | 104.09 | 95.53–136.09 | 2.37 | 1.90–4.06 | 0.55 | 0.41–0.70 |
| MS | 0.23 | 0.12–0.31 | 92.39* | 80.96–103.01 | 2.25 | 1.80–2.94 | 0.45 | 0.36–0.61 | |
| CIS | 0.22 | 0.14–0.27 | 88.83* | 68.64–95.79 | 3.86 | 1.58–3.88 | 0.51 | 0.49–0.56 | |
| C1s | Control | 1.61 | 1.35–1.93 | 153.57 | 91.54–172.44 | 11.74 | 8.83–18.22 | 2.77 | 1.87–4.01 |
| MS | 1.62 | 1.35–2.47 | 126.23 | 111.28–142.78 | 12.63 | 9.09–16.31 | 2.53 | 1.77–3.77 | |
| CIS | 2.35 | 1.35–2.74 | 125.37 | 122.17–146.31 | 8.20 | 6.60–25.71 | 2.70 | 2.00–3.64 | |
| Clusterin | Control | 1.67 | 0.85–2.38 | 33.15 | 27.02–41.03 | 41.10 | 21.16–92.52 | 8.51 | 4.90–18.39 |
| MS | 1.73 | 1.14–2.32 | 40.71 | 29.55–53.51 | 41.05 | 21.36–82.07 | 8.69 | 4.33–16.21 | |
| CIS | 1.45 | 1.02–2.97 | 42.43** | 30.09–52.03 | 81.52 | 46.14–138.01 | 11.56 | 8.91–22.71 | |
| FI | Control | 0.14 | 0.12–0.18 | 17.89 | 12.44–22.54 | 7.88 | 5.37–9.62 | 2.19 | 1.28–2.47 |
| MS | 0.16 | 0.10–0.22 | 16.35 | 14.44–19.08 | 9.55 | 5.56–14.91 | 1.67 | 0.83–3.98 | |
| CIS | 0.26 | 0.15–0.35 | 15.50 | 13.07–20.87 | 17.24 | 3.36–19.29 | 2.21 | 0.49–4.44 | |
| TCC | Control | 4.26 | 0.00–13.55 | N/A | N/A | N/A | N/A | N/A | N/A |
| MS | 5.92 | 1.05–21.51 | N/A | N/A | N/A | N/A | N/A | N/A | |
| CIS | 2.99 | 0.00–74.31 | N/A | N/A | N/A | N/A | N/A | N/A | |
Cerebrospinal fluid (CSF) and plasma values shown in mg/l for factor B (FB), C9, C1s, clusterin and factor I (FI) and mAU/ml for TCC. CSF:plasma ratio, CSF mg/l / Serum g/l; Calculated index, calculated by substituting the specified complement protein for IgG in the Ig Index; Med, median; IQR, interquartiler range; CIS, clinically isolated syndrome. * p=0.01, ** p=0.03.
In order to examine the relationship between levels of complement proteins in CSF and blood–CSF barrier (BCB) integrity, correlation of CSF:plasma complement concentrations with the CSF:serum albumin ratio was assessed21,22 (Table 8). Both fB (r=0.38; p=0.004) and C9 (r=0.65; p<0.001) correlated with CSF:serum albumin ratio, suggesting that intrathecal consumption was partially compensated by leakage across the BCB. CSF C1s (r=0.35; p=0.011) and clusterin (r=0.36; p=0.007) also correlated with CSF:serum albumin ratio, demonstrating effects of BCB leak; indeed, levels of C9, C1s and clusterin were all significantly higher in patients with elevated CSF:serum albumin ratios compared with those with values in the normal range (Table 8). The relationship of CSF complement protein levels with IgG index, a measure of intrathecal IgG synthesis and a suggested measure of disease activity,23 was also tested. Levels of fB (r=0.41; p=0.001) and C9 (r=0.46; p<0.001) showed moderate correlation but none of the other parameters did; levels of fB and C9 were significantly higher in patients with elevated IgG index compared with those with values in the normal range (Table 8), implying a relationship with disease activity. Levels of CSF fI and TCC showed no correlation with either CSF:serum albumin ratio or IgG index.
Table 8.
Correlation of cerebrospinal fluid:plasma complement quotient with IgG index and albumin quotient.
| Correlation |
Normal |
Raised |
||||||
|---|---|---|---|---|---|---|---|---|
| r | p | Mean CSF level | SD | Mean CSF level | SD | p | ||
| FB | IgG Index | 0.41 | 0.001 | 0.74 | 0.41 | 0.92 | 0.33 | 0.056 |
| Albumin ratio | 0.38 | 0.004 | 0.79 | 0.39 | 0.96 | 0.31 | 0.173 | |
| C9 | IgG Index | 0.46 | <0.001 | 0.21 | 0.11 | 0.31 | 0.18 | 0.010 |
| Albumin ratio | 0.65 | <0.001 | 0.23 | 0.11 | 0.42 | 0.22 | 0.013 | |
| C1s | IgG Index | 0.11 | 0.423 | 1.69 | 0.59 | 1.82 | 0.75 | 0.468 |
| Albumin ratio | 0.35 | 0.011 | 1.67 | 0.66 | 2.16 | 0.57 | 0.024 | |
| Clusterin | IgG Index | 0.22 | 0.110 | 1.57 | 0.95 | 1.86 | 0.75 | 0.175 |
| Albumin ratio | 0.36 | 0.007 | 1.58 | 0.83 | 2.28 | 0.82 | 0.011 | |
| Factor I | IgG Index | 0.02 | 0.860 | 0.16 | 0.11 | 0.19 | 0.12 | 0.375 |
| Albumin ratio | 0.03 | 0.850 | 0.17 | 0.11 | 0.19 | 0.13 | 0.674 | |
| TCC | IgG Index | 0.19 | 0.210 | 10.61 | 13.22 | 14.95 | 19.85 | 0.385 |
| Albumin ratio | –0.11 | 0.482 | 12.96 | 14.13 | 10.60 | 25.81 | 0.717 | |
Correlations are shown between cerebrospinal fluid (CSF):plasma complement ratio and either the IgG index or CSF:serum albumin ratio (expect for TCC where plasma levels were not detectable and correlation is shown with CSF TCC). Normal and raised refer IgG index or CSF:serum albumin ratio in reference to their normal laboratory values; a raised value indicates either intrathecal IgG synthesis (raised IgG index) or breakdown of the blood–CSF barrier (raised CSF:serum albumin ratio). The p-value refers to the difference between mean CSF complement levels when the IgG index or CSF:serum albumin ratio is normal or raised. FB, factor B; FI, factor I; TCC, terminal complement complex.
Examination of disease characteristics showed weak to moderate correlation of CSF fB, C9, C1s and clusterin with age (fB r= 0.23, p=0.068; C9 r=0.39, p=0.002; C1s r=0.23, p=0.079; clusterin r=0.38, p=0.002); however, no correlation was seen with disease duration. CSF C9 showed a weak correlation with disability measured by EDSS (r=0.33, p=0.03).
Discussion
Although MS is a predominantly T-cell mediated disease, it is well established that complement plays an important role. We have previously demonstrated both systemic and local concentration changes for complement regulator fH15 and generation of the activation fragment, C4a.16 Here, analysis of local and systemic complement components and activation fragments in MS patients is extended with the aim of clarifying the contribution of complement to disease processes, and combined complement profiles and phenotype models are examined to identify biomarkers of disease and disease course.
Given the complex and variable immunopathogenesis of MS, the use of combinations of plasma analytes to develop informative biomarkers of disease and disease course is likely to be more instructive than using isolated measurement. The model developed here for distinguishing MS from controls used a combination of plasma complement components C3, C4, C9 and regulators fH and C1inh to give a predictive probability of 97%. The use of this model in the clinical setting is limited in its current form in terms of distinguishing MS from other confounding diagnoses or CIS; however, analysis using additional and larger patient subgroups and clinically relevant control groups may clarify this. Additional complement profiles to distinguish disease subgroups were not established. No measured component other than fH was specific for disease progression, and although C9, C4a and fH all showed differential changes in relapse, when combined, they did not produce a reliable predictive model. This may have been because of innacuracies in the clinical classification of relapse and progression, or it may be that other complement proteins or inflammatory markers would be more useful in establishing biomarker profiles. However, the addition of complement measurements to objective clinical characteristics, disease duration and age, to distinguish disease state was of value, with a combined model predictive probability for relapse of 73%. We have therefore established the potential use of complement profiles as plasma biomarkers for disease, although there is a need for this work to be replicated by independent groups to confirm utility of these models in clinical practice, which would be best achieved in an independent prospective cohort with parallel MR data.
Systemic and local consumption of complement components was demonstrated throughout MS disease course, with reduced levels of both plasma fB and plasma and CSF C9 in the total MS population and increased systemic production in acute relapse. This was associated with raised plasma C3 and C4 levels in MS in the absence of any other evidence of an acute phase reponse, demonstrated by normal levels of CRP. We have previously demonstrated raised levels of C4a, an activation fragment of C4, isolated to acute relapse, and raised CSF C4a in the total MS population examined.16 These results imply continuous activation of complement with dynamic effects that are both local and systemic. The observed changes in complement regulators fH and C1inh are in concordance with this suggestion.
Two previous studies found no significant change in plasma C9 in MS patients;12,24 however, these studies were limited in terms of patient numbers and underpowered to detect change. Local reduction of C9 levels shown here is consistent with previous studies;12,24 however, given the nature of our CSF control group, containing patients with other neurological and inflammatory conditions, there may have been more complement activation in the controls than would be seen in normal healthy controls, limiting the difference in C9 observed. Interestingly, CSF C9 in our study did show some degree of correlation with disability, previously only shown in CSF TCC measurements in a single study.13 Further work with extended analysis of CSF C9 and/or TCC in MS disease subgroups and patients with CIS may be useful in determining whether these changes in terminal pathway components and activation products correlating with disability could be clinically informative.
In vitro studies have established not only that CNS cells produce all complement components necessary for complement lysis,25–27 but also that complement is a tissue-damaging factor in demyelination and neurodegeneration.28–30 However, it is still unclear how this relates to in vivo pathology, and whether complement proteins seen in MS white matter plaques are locally produced or are a result of BCB breakdown. This study demonstrates that the majority of complement proteins, including C9, C1s, fB, fH and clusterin, leak into the CSF at times of BCB breakdown, as well as suggesting local biosynthesis of selected components including fB and C9. Evidence from this study therefore suggests that both locally and systemically synthesised complement contributes to the disease process in MS. Given that the vast majority of complement synthesis is hepatic, it is possible that systemically targeted anti-complement therapies may significantly reduce the extent of local complement attack within the CNS and be of use in patients with MS in addition to more traditional adaptive immune-targeted therapies.
Footnotes
Funding: This work was supported by the Multiple Sclerosis Society (UK) [grant number 884/08].
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 2007; 8: 913–919 [DOI] [PubMed] [Google Scholar]
- 2. Hirst C, Ingram G, Swingler R, Compston DA, Pickersgill T, Robertson NP. Change in disability in patients with multiple sclerosis: a 20-year prospective population-based analysis. J Neurol Neurosurg Psychiatry 2008; 79: 1137–1143 [DOI] [PubMed] [Google Scholar]
- 3. Furby J, Hayton T, Altmann D, et al. A longitudinal study of MRI-detected atrophy in secondary progressive multiple sclerosis. J Neurol 2010; 257: 1508–1516 [DOI] [PubMed] [Google Scholar]
- 4. Hutchinson M. Natalizumab: A new treatment for relapsing–remitting multiple sclerosis. Ther Clin Risk Manag 2007; 3: 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirst CL, Pace A, Pickersgill TP, et al. Campath 1-H treatment in patients with aggressive relapsing remitting multiple sclerosis. J Neurol 2008; 255: 231–238 [DOI] [PubMed] [Google Scholar]
- 6. Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. Interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801 [DOI] [PubMed] [Google Scholar]
- 7. Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol 2008; 7: 852–858 [DOI] [PubMed] [Google Scholar]
- 8. Racke MK. The role of B cells in multiple sclerosis: rationale for B-cell-targeted therapies. Curr Opin Neurol 2008; 21 Suppl 1: S9–S18 [DOI] [PubMed] [Google Scholar]
- 9. Ingram G, Hakobyan S, Robertson NP, Morgan BP. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin Exp Immunol 2009; 155: 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol 1996; 6: 259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumsden CE. The immunogenesis of the multiple sclerosis plaque. Brain Res 1971; 28: 365–390 [DOI] [PubMed] [Google Scholar]
- 12. Compston DA, Morgan BP, Oleesky D, Fifield R, Campbell AK. Cerebrospinal fluid C9 in demyelinating disease. Neurology 1986; 36: 1503–1506 [DOI] [PubMed] [Google Scholar]
- 13. Sellebjerg F, Jaliashvili I, Christiansen M, Garred P. Intrathecal activation of the complement system and disability in multiple sclerosis. J Neurol Sci 1998; 157: 168–174 [DOI] [PubMed] [Google Scholar]
- 14. Barnum SR, Szalai AJ. Complement and demyelinating disease: no MAC needed? Brain Res Rev 2006; 52: 58–68 [DOI] [PubMed] [Google Scholar]
- 15. Ingram G, Hakobyan S, Hirst CL, et al. Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain 2010; 133: 1602–1611 [DOI] [PubMed] [Google Scholar]
- 16. Ingram G, Hakobyan S, Robertson NP, Morgan BP. Elevated plasma C4a levels in multiple sclerosis correlate with disease activity. J Neuroimmunol 2010; 223: 124–127 [DOI] [PubMed] [Google Scholar]
- 17. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127 [DOI] [PubMed] [Google Scholar]
- 18. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983; 33(11): 1444–1452 [DOI] [PubMed] [Google Scholar]
- 19. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009; 73: 1914–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hakobyan S, Harris CL, Tortajada A, et al. Measurement of factor H variants in plasma using variant-specific monoclonal antibodies: application to assessing risk of age-related macular degeneration. Invest Ophthalmol Vis Sci 2008; 49: 1983–1990 [DOI] [PubMed] [Google Scholar]
- 21. Eeg-Olofsson O, Link H, Wigertz A. Concentrations of CSF proteins as a measure of blood brain barrier function and synthesis of IgG within the CNS in ‘normal’ subjects from the age of 6 months to 30 years. Acta Paediatr Scand 1981; 70: 167–170 [DOI] [PubMed] [Google Scholar]
- 22. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. II. Relation of the concentration of the proteins in serum and cerebrospinal fluid. Scand J Clin Lab Invest 1977; 37: 391–396 [DOI] [PubMed] [Google Scholar]
- 23. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest 1977; 37: 397–401 [DOI] [PubMed] [Google Scholar]
- 24. Morgan BP, Campbell AK, Compston DA. Terminal component of complement (C9) in cerebrospinal fluid of patients with multiple sclerosis. Lancet 1984; 2: 251–254 [DOI] [PubMed] [Google Scholar]
- 25. Gasque P, Julen N, Ischenko AM, et al. Expression of complement components of the alternative pathway by glioma cell lines. J Immunol 1992; 149: 1381–1387 [PubMed] [Google Scholar]
- 26. Gasque P, Ischenko A, Legoedec J, Mauger C, Schouft MT, Fontaine M. Expression of the complement classical pathway by human glioma in culture. A model for complement expression by nerve cells. J Biol Chem 1993; 268: 25068–25074 [PubMed] [Google Scholar]
- 27. Gasque P, Fontaine M, Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol 1995; 154: 4726–4733 [PubMed] [Google Scholar]
- 28. Piddlesden SJ, Morgan BP. Killing of rat glial cells by complement: deficiency of the rat analogue of CD59 is the cause of oligodendrocyte susceptibility to lysis. J Neuroimmunol 1993; 48: 169–175 [DOI] [PubMed] [Google Scholar]
- 29. Wren DR, Noble M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in absence of antibody. Proc Natl Acad Sci U S A 1989; 86: 9025–9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scolding NJ, Morgan BP, Houston WA, Linington C, Campbell AK, Compston DA. Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature 1989; 339: 620–622 [DOI] [PubMed] [Google Scholar]


