Abstract
Quantitative sensory testing (QST) has become commonly used for the assessment of pain in subjects with clinical conditions. However, there is no consensus about which type of QST is the best predictor of clinical pain responses.
The purposes of this study were to determine a) the QST measure with strongest association with clinical pain intensity, and b) if the QST measure continued to predict clinical pain intensity in a model including relevant psychological factors.
Fifty-nine patients seeking treatment for shoulder pain underwent experimental pain assessment involving heat and pressure stimuli. The patients also completed validated questionnaires for pain intensity, pain catastrophizing, anxiety, and depression. The 5th pain rating in a series of suprathreshold heat pain stimuli accounted for a significant amount of variance in clinical pain intensity, with no other QST measure contributing to the model. The 5th pain rating remained a significant contributor to clinical pain intensity when psychological factors were included in the model. Furthermore, subjects with elevated 5th pain rating, pain catastrophizing, and depression scores had higher clinical pain intensity ratings in pre and post operative assessments. These data suggest that assessment of pain should include suprathreshold heat stimuli and psychological factors separately, and a combination of these factors may be predictive of pain intensity outcomes.
Keywords: Suprathreshold heat assessment, temporal summation, shoulder pain, pain catastrophizing, depression
Introduction
Quantitative sensory testing (QST) has become increasingly applied to the assessment of pain in subjects with clinical conditions; however, the stimulus modalities applied and perceptual responses assessed vary widely across studies. The most commonly used approaches include measures of pain threshold and tolerance, which provide simple, unidimensional assessments of pain perception. Temporal Summation (TS) of suprathreshold heat pain stimuli, a more dynamic form of QST, occurs when repetitive input over C-fibers induces enhanced responses in dorsal horn (DH) neurons. TS results in the perception of increased pain despite constant or even reduced peripheral afferent input29 and is thus considered a perceptual manifestation of enhanced central excitability. TS has been linked to the development and maintenance of chronic pain 1, 6, 30, and is commonly used for the study of hyperalgesic mechanisms in chronic pain conditions such as fibromyalgia 33, 35, 36 and temporomandibular disorders. 22, 27However, assessment of responses to repetitive suprathreshold heat pain stimuli differs from other methods of QST in that responses to the repeated stimuli yield multiple possible methods of calculating pain sensitivity indices.
Abundant evidence indicates that psychological factors influence the perception of pain and exert significant influence on the development and maintenance of chronic conditions. 17, 21, 39 Moreover, psychological factors modulate pain sensitivity 26, but few investigations have considered the combined influences of psychological factors and experimental pain sensitivity on clinical pain intensity. Therefore, the present study examined the ability of different measures of QST, including previous used methods to calculate suprathreshold heat pain stimuli, to predict clinical pain intensity ratings for subjects with shoulder pain. The present study also determined if the QST measure continued to predict clinical pain intensity after considering relevant psychological factors.
Prior studies show that TS was altered among patients with chronic pain syndromes. 1, 6, 22, 27, 30, 33-35 In addition, measures derived from a dynamic QST (suprathreshold heat pain stimuli) approach are thought to better capture the pain modulatory ability of the central nervous system in comparison with static measures from pressure or heat stimuli (threshold, tolerance). 2 However, there appears to be no consensus in the literature for which QST measure is best associated with clinical pain intensity. Therefore we hypothesized that for patients with shoulder pain: 1) inter-correlations among QST measures would be low to moderate, indicating different experimental pain sensitivity components; 2) measures derived from dynamic QST protocols would have the highest association with shoulder pain intensity in comparison to static measures; and 3) the identified QST measure would continue to be associated with shoulder pain intensity in a multivariate model that included relevant pain-related psychological factors. If these hypotheses are confirmed in a cohort of individuals with shoulder pain it would add further evidence to support QST responses as potential markers of chronic pain.
Materials and Methods
Subjects
This analysis includes data from a prospective cohort of consecutive patients seeking treatment of shoulder disorder, which were recruited from University of Florida’s Orthopaedics Sports Medicine Institute (OSMI). 17 The cohort study was approved by the University of Florida Institutional Review Board, and all subjects enrolled in the study provided informed consent before study participation. The inclusion criteria for being a participant were: (a) between 18 and 85 years of age, (b) complaints of pain limited to anterior, lateral, or posterior shoulder, (c) documented or suspected rotator cuff tendinopathy (evidence from clinical examination or imaging studies) including small (<1 cm), medium (1-3 cm), and large (3-5 cm) tears, (d) documented or suspected adhesive capsulitis (evidence from clinical examination or imaging studies), (e) documented or suspected SLAP (Superior Labrum from Anterior to Posterior) lesion (evidence from clinical examination or imaging studies), and (f) scheduled for arthroscopic surgery. Exclusion criteria were: (a) current complaints of pain greater than the past 3 months involving neck, elbow, hand, low back, hip, knee, or ankle, (b) massive or complete rotator cuff tear (>5 cm), (c) documented shoulder OA or RA, (d) prior shoulder surgery within the past year or currently complaining of pain from prior shoulder surgery, (e) current shoulder fracture, tumor, or infection, (f) previously diagnosed chronic pain disorder (including, but not limited to IBS, fibromyalgia, TMD, CLBP, etc), (g) current psychiatric management, and (h) current gastrointestinal or renal illness.
Measures
All patients completed a standard form to collect demographic and clinical data.
Pain assessments
Clinical pain intensity
Shoulder pain intensity ratings were assessed with the Brief Pain Inventory (BPI)5, which includes a numerical rating scale (NRS) from 0 to 10 for pain intensity. Subjects rate their pain intensity over three conditions, the present pain intensity, the worst pain intensity over the past 24 hours, and the best pain intensity over the past 24 hours. Only the present pain intensity was used for the analyses in this study because it reflected the pain intensity experienced by the subject at the time QST was administered.
Experimental pain sensitivity
Suprathreshold heat pain stimuli
Suprathreshold heat pain stimuli were applied to the thenar eminence of the involved (surgery side) and uninvolved hand with a thermode of 2.5 cm2 surface area by a Medoc Neurosensory Analyzer (Ramat Yishai, Israel). Sequences of 10 consecutive heat pulses of < 1 s duration at interpulse intervals of 0.33 Hz were delivered. 9, 13, 24 Subjects verbally rated the intensity of each heat pulse on a numerical rating scale from 0 = “no pain” to 100 = “ the worst pain imaginable”.13 The procedure was performed two times, the first one using 47°C and the second one using 49°C as thermal intensities. The temperature for the heat pulses rapidly fluctuated (10°C/s) from 41°C as a baseline temperature for interstimulus intervals, to a peak of 47°C the first trial and from 41°C to a peak of 49°C in the second trial.
Traditional indices derived from suprathreshold heat pain stimuli assessment reported in the literature include the use of mean of pain ratings 11, 38, the first pain rating 30 the final pain rating 12, 19, 20, 32, highest pain rating minus the first pain rating 10, and maximal pain rating 29; while others have not reported a specific method of calculation. For this study the most commonly utilized indices from previous studies were computed. All our measures involved data related to the first 5 pulses of suprathreshold heat pain stimuli assessment. Thus, “TS” was obtained by subtracting the first pain rating from the last pain rating as this reflected the slope or the amount of temporal summation obtained. 10 The “mean pain ratings” was the sum of ratings divided by five as this index reflected a baseline stage of hyperalgesia. 11, 38 The “5th pain rating” was the fifth pain rating from the fifth pulse of each trial 12, 19, 20, 32, which is considered to represent a simple measure of suprathreshold heat pain stimuli assessment. 23 The “first pain rating” of each trial was also considered in the analysis as a competing measure of QST 30, because it is thought to be mediated primarily by A-delta fiber input. 25
Heat pain threshold and tolerance
Subjects received a continuously ascending heat stimulus on their involved (surgery side) and uninvolved arms. The stimulus started at 35°C and increased at a rate of 0.5°C/second. Subjects were asked to press a button and then rate their pain with a 0 (no pain) −100 (worst pain imaginable) NRS at the first sensation of pain. Two different trials were performed, and the average of the two temperatures was calculated as the heat pain threshold. In a separate trial subjects were asked to indicate when the heat became so painful that they wished it to stop. Two separate tolerance trials were performed and the average temperature was recorded as heat pain tolerance. Because pain threshold and pain tolerance were highly correlated for these subjects (r = 0.85, p<0.01), only pain tolerance was used in subsequent analyses.
Pressure pain threshold
Pressure pain threshold (PPT) for the involved (surgery side) and uninvolved arm was assessed using a Fischer pressure algometer with a 1 cm diameter probe (Pain Diagnostics and Thermography Inc, Great Neck, NY). The algometer was placed vertically on two different anatomical locations, the acromion of the shoulder (PPacromion) same anatomical area as the clinical pain, and the masseter muscle (PPmasseter) distant anatomical area from the source of clinical pain.
PPT was defined as the amount of force (applied at a rate of 1kg per second) required to produced a sensation of pain distinct from pressure or discomfort. 14 Subjects were asked to say “pain” immediately when a sensation of pain, was felt. At this point, the experimenter immediately retracted the algometer and the value of pressure applied was recorded (Kg). Two PPT measures were performed on each anatomical location (acromion and masseter), and the average was computed.
Psychological measures
Anxiety
Trait anxiety was assessed with the State-Trait Anxiety Index (STAI). 28 Only the 20 item trait portion of the STAI was used for purposes of our study, and the subjects were asked to rate their feelings about each statement on a 4-point scale giving the scale a total range of 20 – 80.
Pain Catastrophizing
Pain catastrophizing was measured by the Pain Catastrophizing Scale (PCS). 37 PCS consists in 13 descriptions of pain experience in the following form, for example: “I feel I can’t go on”, “There’s nothing I can do to reduce the intensity of the pain”. Respondents were asked to indicate whether they agreed with these statements by using a 5-point rating scale (0, 1, 2, 3, 4). A PCS sum score was calculated from all items (range, 0 – 52), with a high score indicating a high level of pain catastrophizing.
Depression
Self-report of depressive symptoms was measured using the Beck Depression Inventory (BDI). 4 The BDI consists of 21 groups of items, which are rated from 0 to 3 in terms of intensity, that assess both the cognitive/affective and neurovegetative symptoms of depression. Subjects are asked to circle the statement in each item group that best describes how they have been feeling in the past week, including today. The ratings are summed to calculate total depression scores, which can range from 0 to 63.
Data analysis
Data analysis was performed in SPSS, Version 17.0 at alpha level of 0.05. Descriptive statistics (mean, standard deviation) were calculated for all variables. The distributions of variables were tested for normality by visual examination and with Kolmogorov-Smirnof test before used in analysis. For analysis purposes measurements from both arms were averaged into one score, because paired t-test shows nonsignificant differences (p > 0.05) between measures in the right side versus left side, and between involved versus uninvolved side.
Correlations among QST and clinical pain intensity
Pearson correlations were calculated between clinical pain intensity, experimental pain sensitivity (TS, mean pain ratings, 5th pain rating, first pain rating, tolerance, PPacromion, and PPmasseter), and psychological measures (PCS, STAI, BDI).
Contributions of QST to clinical pain intensity
Multiple regression models were conducted to assess which QST measures accounted for variance in clinical pain intensity. Each regression model included age and sex in the first step to control for these potentially confounding factors. QST measures (TS, mean pain ratings, 5th pain rating, first pain rating, tolerance, PPacromion, PPmasseter) were then considered in a stepwise manner in the second step of the regression model. Stepwise regression was used because we wanted to create a parsimonious model consisting of QST measures with the strongest association with clinical pain intensity. Different regression models were conducted for each suprathreshold heat pain stimuli measure (TS, mean pain ratings, 5th pain rating). Variance inflation factor (VIF) was also reported for the final model to investigate multicollinearity.
Contributions of QST and psychological factors to clinical pain intensity
After determining which QST measure was associated with clinical pain intensity an additional hierarchical regression model was conducted. This model determined whether psychological measures accounted for variance in clinical pain intensity. Age, sex, and the appropriate QST measure from the previous analyses were entered in the first step. Then, psychological variables (PCS, STAI, BDI) were entered in the second step in a hierarchical manner. VIF was reported for the final model to investigate multicollinearity among the independent variables.
Results
Sample characteristics
A total of 59 patients were included the study. Descriptive statistics for the demographic, clinical and psychological measures from the sample are summarized in Table I.
Table I.
Descriptive data
| Variable | Value | % or SD |
|---|---|---|
| Age | 50.39 | 14.92 |
| Sex - (Male) | 35 | 59.3% |
| (Female) | 24 | 40.7% |
| Race – (Caucasian) | 51 | 89.5% |
| (African American) | 4 | 7.0% |
| (Not specified) | 2 | 3.5% |
| No medication | 29 | 49.2% |
| Pain medication | 27 | 45.8% |
| Involved arm - (Right) | 21 | 35.6% |
| (left) | 38 | 64.4% |
| Pre-operative clinical pain (BPI) | 3.92 | 2.50 |
| STAI | 36.60 | 11.84 |
| PCS | 13.44 | 9.55 |
| BDI | 7.14 | 5.63 |
| Pain tolerance (°C) | 48.03 | 2.22 |
| Pain threshold (°C) | 44.34 | 2.94 |
| Mean pain ratings at 47°C | 41.13 | 26.59 |
| Mean pain ratings at 49°C | 43.84 | 27.59 |
| TS at 47°C | −1.5 | 20.95 |
| TS at 49°C | 1.7 | 12.68 |
| First pain rating at 47°C | 41.77 | 28.09 |
| First pain rating at 49°C | 43.19 | 28.65 |
| Fifth pain rating at 47 °C | 38.78 | 29.11 |
| Fifth pain rating at 49°C | 43.74 | 28.27 |
| Pressure Pain Threshold (Kg)– (PPacromion) | 3 .36 | 1.68 |
| (PPmasseter) | 1.68 | 0.69 |
| Completed post-operative follow-up | 48 | 81.4% |
| Post-operative clinical pain (BPI) | 1.95 | 2.20 |
| No post-operative medication | 31 | 52.5% |
| Post-operative medication | 16 | 27.1% |
PCS: Pain Catastrophizing Scale; STAI: State Trait Anxiety Inventory (trait portion of STAI was used); BPI: Brief Pain Inventory; BDI: Beck Depression Inventory
Correlations among QST and clinical pain intensity
Pearson correlations among QST measures and clinical pain intensity are summarized in Table II. Overall, TS, reflected by the increase in pain ratings across the five trials, had no significant association with clinical pain intensity, had a moderate negative association with tolerance, PPmasseter, and first pain rating. The mean pain ratings had a strong positive association with the first pain rating and the 5th pain rating. In addition, mean pain ratings had a negative moderate to strong association with tolerance and PPacromion, and only the mean pain ratings at 47°C had a positive moderate association with clinical pain intensity. The 5th pain rating had a positive strong association with first pain rating and a positive moderate association with clinical pain intensity. A negative strong to moderate association was found between 5th pain rating and tolerance, PPacromion, and PPmasseter. Because 5th pain rating and the mean pain ratings were highly correlated (r = 0.96), and because the 5th pain rating at 47°C had the highest association with clinical pain intensity (r = 0.46) (Figure 1), only the 5th pain rating at 47°C was considered for further analyses.
Table II.
Pearson correlations among QST variables at 47°C and 49°C and clinical pain intensity
| TS (47°C 49°C) |
First pain rating (47°C 49°C) |
Fifth pain rating (47°C 49°C) |
Tolerance | PPacromion | PPmasseter | C.Pain | |
|---|---|---|---|---|---|---|---|
| Mean at 47°C | 0.10 | 0.94** | 0.96** | −0.55** | −0.34* | −0.27 | 0.44** |
| Mean at 49°C | −0.14 | 0.97** | 0.97** | −0.49** | −0.38* | −0.34* | 0.27 |
| TS at 47°C | −0.29* | 0.44** | −0.33* | −0.12 | −0.34* | 0.25 | |
| TS at 49°C | −0.18 | 0.27 | −0.43** | −0.22 | −0.31* | 0.22 | |
| First pain rating 47°C | 0.73** | −0.37** | −0.26 | −0.14 | 0.36** | ||
| First pain rating 49°C | 0.75** | −0.44** | −0.33* | −0.25 | 0.30* | ||
| Fifth pain rating 47°C | −0.56** | −0.30* | −0.33* | 0.46** | |||
| Fifth pain rating 49°C | −0.59** | −0.40** | −0.39** | 0.34* | |||
| Tolerance | 0.54** | 0.43** | −0.38** | ||||
| PPacromion | 0.64** | −0.35** | |||||
| PPmasseter | −0.28* |
Correlation is significant at the .005 level
Correlation is significant at the .001 level
Mean: Mean pain ratings
PP: Pressure pain
C. Pain: Clinical pain intensity
Figure 1.
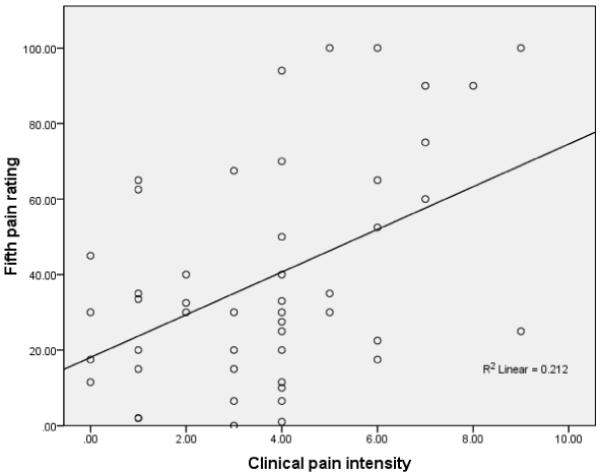
Scatterplot of clinical pain intensity scores vs fifth pain rating at 47°C
Contributions of QST to clinical pain intensity
Hierarchical regression analyses were conducted to determine the contribution of QST measures to clinical pain intensity. Age and sex accounted for 16% of the total variance in clinical pain intensity where only sex appeared as a significant predictor (Table III). Stepwise analysis indicated that the model with 5th pain rating at 47°C contributed an additional 10% variance in clinical pain intensity which was a significant addition to the model (Table III). In this model, sex was no longer a significant predictor. TS and no other QST measure contributed variance to clinical pain intensity in this analysis. VIF shows minimal multicollinearity concerns among the independent variables with the highest value of 1.35.
Table III.
Fifth pain rating at 47°C, significantly contributes to clinical pain intensity
| Variable | R2 | B | SE | β | P-Value |
|---|---|---|---|---|---|
| 1st Model | 0.16 | 0.02 | |||
| Age | −0.02 | 0.02 | −0.10 | 0.48 | |
| Sex | −2.01 | 0.71 | −0.41 | 0.01 | |
| 2nd Model | 0.26 | 0.01 | |||
| Constant | 3.73 | 1.49 | 0.02 | ||
| Age | −0.01 | 0.02 | −0.06 | 0.65 | |
| Sex | −1.18 | 0.75 | −0.24 | 0.12 | |
| Fifth pain rating | 0.03 | 0.01 | 0.36 | 0.02 |
Correlations among QST, psychological factors and clinical pain intensity
There were no statistically significant associations between the 5th pain rating at 47°C and psychological measures (PCS, BDI, STAI) (Table IV). Pearson correlations among psychological variables and clinical pain intensity showed significant associations for PCS (r = 0.46, p<0.01), and BDI (r = 0.29, p<0.05), as expected.
Table IV.
Pearson correlations among fifth pain rating at 47°C, clinical pain intensity and psychological variables
| Clinical pain |
PCS | BDI | STAI | |
|---|---|---|---|---|
| Fifth pain rating | 0.46** | 0.11 | 0.11 | 0.17 |
| Clinical pain | 0.46** | 0.29* | 0.21 | |
| PCS | 0.25 | 0.51** | ||
| BDI | 0.55** |
Correlation is significant at the .005 level
Correlation is significant at the .001 level
PCS: Pain Catastrophizing Scale; BDI: Beck Depression Inventory; STAI: State Trait Anxiety Inventory (trait portion of STAI was used)
Contributions of QST and psychological factors to clinical pain intensity
After accounting for age, sex and 5th pain rating at 47°C, the psychological factors (PCS, STAI, BDI) contributed an additional 17% of the variance in clinical pain intensity with a significant addition to the model (p<0.01) (Table V).
Table V.
Psychological factors and fifth pain rating significantly influence clinical pain intensity
| Variable | R2 | B | SE | β | P-Value |
|---|---|---|---|---|---|
| 1st Model | 0.25 | 0.01 | |||
| Age | −0.01 | 0.02 | −0.05 | 0.70 | |
| Sex | −1.07 | 0.72 | −0.22 | 0.14 | |
| Fifth pain rating | 0.03 | 0.01 | 0.38 | 0.01 | |
| 2nd Model | 0.42 | 0.001 | |||
| Constant | 2.21 | 1.55 | 0.16 | ||
| Age | 0.004 | 0.02 | 0.03 | 0.85 | |
| Sex | −0.56 | 0.70 | −0.12 | 0.43 | |
| Fifth pain rating | 0.03 | 0.01 | 0.38 | 0.01 | |
| PCS | 0.10 | 0.04 | 0.34 | 0.04 | |
| STAI | −0.04 | 0.04 | −0.22 | 0.26 | |
| BDI | 0.13 | 0.06 | 0.33 | 0.04 |
PCS: Pain Catastrophizing Scale; STAI: State Trait Anxiety Inventory (trait portion of STAI was used); BDI: Beck Depression Inventory
In this model, PCS and BDI were the unique psychological factors that contributed significant variance to clinical pain intensity. VIF shows minimal multicollinearity concerns among the independent variables with the highest value of 2.40 for STAI.
To explore whether psychological factors (BDI and PCS) moderated the relationship between 5th pain rating at 47°C and clinical pain intensity, hierarchical regression analyses were conducted. 3 These analyses tested whether psychological factors x 5th pain rating interaction terms accounted for significant variance in clinical pain intensity after the main effects of the psychological factors and 5th pain rating main effect terms had been entered in the model. To reduce multicollinearity we performed a centering process for each predictor variable by subtracting its own mean before entering into the regression model. Interaction terms were then computed by multiplying centered psychological variables (BDI and PCD) with centered 5th pain rating. Interaction terms for BDI x 5th pain rating and PCS x 5th pain rating did not contribute additional variance to clinical pain intensity (p > 0.05).
Contributions of suprathreshold heat pain responses and psychological factors to post-operative pain intensity
Another exploratory analysis was then performed to investigate differences in post-operative pain intensity report (3 months follow-up) based on our previous findings. The PCS, BDI, and 5th pain rating variables were dichotomized at the median value to create crude high and low scores that were added to create a separate risk factor variable. Subjects with low scores (0 and 1) were considered as likely to have low 3 month pain intensity and subjects with higher scores (2 and 3) were considered as likely to have high 3 month pain intensity. As expected, subjects having higher risk factor variables scores had significantly higher [t(57) = −4.10, p <0.001] baseline pain intensity (mean = 5.6, SD =1.9) compared with subjects having low risk factor variable scores (mean = 3.10, SD = 2.3). Additionally, subjects having higher risk factor variable scores had significantly higher [t(46) = −3.58, p <0.001] post-operative pain report (mean = 3.50, SD =2.5) compared with subjects having low risk factor variable scores (mean = 1.27, SD = 1.70). There were no significant differences, however, between these groups for change in pain scores (p > 0.05).
Regression analyses were conducted to examine the contribution of high risk factor variable (high PCS, high BDI, and high 5th pain rating) to post operative clinical pain intensity. After accounting for age, sex and baseline clinical pain intensity, the high risk factor variable contributed an additional 8% of the variance in post operative clinical pain intensity with a significant addition to the model [F(4,47) = 6.32, p< 0.001]. In this model, baseline clinical pain (Beta= 0.47, p<0.01), and the high risk variable (Beta= 0.29, p=0.02) were the significant contributor to post operative clinical pain.
Discussion
This study investigated different measures of QST determined which was most clinically relevant for patients with shoulder pain. As hypothesized correlations among QST measures were generally low to moderate in patients with shoulder pain indicating assessment of different components of pain sensitivity. The 5th pain rating, an index derived from suprathreshold heat pain stimuli assessment was the QST measure with the highest association with shoulder pain intensity. This measure remained associated with shoulder pain intensity in a multivariate model that included psychological factors. The present study provided evidence to support the clinical validity of suprathreshold heat pain stimuli assessment by demonstrating its association with clinical pain intensity in comparison to other QST measures and relevant psychological factors. A recent review 2 suggested that static pain psychophysics such as threshold and tolerance, may provide a limited view on the pain processing system in comparison to dynamic measures, such as TS and descending modulation of pain. Our second hypothesis tested this suggestion as we expected that measures derived from dynamic QST would have the highest association with shoulder pain intensity. Contrary to our hypothesis the 5th pain rating was associated with shoulder pain intensity and this measure does not capture the pain modulatory ability of the central nervous system. Therefore, these data provide evidence suprathreshold heat pain responses were a stronger predictor of clinical pain intensity compared with measures of pain threshold and tolerance.
The results of the current study provide evidence for suprathreshold heat pain response as the strongest QST measure in association with clinical pain intensity, compared to other measures included in this study. These results also confirmed the contribution of catastrophizing and depression as important psychological factors associated with clinical pain. The use of multiple QST measures in healthy subjects or those with chronic pain conditions like FM or LBP has been widely investigated. 2, 7-9, 11, 18, 22, 24, 29 There are fewer studies on QST available for those with shoulder and other upper extremity pain conditions. 16, 17 Therefore, utilization of a sample with shoulder pain and the investigation of different indices derived from a suprathreshold heat pain stimuli assessment, made this study a potentially novel contribution to the literature.
Different indices from suprathreshold heat pain stimuli assessment have been reported in the literature. 10-12, 19, 20, 29, 31, 32, 38, 40 There is no consensus on the thermal pain measure with the highest association with clinical pain. This analysis suggests that when heat pain is evoked with a suprathreshold protocol involving 5 pulses and standardized temperatures, the mean of pain ratings across all 5 pulses and the rating of the fifth pulse were highly correlated, and both showed significant associations with clinical pain intensity. Price et al 23 show that when more than one heat pulse are given with an interpulse interval ≤ 3 seconds, the intensity of first pain decreased with each successive heat pulse, and the second sensation (second pain) increased in pain perception with each successive heat pulse. This study provided evidence that the C-fiber component of the 5th heat pain rating is greater than for the 1st pain rating. Therefore, the stronger association of the 5th pain rating with clinical pain could be because the 5th pain rating is primarily comprised of C-fiber component in comparison to the other heat indices used in this study. This study had a clinical focus, but future investigation could help to identify the mechanisms involved in the relationship between the 5th pain rating and clinical pain intensity.
Several studies have examined the role of psychological factors in pain sensitivity 11, 15, 17, 26; however, our findings in this clinical population revealed little association between psychological factors and QST responses. As expected, our results revealed pain catastrophizing and depression had a strong association with clinical pain intensity and remained unique contributors after controlling for age, sex, and 5th pain rating. Exploratory analyses indicated psychological factors did not moderate the relationship between 5th pain rating and clinical pain intensity. Additionally, longitudinal analysis revealed that subjects having 3 risk factors (high PCS, BDI and 5th pain rating) had significantly higher post-operative pain intensity reports compared with subjects having low risk factors, even when controlling for pre-operative pain intensity. There were, however, no differences in change in pain intensity for these risk groups. Even though psychological factors significantly contributed to clinical pain intensity, results of the multivariate models indicate that 5th pain rating was consistently one of the strongest contributors to clinical pain intensity. Psychological factors did not moderate the relationship, suggesting that assessment of pain should include suprathreshold heat pain stimuli and psychological factors separately.
There is speculation that different pathways exist for the development of idiopathic pain disorders, such as psychological distress and pain amplification.8 Interestingly, the results of the present study show that psychological factors (catastrophizing and depression) and suprathreshold heat pain response contributed separately to clinical pain intensity in patients with shoulder pain. The present study suggests that psychological factors and pain amplification represent independent intermediate phenotypes that are associated with clinical pain severity. Therefore, there might be an overlap in the mechanisms that influence the development of chronic shoulder pain, as these factors were also hypothesized to be involved in the model for other idiopathic pain disorders such as fibromyalgia syndrome or temporomandibular disorders.7
Some important limitations of this study will need to be addressed by future research. First, this clinical sample failed to show a robust slope in TS, evidenced by a small increase in pain across our thermal pulse trials. Lack of a robust slope in TS could be a possible reason why the TS measure did not correlate with clinical pain intensity in this study. Moreover, we only assessed experimental pain sensitivity at baseline, and we do not have corresponding data for the post-operative status. Another limitation is that the cross-sectional nature of the associations presented precludes statements regarding the direction of causality for the association between experimental pain sensitivity and clinical pain intensity. Finally, suprathreshold heat pain assessment through TS was the only dynamic QST measure considered in this study. Future studies should include additional dynamic measures, such as descending modulation of pain, to determine if they uniquely contribute to variance in clinical pain intensity.
Despite these limitations, the current study provides evidence for the clinical relevance of the 5th pain rating of suprathreshold heat pain stimuli assessment based on its association with clinical pain intensity for patients with shoulder pain. The present study also confirmed the contribution of catastrophizing and depression as important psychological factors influencing clinical pain intensity in a potentially novel patient population. Additionally, the study revealed that the combination of psychophysical and psychosocial risk factors significantly predicted post-operative pain reports even after controlling for baseline pain intensity. The present findings suggest that assessing 5th pain rating, pain catastrophizing and depression might play a role in predicting pain intensity outcomes in future longitudinal studies.
Perspective.
The current study provides evidence for a suprathreshold heat pain response as a clinically relevant QST measure for patients with shoulder pain, even after psychological factors were considered. The present findings suggest that the 5th pain rating from a series of suprathreshold stimuli, pain catastrophizing, and depression might play a role in predicting pain intensity outcomes.
Acknowledgments
SZG (PI), RBF, and CV were supported by Grant #AR055899 from NIAMS/NIH while preparing this manuscript.
Michael Moser and Thomas Wright assisted with recruitment.
Warren H. Greenfield, III assisted with recruitment and testing protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this manuscript have no financial or other relationship with any individuals or organizations that constitute a conflict of interest.
References
- 1.Arendt-Nielsen L, Petersen-Felix S. Wind-up and neuroplasticity: is there a correlation to clinical pain? Eur J Anaesthesiol Suppl. 1995;10:1–7. [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–72. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Baron RM, Kenny DA. The Moderator-Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations. Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 4.Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgrad Med. 1972;52:81–5. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 6.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–85. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 7.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 8.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–17. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106:427–37. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–7. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 12.Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007;8:514–20. doi: 10.1111/j.1526-4637.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 13.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–7. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 14.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30:115–26. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 15.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil. 2006;16:95–108. doi: 10.1007/s10926-005-9007-y. [DOI] [PubMed] [Google Scholar]
- 16.George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain. 2007;23:76–84. doi: 10.1097/01.ajp.0000210949.19429.34. [DOI] [PubMed] [Google Scholar]
- 17.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, 3rd, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastie BA, Riley JL, 3rd, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–37. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–8. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Lautenbacher S, Kunz M, Burkhardt S. The effects of DNIC-type inhibition on temporal summation compared to single pulse processing: does sex matter? Pain. 2008;140:429–35. doi: 10.1016/j.pain.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behav Res Ther. 1983;21:401–8. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 22.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 23.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–71. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 24.Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–74. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–56. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 26.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs. 2005;180:69–75. doi: 10.1159/000086200. [DOI] [PubMed] [Google Scholar]
- 28.Spielberger CD, Vagg PR. Psychometric properties of the STAI: a reply to Ramanaiah, Franzen, and Schill. J Pers Assess. 1984;48:95–7. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- 29.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 30.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Robinson ME, Vierck CJ, Jr., Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 32.Staud R, Robinson ME, Vierck CJ, Jr., Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–74. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 33.Staud R. Predictors of clinical pain intensity in patients with fibromyalgia syndrome. Curr Rheumatol Rep. 2004;6:281–6. doi: 10.1007/s11926-004-0036-x. [DOI] [PubMed] [Google Scholar]
- 34.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–96. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–23. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13:12–7. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MBS, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- 38.Vierck CJ, Jr., Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 39.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 40.Weissman-Fogel I, Granovsky Y, Crispel Y, Ben-Nun A, Best LA, Yarnitsky D, Granot M. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain. 2009;10:628–36. doi: 10.1016/j.jpain.2008.12.009. [DOI] [PubMed] [Google Scholar]


