Abstract
NOV-002 (a formulation of disodium glutathione disulfide) modulates signaling pathways involved in tumor cell proliferation and metastasis and enhances anti-tumor immune responsiveness in tumor models. The addition of NOV-002 to chemotherapy has been shown to increase anti-tumor efficacy in animal models and some early phase oncology trials. We evaluated the clinical effects of NOV-002 in primary breast cancer, whether adding NOV-002 to standard preoperative chemotherapy increased pathologic complete response rates (pCR) at surgery, and determined whether NOV-002 mitigated hematologic toxicities of chemotherapy and whether levels of myeloid derived suppressor cells (MDSC) were predictive of response. Forty-one women with newly diagnosed stages II-IIIc HER-2 negative breast cancer received doxorubicin-cyclophosphamide followed by docetaxel (AC→T) every 3 weeks and concurrent daily NOV-002 injections. The trial was powered to detect a doubling of pCR rate from 16% to 32% with NOV-002 plus AC→T (α=0.05, β=80%). Weekly complete blood counts were obtained as well as circulating MDSC levels on day 1 of each cycle were quantified. Of 39 patients with 40 evaluable tumors, 16 achieved a pCR (40%), meeting the primary endpoint of the trial. Lower circulating levels of MDSCs at baseline and cycle 8 were associated with a pCR (P=0.02). Concurrent NOV-002 resulted in pCR rates for AC→T chemotherapy higher than previously reported. Patients with lower levels of circulating MDSCs at baseline and on the last cycle of chemotherapy had significantly higher probability of a pCR (p=0.02). Further evaluation of NOV-002 in a randomized study is warranted.
Keywords: breast cancer, neoadjuvant, phase 2, NOV-002, glutathione analog, MDSC, myeloid derived suppressor cells, pathologic complete response, anthracycline, taxane
Introduction
Key differences in oxidative signalling between normal and cancer cells represent potential therapeutic targets that can be exploited for the rational design of new anticancer agents [1-3]. In addition to their well characterized effects on disruption of cell division, many classical cytotoxic agents can induce oxidative stress in cancer cells by modulating levels of reactive oxygen species (ROS), e.g. superoxide anion radical, hydrogen peroxide, and hydroxyl radicals [2, 4-8]. Breast tumors are particularly sensitive to oxidative stress as they typically have persistently higher levels of ROS than normal cells due to the dysregulation of redox balance because of increased intracellular ROS production or antioxidant protein depletion [9-11]. Several anticancer agents are currently in different stages of clinical development that target cellular redox regulation. The overall cellular redox state is regulated by three systems that modulate cellular redox status by counteracting free radicals and ROS, or by reversing the formation of disulfides; two of these are dependent on glutathione and the third on thioredoxin. NOV-002 is a glutathione disulfide mimetic that has been shown to alter intracellular GSH/GSSG ratio by increasing GSSG levels, creating a mild, transient oxidative intracellular signal and inducing S-glutathionylation [12-14]. Drugs targeting S-glutathionylation may have direct anticancer effects via cell signalling pathways and inhibition of DNA repair, and thus can potentially impact a wide range of signalling pathways [15-18]. NOV-002-induced S-glutathionylation has been shown to have inhibitory effects on tumor cell invasion [19], proliferation and survival[20], and differential effects on myeloid cell lines [21]. NOV-002 has been previously shown to have immunomodulatory properties including increasing levels of circulating T cells in cancer patients receiving chemotherapy as well as modulation of myeloid derived suppressor cells (MDSCs) [22]. When combined with platinum based chemotherapy, NOV-002 has shown promising results in patients with platinum refractory ovarian cancer [23], and mixed results in patients advanced NSCLC [13].
Because chemotherapy is generally less effective in HER-2 negative breast cancer, and least effective in postmenopausal hormone receptor positive patients, we hypothesized that the addition of NOV-002 to standard preoperative chemotherapy would increase the pathologic complete response rates to chemotherapy more than what would be expected with chemotherapy alone. Based on these considerations, we conducted a clinical trial in which women with newly diagnosed stages II-IIIc HER-2 negative breast cancer received doxorubicin and cyclophosphamide (AC) followed by docetaxel (T), i.e. AC→T, in conjunction with daily NOV-002 Here we report the final results of a completed phase II trial in 41 patients with clinical stage II-IIIc HER-2 negative breast cancer. Our primary objectives were to determine whether NOV-002 enhanced pathologic complete response rates (pCR) associated with standard preoperative AC→T chemotherapy, and because of the known immunomodulatory effects of NOV-002, determine whether we could identify biomarkers predictive of pCR.
Patients and methods
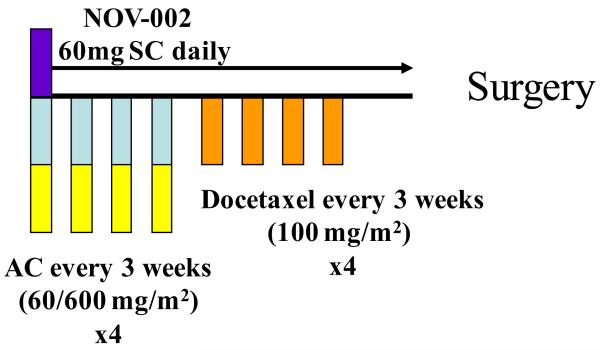
Patients were required to have histologically confirmed HER-2 negative invasive breast cancer by core needle biopsy, with no evidence of metastatic disease except to the ipsilateral axillary lymph nodes and clinical stage II – IIIc (T2-4, N0 or N1, M0 or any T, N1-3, M0) breast cancer (ClinicalTrials.gov identifier NCT00499122). The protocol was reviewed and approved by the local institutional review board at each participating institution, and all patients provided written informed consent. Her-2/neu status was determined by either fluorescent in situ hybridization (FISH) or 0-2+ staining by immunohistochemistry (IHC). Patients received eight 3-week cycles of standard intravenous neoadjuvant chemotherapy consisting of four cycles of.AC (60/600 IV mg/m2) followed by four cycles of T (100 mg/m2). A loading dose of NOV-002 was administered on the day before initiation of chemotherapy 60 mg IV x 2, approximately 3 hours apart. During chemotherapy NOV-002 was administered intravenously 60 mg on day 1 of each cycle over 1 hour approximately 30 minutes prior to infusion of chemotherapy, and then self-administered by patients as 60 mg daily subcutaneous injections on days 2-20 of each cycle in a prefilled syringe (Figure 1).
Figure 1. Treatment schema for NEO-NOVO trial.
All patients underwent either lumpectomy or mastectomy and lymphoscintagraphy (and if indicated axillary lymph node dissections). Patients with biopsy proven axillary lymph node involvement underwent a formal axillary dissection. After definitive surgery patients received additional systemic chemotherapy or endocrine therapy and radiotherapy as clinically indicated by the treating oncologist.
This study was a multicenter open-label, two-stage single arm study to determine the clinical activity and safety of the addition of daily NOV-002 to standard AC→T chemotherapy. The primary endpoint of this study was pathologic complete response (pCR) which was defined as: (i) no metastatic tumor in axillary lymph nodes (ypn0); and (ii) either no invasive tumor in the breast (ypT0) or residual invasive tumor ≤10mm in maximum dimension (ypT1a or ypT1b). The median breast and nodal pCR rate projected for a HER-2 negative population with anthracycline and taxane based chemotherapy was approximately 16% based on historical data. This is also in line with recently reported data for NSABP B27 of pCR rate with AC→T in ER+ HER-2 negative patients was approximately 14% [24, 25]. A Simon two-stage optimal design was used to test the null hypothesis that the addition of NOV-002 to AC→T elicited a pCR rate in a cohort of HER-2 negative patients of ≤16% versus the alternative hypothesis that the observed pCR rate was >32% at a 0.05 level of significance and 80% power. This design specified 19 patients in the first stage and 27 in the second stage, with a requirement of 4 or more pCRs in stage 1 to continue to stage 2. If 12 or more pCRs were observed in the total of 46 patients, then the null hypothesis could be rejected. Due to the threshold for rejecting the null hypothesis being achieved (i.e., more than 12 responses were seen before accruing to the total sample size of 46), the study would stopped early and the null hypothesis rejected
Pathologic response was assessed by two of the coauthors who were breast pathologists for cases at University of Miami Sylvester Comprehensive Cancer Center (UMSCC) [MJ] and at the Medical University of South Carolina Hollings Cancer Center (HCC) [TR]; the specimens were also evaluated for “residual cancer burden” (RCB) as described by Symmans et al [26].
MDSC analysis in peripheral blood was performed on fresh venous blood collected in EDTA-coated vacutainer tubes (BD Biosciences), as previously described [27, 28]. Briefly, 100 μL of blood was combined with 5μL of each monoclonal antibody on a 96-well plate and incubated at room temperature for 30 min. Antibodies were purchased from BD Biosciences: Lin-1 FITC cocktail (CD3, CD14, CD16, CD19, CD20, & CD56), HLA-DR-APC (Clone – L243), CD33-PE (clone WM53), and CD11b-v450 (ICRF44). After incubation, each sample was mixed with 2 mL of 1× lysis buffer (BD Biosciences) and incubated for 15 minutes. Samples were washed with FACS buffer. Pellets were resuspended in 300 μL FACS buffer. The absolute number of MDSCs was calculated as follows: total white blood cell count (cells/μL) × percent MDSCs/100.
Descriptive statistics were used to summarize clinical characteristics. The p-value for addressing the primary objective was calculated based on the observed sample size (N=40) given that the total planned sample size (N=46) was not achieved [29]. The same approach was used for estimating the response rate and its 95% confidence interval. Kaplan-Meier curves were used to demonstrate relapse-free survival by RBC group, and comparisons of the curves, was performed by the log-rank tests. Modeling of hematologic parameters displayed in Figures 3A-3D was implemented using linear mixed effect models. Random intercepts were used to account for correlation of outcomes measured over time within the same individual. Flexible models were used that included main effects of day within cycle and cycle number. Linear, quadratic and cubic terms were included to allow for nonlinearity. Fit of models and assumptions were assessed using graphical displays of fitted model and raw data and residual plots. No hypothesis testing was performed on hematologic parameters and Figures 3A-3D are intended to be descriptive of patterns over time. Boxplots were used to display differences in distributions of MDSC levels in responders and non-responders. Associations between MDSC levels and pCR were assessed using logistic regression, with pCR as the outcome and log of number of cells as the predictor.
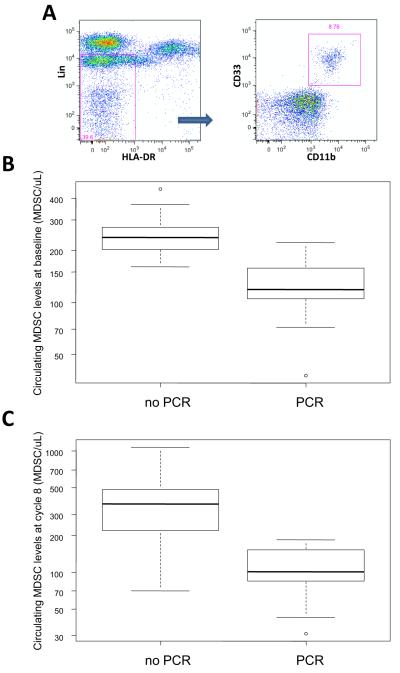
Figure 3. Correlation between myeloid derived suppressor cell (MDSC) levels and pCR in study patients.
(A) FACS gating strategy for MDSCs. Circulating MDSC levels at baseline (B) and on cycle 1 day 8 (C), were significantly higher in patients who didn’t achieve a pCR after preoperative chemotherapy plus NOV-002
Results
Patient characteristics
Between June 4, 2007 and July 16, 2010, three investigational sites enrolled 41 patients into this study. One patient had bilateral breast cancer. Baseline characteristics are summarized in Table 1. Both mean and median ages were 53 years. There were 18 patients (46%) with biopsy proven axillary lymph node involvement. Forty three percent of patients had clinical T3-T4 tumors. Estrogen receptor status was positive in 80% while 16% had ER-/PR-/Her-2- tumors, i.e. triple negative.
Table 1.
ptxBaseline demographics and clinical characteristics
| Characteristic | Number (%) |
|---|---|
| Total Patients Enrolled | N = 41a |
| Age | |
| Mean | 53 |
| Median | 53 |
| 18-49 | 16 (39) |
| 50-64 | 18 (44) |
| ≥65 | 7 (17) |
| Race/Ethnicity | |
| White-Non-Hispanic | 16 (39) |
| Hispanic | 10 (24.3) |
| African American | 13 (31.7) |
| Asian | 2 (4.8) |
| Menopausal Status | |
| Pre-menopausal | 17 (41.5) |
| Post-menopausal | 22 (53.6) |
| Unknown | 2 (4.8) |
| Clinical Tumor Size | |
| T2 | 23 (56) |
| T3 | 15 (36.5) |
| T4 | 3 (7.3) |
| Clinical Node Status | |
| N0 | 23 (56) |
| N1 | 16 (39) |
| N2 | 2 (4.9) |
| Tumor Grade | |
| 1 | 9 (22) |
| 2 | 12 (29) |
| 3 | 18 (44) |
| Not Reported | 2 (5) |
| Tumor Hormone Receptor Status | |
| ER+ and/or PR+ | 35 (85) |
| ER − & PR − | 6 (15) |
one patient with bilateral breast cancer
Pathologic and clinical response
Of 41 enrolled patients, two were not assessable for pathologic response: one patient withdrew consent at Day 8 of Cycle 1; the second patient refused surgery after completion of all planned chemotherapy. Therefore there were 39 assessable patients. Of these, one patient had synchronous triple negative bilateral breast cancer, and had a discordant response with pCR in one side and not in the other, reflected in the denominator of 40 assessable tumors and 39 patients. Of the 40 evaluable tumors, 7 were ypT0 and ypN0, and 8 had a residual invasive primary breast tumor ≤10 mm and ypN0 cancers. The final estimated pCR rate was 39% (95% CI: 25-45%), with a p-value of<0.001, providing strong evidence to reject the null hypothesized pCR rate of 16%. In the minimum residual invasive disease subgroup (ypT0-T1b), the mean residual tumor size was 5.1 mm. When data are analyzed by the RCB score, the RCB 0 frequency was 17.5%. Rates of RCB 1, 2, and 3 were 17.5%, 40%, and 25%, respectively (Table 2). There was good concordance between patients with minimal residual invasive disease, i.e. ypN0 & ypT1a, and an RCB 1, with only 1 patient in this subgroup (out of 8) who was not RCB 1, but instead was classified as RCB 2. We also evaluated the pCR rate by using the definition used in NSABP B27, i.e. no invasive tumor in breast, and the rate was found to be 20% [24, 25]. Interestingly, of the 18 patients with biopsy-proven lymph node involvement, 7 patients (39%) had no residual invasive tumor in axillary nodes after dissection.
Table 2.
Observed pathologic complete responses (pCR)
| Surgically Evaluable Tumors | N = 40 (%) |
| Overall pCRa | 15/40b (37.5) |
| ER+ or PR+ | 11/33 (33.3) |
| ER−, PR− | 4/7b (57) |
| Patients with no residual invasive tumor (ypT0) & N0 | 7 (17.5) |
| Patients with residual invasive tumor ≤1.0cm in greatest dimension & N0 Maximum residual invasive carcinoma [ in mm] |
8 (20) |
| Mean | 5.1 |
| Median | 4.5 |
| Residual Breast Cancer Burden (RCB) | |
| 0 | 7 (17.5) |
| 1 | 7 (17.5) |
| 2 | 16 (40) |
| 3 | 10 (25) |
| pCR B27 definition | 8/40 (20) |
| ER+ or PR+ | 6/33 (18.2) |
| ER−,PR− | 2/7 (28.5) |
| Axillary Conversion Rate (+) → (−) (18 patients with biopsy proven involvement) |
7 (38.8) |
pCR defined as: the absence of any histological evidence of invasive breast cancer in axillary nodes, and either (i) no invasive cancer in the tissue specimen removed from the breast , or (ii) the presence of invasive tumor = 1cm after preoperative treatment determines at definitive breast surgery.
One patient with bilateral breast cancer had pCR in right breast (1.5mm) and 3.5cm in left breast
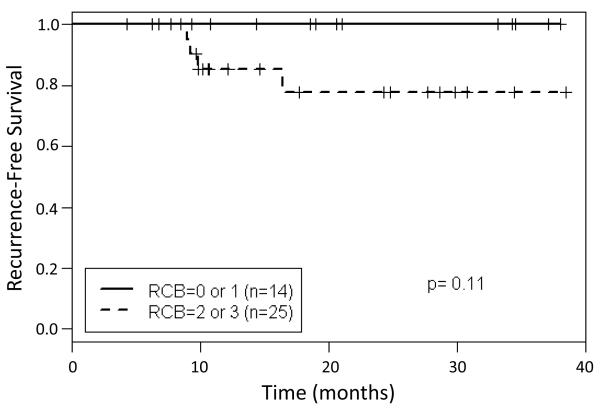
Because hormone receptor positive and HER-2 negative breast tumors are generally least sensitive to chemotherapy, particularly in post-menopausal patients an exploratory analysis was done to evaluate pCR by hormone receptor status [30]. In the 33 evaluable patients with estrogen positive breast cancer, 11 (33%) achieved a pCR (Table 2). Because of the correlation between survival and RCB after neoadjuvant therapy, we sought to determine if patients in this study with RCB 0-1 after systemic therapy with NOV-002 and AC→T had better relapse-free survival (RFS) times than patients with sub-optimal responses to neoadjuvant therapy. We found that patients with documented RCB 0-1 had higher RFS rates than those with RCB 2-3 (Figure 4), however due to small numbers of patients and relatively short follow-up this was not statistically significant (P=0.10).
Figure 4. Kaplan-Meier plots of the probability of recurrence free survival by residual cancer burden (RCB) status.
Overall Toxicities
Chemotherapy plus NOV-002 was well tolerated. A total of 314 chemotherapy cycles were administered. 90% of all patients were able to complete all 8 cycles of planned chemotherapy. Consequently, the addition of NOV-002 to a standard AC→T regimen did not seem to add to the overall toxicities from chemotherapy. The most common non-laboratory grade 1-2 toxicities (adverse events) occurring with a frequency of 25% or more included: nausea 31 (75.6% of patients), fatigue 29 (70.7%), constipation 16 (39%), vomiting 15 (36.6%), sensory neuropathy 11 (26.8%), diarrhea 11 (26.8%). Grade 3-4 serious adverse events (using the National Cancer Institute Common Terminology for Adverse Events, version 3) were uncommon with the most common being febrile neutropenia, which occurred in 4 patients (9.8%) (Table 3).
Table 3.
Overall serious adverse events
| Toxicity | Grade 3, n (%) |
Grade 4, n (%) |
|---|---|---|
| Febrile Neutropenia | 4 (9.8) | 0 |
| Sensory neuropathy | 4 (9.8) | 0 |
| Abdominal Pain | 2 (4.8) | 0 |
| Fatigue | 2 (4.8) | 0 |
| Headache | 1 (2.4) | 0 |
| Cellulitis | 1 (2.4) | 0 |
| Neutropenia | 1 (2.4) | 2 (4.8) |
| Muscular Weakness | 1 (2.4) | 0 |
| Deep Vein Thrombosis | 1 (2.4) | 0 |
| Femoral Artery Occlusion | 1 (2.4) | 0 |
| Constipation | 1 (2.4) | 0 |
| Nausea | 1 (2.4) | 0 |
| Palmar-plantar erythrodysaesthesia syndrome | 6 (14.6) | 0 |
| Pulmonary Embolism | 1 (2.4) | 0 |
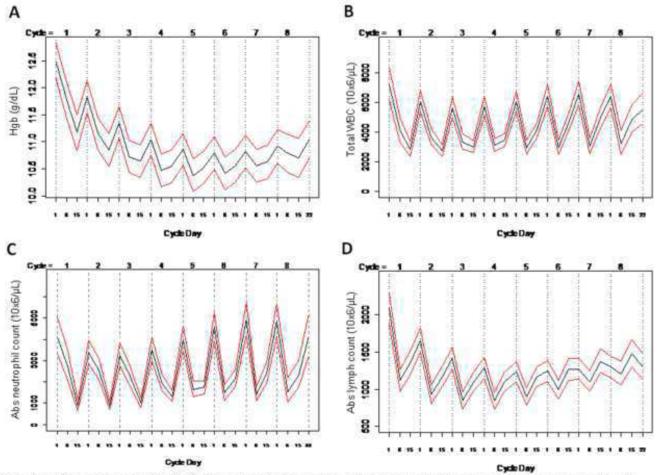
Weekly complete blood counts were drawn on all patients, and prophylactic use of G-CSF was not required. Figure 2A shows estimated average weekly hemoglobin levels throughout the trial with 95% confidence intervals. Hemoglobin concentrations remained above 10 g/dL. Erythropoietin was used in 18 out of 317 cycles of chemotherapy (5.7%) and two patients required blood transfusions during the study. Figure 2 shows estimated average weekly total leukocyte and absolute neutrophil counts (ANC), respectively through all 8 cycles. As expected, there were differences when the nadir occurred during cycles 1-4 (AC) and cycles 5-8 (T), with the former being on day 15 and the latter on day 8. Febrile neutropenia was relatively uncommon occurring in only 4 patients (9.8%). G-CSF was administered in 71 out of 317 cycles (23%) due to neutropenia.
Figure 2. Longitudinal hematologic indices on study patients.
Weekly complete blood counts were obtained on all patients. Data shown for hemoglobin (A), total white blood count (B), absolute neutrophil (C), and lymphocyte (D) counts. Black line represents the average and red lines denote 95% confidence intervals.
Figure 2D shows estimated average weekly absolute lymphocyte counts (ALC) in all patients throughout all 8 cycles (with 95% confidence intervals). The lowest average ALC was observed on day 8 of cycle 5 (802/mm3) which corresponds to grade 1 lymphocytopenia, and compares favorably to prior data with similar chemotherapy regimens in the neoadjuvant setting in breast cancer patients [31].
Myeloid Derived Suppressor Cells (MDSCs) and response (pCR)
Previous research has suggested that NOV-002 may have immunomodulatory properties and in clinical the setting has been shown to be associated with greater number of circulating T-cells [2, 13, 22]. We hypothesized that patients with favorable responses, i.e. pCR, were more likely to have significantly lower levels of MDSCs than non-responders. To test this hypothesis, we evaluated circulating levels of MDSCs prior to starting therapy (baseline) and on day 1 of each cycle of chemotherapy. We have previously reported that elevated levels of MDSC in whole blood defined as: lineage-1 (lin-1)−, HLA-DR−, CD33+, CD11b+ (Figure 3A) are associated with advanced stage and increased metastatic tumor burden and inferior survival [27, 28]. Interestingly, we found that responders, i.e. patients who had a pCR after neoadjuvant chemotherapy plus NOV-002, had significantly lower circulating MDSC levels at baseline than non-responders [257.4 vs. 124.3 MDSC/μL; P<0.001] (Figure 3 B). Moreover, overall MDSC levels were significantly higher on day 1 of the final cycle of chemotherapy in non-responders (Figure 3C). Circulating MDSC levels increased over time in non-responders, during chemotherapy. By contrast, MDSC levels remained unchanged relative to overall pretreatment levels in responders (data not shown). Circulating MDSC levels were found to be significantly higher in patients with residual disease compared to those with pCR on day 1 of cycles 2-4 [534.2 vs. 207.8; P=0.02] and cycles 5-8 [363.7 vs. 171.5; P=0.006]. On univariate analysis, circulating MDSC levels at baseline and cycle 8 were significantly lower in patients with pCR versus not (p=0.02). By contrast only high tumor grade was found to correlate with pCR on UVA, but not hormone receptor status or the absence of clinically apparent nodal involvement at initial diagnosis.
Discussion
Neoadjuvant chemotherapy has been shown to downstage malignant breast tumors, and thereby rendering them amenable to breast conserving treatment (BCT). Neoadjuvant treatment also permits in situ assessment of the tumor’s responsiveness to chemotherapy. Multiple studies have demonstrated a strong correlation in breast cancer patients between pathologic or near pathologic complete responses after neoadjuvant chemotherapy and disease free and overall survival [26, 32-38].
We hypothesized that adding NOV-002 to standard AC→T chemotherapy would increase the pCR rate in women with HER-2 negative breast cancer. Our results when compared to NSABP B-27 trial, which utilized the same chemotherapy regimen, are favorable. In the B27 trial, the addition of preoperative docetaxel (group II) to AC increased pCR rates from 13.7% (group I) to 26.1% [24]. However, HER-2 status was unknown in this trial, which would impact on pCR rates as tumors that overexpress HER-2 are generally more chemosensitive [39, 40]. Other neoadjuvant studies have also reported pCR rates that are lower than those observed in our study with anthracycline and docetaxel based chemotherapy. The GEPARDUO study, a randomized controlled trial evaluating doxorubicin, cyclophosphamide, and docetaxel in the neoadjuvant setting, reported an overall pCR rate for sequential AC→T of 14.3% [41].
We observed a higher pCR rate within our 34 evaluable hormone receptor positive patients (32%) than what would be expected with AC→T chemotherapy alone. The NSABP developed the AC→T regimen, and in NSABP-B27, ER+ patients had a pCR rate of 8.3% [42]. Mazouni et al. reported in a pooled analysis of 1079 patients, a pCR rate of 8.8% in ER+ patients compared to 29% in ER- patients who underwent taxane based neoadjuvant chemotherapy [38]. In a retrospective study, Guarneri et al. observed a similar 8% pCR rate in hormone receptor positive tumors compared to 24% in hormone receptor negative tumors [43].
Since the introduction of RCB is relatively recent, we used pCR as our primary endpoint, however we did analyze the data with the RCB index and found good concordance between patients with minimal residual invasive disease and RCB-1. In a recently published neoadjuvant trial in women with HER-2 negative breast cancer, the proportion of patients with RCB-0 and RCB-1, after 4 cycles of docetaxel based chemotherapy, was 9.9% and 5.9%, respectively [39]. Our results after 8 cycles of therapy for RCB-0 of 17.5% compare favorably in a similar patient population.
NOV-002 was well tolerated and did not appear to add to the toxicities related to AC→T chemotherapy. The Hematologic toxicities were less than expected [44]. Modulation of intracellular glutathione levels (GSG/GSSG) or of glutathione S-transferase (GST) has been shown to increase myeloproliferative activity. An example of this is TLK199, also known as Telintra, a peptidomimetic inhibitor of GSTπ [45]. Similarly, some prior clinical studies in the lung cancer setting with NOV-002 plus cytotoxic chemotherapy have reported that NOV-002 was associated with better tolerability of chemotherapy and reduced hematologic toxicities [13, 46]. Therefore, one of the aims of this study was to determine whether neutropenia would improve over time, something not to be expected necessarily with AC→T chemotherapy, and whether hemoglobin levels and rates of hematologic toxicities compared favorably with previously published reports with this regimen in the neoadjuvant setting. Only 4 patients (9.8%) experienced febrile neutropenia and no prophylactic use of G-CSF was required. G-CSF was administered in 71 out of 317 chemotherapy cycles (23%). By comparison, patients in the NSABP B-27 trial who received both AC and docetaxel had a 21.2% rate of febrile neutropenia required G-CSF support with 18% and 21% of all cycles of AC and docetaxel chemotherapy, respectively[24]. Mean hemoglobin levels in patients remained above 10 g/dL, despite the fact that erythropoietin was used in only 18 of 317 cycles of chemotherapy (5.7%).
We also evaluated weekly absolute lymphocyte counts to determine what impact daily NOV-002 injections would have on the severe lymphopenia expected with anthracycline and taxane based chemotherapy. It has been previously reported that the addition of NOV-002 to platinum based chemotherapy in patients with stage IIIB/IV NSCLC resulted in significantly increased lymphocyte counts in patients relative to patients receiving chemotherapy alone [13, 46]. The lowest average ALC was observed on day 8 of cycle 5 (802/mm3), which corresponds to grade 1 lymphopenia.
Because of the previously reported immunomodulatory effects of NOV-002 [13], specifically on T-cells, and the increasing body of literature on anthracyclines eliciting immunogenic cell death [47, 48], we hypothesized that patients with the highest pretreatment myeloid derived suppressor cell (MDSC) levels in circulation would benefit the least from NOV-002 plus standard chemotherapy. MDSCs have been recognized as important factor in tumor mediated T-cell suppression [49, 50]. MDSCs accumulate due to tumor-derived factors that lead to migration of marrow precursors to tumor site. Circulating MDSCs have been shown to correlate with more advanced clinical stage and poorer overall prognosis due to their important role in suppressing the innate and adaptive antitumor immunity and promoting tumor progression and metastasis [27, 28, 51]. Moreover, a recent study in patients with advanced kidney cancer or melanoma receiving immunotherapy with IL-2 showed that patients with increased levels of MDSCs either prior to starting therapy or post therapy had less favorable outcomes [52]. Similarly we found in our study that patients who had a pCR after chemotherapy plus NOV-002 were most likely to have lower levels of circulating MDSCs both pre and post chemotherapy.
The primary limitation of this typical phase II study is the absence of a randomized control arm of patients who received chemotherapy alone without NOV-002 and a small sample size of 40 patients. Consequently, it remains unclear whether the addition of NOV-002 to chemotherapy is the causal factor that resulted in higher pCR rates than would be expected with AC→T chemotherapy in women with HER-2 negative breast cancer, and lower hematologic toxicities. It also remains unclear if circulating MDSCs were predictive of patients likely to benefit from neoadjuvant anthracycline and taxane based chemotherapy in general, or to specifically predict patients likely to benefit from the addition of NOV-002 to chemotherapy.
In conclusion, NOV-002 in combination with neoadjuvant AC→T in patients with HER-2 negative breast cancer was well tolerated and resulted in a very favorable pCR rate. This was particularly notable in the patients with hormone receptor positive breast cancer. This study establishes that further investigation in large-scale-phase III trials of NOV-002 in conjunction with neoadjuvant chemotherapy in breast cancer is warranted.
Acknowledgments
Grant Support
K23-CA148893-01
The research presented in this article was also supported in part by the Biostatistics Shared Resource as part of the Hollings Cancer Center at the Medical University of South Carolina which is funded by a Cancer Center Support Grant P30 CA138313.
Footnotes
Conflicts of Interest
Alberto J. Montero has previously received grant funding from Novelos Therapeutics.
K Schuhwerk was a former employee of Novelos Therapeutics, and owns Novelos stock.
The other authors have no conflict of interest to declare .
References
- 1.Cabello CM, Lamore SD, Bair WB, 3rd, Davis AL, Azimian SM, Wondrak GT. DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma. Free radical research. 2011;45(3):276–292. doi: 10.3109/10715762.2010.526766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71(11):1385–1396. doi: 10.2165/11592590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxidants & redox signaling. 2009;11(12):3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science (New York, NY) 1997;275(5306):1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 5.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer research. 2005;65(3):948–956. [PubMed] [Google Scholar]
- 6.Rodrigues MS, Reddy MM, Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxidants & redox signaling. 2008;10(10):1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 7.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxidants & redox signaling. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer cell. 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Glorieux C, Dejeans N, Sid B, Beck R, Calderon PB, Verrax J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochemical pharmacology. 2011 doi: 10.1016/j.bcp.2011.06.007. Journal Article. [DOI] [PubMed] [Google Scholar]
- 10.He C, Tamimi RM, Hankinson SE, Hunter DJ, Han J. A prospective study of genetic polymorphism in MPO, antioxidant status, and breast cancer risk. Breast cancer research and treatment. 2009;113(3):585–594. doi: 10.1007/s10549-008-9962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JC, Lu MC, Lee CL, Chen GY, Lin YY, Chang FR, Wu YC. Selective targeting of breast cancer cells through ROS-mediated mechanisms potentiates the lethality of paclitaxel by a novel diterpene, gelomulide K. Free radical biology & medicine. 2011;51(3):641–657. doi: 10.1016/j.freeradbiomed.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer research. 2008;68(8):2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert opinion on investigational drugs. 2008;17(7):1075–1083. doi: 10.1517/13543784.17.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend DM, Tew KD. Pharmacology of a mimetic of glutathione disulfide, NOV-002. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2009;63(2):75–78. doi: 10.1016/j.biopha.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biological chemistry. 2006;387(10-11):1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 16.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Current pharmaceutical design. 2006;12(34):4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 17.Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free radical biology & medicine. 2011;51(2):299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Molecular interventions. 2007;7(6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumireddy K, Pazoles C, Vulfson E. Inhibition of tumor cell invasion and ErbB2/PI3K signalling pathways by the glutathione disulfide-mimetic NOV-002; Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009; Abstract. [Google Scholar]
- 20.Townsend DM, Bowers R, Pazoles CJ. NOV-002 suppresses tumor cell growth by modulating redox-sensitive cell signaling. Mol Cancer Ther. 2009;8(12) [Google Scholar]
- 21.Bowers R, Townsend D, Manevich Y. The redox modulator NOV-002 inhibits proliferation of ovariantumor cells but increases proliferation of myeloid cells; Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; 2010; Abstract. [Google Scholar]
- 22.Montero AJ, Naga O, Xu M. Nov-002, a cellular redox modulator, enhances the antitumor effect of adoptively transferred T cells in a murine melanoma model. Mol Cancer Ther. 2009;8(12 Suppl. 1) [Google Scholar]
- 23.Krasner CN, Seiden MV, Penson RT. NOV-002 plus carboplatin in platinum-resistant ovarian cancer. J Clin Oncol. 2008;26 Abstract. [Google Scholar]
- 24.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of clinical oncology. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Bear HD, Anderson S, Smith RE, Geyer CE, Jr., Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(13):2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 26.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy: CII. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011 doi: 10.1182/blood-2010-12-325753. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Statistics in medicine. 2008;27(16):3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N, Vrancken Peeters MT. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Annals of surgical oncology. 2010;17(9):2411–2418. doi: 10.1245/s10434-010-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolaney SM, Najita J, Winer EP, Burstein HJ. Lymphopenia associated with adjuvant anthracycline/ taxane regimens. Clinical breast cancer. 2008;8(4):352–356. doi: 10.3816/CBC.2008.n.041. [DOI] [PubMed] [Google Scholar]
- 32.Ayers M, Symmans WF, Stec J, Damokosh AI, Clark E, Hess K, Lecocke M, Metivier J, Booser D, Ibrahim N, et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. Journal of clinical oncology. 2004;22(12):2284–2293. doi: 10.1200/JCO.2004.05.166. [DOI] [PubMed] [Google Scholar]
- 33.Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, Ollila DW, Klauber-DeMore N, Halle J, Sawyer L, Moore DT, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. Journal of the National Cancer Institute. 2005;97(15):1137–1142. doi: 10.1093/jnci/dji206. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, Kau SW, Huang EH, Morandi P, Ocana A, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(28):7098–7104. doi: 10.1200/JCO.2005.11.124. [DOI] [PubMed] [Google Scholar]
- 36.Heys SD, Sarkar T, Hutcheon AW. Primary docetaxel chemotherapy in patients with breast cancer: impact on response and survival. Breast cancer research and treatment. 2005;90(2):169–185. doi: 10.1007/s10549-004-1001-0. [DOI] [PubMed] [Google Scholar]
- 37.Kuroi K, Toi M, Tsuda H, Kurosumi M, Akiyama F. Issues in the assessment of the pathologic effect of primary systemic therapy for breast cancer. Breast cancer (Tokyo, Japan) 2006;13(1):38–48. doi: 10.2325/jbcs.13.38. [DOI] [PubMed] [Google Scholar]
- 38.Mazouni C, Kau SW, Frye D, Andre F, Kuerer HM, Buchholz TA, Symmans WF, Anderson K, Hess KR, Gonzalez-Angulo AM, et al. Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2007;18(5):874–880. doi: 10.1093/annonc/mdm008. [DOI] [PubMed] [Google Scholar]
- 39.Glück S, Ross JS, Royce M, McKenna EF, Jr., Perou CM, Avisar E, Wu L. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine +/− trastuzumab. Breast cancer research and treatment. 2011 doi: 10.1007/s10549-011-1412-7. Journal Article. [DOI] [PubMed] [Google Scholar]
- 40.Glück S, McKenna EF, Jr., Royce M. XeNA: capecitabine plus docetaxel, with or without trastuzumab, as preoperative therapy for early breast cancer. International journal of medical sciences. 2008;5(6):341–346. doi: 10.7150/ijms.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Minckwitz G, Raab G, Caputo A, Schutte M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann H, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(12):2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 42.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. Journal of the National Cancer InstituteMonographs. 2001;30(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 43.Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. Journal of clinical oncology. 2006;24(7):1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 44.Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. The lancet oncology. 2005;6(4):229–239. doi: 10.1016/S1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- 45.Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. The Journal of pharmacology and experimental therapeutics. 2001;298(1):339–345. [PubMed] [Google Scholar]
- 46.Pazoles CJ, Gerstein H. NOV-002, a chemoprotectant/ immunomodulator, added to first-line carboplatin/paclitaxel in advanced non-small cell lung cancer (NSCLC): a randomized phase 1/2, open-label, controlled study. J Clin Oncol. 2006;24(18S) [Google Scholar]
- 47.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature medicine. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 48.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Current opinion in immunology. 2008;20(5):504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182(10):6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 50.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer immunology, immunotherapy: CII. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, Dunn M, Urbas P, Daud A, DeConti R, et al. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. Journal of immunotherapy. 2010;33(8):817–827. doi: 10.1097/CJI.0b013e3181ecccad. [DOI] [PubMed] [Google Scholar]