SUMMARY
SETTING
Primary health clinics in Vitoria, Espirito Santo, Brazil.
OBJECTIVE
To identify risk factors associated with patient and health care delays among patients seeking care at primary health clinics.
METHODS
A prospective study among tuberculosis (TB) patients diagnosed in Vitoria between 1 January 2003 and 30 December 2007. A questionnaire ascertained the date of onset and duration of TB symptoms and medical records were reviewed. Between-group distributions of delay were compared and multivariate logistic regression was performed.
RESULTS
Of 304 patients, 296 (97%) reported at least one TB symptom presenting for the first time to a qualified health service; 244 (80%) reported cough > 3 weeks. Median health care delay was 30 days (range 5–68), and median total delay was 110 days (range 26–784). Multivariate analysis revealed any cough (ORadj 7.35, 95%CI 2.40–22.5) and weight at TB diagnosis < 60 kg (ORadj 5.92, 95%CI 1.83–19.1) to be associated with patient delay of ≥30 days. Factors increasing risk of prolonged delay (≥90 days) were age ≥30 years (ORadj 1.93, 95%CI 1.09–3.43) and chest pain (ORadj 2.42, 95%CI 1.29–4.53).
CONCLUSION
Improving health care workers’ education regarding TB symptoms and implementing active case finding in targeted populations may reduce delays.
Keywords: tuberculosis, epidemiology, delayed diagnosis
THE PRIMARY GOAL of tuberculosis (TB) control programs is to minimize transmission within the community and reduce TB incidence by detecting and treating active TB disease as early as possible. A major contributor to ongoing transmission is delay in diagnosis of TB. Diagnostic delays worsen disease prognosis at the individual level and amplify transmission within the community.1,2
The current agenda in global TB control is to develop better and faster tools for diagnosing TB once a symptomatic patient is found or presents to the health care system, and to provide appropriate therapy. Reducing delays in accessing care for symptomatic patients is of considerable public health importance. Studies in all regions of the world have investigated patient and health care delays for TB diagnosis, and findings differ by country, setting and population.3-14
After the declaration of a state of emergency in TB control by the World Health Organization (WHO) in 1993, the Brazilian Ministry of Health changed the structure of the Brazilian TB program. The vertical program was decentralized and city health departments were made responsible for TB diagnosis and treatment. In 2004, TB was incorporated into the Family Health Unit programs, which are now the primary implementers of DOTS.15,16
Brazil follows the WHO-recommended passive case-finding guidelines, in which people with TB-related symptoms are identified when they seek care at a basic health clinic and are then referred to the specialized TB primary care unit for diagnosis, treatment and management. Early detection of TB is dependent upon patients perceiving the need to seek care, then presenting to a health clinic and being properly diagnosed. A recent study among community health care workers in Brazil found that numerous flaws were observed in TB knowledge and control measures among community health workers.17 Timely and accurate TB diagnosis and treatment are vital, and prolonged delays can cause more infections per case.18
In 2000, the Nucleo de Doenças Infecciosas from the Universidade Federal do Espírito Santo (UFES) organized a network of mycobacteriology laboratories in four municipalities of the metropolitan area of Vitoria to connect local TB clinics in an effort to improve case detection and expedite treatment. Although it is not standard practice in Brazil, the city of Vitoria is currently the only place in the country where cultures are routinely performed for all suspected TB cases in an effort to increase case detection. A centralized electronic registry system has also been developed, which can be accessed through the internet by the TB clinics. Despite these efforts, the number of reported cases in the State has remained stable over the past 10 years.15
Identifying the prevalence of prolonged delays among TB patients and associated factors can help TB control programs and health care providers to improve TB diagnosis and treatment.19 We conducted a study to determine the extent of patient and health care delays for TB at primary care units in Vitoria City, Espirito Santo, Brazil.
METHODS
In a prospective study between 1 January 2003 and 31 December 2007, all patients seeking care for TB in the two largest public TB clinics in Vitoria were recruited into the study. These two primary health facilities were responsible for 73% of all reported TB cases during the study period. It should be noted that in Brazil, TB diagnosis and treatment are provided only by the public health system and are free of charge. TB drugs cannot be purchased through the private sector.
Interviewers recorded the date of symptom onset, duration of TB symptoms (cough, fatigue, fever, night sweats and weight loss) and the date of confirmatory laboratory TB diagnosis. All patients received DOTS-based anti-tuberculosis treatment. Medical record data were checked against the interviews to create a conservative timeline of events leading to a TB diagnosis. All patients were followed by their physician, and a second evaluation was conducted at 6 months to assess treatment completion.
The study was approved by the Institutional Review Boards at UFES. All patients provided written informed consent.
Study population and operational definitions
The two main inclusion criteria for all patients were: 1) patients spontaneously seeking care at primary health clinics; and 2) patients on anti-tuberculosis treatment whose diagnosis was based on clinical symptoms and/or a positive direct smear with bacteriological confirmation of infection by a positive culture for Mycobacterium tuberculosis according to standard procedures.20,21 Culture was used as a definitive entry criterion, as a culture conversion at 6 months follow-up was used to define cure.
Patient delay was defined as the period from onset of the first symptom(s) possibly related to pulmonary TB to the date when the patient first contacted qualified primary health care services as a result of the symptoms.
Health system delay was the period from the date of a patient’s first contact with the primary health care service to the date of diagnosis. Total delay was defined as the time from onset of first symptoms to the date of TB diagnosis. Treatment delay was defined as the time from confirmatory laboratory diagnosis until treatment initiation.
Cure was defined as a negative M. tuberculosis culture and a chest radiograph inconsistent with active TB after 6 months of anti-tuberculosis treatment.
Statistical methods
Covariates were divided into two subgroups: demographic factors (e.g., age, race/ethnicity, substance abuse) and clinical factors (e.g., symptoms and existing medical conditions). Median delays of all covariates were calculated and the Mann-Whitney test was used to compare between-group distribution of delays. In addition, univariate logistic regression was performed for each variable of interest, and odds r atios (ORs) with a P < 0.10 were included in the multivariate logistic regression for the calculation of adjusted ORs (ORadj) for two models for patient delay: in the first model delay was categorized as ≥30 days, and in the second model it was categorized as ≥90 days. Health care delay and total delay were analyzed similarly, although each used only the median delay as a cut-off for characterizing patients as delayed or not delayed.
All analyses were conducted using the Stata® statistical package, Version 9 (Stata Corp, College Station, TX, USA, 2001).
RESULTS
Study participants and characteristics
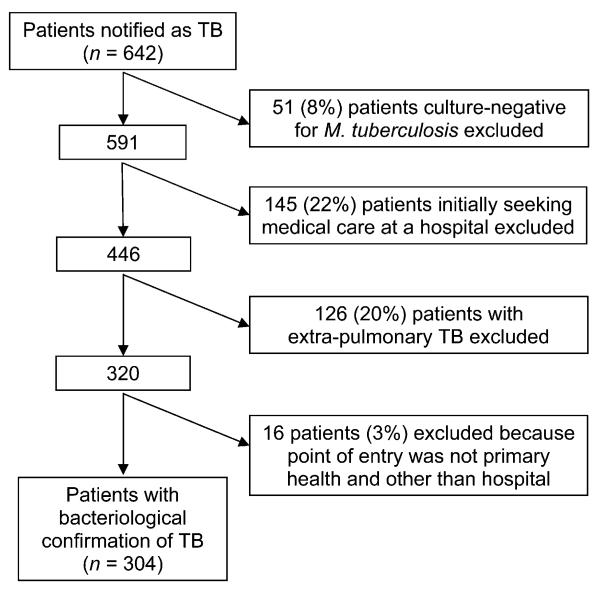
Between 1 January 2003 and 30 December 2007, 642 TB patients were reported in Vitoria City. Fiftyone (8%) patients without a positive M. tuberculosis culture were excluded, as were 145 (22%) patients initially seeking medical care from a hospital, and 126 (20%) patients with extra-pulmonary disease and no evidence of pulmonary involvement (Figure 1). A further 16 patients (3%) were excluded because their point of entry was not the primary health program. The final study population consisted of 304 patients with bacteriological confirmation.
Figure 1.
Patient enrollment plan. TB = tuberculosis.
Demographic, clinical and diagnostic characteristics for all patients are summarized in Tables 1 and 2, stratified by type of treatment delay. The median age of all participants was 31 years and 62% were male. Among the 304 study patients, 296 (97%) reported at least one TB symptom. Thirty-one patients (10%) reported 1–3 symptoms, 75 (25%) 4–6 symptoms, 102 (34%) 7–9 symptoms and 88 (29%) ≥10 symptoms. All symptomatic patients had at least one respiratory symptom (cough, shortness of breath, chest pain, hemoptysis and/or sputum production with or without purulent sputum). Two hundred and fortyfour (80%) reported cough > 3 weeks; the median duration of cough for all patients was 76 days. All eight patients reporting no TB symptoms at their first health care visit were later diagnosed by culture.
Table 1.
Demographic factors and patient delays among laboratory confirmed TB patients in Vitoria, Brazil, 2003–2007
| Symptoms and associated illness |
n (%) | Median | P value | Delay ≥ 30 days OR (95%CI) |
P value | Delay ≥ 90 days OR (95%CI) |
P value |
|---|---|---|---|---|---|---|---|
| Age, years | 0.02 | ||||||
| ≥30 | 159 (54) | 90 | 2.00 (0.98–4.08) | 0.06 | 1.54 (0.97–2.45) | 0.07 | |
| <30 | 136 (46) | 60 | 1.00 | 1.00 | |||
| Sex | 0.36 | ||||||
| Male | 184 (62) | 85 | 1.00 | 0.84 | 1.00 | 0.50 | |
| Female | 111 (38) | 63 | 1.08 (0.52–2.22) | 0.85 (0.53–1.36) | |||
| Any education | 0.02 | ||||||
| Yes | 151 (51) | 60 | 0.48 (0.23–1.00) | 0.05 | 0.64 (0.40–1.01) | 0.06 | |
| No | 144 (49) | 90 | 1.00 | 1.00 | |||
| Employed | 0.55 | ||||||
| Yes | 195 (66) | 70 | 0.72 (0.33–1.56) | 0.83 | 0.86 (0.53–1.39) | 0.53 | |
| No | 100 (34) | 90 | 1.00 | 1 .00 | |||
| Marital status | 0.59 | ||||||
| Single | 117 (40) | 78 | 1.00 | 1.00 | |||
| Married | 137 (46) | 84 | 1.70 (0.81–3.57) | 0.16 | 1.04 (0.63–1.70) | 0.88 | |
| Divorced | 37 (13) | 60 | 2.19 (0.61–7.89) | 0.23 | 0.80 (0.38–1.69) | 0.56 | |
| Ever smoked | 0.02 | ||||||
| Yes | 171 (58) | 90 | 3.21 (1.54–6.71) | 0.002 | 1.52 (0.95–2.42) | 0.08 | |
| No | 123 (42) | 60 | 1.00 | 1.00 | |||
| Drink alcohol | <0.01 | ||||||
| Yes | 54 (18) | 90 | 4.27 (0.99–18.4) | 0.05 | 1.42 (0.78–2.56) | 0.25 | |
| No | 241 (82) | 65 | 1.00 | 1.00 | |||
| Recent contact with a TB patient |
0.80 | ||||||
| Yes | 166 (56) | 79 | 1.17 (0.57–2.39) | 0.67 | 0.91 (0.57–1.46) | 0.70 | |
| No | 121 (41) | 75 | 1.00 | 1.00 | |||
TB = tuberculosis; OR = odds ratio; CI = confidence interval.
Table 2.
Clinical characteristics and patient delay among laboratory confirmed tuberculosis patients in Vitoria, Brazil, 2003–2007
| Symptoms and associated illness |
n (%) | Median | P value | Delay ≥ 30 days OR (95%CI) |
P value | Delay ≥ 90 days OR (95%CI) |
P value |
|---|---|---|---|---|---|---|---|
| Fever | 0.56 | ||||||
| Yes | 193 (65) | 65 | 1.83 (0.91–3.70) | 0.09 | 0.76 (0.47–1.23) | 0.27 | |
| No | 102 (35) | 90 | 1.00 | 1.00 | |||
| Night sweats | 0.23 | ||||||
| Yes | 170 (58) | 90 | 1.61 (0.80–3.25) | 0.18 | 1.22 (0.77–1.94) | 0.40 | |
| No | 125 (42) | 65 | 1.00 | 1.00 | |||
| Any cough | <0.001 | ||||||
| Yes | 244 (83) | 90 | 9.19 (4.31–19.6) | <0.001 | 2.96 (1.53–5.76) | 0.001 | |
| No | 51 (17) | 30 | 1.00 | 1.00 | |||
| Chest pain | <0.00 | ||||||
| Yes | 205 (69) | 90 | 1.75 (0.86–3.58) | 0.12 | 2.46 (1.46–4.12) | 0.001 | |
| No | 90 (31) | 60 | 1.00 | 1.00 | |||
| Weight loss | 0.04 | ||||||
| Yes | 224 (76) | 90 | 4.45 (2.16–9.15) | <0.001 | 1.89 (1.09–3.28) | 0.02 | |
| No | 71 (24) | 60 | 1.00 | 1.00 | |||
| Sputum production | <0.001 | ||||||
| Yes | 228 (77) | 90 | 5.64 (2.72–1 1.7) | <0.001 | 2.13 (1.21–3.75) | 0.009 | |
| No | 67 (23) | 45 | 1.00 | 1.00 | |||
| Purulent sputum | 0.04 | ||||||
| Yes | 187 (63) | 90 | 3.16 (1.54–6.48) | 0.002 | 1.29 (0.80–2.08) | 0.29 | |
| No | 108 (37) | 60 | 1.00 | 1.00 | |||
| Shortness of breath | 0.06 | ||||||
| Yes | 139 (47) | 90 | 1.68 (0.81–3.45) | 0.16 | 1.22 (0.77–1.93) | 0.40 | |
| No | 156 (53) | 60 | 1.00 | 1.00 | |||
| Hemoptysis | 0.66 | ||||||
| Yes | 94 (32) | 75.5 | 0.61 (0.30–1.25) | 0.18 | 0.97 (0.59–1.58) | 0.89 | |
| No | 201 (68) | 77 | 1.00 | 1.00 | |||
| Fatigue | 0.07 | ||||||
| Yes | 141 (48) | 90 | 1.51 (0.72–3.15) | 0.27 | 1.23 (0.76–1.97) | 0.40 | |
| No | 133 (45) | 72 | 1.00 | 1.00 | |||
| Cavitary lesion | 0.07 | ||||||
| Yes | 112 | 90 | 1.26 (0.60–2.63) | 0.54 | 1.66 (1.03–2.66) | 0.04 | |
| No | 183 | 60 | 1.00 | 1.00 | |||
| Loss of appetite | 0.20 | ||||||
| Yes | 150 | 84 | 1.74 (0.85–3.54) | 0.13 | 1.13 (0.72–1.79) | 0.59 | |
| No | 145 | 60 | 1.00 | 1.00 | |||
| Diarrhea | 0.67 | ||||||
| Yes | 15 | 75 | 2.00 (0.26–15.7) | 0.51 | 0.93 (0.33–2.62) | 0.89 | |
| No | 280 | 76.5 | 1.00 | 1.00 | |||
| Myalgia | 0.67 | ||||||
| Yes | 18 | 60 | 2.46 (0.32–19.1) | 0.39 | 0.51 (0.19–1.40) | 0.19 | |
| No | 277 | 80 | 1.00 | 1.00 | |||
| Lymphadenopathy | 0.73 | ||||||
| Yes | 1 | 60 | |||||
| No | 294 | 76.5 | |||||
| Bone pain | 0.44 | ||||||
| Yes | 9 | 60 | 1.12 (0.14–9.19) | 0.92 | 0.52 (0.13–2.13) | 0.36 | |
| No | 286 | 77.5 | 1.00 | 1.00 | |||
| Visual problem | 0.18 | ||||||
| Yes | 7 | 90 | 2.72 (0.52–14.2) | 0.24 | |||
| No | 288 | 75 | 1.00 |
OR = odds ratio; CI = confidence interval.
Patient delay
Median patient delays are presented in Tables 1 and 2. The median patient delay for all patients reporting symptoms (n = 295) was 76 days (range 1–730, interquartile range 85 days). Twelve patients (4%) sought care for symptoms within 7 days of onset, 36 (12%) within <30 days, and 143 (48%) waited ≥90 days before seeking medical care.
In univariate analysis, age <30 years, no prior formal education, history of tobacco use, current alcohol consumption, and history of cough, weight loss, sputum production and purulent sputum were carried forward to the multivariate analysis, as they were statistically significant at the ≤0.10 level with patient delays of ≥30 days. In a separate univariate analysis, age <30 years, presence of cavitary lesion, no prior formal education, history of tobacco use, history of cough, chest pain, weight loss and sputum production were carried forward to the multivariate analysis because they were statistically significant at the ≤0.10 level with patient delays of ≥90 days. These were included in the final analysis.
Multivariate analysis revealed cough (ORadj = 6.67, 95% confidence interval [CI] 2.65–16.79) and initial weight <60 kg (ORadj =3.45, 95%CI 1.40–8.48) to be associated with patient delay of ≥30 days. The multivariate model with ≥90 days patient delay found any cough (ORadj =2.58; 95%CI 1.20–5.57) and chest pain (ORadj =2.69 95%CI 1.55–4.66) to be associated with increased risk of patient delay.
Health care delay
The median heath care delay was 30 days (range 5–68). Male sex, higher education, no history of cough, no weight loss, normal chest X-ray, negative acid-fast bacilli smear and lack of sputum production were all associated with health care delays of >30 days (Table 3). Multivariate analysis revealed two characteristics associated with health care delays of >30 days: no history of cough (ORadj = 24.5, 95%CI 5.60–106.9) and normal chest X-ray (ORadj = 2.3, 95%CI 1.29–4.09).
Table 3.
Health care system delays and total delay: diagnostic factors
| Symptoms and associated illness |
n (%) | Health care delay ≥ 30 days OR (95%CI) |
P value | n (%) | Total delay ≥ 110 days OR (95%CI) |
P value |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≥30 | 86 (52.8) | 0.87 (0.54–1.41) | 0.568 | 90 (58.8) | 1.44 (0.89–2.33) | 0.109 |
| <30 | 77 (47.2) | 63 (41.2) | ||||
| Sex | ||||||
| Male | 104 (63.8) | 1.19 (0.73–1.95) | 0.449 | 108 (70.7) | 1.39 (0.85–2.26) | 0.157 |
| Female | 59 (36.2) | 54 (35.3) | ||||
| Any education | ||||||
| Yes | 99 (60.7) | 2.08 (1.28–3.39) | 0.001 | 70 (45.7) | 0.58 (0.36–0.94) | 0.021 |
| No | 64 (39.3) | 83 (54.3) | ||||
| Ever smoked | ||||||
| Yes | 87 (53.4) | 0.70 (0.43–1.13) | 0.130 | 96 (62.7) | 1.61 (0.99–2.62) | 0.038 |
| No | 76 (46.6) | 56 (37.3) | ||||
| Drink alcohol | ||||||
| Yes | 26 (16.0) | 0.76 (0.40–1.44) | 0.374 | 34 (22.2) | 1.87 (0.98–3.62) | 0.040 |
| No | 137 (84.0) | 119 (77.8) | ||||
| Any cough | ||||||
| Yes | 152 (93.2) | 0.19 (0.21–0.93) | 0.022 | 150 (98.0) | 3.54 (0.88–20.3) | 0.044 |
| No | 11 (6.8) | 3 (2.0) | ||||
| Respiratory symptoms | ||||||
| Yes | 153 (93.8) | 0.0 (0.0–1.09) | 0.061 | 152 (99.4) | 1.07 (0.01–84.5) | 0.961 |
| No | 10 (6.2) | 1 (0.6) | ||||
| Sputum production | ||||||
| Yes | 102 (62.6) | 0.18 (0.09–0.35) | 0.000 | 124 (81.0) | 1.87 (1.06–3.31) | 0.019 |
| No | 61 (37.4) | 29 (19.0) | ||||
| Purulent sputum | ||||||
| Yes | 83 (51.0) | 0.35 (0.21–0.59) | 0.000 | 101 (66.0) | 1.42 (0.87–2.33) | 0.131 |
| No | 80 (49.0) | 52 (33.9) | ||||
| Cavitary lesion | ||||||
| Yes | 41 (25.2) | 0.33 (0.19–0.55) | 0.000 | 61 (39.8) | 1.30 (0.79–2.13) | 0.270 |
| No | 122 (74.8) | 92 (60.2) | ||||
| Hemoptysis | ||||||
| Yes | 52 (32.0) | 1.1 (0.65–1.85) | 0.690 | 50 (32.7) | 1.18 (0.70–1.98) | 0.504 |
| No | 111 (68.0) | 103 (67.3) | ||||
| AFB–positive | ||||||
| Yes | 106 (65.0) | 0.56 (0.33–0.96) | 0.027 | 113 (73.8) | 1.39 (0.82–2.36) | 0.183 |
| No | 57 (35.0) | 40 (26.2) | ||||
| Weight loss | ||||||
| Yes | 113 (69.3) | 0.61 (0.34–1.06) | 0.063 | 122 (79.8) | 1.89 (1.08–3.30) | 0.015 |
| No | 50 (30.7) | 31 (20.2) |
OR = odds ratio; CI = confidence interval; AFB = acid–fast bacilli.
Total delay
The median total delay was 110 days (range 26–784). In univariate analysis, higher education, no history of tobacco and alcohol use, no history of cough, no weight loss and sputum production were significantly associated with total delays of >110 days, although no variables were significant on multivariate analysis (Table 3).
The median time between laboratory diagnosis and initiation of treatment was 4 days (range 1–7). Time to treatment did not differ by patient delay or health care delay.
Endpoint analysis
At 6 months treatment follow-up, all patients underwent culture (Löwenstein-Jensen and BACTEC 12B; BACTEC, Rochester, UK), chest radiograph and physical evaluation. Of the 304 patients studied, 297 (98%) had a negative culture on both methods and no findings of pulmonary TB on chest X-ray, indicating cure. Among the seven patients (2%) who did not achieve cure, the median patient delay was 60 days, compared to 76.5 days for those who had reached cure at the end of 6 months (P = 0.093). All 7 patients underwent retreatment and achieved cure.
DISCUSSION
In our study, the median time between initial TB symptoms and first medical consultation, first consultation and diagnosis, and diagnosis and treatment initiation was respectively 76, 30 and 4 days, with a total delay of 110 days. This delay is longer than in a recently published report from Recife, Brazil, and longer than those reported in Viet Nam,22 Thailand,23 South Africa24 and the United States,25 and, other than a study performed in Tanzania (patient delay was 162 days),26 our results exceed all other study results reported in a recent systematic review where patient delay ranged from 7 to 60 days.14 This prolonged delay reflects the strategy of passive case detection in Brazil, and suggests a patient population that can be targeted for intensified case detection efforts.27
Patient delay was independent of sex, which differs from other studies reporting greater delays in women9,23 and in men,28-31 but was significantly reduced among younger TB patients and those who were better educated.7 Although these results were not significant in multivariate analysis, persons with higher education tend to have more flexible schedules, as do younger people, particularly students, and are better able to attend primary care facilities that are often only open during regular work hours. Although education did not reduce time to diagnosis, education of the population, specifically about TB symptoms, might reduce delays.32
Smokers had longer delays in univariate analysis, but this association was not maintained in multivariate analysis. Smoking often leads to delays in TB diagnosis,4 as prolonged cough is attributed to ‘smoker’s cough’ by both patients and health care providers.
Our results are similar to those reported among low- and middle-income countries in a recently published systematic review14 where the average patient delay was 28.4 days, ranging from 2 days in China to 87 days in Pakistan.
The Recife study reported a mean total delay of 142 days and a median of 90 days.27 The authors suggested that although access to health care is not difficult in their urban setting, ease of access did not reduce delays. One possible explanation for the total delay in starting patients on treatment in all studies could be the limited diagnostic facilities and poorly trained personnel at the same clinic level where patients are repeatedly seen for health care.33 In our study, physicians diagnosed patients with longer patient delays more quickly than those with shorter delays, suggesting that disease in these patients had become more clinically apparent at time of presentation. Moreover, patients with abnormal chest radiographs at first presentation and those presenting with cough were diagnosed more quickly, suggesting that physicians were efficiently diagnosing patients with the most advanced disease. The corollary of this finding is that many patients are not being diagnosed when they arrive with early TB symptoms. Physicians need to understand the epidemiology of TB in this population where patients often present early in disease progression, and proper use of currently available TB diagnostics may improve diagnostic efficiency.34
It is postulated that TB control can best be achieved if patient delay is <3 weeks, and ideally <2 weeks.31 In our study, 90% of our patients exceeded this limit, 50% by more than 7 weeks. Our study reports median health system delays of 4 weeks. The total time from symptom onset to TB diagnosis was about 16 weeks, 11 weeks more than is deemed acceptable for smearpositive TB patients, considering that more than 70% of our sample were smear-positive TB patients.35
There was no association between increased delay and treatment type or cure,7 but all of those patients who had not reached cure at 6 months had cavitary lesions at the beginning of treatment. Patients with cavitary disease usually have a higher bacterial load than those without cavities, and may take longer to achieve cure.30 Although cure was not affected by delays in diagnosis in this study, reducing patient delay is still an important component in reducing TB transmission in the community.36
Although 90% of our patients had more than four symptoms and at least one respiratory symptom, significant delays occurred nevertheless. To reduce detection delays, the primary care service should query all patients reporting to a primary health care unit about respiratory symptoms, regardless of their particular health complaint, and, if present, should screen for pulmonary TB. A recent publication from an out-patient center in Rio de Janeiro, Brazil, detected similar rates of TB among patients with a cough of <3 weeks’ duration and those with a cough of ≥3 weeks.37
Despite the availability of culture for all patients in Vitoria, the number of reported cases has been stable over the past 10 years. This stability could reflect two patterns. First, the majority of cases were already seeking care at primary care facilities and being diagnosed adequately by culture, and therefore reducing delays but not changing the total number of notified cases, as there is currently no strategy for active case finding. Second, patients with the longest delays initially sought care at hospitals and were therefore excluded from our analysis. This has been noted in other studies,31,38 and in our study, where 145/642 (22%) were excluded because the entry point was the hospital.
It is more feasible to educate health professionals about TB than the general population. We conducted two studies in the same health care units looking at knowledge of TB, one with heath care workers (HCWs) and the other among nurses and physicians.17,39 The results revealed significant knowledge about TB among nurses and physicians, and a strong lack of knowledge among HCWs. A longer period in employment was positively associated with an increased understanding of the disease and of TB control activities, although cough of >3 weeks was recognized as a symptom of TB by only 27% (28/105) of HCWs. Training in Vitoria is not regular, and only four courses had been conducted over the last 5 years. Unfortunately, there are frequent changes among HCWs, and in this study only 63% had been in their posts for more than 3 years. This highlights the need for more continuing education for HCWs, as they are often the first to encounter patients with TB symptoms but rarely recognize them as being suggestive of TB. By definition, health care delays should be considerably shorter, if basic diagnostic tools and trained staff are available at the primary health clinics. However, in our setting less than 10% of attending medical professionals are physicians trained in chest disease, infectious diseases, internal medicine or family health.
The major constraints to reaching the WHO global TB targets include a lack of qualified human resources, lack of health infrastructure, poor decentralization of the health system, and little coordination with the private sector.40 In our setting, our health care workers need to be better qualified to conduct intensive case-finding programs that will ultimately reduce detection delays and reduce TB transmission at the community level.
Acknowledgements
Financial support: Edital MCT/CNPq/MS-SCTIE-DECIT 25/ 2006-Estudo de Doenças Negligenciadas and ICOHRTA 5 U2R TW006883-02. JEG was supported by the National Institutes of Health grant AI066994. JB was supported by Alex G Booth Fellowship from Harvard College Office of Career Services.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Bjune G. Tuberculosis in the 21st century: an emerging pandemic? Norsk Epidemiologi. 2005;15:133–139. [Google Scholar]
- 3.Ward J, Siskind V, Konstantinos A. Patient and health care system delays in Queensland tuberculosis patients, 1985-1998. Int J Tuberc Lung Dis. 2001;5:1021–1027. [PubMed] [Google Scholar]
- 4.Calder L, Gao W, Simmons G. Tuberculosis: reasons for diagnostic delay in Auckland. N Z Med J. 2000;113:483–485. [PubMed] [Google Scholar]
- 5.Enarson DA, Grzybowski S, Dorken E. Failure of diagnosis as a factor in tuberculosis mortality. Can Med Assoc J. 1978;118:1520–1522. [PMC free article] [PubMed] [Google Scholar]
- 6.Lönnroth K, Thuong LM, Linh PD, Diwan VK. Delay and discontinuity—a survey of TB patients’ search of a diagnosis in a diversified health care system. Int J Tuberc Lung Dis. 1999;3:992–1000. [PubMed] [Google Scholar]
- 7.Lienhardt C, Rowley J, Manneh K, et al. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int J Tuberc Lung Dis. 2001;5:233–239. [PubMed] [Google Scholar]
- 8.Ngamvithayapong J, Yanai H, Winkvist A, Diwan V. Health seeking behaviour and diagnosis for pulmonary tuberculosis in an HIV-epidemic mountainous area of Thailand. Int J Tuberc Lung Dis. 2001;5:1013–1020. [PubMed] [Google Scholar]
- 9.Yamasaki-Nakagawa M, Ozasa K, Yamada N, et al. Gender difference in delays to diagnosis and health care seeking behaviour in a rural area of Nepal. Int J Tuberc Lung Dis. 2001;5:24–31. [PubMed] [Google Scholar]
- 10.Long NH, Johansson E, Lönnroth K, Eriksson B, Winkvist A, Diwan VK. Longer delays in tuberculosis diagnosis among women in Vietnam. Int J Tuberc Lung Dis. 1999;3:388–393. [PubMed] [Google Scholar]
- 11.Godfrey-Faussett P, Kaunda H, Kamanga J, et al. Why do patients with a cough delay seeking care at Lusaka urban health centres? A health systems research approach. Int J Tuberc Lung Dis. 2002;6:796–805. [PubMed] [Google Scholar]
- 12.Rodger A, Jaffar S, Paynter S, Hayward A, Carless J, Maguire H. Delay in the diagnosis of pulmonary tuberculosis, London, 1998–2000: analysis of surveillance data. BMJ. 2003;326:909–910. doi: 10.1136/bmj.326.7395.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran GJ, McCabe F, Morgan MT, Talan DA. Delayed recognition and infection control for tuberculosis patients in the emergency department. Ann Emerg Med. 1995;26:290–295. doi: 10.1016/s0196-0644(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 14.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. Review. BMC Infect Dis. 2009;9:91. doi: 10.1186/1471-2334-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Saúde Ministério, de Vigilância em Saúde Secretaria, Portuguese . Programa Nacional de Controle da Tuberculose. Ministério da Saúde; Brasilia, Brazil: 2004. [Google Scholar]
- 16.Ruffino-Netto A, Villa TCS. Tuberculosis treatment: DOTS implementation in some regions of Brazil background and regional features. Pan American Health Organization; Brasília, Brazil: 2007. [Google Scholar]
- 17.Maciel ELN, Vieira RCA, Milani EC, Brasil M, Fregona G, Dietze R. Community health workers and tuberculosis control: knowledge and perceptions. Cad Saúde Pública [serial on the Internet] 2008;24:1377–1386. doi: 10.1590/s0102-311x2008000600018. http://www.scielo.br/scielo. php?script=sci_arttext&pid=S0102-311X2008000600018&lng=en Accessed November 2008. [DOI] [PubMed] [Google Scholar]
- 18.Sherman LF, Fujiwara PI, Cook SV, Bazerman LB, Frieden TR. Patient and health care system delay in the diagnosis and treatment of tuberculosis. Int J Tuberc Lung Dis. 1999;3:1088–1095. [PubMed] [Google Scholar]
- 19.Farah MG, Rygh JH, Steen TW, Selmer R, Heldal E, Bjune G. Patient and health care system delay in the start of tuberculosis treatment in Norway. BMC Infect Dis. 2006;6:33. doi: 10.1186/1471-2334-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent PT, Kubica GP. Public health mycobacteriology: a guide for the level III laboratory. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA, USA: 1985. [Google Scholar]
- 21.World Health Organization . Laboratory services in tuberculosis control. II. Microscopy. WHO; Geneva, Switzerland: 1998. WHO/TB.98.258. [Google Scholar]
- 22.Huong NT, Vree M, Duong BD, et al. elays in the diagnosis and treatment of tuberculosis patients in Vietnam: a cross-sectional study. BMC Public Health. 2007;7:110. doi: 10.1186/1471-2458-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojpibulstit M, Kanjanakiritamrong J, Chongsuvivatwong V. Patient and health system delays in the diagnosis of tuberculosis in southern Thailand after health care reform. Int J Tuberc Lung Dis. 2006;10:422–428. [PubMed] [Google Scholar]
- 24.Pronyk PM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuber-culosis patients in rural South Africa. Int J Tuberc Lung Dis. 2001;5:619–627. [PubMed] [Google Scholar]
- 25.Golub JE, Bur S, Cronin WA, et al. Patient and health care system delays in pulmonary tuberculosis diagnosis in a low-incidence state. Int J Tuberc Lung Dis. 2005;9:992–998. [PubMed] [Google Scholar]
- 26.Wandwalo ER, Morkve O. Delay in tuberculosis case-finding and treatment in Mwanza, Tanzania. Int J Tuberc Lung Dis. 2000;4:133–138. [PubMed] [Google Scholar]
- 27.dos Santos MA, Albuquerque MF, Ximenes RA, et al. Risk factors for treatment delay in pulmonary tuberculosis in Recife, Brazil. BMC Public Health. 2005;5:25. doi: 10.1186/1471-2458-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altet Gómez MN, Alcaide Megías J, Canela Soler J, et al. Pulmonary symptomatic tuberculosis’ diagnostic delay study. Arch Bronconeumol. 2003;39:146–152. doi: 10.1016/s0300-2896(03)75348-4. [DOI] [PubMed] [Google Scholar]
- 29.Kiwuwa MS, Charles K, Harriet MK. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: a cross-sectional study. BMC Public Health. 2005;5:122. doi: 10.1186/1471-2458-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooi LN. Case-finding for pulmonary tuberculosis in Penang. Med J Malaysia. 1994;49:223–230. [PubMed] [Google Scholar]
- 31.Madebo T, Lindtjorn B. Delay in treatment of pulmonary tuberculosis: an analysis of symptom duration among Ethiopian patients. MedGenMed. 1999 Jun 18;:E6. [PubMed] [Google Scholar]
- 32.Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis. 2006;10:24–30. [PubMed] [Google Scholar]
- 33.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palaci M, Dietze R, Hadad DJ, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol. 2007;45:4064–4066. doi: 10.1128/JCM.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert ML, Van der Stuyft P. Delays to tuberculosis treatment: shall we continue to blame the victim? Trop Med Int Health. 2005;10:945–946. doi: 10.1111/j.1365-3156.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 36.Epstein MD, Schluger NW, Davidow AL, Bonk S, Rom WN, Hanna B. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1998;113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 37.Bastos LG, Fonseca LS, Mello FC, Ruffino-Netto A, Golub JE, Conde MB. Prevalence of pulmonary tuberculosis among respiratory symptomatic subjects in an out-patient primary health unit. Int J Tuberc Lung Dis. 2007;11:156–160. [PubMed] [Google Scholar]
- 38.El-Sony AI, Mustafa SA, Khamis AH, Enarson DA, Baraka OZ, Bjune G. The effect of decentralisation on tuberculosis services in three states of Sudan. Int J Tuberc Lung Dis. 2003;7:445–450. [PubMed] [Google Scholar]
- 39.Maciel EL, de Araújo WK, Giacomin SS, de Jesus FA, Rodrigues PM, Dietze R. Knowledge about tuberculosis by doctors and nurses, who work in the Family Health Strategy in the city of Vitoria, Espirito Santo State: a cross-sectional study. Cien Saude Colet. 2009;14(Suppl 1):S1395–S1402. doi: 10.1590/s1413-81232009000800012. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization . Global tuberculosis control: surveillance, planning, financing. WHO report 2004. WHO; Geneva, Switzerland: 2004. WHO/HTM/ TB/2004.331. [Google Scholar]