Abstract
The canonical Wnt/β-catenin pathway is an ancient and evolutionarily conserved signaling pathway that is required for the proper development of all metazoans, from the basal demosponge Amphimedon queenslandica to humans. Misregulation of Wnt signaling is implicated in many human diseases, making this pathway an intense area of research in industry as well as academia. In this review, we explore our current understanding of the molecular steps involved in the transduction of a Wnt signal. We will focus on how the critical Wnt pathway component, β-catenin, is in a “futile cycle” of constant synthesis and degradation and how this cycle is disrupted upon pathway activation. We describe the role of the Wnt pathway in major human cancers and in the control of stem cell self-renewal in the developing organism and in adults. Finally, we describe well-accepted criteria that have been proposed as evidence for the involvement of a molecule in regulating the canonical Wnt pathway.
Keywords: Wnt signaling, wnt pathways, beta-catenin
Introduction
Wnt signaling plays a fundamental role in the determination of cell fate, proliferation, polarity, and cell death during embryonic development, as well as in tissue homeostasis in adults. The Wnt pathway, named for its ligands, the Wnt family of secreted glycoproteins, was discovered more than 30 years ago, and the historical events that led to the discovery and naming of Wnt ligands highlight its importance in development and in human disease. In 1976, Sharma and Chopra described a Drosophila melanogaster mutant that exhibited reduced or absent wings and halteres (Sharma and Chopra 1976). Based on the mutant phenotype, they named this locus wingless (wg) and suggested that it played an important role in development. A few years later, Nusse and Varmus conducted a forward genetic screen to identify genes in mice that could lead to tumorigenesis (Nusse et al. 1984). Using mouse mammary tumor virus (MMTV) insertion sites, they identified a locus termed int-1, short for integration 1, which induced mouse mammary tumors. Comparative genomic studies revealed that wg and int-1 were homologs, and the names were merged into the mnemonic Wnt (Nusse et al. 1991). Overexpression of int-1 in Xenopus embryos induced the formation of an ectopic axis, demonstrating that it not only acts as an oncogene but also plays a critical role in early axis specification (McMahon and Moon 1989a,b). These studies collectively drew an implicit connection between the physiological role for Wnts in development and a potential pathophysiological role in carcinogenesis.
Forward genetic studies in Drosophila have been crucial in identifying Wnt pathway components. In 1980, Eric Wieschaus and Christiane Nusslein-Volhard identified a series of Drosophila mutants that controlled patterning of the early embryo (Nusslein-Volhard and Wieschaus 1980). This work was a watershed moment in developmental biology, for which they were awarded a Nobel Prize in 1995. The 15-year period after their initial publication produced a number of genetic and molecular studies that elucidated the role of these mutants within various signaling pathways and resulted in the discovery of key members of the Wnt pathway, including armadillo (the vertebrate homolog of β-catenin), dishevelled, shaggy (the vertebrate version of glycogen synthase kinase 3 or GSK3), frizzled, and arrow (Riggleman et al. 1989, 1990; Siegfried et al. 1992; Klingensmith et al. 1994; Bhanot et al. 1996; Wehrli et al. 2000).
The activation of the Wnt signaling pathway on the future dorsal side of the early Xenopus embryo is a critical event in the formation of the Spemann organizer, a tissue-organizing center found in vertebrates (Spemann and Mangold 1938). The role of Wnt in organizer formation was uncovered when mRNA of Wnt-1 and Xwnt8 was injected into Xenopus blastomeres. Ectopic activation of Wnt signaling on the future ventral side of the embryo was shown to induce a second organizer that coordinates the formation of a complete secondary body axis (Smith and Harland 1991; Sokol et al. 1991). Embryonic axis duplication was also found to be induced by overexpression of positive downstream components of the pathway (i.e. Dishevelled (Dsh) and β-catenin) or by inhibiting negative components of the pathway (i.e. inhibiting GSK3 activity or overexpressing dominant-negative Axin) (Dominguez et al. 1995; Guger and Gumbiner 1995; He et al. 1995; Sokol et al. 1995; Fagotto et al. 1999). To date, all major members of the Wnt pathway have been shown to be active in the Xenopus axis specification studies, and this assay represents a powerful tool to validate candidate genes as bona fide activators or inhibitors of Wnt signaling in vertebrates.
Numerous genetic and environmental perturbations of the Wnt pathway can lead to a variety of human diseases, ranging from birth defects to cancers [reviewed in MacDonald et al. (2009)]. One well-established connection between the Wnt pathway and human disease is a genetic lesion that occurs early in the onset of colon cancer. In 1991, a germline mutation in the Wnt pathway component adenomatous polyposis coli (APC) was identified in patients with familial adenomatous polyposis (FAP), a form of hereditary cancer (Kinzler et al. 1991; Nishisho et al. 1991). FAP patients inherit one defective allele of APC, and upon stochastic loss of the second allele develop colon adenomas (polyps) at an early age. These benign polyps frequently acquire other mutations and develop into invasive colon carcinomas. Later studies showed that loss of both APC alleles occurs in the large majority (>80%) of nonhereditary, sporadic colorectal cancers as well (Kinzler and Vogelstein 1996). Following this work, inappropriate activation of Wnt signaling was subsequently found in other cancers, including liver cancer, skin cancer, lung cancer, Wilms’ tumor, prostate cancer, and breast cancer [reviewed in Klaus and Birchmeier (2008); Table I]. A variety of developmental genetic defects were also shown to occur as a result of Wnt pathway misregulation, including defects in limb formation (tetra-amelia), bone ossification, eye vascularization, and tooth development (Gong et al. 2001; Boyden et al. 2002; Lammi et al. 2004; Niemann et al. 2004; Toomes et al. 2004; Xu et al. 2004b). Understanding the basis of the numerous human diseases resulting from misregulation of Wnt signaling and designing therapies for their treatment obviously require a detailed understanding of the molecular mechanism of the Wnt pathway.
Table I.
Wnt pathway alterations in cancer.
| Gene | Type of change | Cancer type | Reference |
|---|---|---|---|
| LRP5 | Missplicing/in-frame deletion/gain of function | Breast cancer | Bjorklund et al. (2009) |
| LRP6 | Upregulation | Breast cancer | Liu et al. (2010) |
| Axin1/2 | Truncation/loss of function | Colorectal cancer | Satoh et al. (2000) and Taniguchi et al. (2002) |
| Hepatocellular carcinoma | Liu et al. (2000) and Lammi et al. (2004) | ||
| Medulloblastoma | Dahmen et al. (2001) | ||
| APC | Truncation/loss of function | Colorectal cancer | Kinzler et al. (1991) and Nishisho et al. (1991) |
| Breast cancer | Bafico et al. (2004) and Ugolini et al. (2001) | ||
| Medulloblastoma | Huang et al. (2000) and Koch et al. (2001) | ||
| Hepatocellular carcinoma | Battagli et al. (2003) | ||
| CTNNB1 (β-catenin) | Missense/in-frame deletion/gain of function | Medulloblastoma | Eberhart et al. (2000) |
| Pancreatic cancer | Pasca di Magliano et al. (2007) | ||
| Hepatocellular carcinoma | de La Coste et al. (1998) | ||
| Colorectal cancer | Korinek et al. (1998) | ||
| CREBP (CBP) | Truncation/loss of function | Lymphoma/leukemia | Teo and Kahn (2011) |
| sFRP1 (secreted Wnt antagonist) | Downregulation by methylation | Breast cancer | Suzuki et al. (2008) |
| Hepatocellular carcinoma | Awakura et al. (2008a) | ||
| Lung cancer | Fukui et al. (2005) | ||
| Colorectal cancer | Suzuki et al. (2002) | ||
| WIF-1 (secreted Wnt antagonist) | Downregulation by methylation | Breast cancer | Wissmann et al. (2003) |
| Lung cancer | Mazieres et al. (2004) | ||
| Kidney cancer | Kawakami et al. (2009) | ||
| DVL | Upregulation | Lung cancer | Uematsu et al. (2003) |
| HSPGs | Upregulation | Pancreatic cancer | Pasca di Magliano et al. (2007) |
| DKK2 (Wnt antagonist) | Downregulation by methylation | Kidney cancer | Hirata et al. (2009) |
| TCF7L2 (TCF4) | Missense/deletion/ truncation/gain of function | Colorectal cancer | Cuilliere-Dartigues et al. (2006) |
| WTX | Truncation/deletion/loss of function | Wilms’ tumor | Ruteshouser et al. (2008) |
The current model of the Wnt pathway
Wnt signals can direct a wide variety of cellular responses in development, physiology, and disease. Originally, it was thought that a variety of cellular responses to Wnt signaling were mediated by the different transcriptional targets modulated in different cellular contexts. This original model, in which Wnt signaling alters transcription, is referred to as “canonical” Wnt signaling. It is now widely accepted that Wnt signaling can also activate distinct pathways that do not involve the nucleus or transcription, but rather signals cytoplasmic changes involving the actin cytoskeleton and intracellular calcium stores. These non-transcriptional Wnt pathways are loosely known as noncanonical Wnt signaling. Some aspects of these alternative modes of Wnt signaling occur via tyrosine kinase receptors (ROR and RYK receptors; Nusse 2008). Noncanonical Wnt signaling is outside the scope of this review, and the reader is referred to several reviews on the topic (Simons and Mlodzik 2008; Lai et al. 2009). Recent studies have questioned the simplicity of even a two-pathway response (van Amerongen et al. 2008). For clarity, we will use the term Wnt/β-catenin signaling to identify what has been commonly referred to as “canonical” signaling. This nomenclature specifies the ligand (Wnt) and the essential downstream transcriptional effector (β-catenin).
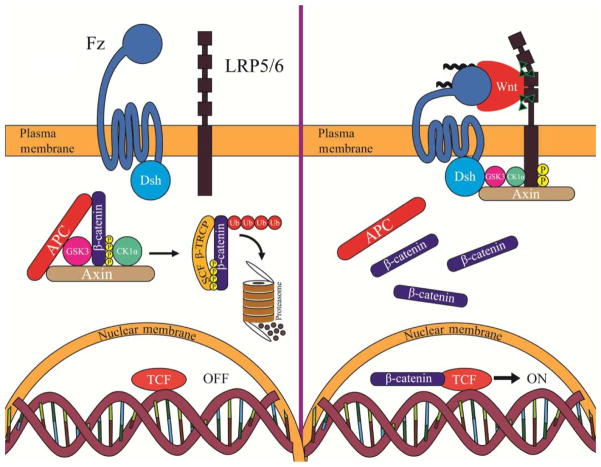
Wnt/β-catenin signal transduction, at its simplest, is a pathway that results in the cytoplasmic protein β-catenin entering the nucleus to modulate transcription. When the pathway is not activated, β-catenin is subject to a “futile cycle” of continual synthesis and destruction by the β-catenin destruction complex, comprised of the scaffold proteins Axin and APC and the kinases GSK3 and casein kinase 1 (CK1) (Figure 1). Wnt signaling removes APC from the complex and relocalizes the other components to the plasma membrane via the adaptor Dsh, thus stabilizing β-catenin which enters the nucleus to mediate transcription (Figure 1). Thus, Wnt/β-catenin signaling can be divided into three general molecular events: (1) surface receptor activation, (2) inhibition of the β-catenin destruction complex, and (3) activation of a Wnt-specific nuclear transcriptional complex. The next sections of this review consider each of these steps more closely.
Figure 1.
The current model of Wnt/β-catenin signaling. (Left panel) In the absence of Wnt, cytoplasmic β-catenin forms a complex with APC, Axin, GSK3, and CK1α. β-Catenin is phosphorylated by CK1α and subsequently phosphorylated by GSK3. The phosphorylated form of β-catenin is recognized by the E3 ubiquitin ligase SCFβ-TRCP, which targets β-catenin for proteasomal degradation. In the absence of nuclear β-catenin, Wnt target genes are repressed. APC, adenomatous polyposis coli; GSK3, glycogen synthase kinase 3; CK1α, casein kinase 1 alpha. (Right panel) In the presence of Wnt ligand, a receptor complex forms between Fz, LRP5/6, and Wnt. The recruitment of Dsh by Fz leads to LRP5/6 phosphorylation by CK1α and GSK3 followed by recruitment of Axin to LRP5/6. The latter disrupts Axin-mediated phosphorylation/degradation of β-catenin, leading to accumulation of β-catenin in the cytoplasm and its translocation to the nucleus, where it acts as a transcriptional co-activator with TCF to activate Wnt-responsive target genes. Fz, Frizzled; Dsh, Dishevelled; TCF, T-cell factor.
Step 1: Surface receptor activation
Wnt ligands
Wnt proteins are cysteine-rich morphogens of ~350–400 amino acids that can act in short- and long-range signaling. There are 19 vertebrate Wnts, and all appear to be able to activate the pathway. The crystal structure of Xenopus Wnt8 (XWnt8) bound to the cysteine-rich domain (CRD) of the mouse Frizzled (Fz) 8 receptor has been solved to 3.25 Å. The Wnt8 structure is bilobular with an N-terminal helical domain and a C-terminal extended β-hairpin stabilized by extensive disulfide bonds. Interactions between XWnt8 and Fz8 occur via extensions from each lobe (“thumb” and “finger”) to grasp the CRD of Fz8 on two distinct sites (Janda et al. 2012). All Wnts contain an N-terminal signal peptide for secretion and are N-linked glycosylated (Smolich et al. 1993; Willert et al. 2003; Takada et al. 2006; Komekado et al. 2007a). N-glycosylation of Wg (Drosophila Wnt homolog) has been shown to be stimulated by lipid modifications (Tanaka et al. 2002). Although an early study suggests that glycosylation is dispensable for Wnt secretion and activity (Mason et al. 1992), more recent studies demonstrate that mutating the glycosylation sites on Wnts blocks their secretion (Komekado et al. 2007b; Kurayoshi et al. 2007).
Wnts contain several charged amino acids and undergo a series of lipid modifications that affect their activity and secretion (Bradley and Brown 1990). Wnts have been shown to undergo acylation at Cys77 and Ser209 with palmitate and palmitoleate, respectively (Willert et al. 2003; Takada et al. 2006). Interestingly, the co-crystal structure of XWnt8–Fz8 CRD indicates that Cys77 is engaged in disulfide bonding, whereas Ser209 is acylated (likely palmitoleic acid). The palmitoleic acid lipid group was shown to dock within a hydrophobic groove on the CRD of Fz8 and, thus, plays a direct role in Wnt–Fz interaction (Janda et al. 2012).
The endoplasmic reticulum (ER)-embedded, multi-pass transmembrane O-acetyltransferase protein Porcupine (Porc) is the enzyme that mediates lipid modifications of Wnt (MacDonald et al. 2009; Port and Basler 2010). Porc was initially identified in Drosophila as a segment polarity gene and was the first gene shown to be required in Wnt-secreting cells (van den Heuvel et al. 1993). Loss of Porc function causes Wnts to accumulate in the ER (van den Heuvel et al. 1993; Kadowaki et al. 1996), whereas Porc over-expression results in a larger fraction of Wnts that are modified by lipids (Galli et al. 2007). It is not clear whether Porc is directly responsible for palmitoylation on Cys77 and Ser209 of Wnts or whether other acetyltransferases are involved. Modified Wnts are translocated from the ER to the Golgi apparatus in a process mediated by the p24 protein family (Buechling et al. 2011; Port et al. 2011). Once in the trans-Golgi network, the seven-pass transmembrane protein Wntless (Wls) is thought to provide further transport of Wnts to the plasma membrane for release outside the cell (Port and Basler 2010). Consistent with the necessary role of Wls in Wnt signaling, loss of Wls resembles a Wnt loss-of-function phenotype (Bänziger et al. 2006). Wls has been shown to bind Wnts at the conserved palmitoylated Ser209, explaining the accumulation of Wnts in the ER of Porc mutants (Herr and Basler 2011). Wls is recycled from the plasma membrane via a multiprotein complex called the retromer. The retromer is responsible for routing Wls into a retrograde pathway that transports transmembrane proteins from endosomes back to the trans-Golgi network (Coudreuse et al. 2006; Port and Basler 2010). In the absence of the retromer complex, Wls is trapped in endosomes and subsequently degraded (Yang et al. 2008). This requirement of the retromer for Wnt signaling can be bypassed by providing additional Wls (Franch-Marro et al. 2008; Port et al. 2008), further confirming the role of the retromer in Wnt secretion via its regulation of Wls (Figure 2).
Figure 2.
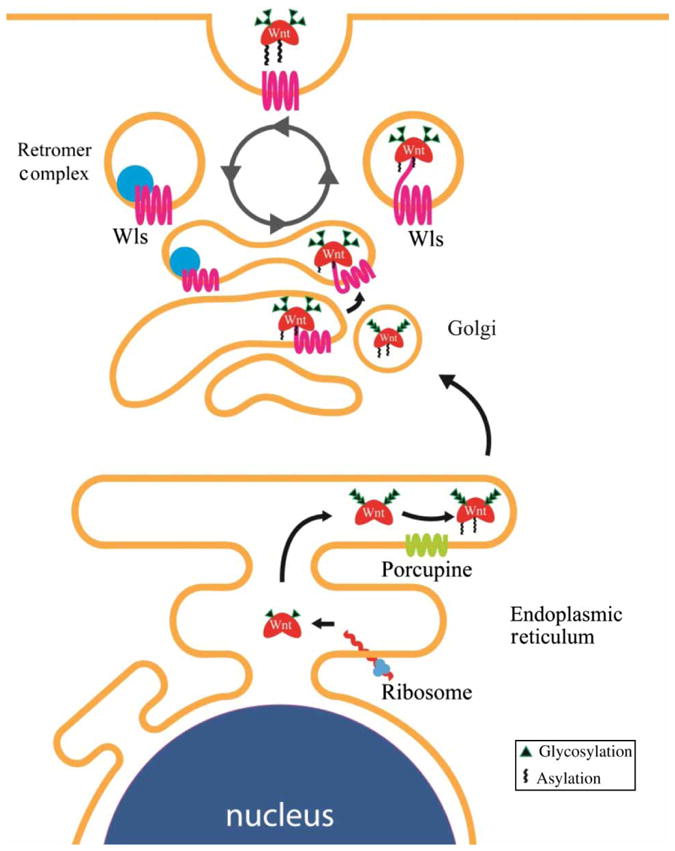
Synthesis and export of Wnt ligand. Wnt ligand undergoes multiple posttranslational modifications in the ER. Glycosylation and palmitoylation of Wnt ligand (the latter mediated by the transmembrane protein Porc) are required for its translocation to the Golgi apparatus. Palmitoylation of Wnt allows it to bind Wls, which provides a mechanism for transportation to the plasma membrane. The retromer complex recycles Wls from the plasma membrane back to the Golgi.
Wnt extracellular transport
Several mechanisms have been proposed for how Wnt ligands traverse the extracellular space to bind their target cells. It is possible that these mechanisms are tissue specific and influenced by the extracellular environment in which the cell resides (Port and Basler 2010). The mechanisms underlying the graded distribution of extracellular Wnt/Wg ligands have been best delineated in Drosophila wing discs, where a gradient of Wg protein patterns the boundary between the developing dorsal and ventral wing surfaces. These studies suggest a restricted diffusion model. In this model, Wg diffuses across cells extracellularly while interacting with receptors and cell-surface heparan sulfate proteoglycans (HSPGs), which act generally as positive regulators of Wg signaling (Strigini and Cohen 2000; Baeg et al. 2004; Han et al. 2005; reviewed in Yan and Lin 2009). HSPGs are comprised of a protein core decorated with long glycosaminoglycan (GAG) chains [reviewed in Hacker et al. (2005)]. The importance of GAG chains of HSPGs has been demonstrated by studies showing that when enzymes involved in heparan sulfate synthesis are mutated, extracellular Wg does not accumulate and Wg signaling is reduced (Binari et al. 1997; Hacker et al. 1997; Haerry et al. 1997; Lin and Perrimon 1999; Han et al. 2004; Takei et al. 2004; reviewed in Lin (2004). The HSPGs most studied as modulators of morphogen activity are glypicans, which are anchored to the cell surface by a glycosylphosphatidylinositol (GPI) anchor. There are two glypicans in Drosophila, known as division abnormally delayed (Dally) and Dally-like protein (Dlp), and both bind Wg in cell culture (Franch-Marro et al. 2005). Dlp overexpression in wing discs leads to extracellular Wg accumulation, whereas Dally has little effect (Baeg et al. 2001; Franch-Marro et al. 2005; Han et al. 2005), suggesting that Dlp binds Wg with higher affinity than Dally. Genetic studies suggest that Dally mainly functions as a co-receptor to present Wg to the Fz2 receptor (Lin and Perrimon 1999).
In contrast to Dally, Dlp has a more complex influence on Wg signaling. Dlp inhibits Wg signaling close to the Wg source (short-range Wg signaling) but promotes the range of Wg signaling distant from the source (long-range Wg signaling) (Kirkpatrick et al. 2004; Kreuger et al. 2004; Franch-Marro et al. 2005); this biphasic activity of Dlp serves to reduce the morphogen gradient. Various models have been proposed to explain Dlp’s biphasic activity. An early model focused on proteolytic cleavage of Dlp by Notum, an α/β-hydrolase expressed at the Wg source, capable of shedding Dlp at its GPI anchor and releasing it from the cell surface. One possibility to account for Dlp biphasic activity is that Notum cleavage converts Dlp into a short-range Wg antagonist (Kreuger et al. 2004). However, in the wing, neither the ectopic expression nor the loss of Notum alters Dlp levels, suggesting that the biphasic activity of Dlp is independent of Notum (Han et al. 2005; Gallet et al. 2008). A more recent model proposes that Dlp mediates transcytosis of Wg from the apical cell surface to the basolateral surface, where it is spread to the next distal cell, so that Dlp effectively siphons Wg away from regions of high Wg expression toward distal regions (Gallet et al. 2008). Another model, based on wing-disc and cell culture studies, suggests that competition between Dlp and Fz2 for binding Wg at the cell surface is responsible for the biphasic activity of Dlp (Yan et al. 2009), such that when Wg concentration is high (in short-range signaling), Dlp sequesters Wg from Fz2, but when Wg concentration is lower (in long-range signaling), Dlp concentrates Wg in the vicinity of Fz2 to promote signaling.
In Drosophila embryos, Wg transport and gradient depend on endocytosis, as the signaling range is compromised in a shibire (dynamin) endocytic pathway mutant (Bejsovec and Wieschaus 1995). Endo-cytosis may provide a means to internalize and recycle Wg, maintaining Wg protein in the cells that secrete it (Pfeiffer et al. 2002). Studies also indicate that endocytosis of Wg and its subsequent degradation restrict its signaling activities (Dubois et al. 2001).
Recent studies have revealed other forms of extracellular Wg ligands. In the Drosophila wing disc, Wg is packed into extracellular lipoprotein particles, which enhances its long-range signaling capacities (Panakova et al. 2005). Interestingly, lipoprotein particles also interact with glypicans (Eugster et al. 2007), raising the possibility that glypicans (Dally and Dlp) and lipoprotein particles act together to regulate Wg movement. Wg has also been shown to be transported on exosomal vesicles across the synapse of the Drosophila larval neuromuscular junction; these exosomes also contain Wls, which is required for Wg trans-synaptic transport (Korkut et al. 2009). Recently, exosomal secretion of Wnts has also been shown to occur in Drosophila and vertebrate cells, suggesting that it may represent a more general mechanism for distribution of active Wnt ligands in the extracellular space (Gross et al. 2012).
HSPGs are conserved from Drosophila to vertebrates. Despite studies confirming the importance of HSPGs in Wnt signaling in vertebrates including Xenopus (Ohkawara et al. 2003), zebra fish (Topczewski et al. 2001), cell culture (Capurro et al. 2005; Sakane et al. 2012), and rodent models (Song et al. 2005; Filmus et al. 2008), the details and mechanisms will need to be explored to gain a better understanding of how extracellular Wnt/Wg gradient is achieved and regulated in different tissue and developmental contexts.
The Fz receptor family
The soluble Wnt ligands bind to members of the Fz (Fz) family of seven transmembrane domain receptors, which have structural similarities to G-protein-coupled receptors (GPCRs). Biochemical evidence indicates that Wnts bind to the CRD of the Fz receptor, and that the affinity of Wnt for Fz is in the low nanomolar range (Bhanot et al. 1996; Hsieh et al. 1999).
The topological similarities of Fz to GPCRs have led to the suggestion that heterotrimeric G proteins may be required for Wnt signal transduction, and several studies propose a link between G proteins and Wnt pathway activation. Genetic studies in Drosophila suggest that Gαo transduces signaling from Fz, and that Gαo interacts with the scaffold protein Axin to promote its localization to the plasma membrane (Katanaev et al. 2005; Egger-Adam and Katanaev 2009). In cultured mammalian cells, depletion of Gαo or Gαq has been shown to inhibit Wnt signaling, possibly via disruption of GSK3β–Axin complexes (Liu et al. 2005). More direct evidence for a role of G proteins in Wnt pathway activation comes from reconstitution studies indicating that Gαo, Gαi2, Gαq, and Gβγ have the capacity to inhibit both β-catenin phosphorylation by GSK3 and β-catenin turnover in Xenopus egg extract. In the case of the latter, it was proposed that Gβγ promotes the recruitment of GSK3 to the plasma membrane to enhance low-density lipoprotein receptor-related protein 6 (LRP6) phosphorylation and activation (Jernigan et al. 2010). Whether heterotrimeric G proteins are bona fide mediators of Wnt ligand-mediated signaling, or whether other pathways act through them to modulate Wnt signaling, remains unclear.
The co-receptor LRP5/6
LRP5 and LRP6 are functionally redundant single-pass transmembrane receptors that act as co-receptors for Wnt ligands (Pinson et al. 2000; Tamai et al. 2000; Wehrli et al. 2000). In Drosophila, there is only one family member, Arrow. In some assays, LRP6 is more potent than LRP5. There are thought to be no qualitative differences in their mechanism of action in mediating Wnt pathway activation, although they likely play different roles during development (He et al. 2004; Mi and Johnson 2005). Biochemical studies of LRP6 indicate that different Wnts may bind to different extracellular domains of the LRP5/6 protein (Bourhis et al. 2010). Specifically, the LRP6 extracellular domain contains four repeating sequences of β-propeller and epidermal growth factor-like (βP–E) domains. The crystal structures of the extracellular LRP6 regions indicate that the βP–E repeats represent two discrete, compact, rigid structures, each containing two βP–E pairs. Wnt9b binds the first two βP–E repeats on the extracellular domain of LRP6, whereas Wnt3a binds the last two βP–E domains (Ahn et al. 2011; Chen et al. 2011; Cheng et al. 2011).
Binding of Wnt ligands to Fz and LRP5/6 results in the production of phosphatidylinositol (4,5)-bispho-sphate (PIP2) (Pan et al. 2008). Increased PIP2 induces oligomerization and clustering of LRP5/6. Although hydrodynamic studies suggest that Fz and LRP6 oligomerize and form clusters of “signalosomes” upon Wnt signaling, the in vivo, physiological significance of such events in Wnt pathway activation remains to be determined (Cong et al. 2004b; Bilić et al. 2007).
Increased PIP2 also induces recruitment of Axin to LRP5/6. This recruitment may be due, in part, to the action of Amer1/WTX (APC membrane recruitment 1 or Wilms tumor gene on the X chromosome), a tumor suppressor mutated in Wilms’ tumor that binds to Axin, CK1γ, and GSK3. Amer1/WTX is recruited to the plasma membrane in a PIP2-dependent manner (Major et al. 2007; Tanneberger et al. 2011).
The interaction between LRP6 and Axin is critical for activation of the Wnt pathway, and the recruitment of Axin and the associated destruction complex to the plasma membrane upon Wnt ligand binding initiates a chain of events that leads to the phosphorylation of the intracellular domain of LRP5/6. This initial recruitment of Axin to LRP6 in a Wnt–Fz-dependent manner is referred to as the “initiation step” of Wnt pathway activation (Baig-Lewis et al. 2007).
The LRP5/6 receptor contains five PPPSPxS motifs on its intracellular domain that are required for signal transmission. Each of these five motifs alone can activate the Wnt/β-catenin pathway: when transferred to heterologous receptors, the PPPSPxS motif is sufficient for pathway activation (Tamai et al. 2004; Zeng et al. 2005b; MacDonald et al. 2009). Mutational analyses of these motifs indicate that they act in a cooperative manner to mediate downstream signaling (MacDonald et al. 2008; Wolf et al. 2008).
The predominant kinases involved in PPPSPxS phosphorylation have been identified as GSK3 and CK1 (Davidson et al. 2005; Zeng et al. 2005b). Wnt binding to LRP5/6 has been shown to induce PPPSP phosphorylation by GSK3, and this event primes LRP6 for subsequent xS phosphorylation by CK1 (Zeng et al. 2005b; Khan et al. 2007b; Pan et al. 2008). Another study, however, suggests that CK1 phosphorylates conserved S/T clusters outside the PPPSPxS motif, and this phosphorylation primes phosphorylation of LRP6 by GSK3 (Davidson et al. 2005). Phosphorylated LRP6 has a high affinity for Axin and promotes further recruitment of cytoplasmic Axin-bound GSK3 complexes to the cell surface (Mao et al. 2001; Zeng et al. 2008). The recruitment of additional Axin-bound GSK3 complexes further promotes the phosphorylation of additional LRP5/6 PPPSP motifs in a positive feedback mechanism and has been referred to as the “amplification step” in Wnt pathway activation (Baig-Lewis et al. 2007). Axin is likely the limiting component of the β-catenin destruction complex (Lee et al. 2003). Once the Axin-bound β-catenin destruction complex is recruited by LRP6, the phosphorylated cytoplasmic domain of LRP6 is capable of directly inhibiting GSK3 activity, blocking β-catenin phosphorylation and subsequent ubiquitin-mediated proteasomal degradation (Cselenyi et al. 2008; Piao et al. 2008; Wu et al. 2009). The capacity of phosphorylated LRP6 to limit GSK3 activity by direct inhibition appears to limit the capacity of Axin-bound GSK3 to promote further LRP6 phosphorylation (amplification step). It is possible that these two events are temporally regulated such that direct inhibition of GSK3 may be blocked during the amplication step. It goes without saying that more experiments need to be performed to resolve this relationship between LRP6/GSK3 amplification and inhibition.
Wnt antagonists and agonists
Several secreted proteins act as agonists or antagonists of Wnt/β-catenin signaling. For secreted antagonists, there are two general classes of proteins. One class, including secreted Fz-related proteins (sFRPs) and Wnt inhibitory factors (WIFs), binds and sequesters Wnt ligands to block their association with Wnt receptors (Bovolenta et al. 2008). Another class of antagonists, consisting of Dkk1 and Wise/SOST family members, binds the co-receptor LRP5/6, blocking interaction with Wnt ligands (Semenov et al. 2001; Mao et al. 2002). Recently, the X-ray crystal structure of the extracellular region of LRP6 bound to a C-terminal domain of Dkk1 has been solved, demonstrating that the C-terminal domain of Dkk1 binds to the last two βP–E domains of LRP6 (Ahn et al. 2011; Cheng et al. 2011). These studies, along with biochemical analyses demonstrating that the N-terminal domain of Dkk1 binds to the first two βP–E domains, provide a structural basis for the broad and potent inhibition of ligand-mediated Wnt signaling by Dkk1 (Bourhis et al. 2010; Ahn et al. 2011). In addition to these extracellular antagonists of Wnt signaling, the Shisa proteins have been shown to trap Fz proteins in the ER and prevent them from reaching the cell surface. Shisa proteins, however, are not specific to Wnt regulation and regulate intracellular trafficking of fibroblast growth factor receptors as well (Yamamoto et al. 2005).
Non-Wnt agonists include Norrin and R-Spondin (Kazanskaya et al. 2004; Kim et al. 2006; Nam et al. 2006; Wei et al. 2007). Norrin appears to be an Fz4-specific ligand that, in complex with LRP5, controls retinal vascular development. Mutations in the Norrin gene, ndp, lead to familial exudative vitreoretinopathy and Norrie disease, both of which are associated with retinal hypovascularization (Xu et al. 2004a). The four R-Spondin genes represent a family of conserved secreted proteins that were initially shown to potentiate the Wnt pathway and are required for muscle development in Xenopus laevis (Kazanskaya et al. 2004). R-Spondin was subsequently found to be a potent mitogen and to induce rapid proliferation of intestinal crypts when injected into mice, a phenotype consistent with Wnt/β-catenin pathway activation (Kim et al. 2005). Consistent with the role of R-Spondins in colorectal cancer, whole-exome and transcriptome sequencing has identified in-frame fusions of R-Spondins (RSPO2 and RSPO3), leading to their overexpression in 10% of colon tumors. Interestingly, these fusions were found in tumors lacking Wnt pathway-activating mutations in APC or β-catenin. Thus, R-Spondins, similar to APC and β-catenin, may represent a major driver of colorectal cancer (Seshagiri et al. 2012).
LGR4/5/6 (leucine-rich repeat-containing GPCRs 4, 5, and 6), known as markers for adult stem cells in the gastrointestinal tract and skin, were shown to be receptors for R-Spondins. How binding of R-Spondins to LGR4/5/6 potentiates Wnt signaling is not clear (Barker et al. 2007; Snippert et al. 2010; Carmon et al. 2011; de Lau et al. 2011; Glinka et al. 2011). Recently, it was shown that the Wnt target genes, RNF43 and ZNRF3, which are related transmembrane E3 ubiquitin ligases, target Fz receptors and LRP6 for degradation to inhibit Wnt signaling (Hao et al. 2012; Koo et al. 2012). The mechanism of R-Spondin action in the Wnt pathway was illuminated by demonstration that ZNRF3 is a receptor for R-Spondin, and that when complexed with LGR4, ZNRF3 activity is inhibited (Hao et al. 2012). Thus, the role of R-Spondins appears to stabilize the Wnt receptors, Fz and LRP6, to promote Wnt signaling.
Recently, the type 1 transmembrane protein, Tiki, was identified in an expression cloning screening for mRNAs that perturbed axis formation in X. laevis embryos (Zhang et al. 2012). Tiki was shown to be a novel metalloprotease that cleaved the N-terminal 8 amino acids of mature Wnt proteins. In vitro, Tiki-mediated cleavage of this N-terminal fragment of Wnts results in the formation of soluble, large oligomeric Wnt complexes due to oxidation and formation of disulfide bonds. Whether or not formation of these large, inactive proteolyzed Wnt complexes is the mechanism of action of Tiki in the Wnt pathway in vivo remains to be elucidated.
The cytoplasmic adaptor Dsh
Dishevelled (Dsh) has long been known to be required genetically in the Wnt/β-catenin pathway (Klingensmith et al. 1994). In vertebrates, there are three Dsh isoforms encoded by distinct genes (Dvl1–3) (Sussman et al. 1994; Yang et al. 1996; Semenov and Snyder 1997). Upon Wnt–receptor interaction, Dsh is phosphorylated and recruited to the cytoplasmic side of the receptor complex (Yanagawa et al. 1995; Semenov and Snyder 1997; Rothbacher et al. 2000). Several studies suggest that physical interaction between the Fz receptor and Dsh is important for transduction of the Wnt signal. Dsh contains three major domains: the DEP, the PDZ, and the DIX domains. Biochemical and structural studies have implicated both the PDZ and the DIX domains of Dsh in binding to the Fz receptor (Wong et al. 2000; Wong et al. 2003; Tauriello et al. 2012). Dsh phosphorylation upon Wnt signaling appears to be independent of LRP6 activation (Gonzalez-Sancho et al. 2004). Dsh and Axin share DIX domains that can polymerize and are required for receptor clustering (Schwarz-Romond et al. 2007a). Loss-of-function studies show that Dsh acts upstream of LRP6 (Tolwinski et al. 2003). Consistent with this observation, Dsh has been shown to bind and activate PI4KIIa and PIP5KI to promote the synthesis of PIP2, which is required to promote oligomerization and clustering of LRP5/6 (Pan et al. 2008). In overexpression studies in Drosophila and Xenopus egg extracts, however, Dsh has been shown to activate β-catenin signaling independently of Arrow/LRP6 (Salic et al. 2000; Wehrli et al. 2000). Although Caenorhabditis elegans has a Dsh homolog, it has no obvious LRP5/6 homolog, suggesting that Dsh may play a more central role in pathway activation in divergent phyla (Phillips and Kimble 2009). The precise involvement of Dsh in receptor activation and Axin recruitment remains to be determined.
Both the stability and the activity of Dsh appear to be regulated by ubiquitination. Three ubiquitin ligases, NEDL1 and ITCH of the HECT-type ligase and KLHL12 of the Cullin3-type ligase, have been implicated in ubiquitinating Dsh to promote its degradation (Miyazaki et al. 2004; Angers et al. 2006; Wei et al. 2012). One study implicates the Naked2 protein as a necessary co-factor for Dsh ubiquitination (Hu et al. 2010). Finally, the deubiquitinating enzyme, CYLD (encoded by the familial cylindromatosis tumor suppressor gene), has been shown to be a negative regulator of Wnt signaling (Tauriello et al. 2010). CYLD was shown to remove a regulatory Lys63-linked ubiquitin from Dsh. Thus, ubiquitination of Dsh via Lys63 linkages appears to be necessary for efficient activation of signaling by Dsh. The ubiquitin ligase that mediates Lys63-linked ubiquitination of Dsh, however, is still unknown.
Step 2: Inhibition of the β-catenin destruction complex
The β-catenin destruction complex is a macromolecular machine that efficiently acts to phosphorylate β-catenin, targeting it for degradation. We will first describe the players involved in the formation of the β-catenin destruction complex (Figure 1) and follow with our current understanding of the behavior of the pathway upon receptor activation.
The transcriptional regulator β-catenin
β-Catenin is the primary effector of Wnt signaling. In the absence of signaling, the destruction complex targets β-catenin for ubiquitin-mediated proteasome degradation by SCFβ-TRCP, a member of the Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex. In the presence of signaling, β-catenin is spared destruction and translocates from the cytoplasm to the nucleus to activate signaling. Although other substrates of the destruction complex have been identified in addition to β-catenin, their physiological significance is still unclear. β-Catenin was first identified in Drosophila as the segment polarity gene armadillo and also as a component of the adherens junction in Xenopus (Nusslein-Volhard and Wieschaus 1980; McCrea et al. 1991). The structure of β-catenin consists of a central core of 12 helical 42 amino acid armadillo repeats that form a superhelical structure (Huber et al. 1997). Analysis of full-length β-catenin protein indicates that the N- and C-terminal domains are unstructured and form dynamic interactions with the armadillo repeats of the protein (Xing et al. 2008). Notably, the armadillo repeats form a positively charged groove that mediates the interaction of β-catenin with other components of the Wnt pathway [e.g. APC, Axin, and T-cell factor (TCF)/LEF] as well as with E-cadherin (Huber et al. 1997; Graham et al. 2000; Huber and Weis 2001; Xing et al. 2003; Xing et al. 2004).
The cellular factors that coordinate whether nascent β-catenin mediates Wnt target gene transcription or plays a structural role in maintaining integrity of the adherens junction are not completely understood. A large number of studies using a variety of model systems have shown that overexpression of cadherins is sufficient to inhibit Wnt target gene transcription and to promote relocalization of β-catenin to the membrane (Heasman et al. 1994; Sanson et al. 1996; Sadot et al. 1998; Shtutman et al. 1999; Gottardi et al. 2001; Stockinger et al. 2001). Furthermore, evidence to support potential influence of cadherins on Wnt signaling comes from studies demonstrating that proteolytic cleavage of cadherins by proteases such as ADAM10 and presenilin-1/γ-secretase is sufficient to release bound β-catenin, to increase soluble cytoplasmic β-catenin, and to activate Wnt target gene transcription (Marambaud et al. 2002; Maretzky et al. 2005; Reiss et al. 2005; Uemura et al. 2006). Multiple studies in which E-cadherin is knocked down in cells with wild-type Wnt pathway components, however, fail to demonstrate activation of Wnt signaling, suggesting a lack of a significant interaction between cadherin-mediated cell adhesion and Wnt signaling, compensatory regulation of β-catenin levels with E-cadherin, or that turnover of β-catenin by the degradation complex in the wild-type situation is capable of compensating for the increased flux of β-catenin (Kuphal and Behrens 2006; Herzig et al. 2007). Support for the existence of distinct pools of β-catenin comes from a study demonstrating that β-catenin can exist as a monomeric or dimeric form bound to α-catenin (Gottardi and Gumbiner 2004). Biochemical studies indicate that the monomeric form preferentially participates in Wnt signaling, whereas the dimeric form preferentially binds cadherins. Surprisingly, little is known about the mechanism of β-catenin nuclear translocation.
The scaffold protein Axin, the limiting component of the destruction complex
The scaffold protein Axin is a critical component of the β-catenin destruction complex, acting as a limiting negative regulator of Wnt/β-catenin signaling. It was first identified as the gene product of the locus fused in mice (Zeng et al. 1997). Axin plays a role as a scaffold protein that directly binds to many of the other components of the destruction complex and brings them within close proximity to each other (Figure 1). The sites of interactions have been visualized in co-crystal structures of Axin and APC proteins (Spink et al. 2000), Axin and β-catenin (Xing et al. 2003), and Axin and GSK3β (Dajani et al. 2003). Studies of Axin in fly embryos suggest that Axin complexes may form oligomers in vivo, and that Axin may also act as a cytoplasmic anchor to restrict armadillo/β-catenin import into the nucleus (Tolwinski and Wieschaus 2001; Peterson-Nedry et al. 2008). Axin was initially found to be present at low concentrations and is the limiting component of the β-catenin degradation complex in Xenopus (Lee et al. 2003). A long-standing puzzle about Wnt signaling is how it maintains specificity because many Wnt components also play biological roles in other cellular processes; for example, GSK3 is a node for many types of cell signaling (Forde and Dale 2007). The low concentration of Axin has been proposed to isolate the Wnt pathway from affecting other intracellular pathways (Lee et al. 2003). The low level of intracellular Axin is due, in part, to its ubiquitin-mediated turnover that is promoted by LRP5/6 (Yamamoto et al. 1999; Mao et al. 2001; Tolwinski et al. 2003; Kofron et al. 2007; Cselenyi et al. 2008). Degradation of Axin has been shown to be regulated by GSK3 phosphorylation, which inhibits its rate of degradation (Yamamoto et al. 1999). In addition, the turnover of Axin requires the tumor suppressor APC, and studies in Xenopus egg extract, as well as in flies, suggest that this may represent a mechanism to compensate for fluctuations in levels of APC in order to maintain low levels of β-catenin in cells (Lee et al. 2003).
Smad ubiquitin regulatory factor 2 (Smurf2) has recently been shown to be an E3 ubiquitin ligase that targets Axin for degradation (Kim and Jho 2010). Due to its key role in Wnt signaling, it is likely that Axin is tightly regulated. Tankyrase has been shown to promote poly(ADP-ribosyl)ation (PARsylation) and ubiquitination and further degradation of Axin through the addition of poly(ADP-ribose) moieties onto proteins through PARsylation (Huang et al. 2009a). The importance of Axin turnover is demonstrated by the identification of tankyrase inhibitors IWR-1 and XAV939 that have been shown to potently inhibit Wnt signaling by increasing the steady-state level of Axin (Chen et al. 2009; Huang et al. 2009a). These tankyrase inhibitors prevent PARsylation of Axin and thus reduce Axin turnover. RNF146 has been identified as the poly(ADP-ribose)-directed E3 ubiquitin ligase that ubiquitinates Axin (Callow et al. 2011; Zhang et al. 2011). RNF146 binds directly to the covalently linked poly(ADP-ribose), targeting Axin for degradation and maintaining low steady-state levels of Axin. A deubiquitinating enzyme, ubiquitin-specific protease (USP)34, has been identified to catalyze the deubiquitination of Axin and increase steady-state levels of Axin in cells (Lui et al. 2011). In contrast to PARsylation, SUMOylation of Axin at its C-terminus has been shown to confer stability by inhibiting Axin ubiquitination (Kim et al. 2008). Recently, quantitative measurements of Axin concentration in a variety of mammalian cells suggest that its levels vary significantly to alter the dynamics of Wnt signaling (Tan et al. 2012). Thus, the regulation of Axin levels and stability may be a major mechanism by which cells control the response to Wnt signals.
The kinase GSK3
GSK3 is a ubiquitous serine/threonine protein kinase involved in numerous cellular processes (Forde and Dale 2007). Antagonizing GSK3 activity is central to all models of Wnt signaling mechanisms. The homolog of GSK3 in Drosophila is shaggy, aka zeste white 3 (Siegfried et al. 1992). In mammals, there are two distinct genes, α and β, that are likely to have redundant functions in the Wnt pathway (Doble et al. 2007). GSK3 was first identified for its role in the regulation of glucose metabolism, targeting muscle glycogen synthase (Embi et al. 1980). GSK3 has both positive and negative roles in Wnt signal transduction, which will be described in further detail later in this review. GSK3 often recognizes substrates that have been previously phosphorylated (primed), and thus GSK3 is often found to act in concert with other kinases. β-Catenin phosphorylation by GSK3 (at Ser33, Ser37, and Thr41) leads to its ubiquitin-mediated degradation (Peifer et al. 1994; Yost et al. 1996). The crystal structure of GSKβ has been solved and conforms to a typical protein kinase bilobed structure topology consisting of an amino-terminal β-sheet domain linked to a carboxy-terminal α-helical domain (Dajani et al. 2001; Haar et al. 2001). Similar to other activated kinases, the structure of unphosphorylated GSK3β shows an activation loop that is responsible for its unique priming mechanism (Haar et al. 2001). Furthermore, phosphorylation of Ser9, which has been shown to inhibit GSK3 activity, is predicted to act in an auto-inhibitory fashion by blocking access to the catalytic site (Cross et al. 1995; Dajani et al. 2001). In addition to β-catenin, other major Wnt pathway substrates of GSK3 include APC, Axin, and LRP6 (Rubinfeld et al. 1996; Willert et al. 1999; Zeng et al. 2005a).
The kinase CK1α
The CK1 family of kinases is comprised of a group of serine/threonine kinases encoded by seven distinct genes in mammals (α, β, γ1, γ2, γ3, δ, and ε; although β was identified in bovine and has not been found in humans) (Knippschild et al. 2005; Price 2006). As with GSK3, CK1 is a widely expressed family of kinases with a large number of substrates. All CK1 members have highly similar catalytic domains, but differ significantly in both the length and the sequence of their C-terminal non-catalytic domains. CK1α, with its short (~24 amino acid) C-terminal domain, appears to be an outlier compared with the other family members, which have much longer C-terminal tails (~200 amino acids). CK1α, γ, δ, and ε have been implicated in positively regulating the Wnt pathway by phosphorylating Dsh, LRP5, TCF/LEF, and Axin (Yanagawa et al. 1995; Peters et al. 1999; Sakanaka et al. 1999; Kishida et al. 2001; Lee et al. 2001; Gao et al. 2002; Cong et al. 2004a; Swiatek et al. 2004; Zeng et al. 2005a; Zhang et al. 2006). In contrast, CK1 family members have also been implicated as negative regulators of the Wnt pathway by phosphorylating β-catenin, APC, Axin, and TCF/LEF (Kishida et al. 2001; Rubinfeld et al. 2001; Liu et al. 2002; Gao et al. 2002; Hammerlein et al. 2005). CK1α has been proposed to be the in vivo priming kinase for GSK3 and phosphorylates β-catenin at Ser45 (Liu et al. 2002). Consistent with this “dual kinase” model, two independent genome-wide Drosophila S2 RNAi screens identified CK1α as essential for suppressing Wnt/Wg signaling in the unstimulated state (Lum et al. 2003; DasGupta et al. 2005). Recently, the activation of CK1α has emerged as a potential therapeutic drug target, and a recent study reported that the antihelminthic drug, pyrvinium, inhibits Wnt signaling by activating CK1α to enhance β-catenin phosphorylation and degradation (Thorne et al. 2010).
The scaffold protein APC
APC is a scaffold protein consisting of 2843 amino acids with a mass of approximately 310 kDa, and it acts as a negative regulator of Wnt/β-catenin signaling. Vogelstein and colleagues first identified the gene in 1991 as the site of a mutation found in FAP, a familial form of colon cancer (Kinzler et al. 1991; Nishisho et al. 1991). APC plays diverse roles in cellular functions, including Wnt signaling, migration, mitotic spindle alignment, and apoptosis, which are likely carried out through different APC subpopulations (Faux et al. 2008). The C-terminal third of APC contains a region involved in microtubule binding that interacts with the proteins EB1 and Discs large (Su et al. 1995; Matsumine et al. 1996). These regions have been demonstrated to be involved in microtubule dynamics in mitosis and cell migration and are thought to be independent of the role of APC in Wnt signaling (Nathke 2006). The connection between APC and Wnt signaling was identified in a series of studies that showed that APC binds to β-catenin, and that mutations in APC caused elevated levels of β-catenin in cancer cells (Rubinfeld et al. 1993; Su et al. 1993). APC binds β-catenin, GSK3, and Axin in several regions within the central portion of the protein (Rubinfeld et al. 1996; Ikeda et al. 1998; Itoh et al. 1998; Fagotto et al. 1999). Despite numerous studies published on the function of APC, a clear mechanistic picture of the role of APC in regulating the Wnt pathway remains elusive (Cadigan and Peifer 2009; MacDonald et al. 2009). Indeed, loss of APC leading to elevated β-catenin levels and activation of the Wnt pathway can be overcome by overexpression of Axin, which is the limiting component of the β-catenin destruction complex (Lee et al. 2003). Furthermore, overexpression of an Axin mutant lacking its APC binding domain (RGS) is capable of promoting β-catenin degradation and inhibiting Wnt signaling to a similar extent as overexpression of wild-type Axin (Hart et al. 1998).
How APC acts as a negative regulator of Wnt/β-catenin signaling is something of a mystery. Several models have been proposed, including (1) exporting β-catenin from the nucleus (Hamada and Bienz 2004), (2) repressing Wnt target genes (Sierra et al. 2006), (3) retaining β-catenin in the cytoplasm (Tolwinski and Wieschaus 2001; Ahmed et al. 2002), (4) targeting the β-catenin destruction complex to the cell cortex, where its E3 ubiquitin ligase (SCFβ-TRCP) resides (Nathke et al. 1996; McCartney et al. 1999; Yu et al. 1999), (5) coordinating the phosphorylation and release of β-catenin from the destruction complex to allow its ubiquitination (Kimelman and Xu 2006), (6) blocking dephosphorylation of β-catenin by protein phosphatase 2A (PP2A) (Su et al. 2008), and (7) shielding the β-catenin degradation complex from the inhibitory action of Dsh (Mendoza-Topaz et al. 2011).
None of the proposed models for the role of APC in the Wnt pathway are mutually exclusive, although the most compelling experimental evidence (4–6) strongly supports APC’s role in negatively regulating steady-state levels of cytoplasmic β-catenin. It is likely that APC, similar to other components of the Wnt pathway (e.g. GSK3 and CK1), may participate in multiple events in the Wnt signaling pathway.
Several kinases, including CK1, protein kinase A (PKA), and GSK3, have been shown to phosphorylate APC (Rubinfeld et al. 1996; Morin et al. 1997). Phosphorylation of APC by GSK3 was shown to enhance binding of β-catenin by APC (Rubinfeld et al. 1996; Salic et al. 2000). In addition to phosphorylation, the APC protein is ubiquitinated. In cells, APC is stabilized by the COP9 signalosome-associated deubiquitinase, USP15, which binds to the β-catenin degradation complex (Huang et al. 2009b). The deubiquitinase Trabid, which removes K63-linked regulatory ubiquitination chains from APC, has been identified as a positive regulator of Wnt signaling (Tran et al. 2008). Although it is not clear if APC is the sole target of Trabid, this finding is consistent with the suggestion that K63-linked ubiquitination of APC antagonizes Wnt signaling, presumably by potentiating APC activity. The E3 ligase that mediates ubiquitination of APC and the exact mechanism by which these K63-linked ubiquitin chains regulate APC activity are unknown.
Other destruction complex factors: PP2A and PS1
Our understanding of the molecular machinery involved in β-catenin destruction is far from complete. Many other components have been implicated in preliminary molecular studies but have not received a thorough structural or biochemical analysis. Studies in cultured cells, Xenopus egg extracts, and Xenopus embryos have shown both a positive and negative role for the heterotrimeric phosphatase PP2A. The PP2A catalytic C subunit dephosphorylates both Axin and APC in vitro and cooperates with Dsh to induce secondary body axes in Xenopus embryos (Hsu et al. 1999; Willert et al. 1999; Ratcliffe et al. 2000). In contrast, the PP2A subunits (A, B56α, and C) each possess ventralizing activity in Xenopus embryos and are required for β-catenin degradation in Xenopus egg extract (Li et al. 2001; Gao et al. 2002). Furthermore, overexpression of the B56 subunit of PP2A has been shown to inhibit Wnt signaling by dephosphorylating key regulatory proteins in the β-catenin degradation complex (Seeling et al. 1999). These studies suggest negative regulation of the Wnt pathway by PP2A. It is possible that different PP2A heterotrimers have multiple targets, through either different regulatory proteins or multiple sites on a target protein. Thus, the phosphatase activity of PP2A mirrors that of the kinases CK1 and GSK3 in that it acts as both a positive and a negative regulator of the pathway, depending on its site of action.
Presinilin 1 (PS1), the catalytic subunit of the protease γ-secretase that has been implicated in Notch signaling and Alzheimer’s disease, has also been shown to inhibit Wnt signaling (Killick et al. 2001; Kang et al. 2002). PS1 appears to regulate β-catenin stability by acting as a scaffold protein in a similar fashion to Axin to promote phosphorylation of β-catenin by GSK3, targeting it for ubiquitin-mediated degradation. When PS1 acts as a scaffold, PKA appears to be the priming kinase for β-catenin phosphorylation by GSK3 (Kang et al. 2002), whereas CK1 is the priming kinase in the context of an Axin scaffold. APC may not be required for PS1-mediated degradation of β-catenin. Interestingly, the capacity of PS1 to promote β-catenin degradation is dependent on E-cadherin, potentially linking the regulation of cell adhesion to Wnt signaling (Serban et al. 2005).
The phosphatases PP2C and PP1 have also been implicated as positive regulators of Wnt signaling by opposing the phosphorylation of Axin by CK1, possibly leading to decreased association between Axin and GSK3 (Strovel et al. 2000; Luo et al. 2007). Amer1/WTX, in addition to its role in mediating receptor signaling, can bind β-catenin, Axin, APC, and β-TRCP to stimulate β-catenin degradation in cultured mammalian cells and Xenopus egg extract (Major et al. 2007). However, its precise biochemical role in this process to date is unclear. The ankyrin protein Diversin was identified as a negative regulator of Wnt signaling that acts by recruiting CK1ε to Axin in order to promote the efficient priming of β-catenin for GSK3 phosphorylation (Schwarz-Romond et al. 2002). Subsequent studies suggest that these sites of β-catenin destruction mediated by Diversin occur at centrosomes (Itoh et al. 2009).
The β-catenin futile cycle
The ubiquitous expression of β-catenin and other pathway members suggests that all metazoan cells express the β-catenin destruction complex. Although commonly described as having cytoplasmic localization, the core components can also be found in the nucleus where the β-catenin destruction complex is likely to reside and function (Bienz 2002; Cong and Varmus 2004; Wiechens et al. 2004; Sierra et al. 2006). This machine is in a constitutively active state and contains a number of enzymes that target β-catenin for degradation. Thus, in the absence of a Wnt signal, β-catenin is caught in what appears to be a futile cycle of synthesis followed by rapid destruction. Such futile cycles in signaling pathways have been proposed to allow the system to exhibit more complex behavior patterns (e.g. stochastic switching bistability) (Samoilov et al. 2005). Axin is the scaffold protein that nucleates the formation of the β-catenin destruction complex. It binds with high affinity to the two kinases GSK3 and CK1α. A well-defined α-helix in the central portion of Axin anchors GSK3, whereas the site of CK1 binding has been mapped to a more C-terminal region of Axin (Dajani et al. 2003; Sobrado et al. 2005). APC binds the RGS domain of Axin, an N-terminal region that has structural homology to domains found in regulators of G-protein signaling (Spink et al. 2000).
β-Catenin enters the complex by binding both 15 amino acid repeats of APC and a single α-helix on Axin on the C-terminal side of the GSK3 binding site. The exact order and kinetics of binding are unknown, and it is unclear whether this binding is ordered or stochastic (Lee et al. 2003).
Evidence suggests that phosphorylation of Axin, likely by GSK3, increases its affinity for β-catenin (Willert et al. 1999). Upon binding to Axin, the N-terminal region of β-catenin becomes positioned for rapid phosphorylation by CK1 at serine 45. This creates a priming site for subsequent and successive phosphorylation of β-catenin by GSK3 at Thre41, Ser37, and Ser33 (Amit et al. 2002; Liu et al. 2002). Twenty amino acid repeats on APC are also phosphorylated by CK1 and GSK3 (Ha et al. 2004). This phosphorylation increases the affinity of the 20 amino acid repeats for β-catenin by 140-fold and competes β-catenin off of the α-helix binding site on Axin. Based on these observations, it has been proposed that APC phosphorylation triggers β-catenin removal from Axin, allowing a new β-catenin species to enter the destruction complex (Kimelman and Xu 2006). The action of APC is also thought to prevent the action of the phosphatase, PP2A, from acting on the phosphorylated β-catenin (Su et al. 2008). Once β-catenin is phosphorylated at serines 33 and 37, a destruction consensus sequence is recognized by β-TRCP, a specificity subunit of the SCF ubiquitin ligase (Jiang and Struhl 1998; Kitagawa et al. 1999; Lagna et al. 1999; Liu et al. 1999; Marikawa and Elinson 1998). Binding of SCFβ-TRCP to β-catenin catalyzes its polyubiquitination (via K48 linkages) and subsequent degradation through the proteasome. These events ensure that newly synthesized, free cytosolic levels of β-catenin are kept below the threshold necessary for gene regulation. Recently, the HECT domain containing E3 ligase, EDD, has been shown to ubiquitinate β-catenin, inhibiting its degradation (Hay-Koren et al. 2010). Although the mechanism by which ubiquitination of β-catenin by EDD inhibits its turnover is unclear, it may involve, in part, ubiquitination of β-catenin via non-K48 linkages (K29 or K11). Furthermore, studies need to be performed in order to better understand the physiological relevance of this mode of regulation by EDD and its role in Wnt signal transduction.
Pathway behavior on activation
Elevation of β-catenin levels in response to the presence of Wnt is a hallmark of the Wnt/β-catenin pathway. The precise mechanism of destruction complex inhibition is under intense investigation and a number of proposed mechanisms exist. At the core of all the current models is the inhibition of GSK3 anti-catenin activity. This has been proposed to arise through several distinct mechanisms: (1) dissociation of components of the β-catenin destruction complex, (2) phosphorylation of GSK3 at Ser9, which inhibits its activity, (3) LRP6 binding and direct inhibition of GSK3 activity against β-catenin, (4) Axin degradation upon activation of signaling, which prevents formation of the destruction complex necessary for GSK3 to phosphorylate β-catenin, and (5) global inhibition of GSK3 activity via its sequestration in multivesicular bodies (MVBs). We discuss these models individually below.
Several studies have described Axin–GSK3 dissociation upon signaling (Gao et al. 2002; Liu et al. 2002; Logan and Nusse 2004; Luo et al. 2007). Early studies seem to suggest that Dsh recruits GSK3 binding protein (GBP) to Axin where GSK3 is directly inhibited and dissociates from Axin (Yost et al. 1998; Farr et al. 2000). One caveat to these studies is the apparent lack of requirement for GBP in Wnt signaling in Drosophila and mice. Drosophila does not have a GBP ortholog, and genetic knockout studies in mice show no requirement for GBP in development or Wnt signaling (van Amerongen et al. 2005). In contrast to these studies, other studies have shown that the complex remains intact and localizes to the Fz/LRP6 co-receptors rapidly after Wnt stimulation (Mao et al. 2001; Yamamoto et al. 2006; Bilić et al. 2007; Hendriksen et al. 2008).
GSK3α/β is phosphorylated at Ser9 and Ser21, and when phosphorylated at these sites, kinase activity is greatly reduced (Sutherland et al. 1993; Cross et al. 1995). Wnt-induced stimulation of Ser9/21 phosphorylation has been speculated about by several groups (Ding et al. 2000; Yokoyama and Malbon 2007). However, studies in mice in which non-phosphorylatable forms of GSK3α/β were knocked-in produced animals with no developmental defects and no observable perturbation in Wnt pathway activation (McManus et al. 2005).
A number of mammalian cell culture and biochemical studies suggest that the β-catenin destruction complex moves to the cell surface upon Wnt stimulation, and this migration is followed by the direct inhibition of GSK3 by LRP6 (Yamamoto et al. 2006; Bilić et al. 2007; Cselenyi et al. 2008; Hendriksen et al. 2008; Piao et al. 2008; Wu et al. 2009). The collective evidence from these studies suggests a mechanism in which ligand-stimulated LRP6 phosphorylation by GSK3 and CK1 creates a docking site for Axin. This phosphorylation event in turn recruits the Axin–GSK3 complex to the receptor. Recruitment of Axin–GSK3 to phosphorylated LRP6 has been proposed to be facilitated in part by Dsh, which binds directly to the Fz receptor and Axin (Schwarz-Romond et al. 2007b). A problem for this model, however, is the requirement for GSK3 to phosphorylate the PPPSPxS motifs on LRP5/6 to create docking sites for Axin–GSK3 (amplification step) and the fact that phosphorylated PPPSPxS motifs can directly inhibit GSK3 activity, further limiting LRP6 phosphorylation. High-resolution structural data are essential to clarify this model and to explain how PPPSPxS motifs are sufficient for both Axin docking and GSK3 inhibition.
A compendium of studies from cell culture, Xenopus embryos and egg extracts, and Drosophila suggest that a conserved and critical event is the degradation of Axin upon Wnt signaling (Yamamoto et al. 1999; Tolwinski et al. 2003; Kofron et al. 2007; Cselenyi et al. 2008). Because Axin is a concentration-limiting factor, regulation of Axin stability is likely to have a dramatic effect on signaling (Lee et al. 2003). Genetic studies in Drosophila designed to elucidate the requirement of Axin regulation show that flies genetically null for GSK3 have elevated levels of β-catenin as expected, yet unexpectedly they still have near wild-type Wg-based embryonic patterning that is dependent on dynamic levels of Axin. These results indicate that regulation of Axin stability is sufficient to pattern the embryo, as β-catenin nuclear translocation only occurs when Axin levels are low, and Axin may act as a cytoplasmic anchor to regulate β-catenin-mediated transcription (Tolwinski et al. 2003). Other work has shown that Axin degradation appears to lag behind β-catenin stabilization and is not necessary for β-catenin stabilization (Willert et al. 1999; Hino et al. 2005; Cselenyi et al. 2008). These studies promote the model that the block in β-catenin destruction that occurs upon Wnt signaling does not require Axin turnover, and that degradation of Axin is a distinct event that may modulate the character of the response.
It has been proposed that binding of a Wnt ligand to its receptors regulates GSK3 activity by promoting its sequestration into MVBs (Taelman et al. 2010). Using elegant biochemistry and electron microscopy, it was shown that large oligomeric complexes (signalosomes) form in response to Wnt signaling; these signalosomes associate with the late endocytic markers Rab7 and Vps, eventually giving rise to MVBs. It was shown that GSK3 is sequestered into the lumen of these MVBs, thus blocking its capacity to act in the cytoplasm to target substrates such as β-catenin. Since GSK3 is involved in phosphorylating numerous proteins, it is predicted that a significant number of cellular proteins would be affected by Wnt signaling. This appears to be the case as the authors show by protein labeling experiments that Wnt signaling affects the turnover of 20% of all cellular proteins. Given the timing of the observations (hours after activation of the Wnt pathway), whether or not these effects are required for Wnt signaling or whether they represent a more complex modulation of the pathway, similar to Axin degradation, remains to be determined. In addition, because GSK3 is involved in multiple signaling pathways, isolating the effect of Wnt signaling from other GSK3-mediated pathways is difficult. Studies of immunoprecipitated Axin complexes from Wnt-stimulated cells suggest that β-catenin is phosphorylated, ubiquitinated, and degraded via the proteosome within these Axin complexes (Li et al. 2012). Thus, it was proposed, in contrast to the prevailing model, that Wnt signaling does not affect GSK3 activity, but, rather, ubiquitination of β-catenin. The incapacity to ubiquitinate and degrade phosphorylated β-catenin was proposed to “tie up” the degradation complex. Inconsistent with this model, however, is the recent dynamic analysis of Wnt pathway activation (as assessed by quantitative measurements of distinct species of the β-catenin degradation complex) that strongly indicates that activation of the pathway by Wnt ligands results in inhibition of β-catenin degradation at, or upstream of, its phosphorylation (Hernandez et al. 2012). Clearly, more studies need to be performed to support these opposing models of Wnt pathway activation.
Step 3: Activation of a Wnt-specific nuclear transcriptional complex
Nuclear translocation of β-catenin
β-Catenin is normally constitutively transcribed and translated. Thus, a signal-induced block in proteolysis leads to rapid rise in cytosolic β-catenin protein levels upon receptor activation. In addition to increased cytosolic accumulation, β-catenin also accumulates in the nucleus. Remarkably, studies indicate that it is not the absolute increase in concentration of β-catenin that results in activation of a Wnt transcriptional program (Goentoro and Kirschner 2009). Rather, it is the relative fold change (~2 ×) in the concentration of β-catenin that is recognized by the Wnt transcriptional machinery. Such a mechanism is expected to minimize cell–cell variation caused by fluctuations in the basal level of β-catenin as well as allowing for activation of the transcriptional program when the signal is sufficiently elevated in proportion to the background noise (Goentoro et al. 2009).
β-Catenin does not contain any recognizable nuclear localization signal (NLS) or nuclear export signal (NES). Nuclear accumulation of β-catenin has been attributed to its cytoplasmic retention, nuclear retention, and nuclear export. Nuclear entry of β-catenin is thought to be independent of classic import factors (e.g. RanGTPase and importins) (Fagotto et al. 1998; Yokoya et al. 1999). The armadillo repeats of β-catenin, however, are structurally related to importin-β HEAT repeats and may interact directly with the nuclear pore complex during nuclear entry (Kutay et al. 1997; Malik et al. 1997). Recent data suggest that armadillo repeats bind directly to the nucleoporins Nup62, Nup153, and Ran binding protein 2 (RanBP2)/Nup358 (Sharma et al. 2011). Surprisingly, deletion of β-catenin armadillo repeats 3–6 in Drosophila, which presumably disrupts binding to major cytoplasmic Wnt components, results in constitutive nuclear localization of the armadillo/β-catenin mutant (Orsulic and Peifer 1996). Based on this result, it has been suggested that β-catenin is normally regulated by its cytoplasmic retention. Consistent with this data, both cadherins and Axin have been shown to sequester β-catenin in the plasma membrane and cytoplasm, respectively (Heasman et al. 1994; Fagotto et al. 1996; Sanson et al. 1996; Sadot et al. 1998; Shtutman et al. 1999; Gottardi et al. 2001; Tolwinski and Wieschaus 2001). The nuclear proteins TCF, Pygopus, and BCL9 (described below) have been proposed to similarly act as anchors for β-catenin in the nucleus (Tolwinski and Wieschaus 2001; Townsley et al. 2004; Krieghoff et al. 2006). It has been proposed that nuclear export of β-catenin may play a critical role in Wnt signaling, and Axin, APC, and RanBP3 have been implicated in regulating the export of β-catenin from the nucleus (Henderson and Fagotto 2002; Cong and Varmus 2004; Hendriksen et al. 2005). Finally, Rac1 GTPase and Jun N-terminal kinase 2 (JNK2) have also been shown to promote β-catenin nuclear localization upon Wnt signaling, although the mechanism by which this is accomplished is unclear (Wu et al. 2008).
β-Catenin-mediated gene transcription
Wnt-induced nuclear β-catenin accumulation leads to an interaction with the TCF/LEF family of DNA-bound transcription factors that are critical for Wnt-mediated gene regulation (Behrens et al. 1996; Molenaar et al. 1996). Invertebrates appear to have only one TCF gene, whereas mammals have four: TCF1, LEF1, TCF3, and TCF4. In the absence of β-catenin, TCF interacts with the co-repressor Groucho/transducin-like enhancer (Gro/TLE1–3 in vertebrates) to repress gene transcription. TCF binds at the DNA consensus sequence CCTTTGWW (W can be either T or A), termed the Wnt-responsive element (WRE). It has been predicted that there are greater than 6000 high-confidence WREs in at least one studied colorectal cancer cell line, and these WREs collectively regulate 300–400 genes (Hatzis et al. 2008).
Several studies suggest that TCF proteins are phosphorylated to regulate Wnt signaling. CK1 has been shown to phosphorylate TCF to positively and negatively regulate its interaction with β-catenin (Lee et al. 2001; Hammerlein et al. 2005). The Nemo-like kinase (NLK) has been shown to phosphorylate TCF and inhibit Wnt signaling by reducing the affinity of β-catenin–TCF for DNA (Ishitani et al. 1999, 2003; Smit et al. 2004). In Caenorhabditis elegans, phosphorylation of TCF (POP-1) by the NLK homolog, LIT-1, promotes its export from the nucleus (Lo et al. 2004). Finally, phosphorylation of LEF-1, TCF4, and TCF3 by the homeodomain-interacting protein kinase 2 has been shown to promote their dissociation from Wnt target gene promoter (Hikasa et al. 2010; Hikasa and Sokol 2011). TCF proteins are also modified by ubiquitination. The NLK-associated RING finger protein promotes the ubiquitination of TCF/LEF, targeting it for degradation in a manner dependent on NLK activity (Yamada et al. 2006). Similarly, the deubiquitinase USP4, which deubiquitinates K48 and K63 ubiquitin linkages, has been shown to act on TCF4 to inhibit Wnt signaling (Zhao et al. 2009). In the canonical model of Wnt target gene activation, displacement of Gro/TLE by β-catenin converts TCF/LEF into a transcriptional activator. Elegant in vitro studies indicate that β-catenin is capable of simply displacing Gro/TLE to compete for TCF/LEF binding (Daniels and Weis 2005). This simple competition, however, may not always be the case. Recently, the X-linked inhibitor of apoptosis was shown to monoubiquitinate Gro/TLE, decreasing its affinity for TCF/LEF and allowing for unrestricted binding of β-catenin to TCF/LEF (Hanson et al. 2012).
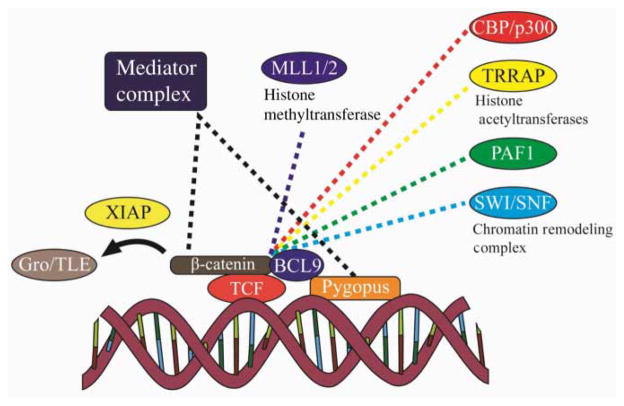
Two notable nuclear co-factors found in Drosophila and vertebrates, Pygopus (Pygo) and BCL9, have been shown to facilitate Wnt pathway-mediated transcription (Figure 3) (Belenkaya et al. 2002; Parker et al. 2002; Thompson et al. 2002). BCL9 binds and bridges β-catenin with the Pygo protein. Pygo has been shown to bind the multiprotein transcriptional co-activator mediator complex and contains a plant homology domain that interacts with dimethylated histone 3 lysine 4, an indicator of transcriptional activation (Fiedler et al. 2008). Studies in Drosophila indicate that TCF, β-catenin, BCL9, and Pygo represent a core transcriptional complex that is necessary for Wnt-mediated gene transcription. In mammals, the situation is more complex, and there appears to be functional redundancy and cell type-specific roles for BCL9 and Pygo (Schwab et al. 2007; Sustmann et al. 2008).
Figure 3.
Nuclear TCF/β-catenin transcriptional complexes. Upon Wnt/β-catenin signaling, DNA-bound TCF/β-catenin recruits many other transcriptional complexes to Wnt target genes. Dotted lines represent interactions between the transcriptional complexes and β-catenin. During active Wnt target gene transcription, the co-repressor Gro/TLE cycles on and off of β-catenin in an XIAP-dependent manner with the other transcriptional complexes. Gro/TLE, Groucho/transducin-like enhancer of split.
A role in extensive chromatin remodeling at TCF/ β-catenin-bound promoter regions appears to be an important aspect of Wnt signaling. In addition to Pygo and BCL9, β-catenin interacts with an extensive set of chromatin remodeling complexes that include the p300/CBP and TRRAP/TIP60 histone acetyltransferases, the SWI/SNF family of ATPases, which are important for chromatin remodeling, the PAF1 complex, which is involved in transcription elongation and histone modification, and the MLL1/2 histone methyltransferases (Willert and Jones 2006; Mosimann et al. 2009). Although it is known that most of these complexes interact with the C-terminal end of β-catenin, the order and timing of these interactions remain unclear. The importance of these interactions is highlighted by studies demonstrating that the small molecule inhibitor of β-catenin–CBP interaction, ICG-001, inhibits Wnt signaling in vitro and in vivo (Eguchi et al. 2005; Henderson et al. 2010).
It is important to note an often-underappreciated role for TCF/β-catenin signaling in transcriptional repression. Studies have shown a number of potential mechanisms of repression, including competition of TCF/β-catenin with transcriptional activators, recruitment of co-repressors to WREs, or TCF binding to a novel consensus sequence that specifically mediates repression (Piepenburg et al. 2000; Jamora et al. 2003; Kahler and Westendorf 2003; Theisen et al. 2007; Blauwkamp et al. 2008). In addition, there are a plethora of studies showing that many DNA-binding transcription factors (e.g. Smad3, AP-1, RXR, and Kaiso) bind to β-catenin to activate or repress Wnt/β-catenin target genes. In light of the large number of TCF binding sites and numerous transcriptional co-regulators, it is clear that the Wnt/β-catenin gene expression program is vast and induces dramatic changes in the physiological state of the cell, many of which are still not well understood (Cadigan 2012). Thus, Wnt signaling leads to a transcriptional program that includes downregulation and upregulation, rather than to simply transcriptional activation.
Wnt signaling and cancer
The Wnt signaling pathway plays a critical role in many human diseases, and its misregulation is implicated in numerous types of cancer. Inappropriate Wnt pathway activation has been implicated in nearly all the major solid human cancers. Gain-of-function mutations of β-catenin that block its phosphorylation by GSK3 occur at positions Ser33, Ser37, and Thr41 (Peifer and Polakis 2000; Laurent-Puig et al. 2001). Mutations of Ser45, the CK1α priming site on β-catenin, have also been found in cancers. In addition, to these point mutations, N-terminal in-frame deletions of β-catenin (which remove the GSK3 and CK1α phosphorylation sites) have also been described in cancers (Peifer and Polakis 2000; Laurent-Puig et al. 2001). All of these β-catenin mutations prevent its phosphorylation by GSK3 and/or CKIα and its subsequent ubiquitin-mediated degradation. These gain-of-function mutations of β-catenin often occur separately from mutations in other Wnt pathway components. Nonhereditary APC mutations in cancers leading to premature truncation occur frequently at two hot spots (codons 1309 and 1450) (Polakis 1995; Segditsas and Tomlinson 2006). These truncation mutations disrupt APC’s binding to Axin and β-catenin and results in decreased activity of the β-catenin degradation complex. Mutations in Axin, the limiting component of the β-catenin degradation complex, have also been found in human cancer, and both missense and nonsense mutations have been described (Liu et al. 2000; Satoh et al. 2000; Lammi et al. 2004).
Perhaps, the best-studied role of Wnt signaling is in colorectal cancer, which we discussed earlier in this review. In ~90% of sporadic colorectal cancers, an activating mutation in a component of the Wnt pathway leads to the formation of colorectal carcinomas, with 80% of the mutations located in the destruction complex protein APC (reviewed in Peifer and Polakis 2000; Polakis 2000). The epithelium of the small intestine is ordered into villi and crypts of Lieberkühn. Proliferative stem and progenitor cells are found in the bottom two-thirds of the crypts, with differentiated cells continuously moving upward the tips of the villi (Heath 1996; Reya and Clevers 2005). The Wnt signaling pathway is the dominant force in controlling cell fate along the crypts (Reya and Clevers 2005), and it is required for maintenance of the crypt progenitor phenotype (Korinek et al. 1998). An inactivating mutation in APC, resulting in inappropriate activation of the Wnt pathway, expands the progenitor populations; an APC mutation is typically the earliest event in a series of mutations that occur during progression to metastatic colorectal cancer (Rubinfeld et al. 1996; Markowitz and Bertagnolli 2009). The mechanism by which Wnt signaling mediates proliferation in colorectal cancers has been proposed to be mediated in a large part by its induction of c-Myc expression and possibly by stabilizing the oncogene RAS (Sansom et al. 2007; Jeong et al. 2012).
Wnt pathway activation has also been implicated in breast cancer. As mentioned earlier, wnt (identified as int-1) was originally identified in mammals as a tumorigenic oncogene in a forward genetic screen using MMTV integration sites (Nusse et al. 1984). Interestingly, although Wnt signaling appears to be elevated in many breast cancer lines, mutations in components of the destruction complex are extremely rare (Bafico et al. 2004). Instead, activation of the Wnt pathway in breast carcinomas appears to be due to increased expression of the secreted Wnt ligands (autocrine signaling), as well as decreased expression of soluble Wnt inhibitors, such as WIF1 and sFRP1 (Ugolini et al. 2001; Bafico et al. 2004). Such breast cancers are particularly associated with the triple-negative type (i.e. does not express the genes for estrogen receptor, progesterone receptor, and Her2/ neu) and are predicted to have a poorer prognosis (Geyer et al. 2010; Khramtsov et al. 2010). In fact, β-catenin and LRP6 have been proposed to be prognostic markers for breast cancer, particularly the triple-negative type (Kinzler et al. 1991; Lin et al. 2000; Liu et al. 2010).
In lung cancer, similar to breast cancer, activating mutations in β-catenin and APC are rare, and the Wnt pathway is activated upstream of β-catenin (Ohgaki et al. 2004). At least three mechanisms of canonical Wnt activation in lung cancer have been described: (1) autocrine Wnt signaling (non-small cell lung cancer), (2) overexpression of positive Wnt mediators such as Dsh, and (3) repression of Wnt antagonists (e.g. WIF-1) (Van Scoyk et al. 2008; Akiri et al. 2009). Wnt signaling in lung cancer has also been associated with increased metastatic capacity (Nguyen et al. 2009).
Wnt signaling has also been shown to play a prominent role in liver cancer (Peifer and Polakis 2000; Polakis 2000; Laurent-Puig et al. 2001). β-Catenin activating mutations are present in up to 50% of all hepatic tumors (de La Coste et al. 1998). Another common activating Wnt mutation in liver cancer occurs in the negative regulator Axin (Satoh et al. 2000; Taniguchi et al. 2002). These β-catenin and Axin mutations alone are not believed to be sufficient to cause spontaneous hepato-cellular carcinoma, but likely act in concert with other cellular signaling pathways to promote hepato-cellular carcinogenesis.
Wnt signaling has also been shown to play an important role in less prevalent cancers. Increased β-catenin levels have been shown to be present in a majority of pancreatic carcinomas (Zeng et al. 2006; Pasca di Magliano et al. 2007). Altered β-catenin levels are thought to result in loss of differentiation, as well as the progression of pancreatic carcinoma (Lowy et al. 2003). In addition, HSPGs, which positively regulate Wnt signaling, are also overexpressed in pancreatic carcinomas (Su et al. 2001; Iacobuzio-Donahue et al. 2003; Li et al. 2005; Nawroth et al. 2007). Sporadic medulloblastomas are also often affected by misregulated Wnt signaling. Mutations that activate the Wnt pathway (primarily in APC, Axin, and β-catenin) occur in ~15% of sporadic medulloblastomas (Huang et al. 2000; Koch et al. 2001). The Wnt pathway also plays a role in renal cell carcinoma. Although β-catenin activating mutations are rare (Kim et al. 2000), elevated β-catenin levels have been shown to induce renal tumors in mice (Saadi-Kheddouci et al. 2001; Qian et al. 2005; Sansom et al. 2005). Promoter hypermethylation-associated silencing of the APC gene, as well as genes encoding the secreted Wnt inhibitors sFRP1, Dkk2, and WIF-1, has been proposed to play a role in renal cell carcinogenesis (Battagli et al. 2003; Awakura et al. 2008b; Kawakami et al. 2009).
The Wnt pathway plays an important role in the maintenance of hematopoietic stem and progenitor cells, suggesting that oncogenic growth in some leukemia is Wnt dependent. High levels of Wnts have also been detected in acute lymphoblastic leukemia (Khan et al. 2007a). Similarly, elevated Wnt signaling in granulocyte–macrophage progenitor cells obtained from chronic myeloid leukemia patients has enhanced leukemic potential (Jamieson et al. 2004). Ovarian cancer lines have also been shown to exhibit elevated Wnt signaling (Bafico et al. 2004). Finally, the Wnt pathway controls the fate and proliferation of cells in hair follicles, which are derived from the bulge stem cells located in the bulge niche. The vast majority of human hair follicle tumors (pilomatricomas) have mutations in the N-terminus of β-catenin that affect its degradation (Chan et al. 1999).
The cell cycle has been shown to control signaling through the Wnt pathway via regulation of LRP6 and Axin levels (Davidson et al. 2009; Hadjihannas et al. 2012). It is tempting to speculate that aberrant Wnt signaling independent of cell cycle control may play a role in the genesis of Wnt-driven cancer.
Wnt signaling and stem cell control
Embryonic stem cells (ESCs) are pluripotent stem cells taken from the inner cell mass. They can give rise to any cell type from the three germ cell layers (endoderm, mesoderm, and ectoderm) (Tesche and Gerber 2010). The isolation of murine ESCs (mESCs) and human ESCs (hESCs) generated great excitement in the scientific community. However, hESCs are resistant to nonviral genetic manipulation, greatly limiting their application for the study of human development and disease. Therefore, most of the studies have been carried out in mESCs. Although some progress has been made toward the identification of the factors required to maintain ESCs in an undifferentiated state, only a few have been identified, including leukemia inhibitory factor (Smith et al. 1988; Williams et al. 1988) and bone morphogenetic protein 4 (BMP4) (Ying et al. 2003). The prevalent model shows that Wnt signaling is required to maintain ESC self-renewal; however, it is not clear whether the Wnt pathway is sufficient for this process.
In mESCs, TCF3 is the predominant TCF/LEF transcription factor and behaves as a repressor of Wnt signaling (Pereira et al. 2006; Yi et al. 2008). TCF3, in complex with other co-repressors such as Groucho, represses the expression of pluripotency-related genes under the control of Oct4. Upon activation of Wnt signaling, either the interaction of β-catenin with TCF3 or the phosphorylation of TCF3 upon binding β-catenin induces the expression of TCF3 target genes (Wray and Hartmann 2012). Also, it has been shown that a TCF1/β-catenin complex activates the expression of Wnt target genes, probably enhancing the expression of pluripotency-related genes (Yi et al. 2011). It is possible that noncanonical Wnt signaling plays a positive role on the pluripotency network.
The gene expression profile and growth factor requirements of hESCs suggest that they are more similar to mouse epiblast stem cells (derived from postimplantation mouse embryos) than mESCs (Brons et al. 2007; Tesar et al. 2007). Therefore, it cannot be assumed that the findings in mESCs also apply to hESCs. Activation of the canonical Wnt pathway has been shown to inhibit the differentiation of hESCs (Sato et al. 2004). However, blocking the activity of Wnt ligands using antagonists does not induce differentiation (Dravid et al. 2005), suggesting that Wnt signaling is not required for hESC maintenance.
Induced pluripotent stem cells (iPSCs) exhibit properties similar to ESCs, but iPSCs can be generated from fully differentiated somatic cells. iPSCs closely resemble ESCs in gene expression, epigenetic signature, and their unlimited capacity to undergo proliferation in vitro while maintaining their pluripotency (Li and Ding 2010; Okita and Yamanaka 2010). iPSCs can be generated by the addition of several combinations of the transcription factors Oct4, Sox2, Klf4, and c-Myc using retroviral- or lentiviral-based vectors (Takahashi and Yamanaka 2006; Takahashi et al. 2007). It is possible to vary the number of transcription factors used during reprogramming. In the absence of c-Myc, Wnt3a-conditioned media was reported to enhance iPSC reprogramming (Marson et al. 2008), further showing the requirement of Wnts in stimulating pluripotency.
Several studies support a role for Wnt in lineage decisions and cell fate in embryonic and adult stem cells. For instance, Wnts promote differentiation of neural stem cells in the mouse neocortex (Hirabayashi et al. 2004). Another study reports that Wnt signaling is required in neuronal specification in the dorsal spinal cord (Muroyama et al. 2002). During muscle injury, Wnts promote myogenic specification and are involved in muscle regeneration (Polesskaya et al. 2003). It is possible that the differential responses to stimuli and the differential activation of specific sets of Wnt target genes are mediated by cell type-specific intrinsic properties. Also, a given cell might acquire changes in these intrinsic properties over time. Thus, at different stages of development, Wnt can promote either self-renewal or lineage commitment (Kléber and Sommer 2004).
The effect of Wnt signaling on a particular type of stem cell depends on its microenvironment. Thus, Wnt signaling interacts with other signaling pathways, and the output is determined by their combined activity (Kléber and Sommer 2004). For example, in the nervous system, Wnt signaling and BMP signaling cooperatively regulate Emx2 expression in the dorsal telencephalon (Theil et al. 2002), whereas Wnt and BMP have antagonistic functions in establishing neuronal and glial lineages, as opposed to melanocytes in the neural crest (Jin et al. 2001).
In Drosophila, Wg regulates stem cells during development in many different tissues. Several studies have revealed conserved mechanisms of Wg regulating Drosophila gut stem cells, similar to Wnt regulation of mammalian stem cells in the intestinal crypts. Wg signaling is required and sufficient for self-renewal and/or proliferation of stem cells in the Drosophila midgut, a functional equivalent of mammalian small intestine (Lin et al. 2008; Lee et al. 2009). Here, the Wg ligand is produced by circular muscles, which are separated from the stem cells by a basement membrane through which the Wg ligand apparently moves (Lin et al. 2008). Wg is also expressed in another gut stem cell, the hindgut stem cells at the midgut/hindgut boundary, to maintain them in a slow-cycling, self-renewal state (Takashima et al. 2008). Wg signaling also regulates other types of stem cells in Drosophila. For example, Wg signaling regulates the self-renewal of gastric stem cells in the stomach (Strand and Micchelli 2011), follicle stem cells in the ovary (Song and Xie 2003), and stem-like hematopoietic precursor cells in the lymph gland (Sinenko et al. 2009).
Assaying the Wnt pathway in vitro and in vivo
It has been nearly three decades since the discovery of Wnt. The pathway has seemingly become more complex, and every year additional proteins are identified to be critical for transduction of a Wnt signal or to impinge on the Wnt pathway. Given our current understanding of the pathway, it is now possible to establish criteria which one would consider strong evidence that a given protein or small molecule is involved in Wnt signaling. We can divide these criteria into three broad categories: biochemical alterations in key Wnt components in the plasma membrane and cytoplasm, transcriptional changes in response to Wnt signaling, and perturbations in Wnt pathway-driven developmental processes. For the sake of simplicity, we focus on proteins/small molecules that act positively in the Wnt pathway, although we expect negative regulators to have opposite effects. We describe the most common assays below, although this list is by no means comprehensive.
Biochemical evidence for participation in Wnt signaling in the plasma membrane and cytoplasm
-
Binding of secreted Wnt ligand to the co-receptors LRP5/6 and Frizzled is the initial event for Wnt pathway activation. Activation of the Wnt pathway at the level of the plasma membrane can be assessed in the following manner:
Phosphorylation of Dishevelled upon Wnt activation of Frizzled. Dishevelled phosphorylation can be assessed by mobility shift on SDS-PAGE (Yanagawa et al. 1995).
Phosphorylation of LRP5/6 by GSK3 and/or CK1 that occurs rapidly upon Wnt pathway activation. LRP6 phosphorylation can be detected by anti-phospho-LRP6-specific antibodies (Zeng et al. 2005a).
Recruitment of the scaffold protein Axin (and associated GSK3) to the plasma membrane pool by activated LRP6. Axin recruitment can be observed by immunoblotting plasma membrane fractions for Axin and/or GSK3 (Mao et al. 2001).
Formation of signalosomes upon Wnt–receptor engagement. Frizzled–LRP5 complexes can be assessed by immunoprecipitation followed by immunoblotting. LRP6 oligomers can be assessed by sucrose density gradient centrifugation (Bilić et al. 2007).
-
Inhibition of the β-catenin destruction complex represents the central feature of Wnt pathway activation. Effects on the β-catenin destruction complex represent strong biochemical evidence for participation in the Wnt pathway and can be assessed as follows:
Accumulation of intracellular β-catenin levels. This can be readily assessed by immunoblotting the cytoplasmic fraction or by performing immunofluorescence studies. Because Wnt stimulation also leads to β-catenin accumulation in the nucleus, increased nuclear β-catenin can similarly be assessed (Morin et al. 1997).
The degradation of β-catenin requires its phosphorylation by GSK3, and Wnt pathway activation inhibits GSK3 phosphorylation of β-catenin (Peifer et al. 1994; Yost et al. 1996). Antibodies that recognize the GSK3-phosphorylated and GSK3-deposphorylated form of β-catenin can be used to assess Wnt pathway activation at this step, in tissues or on immunoblots.
In many, but not all, instances, Axin degradation is stimulated in response to Wnt signaling. Thus, a decreased steady-state level of cytoplasmic Axin, when it occurs, is an indicator of active Wnt signaling (Yamamoto et al. 1999).
GSK3 activity has been shown to be inhibited upon Wnt pathway activation. Recently, a reporter protein for GSK3 activity has been described in which GFP is fused to three GSK3 phosphorylation sites (Taelman et al. 2010). Stability of this protein is regulated by GSK3 activity, and activation of the Wnt pathway inhibits its degradation.
Transcriptional changes in response to Wnt signaling
-
Activation of Wnt/β-catenin ultimately leads to alterations in a Wnt-specific transcriptional program. Wnt-mediated transcriptional response can be assessed in the following manner:
Activation of a luciferase transcriptional reporter or TOPflash (Molenaar et al. 1996; van de Wetering et al. 1997). The TOPflash reporter consists of multimerized TCF/Lef-binding sites fused to the luciferase gene. Luciferase activity is proportional to the strength of the Wnt-mediated transcriptional activation. Although normally cell based, this reporter has also been used in Xenopus (Kiecker and Niehrs 2001). A similar approach using GFP signal as a readout has also been described (Dorsky et al. 2002).
Regulation of endogenous Wnt target genes. Well-characterized Wnt pathway target genes, such as axin2, naked2, siamois, Xnr3, en, and distal-less will show a change in mRNA transcription in response to Wnt transcriptional activation. Although Wnt signaling is often associated with target gene activation, it is worth noting that there are instances in which Wnt signaling can also result in the suppression of gene transcription (Hoverter and Waterman 2008; Cadigan 2012). Regardless, these changes can be readily assessed by real-time PCR, microarray, or tissue staining.
Recruitment of β-catenin onto WRE. The dynamic recruitment of β-catenin onto Wnt target promoters can be determined by chromatin immunoprecipitation assays (Blauwkamp et al. 2008; Hatzis et al. 2008).
-
Wnt/β-catenin signaling plays critical roles in metazoan development. Thus, alteration in Wnt signaling will result in notable stereotypic effects on the developing organism. These changes can be used as a readout to assess activation or inhibition of Wnt signaling in an in vivo setting.
The Wnt pathway plays a crucial role in the development of the Xenopus embryonic axis. In the early Xenopus embryo, ectopic activation of Wnt signaling leads to formation of a secondary body axis. Conversely, inhibition of Wnt signaling leads to embryos with reduced anterior–dorsal structures (Smith and Harland 1991; Sokol et al. 1991).
In Drosophila, Wg establishes segment polarity during embryogenesis, which can be assessed either by reporter expression or by patterning of the cuticle into regions of naked cuticle or hair-like projections called denticles (Nusslein-Volhard and Wieschaus 1980). Upon decreased wg function, embryos are covered with a lawn of denticles. Conversely, enhanced wg function results in embryos with naked cuticles (Sampedro et al. 1993; Sanson et al. 1999). In the wing disc, Wg patterns the dorsal–ventral boundary of the future wing blade (Couso et al. 1993); Wg pathway activation results in activation of reporter genes senseless, a short-range target, and distal-less, a long-range target, both of which can be detected by antibody staining (Sampedro et al. 1993; Sanson et al. 1999).
In Caenorhabditis elegans, the posterior migration of QL neuroblast progeny, QL.d, is regulated by canonical Wnt signaling and controls the expression of the mab-5 gene (reviewed by Eisenmann 2005). Inhibition of Wnt signaling in Caenorhabditis elegans causes anterior migration of QL.d cells as well as loss of mab-5 expression. Other developmental processes regulated by Wnt signaling in worms include vulval development and endoderm induction.
In mice, mutations in the Wnt pathway result in distinct phenotypes, including defects in midbrain–hindbrain formation, bone formation, kidney development, and lung development. In addition, hyperactivation of the pathway induces formation of tumors in various tissues, most notably, adenomas of the gastrointestinal tract (Wang et al. 2012).
Perspectives/future directions
Our understanding of Wnt/β-catenin signaling has broadened significantly over the last several years. Particularly exciting is the emerging role of E3 ubiquitin ligases and deubiquitinases in regulating Wnt pathway. Ubiquitination of Wnt pathway components occurs at all levels of the Wnt pathway, from receptor activation to nuclear transcription. Ubiquitination may represent a regulatory mechanism that plays an absolutely critical role in regulating pathway activation, similar to the critical role that has been shown for kinases. The application of biochemical techniques such as reconstitution of key steps using purified components provides strong demonstration of sufficiency. One important goal will be to reconstitute the pathway from ubiquitin-mediated degradation of β-catenin to receptor-mediated inhibition of β-catenin turnover. Such an approach has been performed for the G-protein-coupled systems in which ligands, receptors, G proteins, and effectors have been reconstituted in vitro, demonstrating sufficiency in pathway activation and confirming the prevalent model of G-protein action (Gilman 1987). Although this feat would likely be much more difficult because many more components are involved, it would provide invaluable insight into the central mechanism of β-catenin degradation and its signal-dependent inhibition. Newly resolved structures of Wnt pathway components, as evidenced by the recent structure determination of XWnt8 bound to the CRD of Fz8, will allow us to look at protein–protein interactions, as well as binding and conformational changes that might provide a better understanding of how individual proteins respond to Wnt signaling. A particularly important structure would be that of the β-catenin degradation complex as it may illuminate our understanding of this central step in the pathway: the mechanism of β-catenin degradation, the role of APC, and how pathway activation inhibits this complex. A similar biochemical and structural approach may be required for the β-catenin transcriptional activation complex, of which even less is known.
Another exciting development in the Wnt field is the increasing use of quantitative mathematical modeling to predict the behavior of the Wnt pathway and its capacity to change over time or in response to specific stimuli or perturbations. Such studies should provide insight into how the Wnt pathway can be “adapted” to play distinct roles in a broad number of developmental and physiological processes. Specifically, modeling may be informative as to how different factors can markedly alter the output of the system to allow the pathway to perform a legion of specific developmental and/or physiological tasks or, in pathological situations, lead to human disease. Some factors that might be investigated by modeling include cross talk with other pathways, changes in pathway composition, changes in pathway concentrations, and alterations in intramolecular interactions. It is likely that a better understanding of the Wnt/β-catenin pathway will have major impact on the field of developmental biology, regenerative (stem cell) medicine, and cancer research. It is thus very exciting that in the last few years many small molecule inhibitors (Porc inhibitors, tåankyrase inhibitors, CK1α activators, to name a few) that inhibit the Wnt pathway in vitro and in animal models have been identified. In the next several years, it is likely that the long journey from the first identification of the Wnt pathway over 30 years ago to the application of a Wnt inhibitor for the treatment of a human disease will come to fruition. These are very interesting times in the Wnt field indeed.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was supported by NCI GI SPORE P50 CA95103 and NIH 1 R01 GM081635-05 (E. Lee); NIH R01 GM073883 (A. Page-McCaw); NIH 5T32CA11992504 (H.A. Wallace); American Heart Association 12 PRE 6590007 (T.W. Chen).
References
- Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129:1751–1762. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- Ahn Victoria E, Chu Matthew L-H, Choi H-J, Tran D, Abo A, Weis William I. Structural basis of Wnt signaling inhibition by Dickkopf Binding to LRP5/6. Dev Cell. 2011;21:862–873. doi: 10.1016/j.devcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Awakura Y, Nakamura E, Ito N, Kamoto T, Ogawa O. Methylation-associated silencing of SFRP1 in renal cell carcinoma. Oncol Rep. 2008a;20:1257–1263. [PubMed] [Google Scholar]
- Awakura Y, Nakamura E, Ito N, Kamoto T, Ogawa O. Methylation-associated silencing of SFRP1 in renal cell carcinoma. Oncol Rep. 2008b;20:1257–1263. [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and heparan sulfate proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Baig-Lewis S, Peterson-Nedry W, Wehrli M. Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Dev Biol. 2007;306:94–111. doi: 10.1016/j.ydbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Battagli C, Uzzo RG, Dulaimi E, Ibanez de Caceres I, Krassenstein R, Al-Saleem T, Greenberg RE, Cairns P. Promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Cancer Res. 2003;63:8695–8699. [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Wieschaus E. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics. 1995;139:309–320. doi: 10.1093/genetics/139.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J. Pygopus encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bienz M. The subcellular destinations of APC proteins. Nat Rev Mol Cell Biol. 2002;3:328–338. doi: 10.1038/nrm806. [DOI] [PubMed] [Google Scholar]
- Bilić J, Huang Y-L, Davidson G, Zimmermann T, Cruciat C-M, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes Dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Bjorklund P, Svedlund J, Olsson AK, Akerstrom G, Westin G. The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One. 2009;4:e4243. doi: 10.1371/journal.pone.0004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Lopes SMCdS, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Buechling T, Chaudhary V, Spirohn K, Weiss M, Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO Rep. 2011;12:1265–1272. doi: 10.1038/embor.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM. TCFs and Wnt/beta-catenin signaling: More than one way to throw the switch. Curr Top Dev Biol. 2012;98:1–34. doi: 10.1016/B978-0-12-386499-4.00001-X. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harb Perspect Biol. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow MG, Tran H, Phu L, Lau T, Lee J, Sandoval WN, Liu PS, Bheddah S, Tao J, Lill JR, Hongo J-A, Davis D, Kirkpatrick DS, Polakis P, Costa M. Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One. 2011;6:e22595. doi: 10.1371/journal.pone.0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bubeck Bryan D, MacDonald T, Liang W-X, Mao J-H, Malinauskas T, Llorca O, Aricescu AR, Siebold C, He X, Jones EY. Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev Cell. 2011;21:848–861. doi: 10.1016/j.devcel.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, Xu W. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004a;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004b;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Coudreuse DYM, Roël G, Betist MC, Destrée O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of β-catenin. Proc Nat Acad Sci. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuilliere-Dartigues P, El-Bchiri J, Krimi A, Buhard O, Fontanges P, Flejou JF, Hamelin R, Duval A. TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene. 2006;25:4441–4448. doi: 10.1038/sj.onc.1209471. [DOI] [PubMed] [Google Scholar]
- Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sörensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 2001;61:7039–7043. [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3 beta: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 2003;22:494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–833. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 [gamma] couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Nat Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/β-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol. 2000;59:333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- Egger-Adam D, Katanaev VL. The trimeric G protein Go inflicts a double impact on axin in the Wnt/frizzled signaling pathway. Dev Dyn. 2009;239:168–183. doi: 10.1002/dvdy.22060. [DOI] [PubMed] [Google Scholar]
- Eguchi M, Nguyen C, Lee SC, Kahn M. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem. 2005;1:467–472. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM. WormBook, editor. The C. elegans Research Community. WormBook; Jun 25, 2005. Wnt signaling. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Eugster C, Panakova D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Gluck U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux MC, Coates JL, Catimel B, Cody S, Clayton AHA, Layton MJ, Burgess AW. Recruitment of adenomatous polyposis coli and |[beta]| -catenin to axin-puncta. Oncogene. 2008;27:5808–5820. doi: 10.1038/onc.2008.205. [DOI] [PubMed] [Google Scholar]
- Fiedler M, Sanchez-Barrena MJ, Nekrasov M, Mieszczanek J, Rybin V, Muller J, Evans P, Bienz M. Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Mol Cell. 2008;30:507–518. doi: 10.1016/j.molcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde JE, Dale TC. Glycogen synthase kinase 3: A key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Marchand O, Piddini E, Ricardo S, Alexandre C, Vincent JP. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132:659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent J-P. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci USA. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. Beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2010;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell. 2009;36:872–884. doi: 10.1016/j.molcel.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goentoro L, Shoval O, Kirschner MW, Alon U. The incoherent feedforward loop can provide fold-change detection in gene regulation. Mol Cell. 2009;36:894–899. doi: 10.1016/j.molcel.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol Cell Biol. 2004;24:4757–4768. doi: 10.1128/MCB.24.11.4757-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. 2000;103:885–896. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. Beta-Catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Ha N-C, Tonozuka T, Stamos JL, Choi H-J, Weis WI. Mechanism of phosphorylation-dependent binding of APC to [beta]-catenin and its role in [beta]-catenin degradation. Mol Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Haar Et, Coll JT, Austen DA, Hsiao H-M, Swenson L, Jain J. Structure of GSK3|[beta]| reveals a primed phosphorylation mechanism. Nat Struct Mol Biol. 2001;8:593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- Hacker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–3573. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Hadjihannas MV, Bernkopf DB, Bruckner M, Behrens J. Cell cycle control of Wnt/beta-catenin signalling by conductin/axin2 through CDC20. EMBO Rep. 2012;13:347–354. doi: 10.1038/embor.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerry TE, Heslip TR, Marsh JL, O’Connor MB. Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- Hamada F, Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Dev Cell. 2004;7:677–685. doi: 10.1016/j.devcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Hammerlein A, Weiske J, Huber O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell Mol Life Sci. 2005;62:606–618. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Hanson AJ, Wallace HA, Freeman TJ, Beauchamp RD, Lee LA, Lee E. XIAP monoubiquitylates Groucho/TLE to promote canonical Wnt signaling. Mol Cell. 2012;45(5):619–628. doi: 10.1016/j.molcel.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, Versteeg R, Cuppen E, van de Wetering M, Clevers H, Stunnenberg HG. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28:2732–2744. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Koren A, Caspi M, Zilberberg A, Rosin-Arbesfeld R. The EDD E3 ubiquitin ligase ubiquitinates and up-regulates beta-catenin. Mol Biol Cell. 2010;22:399–411. doi: 10.1091/mbc.E10-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20:139–146. doi: 10.1006/cbir.1996.0018. [DOI] [PubMed] [Google Scholar]
- Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 2002;3:834–839. doi: 10.1093/embo-reports/kvf181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M. RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. J Cell Biol. 2005;171:785–797. doi: 10.1083/jcb.200502141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N, Offerhaus GJ, Fagotto F, Fornerod M. Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. J Cell Sci. 2008;121:1793–1802. doi: 10.1242/jcs.025536. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Klein AM, Kirschner MW. Kinetic responses of beta-catenin specify the sites of Wnt control. Science. 2012;338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2011;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G. Tumor progression induced by the loss of E-cadherin independent of beta-catenin/Tcf-mediated Wnt signaling. Oncogene. 2007;26:2290–2298. doi: 10.1038/sj.onc.1210029. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem. 2011;286:12093–12100. doi: 10.1074/jbc.M110.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/B-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, Ishii N, Dahiya R. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–5687. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- Hoverter NP, Waterman ML. A Wnt-fall for gene regulation: Repression. Sci Signal. 2008;1:pe43. doi: 10.1126/scisignal.139pe43. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci USA. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- Hu T, Li C, Cao Z, Van Raay TJ, Smith JG, Willert K, Solnica-Krezel L, Coffey RJ. Myristoylated Naked2 antagonizes Wnt-beta-catenin activity by degrading Dishevelled-1 at the plasma membrane. J Biol Chem. 2010;285:13561–13568. doi: 10.1074/jbc.M109.075945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H. APC mutations in sporadic medulloblastomas. Am J Pathol. 2000;156:433–437. doi: 10.1016/S0002-9440(10)64747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009a;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Huang X, Langelotz C, Hetfeld-Pechoc BK, Schwenk W, Dubiel W. The COP9 signalosome mediates beta-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol. 2009b;391:691–702. doi: 10.1016/j.jmb.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq R, Jaffee E, Ryu B, Jones J, Eshleman JR, Yeo CJ, Cameron JL, Kern SE, Hruban RH, Brown PO, Goggins M. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3[beta] and [beta]-catenin and promotes GSK-3[beta]-dependent phosphorylation of [beta]-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and [beta]-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Itoh K, Jenny A, Mlodzik M, Sokol SY. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci. 2009;122:3791–3798. doi: 10.1242/jcs.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WJ, Yoon J, Park JC, Lee SH, Kaduwal S, Kim H, Yoon JB, Choi KY. Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Sci Signal. 2012;5:ra30. doi: 10.1126/scisignal.2002242. [DOI] [PubMed] [Google Scholar]
- Jernigan KK, Cselenyi CS, Thorne CA, Hanson AJ, Tahinci E, Hajicek N, Oldham WM, Lee LA, Hamm HE, Hepler JR, Kozasa T, Linder ME, Lee E. Gbetagamma activates GSK3 to promote LRP6-mediated beta-catenin transcriptional activity. Science Signal. 2010;3:ra37. doi: 10.1126/scisignal.2000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233:22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J Biol Chem. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphoryl-ation of beta-catenin independent of axin: Implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Hirata H, Yamamura S, Kikuno N, Saini S, Majid S, Tanaka Y, Kawamoto K, Enokida H, Nakagawa M, Dahiya R. Functional significance of Wnt inhibitory factor-1 gene in kidney cancer. Cancer Res. 2009;69:8603–8610. doi: 10.1158/0008-5472.CAN-09-2534. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007a;138:338–348. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- Khan Z, Vijayakumar S, de la Torre TV, Rotolo S, Bafico A. Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor. Mol Cell Biol. 2007b;27:7291–7301. doi: 10.1128/MCB.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Killick R, Pollard CC, Asuni AA, Mudher AK, Richardson JC, Rupniak HT, Sheppard PW, Varndell IM, Brion JP, Levey AI, Levy OA, Vestling M, Cowburn R, Lovestone S, Anderton BH. Presenilin 1 independently regulates beta-catenin stability and transcriptional activity. J Biol Chem. 2001;276:48554–48561. doi: 10.1074/jbc.M108332200. [DOI] [PubMed] [Google Scholar]
- Kim S, Jho E-h. The protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2) J Biol Chem. 2010;285:36420–36426. doi: 10.1074/jbc.M110.137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kang YK, Kim JB, Han SA, Kim KI, Paik SR. Beta-catenin expression and mutational analysis in renal cell carcinomas. Pathol Int. 2000;50:725–730. doi: 10.1046/j.1440-1827.2000.01111.x. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kim K-A, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Chia IV, Costantini F. SUMOylation target sites at the C terminus protect Axin from ubiquitination and confer protein stability. FASEB J. 2008;22:3785–3794. doi: 10.1096/fj.08-113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Xu W. Beta-catenin destruction complex: Insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, Boguski MS, Altschul SF, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Dimitroff BD, Rawson JM, Selleck SB. Spatial regulation of Wingless morphogen distribution and signaling by Dally-like protein. Dev Cell. 2004;7:513–523. doi: 10.1016/j.devcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, Asashima M, Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. J Biol Chem. 2001;276:33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Koch A, Waha A, Tonn JC, Sörensen N, Berthold F, Wolter M, Reifenberger J, Hartmann W, Friedl W, Reifenberger G, Wiestler OD, Pietsch T. Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer. 2001;93:445–449. doi: 10.1002/ijc.1342. [DOI] [PubMed] [Google Scholar]
- Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, Heasman J. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007a;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007b;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, Van Donselaar E, Huls G, Peters PJ, Clevers H, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J Cell Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- Kuphal F, Behrens J. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Exp Cell Res. 2006;312:457–467. doi: 10.1016/j.yexcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515–523. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Gorlich D. Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex. EMBO J. 1997;16:1153–1163. doi: 10.1093/emboj/16.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagna G, Carnevali F, Marchioni M, Hemmati-Brivanlou A. Negative regulation of axis formation and Wnt signaling in Xenopus embryos by the F-box/WD40 protein beta TrCP. Mech Dev. 1999;80:101–106. doi: 10.1016/s0925-4773(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Lai SL, Chien AJ, Moon RT. Wnt/Fz signaling and the cytoskeleton: Potential roles in tumorigenesis. Cell Res. 2009;19:532–545. doi: 10.1038/cr.2009.41. [DOI] [PubMed] [Google Scholar]
- Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenter-ology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Krüger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31:36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kleeff J, Abiatari I, Kayed H, Giese NA, Felix K, Giese T, Büchler MW, Friess H. Enhanced levels of Hsulf-1 interfere with heparin-binding growth factor signaling in pancreatic cancer. Mol Cancer. 2005;4:14. doi: 10.1186/1476-4598-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. Beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3β associations that regulate β-catenin stabilization are mediated by Gα proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MC, Gay F, Odom R, Shi Y, Lin R. Phosphorylation by the beta-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell. 2004;117:95–106. doi: 10.1016/s0092-8674(04)00203-x. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lowy AM, Fenoglio-Preiser C, Kim OJ, Kordich J, Gomez A, Knight J, James L, Groden J. Dysregulation of beta-catenin expression correlates with tumor differentiation in pancreatic duct adenocarcinoma. Ann Surg Oncol. 2003;10:284–290. doi: 10.1245/aso.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Lui TTH, Lacroix C, Ahmed SM, Goldenberg SJ, Leach CA, Daulat AM, Angers S. The ubiquitin-specific protease USP34 regulates Axin stability and Wnt/β-catenin signaling. Mol Cell Biol. 2011;31:2053–2065. doi: 10.1128/MCB.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Yokota C, Tamai K, Zeng X, He X. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J Biol Chem. 2008;283:16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Malik HS, Eickbush TH, Goldfarb DS. Evolutionary specialization of the nuclear targeting apparatus. Proc Natl Acad Sci USA. 1997;94:13738–13742. doi: 10.1073/pnas.94.25.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, III, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Elinson RP. Beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech Dev. 1998;77:75–80. doi: 10.1016/s0925-4773(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JO, Kitajewski J, Varmus HE. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol Biol Cell. 1992;3:521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, Kawahara T, Kobayashi S, Okada M, Toyoshima K, Akiyama T. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, Reguart N, Rosell R, McCormick F, Jablons DM. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, Bejsovec A. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989a;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. int-1—A proto-oncogene involved in cell signalling. Development. 1989b;107(suppl):161–167. doi: 10.1242/dev.107.Supplement.161. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Topaz C, Mieszczanek J, Bienz M. The adenoma-tous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin’s interaction with Dishevelled. Open Biol. 2011;1:110013. doi: 10.1098/rsob.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Johnson GVW. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95:328–338. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Fujita T, Ozaki T, Kato C, Kurose Y, Sakamoto M, Kato S, Goto T, Itoyama Y, Aoki M, Nakagawara A. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J Biol Chem. 2004;279:11327–11335. doi: 10.1074/jbc.M312389200. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: Regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J-S, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nathke I. Cytoskeleton out of the cupboard: Colon cancer and cytoskeletal changes induced by loss of APC. Nat Rev Cancer. 2006;6:967–974. doi: 10.1038/nrc2010. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS One. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kros JM, Okamoto Y, Gaspert A, Huang H, Kurrer MO. APC mutations are infrequent but present in human lung cancer. Cancer Lett. 2004;207:197–203. doi: 10.1016/j.canlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- Okita K, Yamanaka S. Induction of pluripotency by defined factors. Exp Cell Res. 2010;316:2565–2570. doi: 10.1016/j.yexcr.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Choi S-C, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, Abrams CS, Sokol SY, Wu D. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJA, Deramaudt T, Segara D, Dawson AC, Kench JG, Henshall SM, Sutherland RL, Dlugosz A, Rustgi AK, Hebrok M. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: Roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Peterson-Nedry W, Erdeniz N, Kremer S, Yu J, Baig-Lewis S, Wehrli M. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev Biol. 2008;320:226–241. doi: 10.1016/j.ydbio.2008.05.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kimble J. A new look at TCF and beta-catenin through the lens of a divergent C. elegans Wnt pathway. Dev Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S, Lee S-H, Kim H, Yum S, Stamos JL, Xu Y, Lee S-J, Lee J, Oh S, Han J-K, Park B-J, Weis WI, Ha N-C. Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signaling. PLoS One. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenburg O, Vorbruggen G, Jackle H. Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol Cell. 2000;6:203–209. [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Polakis P. Mutations in the APC gene and their implications for protein structure and function. Curr Opin Genet Dev. 1995;5:66–71. doi: 10.1016/s0959-437x(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45 + adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Port F, Basler K. Wnt trafficking: New insights into Wnt maturation, secretion and spreading. Traffic. 2010;11:1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- Port F, Hausmann G, Basler K. A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO Rep. 2011;12:1144–1152. doi: 10.1038/embor.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Price MA. CKI, there’s more than one: Casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- Qian C-N, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–3945. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- Ratcliffe MJ, Itoh K, Sokol SY. A positive role for the PP2A catalytic subunit in Wnt signal transduction. J Biol Chem. 2000;275:35680–35683. doi: 10.1074/jbc.C000639200. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Riggleman B, Wieschaus E, Schedl P. Molecular analysis of the armadillo locus: Uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 1989;3:96–113. doi: 10.1101/gad.3.1.96. [DOI] [PubMed] [Google Scholar]
- Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscrip-tionally regulated by wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Tice DA, Polakis P. Axin-dependent phos-phorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J Biol Chem. 2001;276:39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- Ruteshouser EC, Robinson SM, Huff V. Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer. 2008;47:461–470. doi: 10.1002/gcc.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase iepsilon in the wnt pathway: Regulation of beta-catenin function. Proc Natl Acad Sci USA. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane H, Yamamoto H, Matsumoto S, Sato A, Kikuchi A. Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. J Cell Sci. 2012;125:449–460. doi: 10.1242/jcs.091876. [DOI] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: Reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Samoilov M, Plyasunov S, Arkin AP. Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations. Proc Natl Acad Sci USA. 2005;102:2310–2315. doi: 10.1073/pnas.0406841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Johnston P, Lawrence PA. A role for wingless in the segmental gradient of Drosophila? Development. 1993;117:677–687. doi: 10.1242/dev.117.2.677. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Griffiths DFR, Reed KR, Winton DJ, Clarke AR. Apc deficiency predisposes to renal carcinoma in the mouse. Oncogene. 2005;24:8205–8210. doi: 10.1038/sj.onc.1208956. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Sanson B, Alexandre C, Fascetti N, Vincent JP. Engrailed and hedgehog make the range of Wingless asymmetric in Drosophila embryos. Cell. 1999;98:207–216. doi: 10.1016/s0092-8674(00)81015-6. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Schwab KR, Patterson LT, Hartman HA, Song N, Lang RA, Lin X, Potter SS. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC Biol. 2007;5:15. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007a;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007b;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–310. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Serban G, Kouchi Z, Baki L, Georgakopoulos A, Litterst CM, Shioi J, Robakis NK. Cadherins mediate both the association between PS1 and beta-catenin and the effects of PS1 on beta-catenin stability. J Biol Chem. 2005;280:36007–36012. doi: 10.1074/jbc.M507503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J Biol Chem. 2011;287:819–831. doi: 10.1074/jbc.M111.299099. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: From fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem. 2004;279:17232–17240. doi: 10.1074/jbc.M307801200. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Smolich BD, McMahon JA, McMahon AP, Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Sobrado P, Jedlicki A, Bustos VH, Allende CC, Allende JE. Basic region of residues 228-231 of protein kinase CK1alpha is involved in its interaction with axin: Binding to axin does not affect the kinase activity. J Cell Biochem. 2005;94:217–224. doi: 10.1002/jcb.20350. [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–1647. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005;280:2116–2125. doi: 10.1074/jbc.M410090200. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Uber die Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. W Roux Arch Entw Mech Org. 1938;100:599–638. [Google Scholar]
- Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M, Micchelli CA. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc Natl Acad Sci USA. 2011;108:17696–17701. doi: 10.1073/pnas.1109794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Strovel ET, Wu D, Sussman DJ. Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem. 2000;275:2399–2403. doi: 10.1074/jbc.275.4.2399. [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Su L-K, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H, Schultz PG, Powell SM, Moskaluk CA, Frierson HF, Jr, Hampton GM. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res. 2001;61:7388–7393. [PubMed] [Google Scholar]
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Sustmann C, Flach H, Ebert H, Eastman Q, Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Mol Cell Biol. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: New kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Toyota M, Caraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, Mori M, Hirata K, Imai K, Shinomura Y, Baylin SB, Tokino T. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec J-L, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A Mechanism for Wnt Coreceptor Activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tan CW, Gardiner BS, Hirokawa Y, Layton MJ, Smith DW, Burgess AW. Wnt signalling pathway parameters for mammalian cells. PLoS One. 2012;7:e31882. doi: 10.1371/journal.pone.0031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of Wingless in the endoplasmic reticulum. J Biol Chem. 2002;277:12816–12823. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, Ross JA, Liu W. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, Schulte G, Bryja V, Behrens J. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J. 2011;30:1433–1443. doi: 10.1038/emboj.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DV, Haegebarth A, Kuper I, Edelmann MJ, Henraat M, Canningavan Dijk MR, Kessler BM, Clevers H, Maurice MM. Loss of the tumor suppressor CYLD enhances Wnt/beta-catenin signaling through K63-linked ubiquitination of Dvl. Mol Cell. 2010;37:607–619. doi: 10.1016/j.molcel.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, Noutsou M, Rudiger SG, Schwamborn K, Schambony A, Maurice MM. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci USA. 2012;109:E812–E820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv Drug Deliv Rev. 2011;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Tesche LJ, Gerber DA. Tissue-derived stem and progenitor cells. Stem Cells Int. 2010;2010:824876. doi: 10.4061/2010/824876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Rüther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Theisen H, Syed A, Nguyen BT, Lukacsovich T, Purcell J, Srivastava GP, Iron D, Gaudenz K, Nie Q, Wan FY, Waterman ML, Marsh JL. Wingless directly represses DPP morphogen expression via an armadillo/TCF/Brinker complex. PLoS One. 2007;2:e142. doi: 10.1371/journal.pone.0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM, Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1? Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development. 2001;128:2107–2117. doi: 10.1242/dev.128.11.2107. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GC, Downey LM, Zhang K, Inglehearn CF. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- Tran H, Hamada F, Schwarz-Romond T, Bienz M. Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 2008;22:528–542. doi: 10.1101/gad.463208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: Evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Bito H, Ninomiya H, Sugimoto H, Kinoshita A, Shimohama S. Activity-dependent regulation of beta-catenin via epsilon-cleavage of N-cadherin. Biochem Biophys Res Commun. 2006;345:951–958. doi: 10.1016/j.bbrc.2006.04.157. [DOI] [PubMed] [Google Scholar]
- Ugolini F, Charafe-Jauffret E, Bardou VJ, Geneix J, Adélaïde J, Labat-Moleur F, Penault-Llorca F, Longy M, Jacquemier J, Birnbaum D, Pébusque MJ. WNT pathway mammary carcinogenesis: Loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20:5810–5817. doi: 10.1038/sj.onc.1204706. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nawijn M, Franca-Koh J, Zevenhoven J, van der Gulden H, Jonkers J, Berns A. Frat is dispensable for canonical Wnt signaling in mammals. Genes Dev. 2005;19:425–430. doi: 10.1101/gad.326705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Scoyk M, Randall J, Sergew A, Williams LM, Tennis M, Winn RA. Wnt signaling pathway and lung disease. Transl Res. 2008;151:175–180. doi: 10.1016/j.trsl.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: Lessons from mouse genetics. Cold Spring Harb Perspect Biol. 2012;4:a007963. doi: 10.1101/cshperspect.a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Wei W, Li M, Wang J, Nie F, Li L. The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol Cell Biol. 2012;19:3903–3912. doi: 10.1128/MCB.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechens N, Heinle K, Englmeier L, Schohl A, Fagotto F. Nucleo-cytoplasmic shuttling of Axin, a negative regulator of the Wnt-beta-catenin Pathway. J Biol Chem. 2004;279:5263–5267. doi: 10.1074/jbc.M307253200. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh J-C, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A, Pilarsky C. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201:204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- Wolf J, Palmby TR, Gavard J, Williams BO, Gutkind JS. Multiple PPPS/TP motifs act in a combinatorial fashion to transduce Wnt signaling through LRP6. FEBS Lett. 2008;582:255–261. doi: 10.1016/j.febslet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Mao J, Nguyen JT, Srinivas S, Zhang W, Liu B, Li L, Wu D, Zheng J. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat Struct Biol. 2000;7:1178–1184. doi: 10.1038/82047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J, Hartmann C. WNTing embryonic stem cells. Trends Cell Biol. 2012;22:159–168. doi: 10.1016/j.tcb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Le Trong I, Hinds TR, Stenkamp R, Kimelman D, Xu W. Crystal structure of a beta-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol Cell. 2004;15:523–533. doi: 10.1016/j.molcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length beta-catenin. Structure. 2008;16:478–487. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004a;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004b;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo-Miura J, Kawachi K, Natsume T, Shibuya H. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) J Biol Chem. 2006;281:20749–20760. doi: 10.1074/jbc.M602089200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell. 2009;17:470–481. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lijam N, Sussman DJ, Tsang M. Genomic organization of mouse Dishevelled genes. Gene. 1996;180:121–123. doi: 10.1016/s0378-1119(96)00430-1. [DOI] [PubMed] [Google Scholar]
- Yang P-T, Lorenowicz MJ, Silhankova M, Coudreuse DYM, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nature Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q-L, Nichols J, Chambers I, Smith A. BMP Induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Yokoya F, Imamoto N, Tachibana T, Yoneda Y. Beta-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol Biol Cell. 1999;10:1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Malbon CC. Phosphoprotein phosphatase-2A docks to Dishevelled and counterregulates Wnt3a/beta-catenin signaling. J Mol Signal. 2007;2:12. doi: 10.1186/1750-2187-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Yu X, Waltzer L, Bienz M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat Cell Biol. 1999;1:144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005a;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005b;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, Malhotra V, Sood N, Midda V, Monga DK, Kokkinakis DM, Monga SPS. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh J-C, He X. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jia J, Wang B, Amanai K, Wharton KA, Jr, Jiang J. Regulation of wingless signaling by the CKI family in Drosophila limb development. Dev Biol. 2006;299:221–237. doi: 10.1016/j.ydbio.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Mickanin C, Feng Y, Charlat O, Michaud GA, Schirle M, Shi X, Hild M, Bauer A, Myer VE, Finan PM, Porter JA, Huang S-MA, Cong F. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 2011;13:623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C, Macdonald BT, Singh S, Coburn KL, Cheong SM, Zhang MM, Ye QZ, Hang HC, Steen H, He X. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Schlesiger C, Masucci MG, Lindsten K. The ubiquitin specific protease 4 (USP4) is a new player in the Wnt signalling pathway. J Cell Mol Med. 2009;13:1886–1895. doi: 10.1111/j.1582-4934.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]