Abstract
Objectives
The aim of the present study was to estimate the incidence and spontaneous clearance rate of Helicobacter pylori infection and the effect of some variables on these outcomes in schoolchildren.
Methods
From May 2005 to December 2010, 718 schoolchildren enrolled in 3 public boarding schools in Mexico City participated in the follow-up. At the beginning of the study and every 6 months thereafter, breath samples were taken to detect H pylori infection; blood samples and anthropometric measurements were taken to evaluate nutritional status. Data on sociodemographic characteristics were collected.
Results
The prevalence of H pylori infection was 38%. The incidence rate was 6.36%/year. Schoolchildren with anemia or iron deficiency at the beginning of the study (who received iron supplements) showed a higher infection acquisition rate than those with normal iron nutritional status, hazard ratio (HR) 12.52 (95% confidence interval [CI] 4.01%–39.12%), P <0.001 and HR 2.05 (95% CI 1.09%–3.87%), P = 0.027, respectively. The spontaneous clearance rate of the infection was 4.74%/year. The spontaneous clearance rate was higher in children who had iron deficiency (who received iron supplements), HR 5.02 (95% CI 1.33%–18.99%), P = 0.017, compared with those with normal nutritional iron status. It was lower in schoolchildren with ≥2 siblings compared with schoolchildren with 1 or no siblings, HR 0.23 (95% CI 0.08%–0.63%), P = 0.004.
Conclusions
H pylori infection status is dynamic in schoolchildren. Variables related to health status and infection transmission, such as iron status and number of siblings, are important for the incidence and spontaneous clearance of H pylori infection.
Keywords: H pylori infection, incidence rate, Mexican schoolchildren, spontaneous clearance rate
Helicobacter pylori infection in children in developing countries is usually acquired during the first 10 years of life (1). In these countries, >80% of adults are colonized with H pylori, and >50% of children are infected at 10 years of age as compared with 30% of adults and 10% of children in developed countries (2). This has been related to socioeconomic status (SES) and sanitary conditions; even in populations in the same country, low SES is associated with infection acquisition (3,4). In the third National Health and Nutrition Examination Survey conducted in the United States, a 25% prevalence of H pylori infection was found in children and young adults between 6 and 19 years. In the Mexican American population, prevalence was 42%; prevalence was higher in children from a low SES, in those whose mothers had a lower education level, and in those living in crowded conditions (5). These risk factors have been reported in locations such as Bolivia, Mexico, and in towns along the US border with Mexico (3,4). A seroepidemiological national survey in 1988 in Mexico found a national prevalence of 66% in the general population, 20% in children younger than 1 year, and 50% in children younger than 10 years. There were differences in prevalence, depending on the economic development of the regions (86.1% prevalence southeastern Mexico, 47.1% in the northeast) (6).
It is commonly thought that once the H pylori infection is acquired, it evolves toward persistent chronic infection (2,7) and that spontaneous clearance is relatively rare (1,2,4); however, in a study of children in which prevalence by age was reported in intervals of 1 year; no increase in prevalence by age was observed (3). This suggests that transient H pylori infection is not uncommon in children (2,8,9). In 6- to 24-month-old children in Ciudad Juarez, Mexico, and El Paso, Texas, researchers found 80% spontaneous reversion of the infection (8,9).
H pylori infection has an important effect on public health. In adults, gastrointestinal diseases such as chronic active gastritis, peptic ulcer, and gastric cancer are etiologically associated with H pylori infection (10,11); however, the natural history of this frequently asymptomatic infection remains poorly understood, particularly in relation to acquisition and spontaneous clearance. It is known that the infection is mainly acquired during childhood, but the specific age of acquisition and the factors associated with its persistence are unknown (1,4). The aim of the present study was to estimate the incidence and spontaneous clearance rate of H pylori infection in schoolchildren of low SES in Mexico City and the effect of some factors of interest related to these outcomes.
METHODS
Study Population
In the baseline stage of the study, 940 schoolchildren between 6 and 13 years participated; of these, 718 had at least 1 follow-up 6 months after the baseline measurement. H pylori infection status and iron nutritional status were evaluated every 6 months. The children included in the study were enrolled in 3 public elementary boarding schools in Mexico City. The children remain at school 5 days per week and go home on weekends and vacations. The boarding school program is offered by the Secretary of Education and is oriented toward children of low SES.
The study started in May 2005 and the follow-up ended in December 2010. It was reviewed and authorized by the ethics and investigation committees of the Instituto Mexicano del Seguro Social and was also authorized by the secretary of public education of Mexico. The parents signed an informed consent form authorizing their children’s participation; additionally, children older than 7 years were asked to give their assent to participate in the study.
Diagnosis and Follow-up of H pylori Infection
The infection diagnosis was performed by means of a breath test, using urea labeled with 13C (13C urea breath test [UBT]). A difference ≥5 per thousand between ratio values 13CO2/12CO2 at baseline and 30 minutes postintake of 50 mg of 13C-urea was considered a positive test for H pylori. At the time the samples were taken, the schoolchildren had been fasting 8 hours and they drank 100 mL of water with 2 g of citric acid before the basal sample collection. The samples were collected in 10-mL tubes (Exatainers, Labco Ltda, High Wycombe, UK); the tubes were capped immediately after breath collection. The samples were stored at room temperature and were analyzed within 1 month after their collection by a mass spectrometer (BreathMat-plus, Finnigan MAT, Bremen, Germany). The schoolchildren had not received antibiotic treatments, bismuth salts, proton pump inhibitors, or sucralfate during the previous month. The sensitivity and specificity of this test in schoolchildren are >90% (8,12–14). The stability and reproducibility of the analyses of the breath samples are not affected by short- and long-term sample storage (15,16).
Infection-related Factors
To estimate the effect of variables related to infection status, parents were asked about the children’s socioeconomic and health characteristics. SES was assessed using variables such as mother’s education level, number of siblings of the participants, and crowding at home. Anthropometric measurements of weight and height were taken, and height-for-age z score was determined (17). Body mass index (BMI) (weight in kg/height in m2) was calculated by age and sex using the cutoff points proposed by Cole et al (18,19). Blood samples were taken every 6 months to assess the children’s iron nutritional status. Children with iron deficiency and/or anemia (20,21) at the beginning of the study or during the follow-up received daily iron supplements for 3 months. No eradication therapy for the infection was provided during the study because practice guidelines about H pylori infection in children had not approved giving such treatment for asymptomatic children (22).
Statistical Analysis
Infection Prevalence and Effect of Some Variables
With baseline information, infection prevalence was estimated according to the variables reported as associated with the infection. The association of these variables with the presence of H pylori infection was estimated using logistic regression models. Based on those findings, an assessment was made whether to include random intercepts to take into account errors correlation caused by cluster of siblings or school cluster (23).
Child-oriented variables were age at baseline measurement (younger than 9 or from 9–13 years); sex; anthropometric indicators of height for age, categorized as children with normal nutritional status (z score ≥−1) and children with slight or moderate malnutrition (z score <−1) (17); BMI, classifying children with normal BMI or those who are overweight or obese by age and sex, using cutoff points suggested by Cole et al (18,19); and iron nutritional status, based on hemoglobin and serum ferritin concentration, classifying schoolchildren with normal iron status (hemoglobin adjusted by altitude ≥11.5 g/dL for children younger than 12 years and ≥12 g/dL for children 12 years or older, and ferritin ≥12 ng/mL), children with iron deficiency without anemia (ferritin < 12 ng/mL) and normal concentration of hemoglobin by age, and schoolchildren with anemia (hemoglobin adjusted by altitude <11.5 g/dL for children younger than 12 years and < 12 g/dL for children of 12 years or more) (20,21).
Other factors were also considered: SES, according to mother’s education (secondary or less and middle school or more); possible infection transmission due to number of siblings (1 or no siblings and 2 or more siblings) and crowding (<3 people/room or 3 or more people/room); and possible interactions between variable pairs (crowding and mother’s education level, iron nutritional status and height for age, iron nutritional status, crowding).
H pylori Infection Status During Follow-up
Schoolchildren with at least 2 assessments of H pylori infection (at baseline and 6 months after) were included in these analyses. The follow-up of children was planned until they finished elementary school or until December 2010, when the study concluded. From the breath tests during follow-up, the H pylori infection was classified as always positive (positive breath test result in all of the measurements), always negative (negative breath test result in all of the measurements), or variable (positive and negative breath tests results). The percentage of children with different infection types and time of follow-up were calculated by age group. When changes in infection status occurred, the percentages of children with infection acquisition and those who showed spontaneous clearance were calculated.
Incidence and Spontaneous Clearance of H pylori Infection
From the baseline results about H pylori status, the schoolchildren were categorized into 1 of 2 follow-up groups: One evaluated the incidence of the infection and the other evaluated the spontaneous clearance of the infection. In children without H pylori infection at the beginning of the study, the incidence rate was calculated by dividing the number of new cases by the time of follow-up of the individuals at risk. Likewise, the spontaneous clearance rate of the infection in children who showed H pylori infection at the beginning of the study was calculated by dividing the number of new cases by the time of follow-up of the individuals at risk. If data were missing during follow-up, both for acquisition and spontaneous clearance of the infection, the time at risk was calculated, assuming that the schoolchild did not show changes in his or her infection status in the missing measurements. The time at risk or time to the event was calculated as the difference between the date of study beginning—first 13C-UBT to detection of H pylori—and the date when the event—H pylori acquisition or spontaneous clearance of H pylori—was detected. Observations without the event during the study were censored, and the time at risk was calculated as the difference between the first 13C-UBT to H pylori detection and the date of the last follow-up or date of the study end.
Effect of the Variables Related to the Incidence and Spontaneous Clearance of H pylori Infection
We calculated the rates of acquisition and spontaneous clearance of H pylori by clinical and demographic variables of the study population. We compared these rates by means of crude hazard ratios (HR). In addition, a multivariate analysis was performed, using Cox proportional hazards models, to estimate the effect of the factors related to the acquisition of the infection and to the spontaneous clearance of the infection. It was necessary to include robust standard errors in the Cox proportional hazards models because of the correlation between the observations due to the presence of siblings in the sample. Possible interactions (iron nutritional status and z score of height for age, mother’s education level and iron nutritional status, and sex and iron nutritional status) were evaluated. Diagnosis of the models was performed (24). The statistical analysis was carried out using the statistical package STATA version 11 (StataCorp, College Station, TX).
RESULTS
At baseline measurement, 940 of the 1180 invited children participated. More than half (58%) of the participants were girls (1 of the boarding schools is for girls only). The median age of the schoolchildren at the beginning of the study was 9.0 (5.8–13.8) years. Seventeen percent of them (n = 164) had anemia or iron deficiency without anemia, 0.9% (n = 8) had anemia, and 16.7% (n = 156) had iron deficiency without anemia. Twenty-six percent of the schoolchildren showed slight or moderate malnutrition according to the height-for-age indicator, and 24% were overweight or obese according to BMI. More than two-thirds (68.4%) of the participants’ mothers had secondary education or less, and 31.6% had some middle school education or more.
Prevalence of H pylori Infection and Associated Variables
At baseline measurement, 38% (95% CI 34.9%–41.1%) of the schoolchildren had H pylori infection. Prevalence of the infection was higher in children older than 9 years in relation to children 8 years or younger and in schoolchildren with height for age lower than −1 standard deviation in relation to those with normal height for age. Children with ≥2 siblings showed a higher prevalence of the infection than children without siblings or with only 1 sibling, and children with crowding at home showed higher prevalence than those without crowding (Table 1) (17–19). In the multivariate analysis, the variables that were significantly associated with the presence of infection were age ≥9 years (odds ratio [OR] 1.6, 95% CI 1.03%–2.5%; P = 0.036), slight or moderate malnutrition (OR 2.17, 95% CI 1.25%–3.74%; P = 0.006), and crowding (OR 1.72, 95% CI 1.06%–2.8%; P = 0.029), after adjusting for sex, iron status, BMI, number of siblings, and mother’s education level.
TABLE 1.
Prevalence of Helicobacter pylori infection by study population characteristics: baseline data
| Characteristics, n | Prevalence of H pylori infection
|
Relation between baseline characteristics and H pylori infection
|
|||
|---|---|---|---|---|---|
| % | 95% CI | OR* | 95% CI | P | |
| Age group, N = 940 | |||||
| 5–8 y, n = 454 | 34.6 | 30.2–39.0 | Ref | ||
| 9–13 y, n = 486 | 41.2 | 36.8–45.5 | 1.56 | 1.04–2.33 | 0.030 |
| Sex, N = 940 | |||||
| Male, n = 394 | 40.9 | 36.0–45.7 | Ref | ||
| Female, n = 546 | 35.9 | 31.9–39.9 | 0.77 | 0.52–1.16 | 0.217 |
| Iron status, N = 932 | |||||
| Normal, n = 768† | 36.6 | 33.2–40.0 | Ref | ||
| Iron deficiency and/or anemia, n = 164‡ | 45.1 | 37.4–52.8 | 1.47 | 0.88–2.45 | 0.141 |
| Height for age, N = 837§ | |||||
| Normal, n = 618 | 34.0 | 30.2–37.7 | Ref | ||
| ≤1 SD, n = 219 | 47.5 | 40.8–54.2 | 2.58 | 1.51–4.40 | <0.001 |
| Body mass index, N = 836|| | |||||
| Normal, n = 637 | 39.2 | 35.4–43.0 | Ref | ||
| Overweight or obese, n = 199 | 32.2 | 25.6–38.7 | 0.68 | 0.41–1.11 | 0.121 |
| No. siblings, N = 871 | |||||
| 0 or 1, n = 466 | 34.5 | 30.2–38.9 | Ref | ||
| ≥2, n = 405 | 42.2 | 37.4–47.0 | 1.68 | 1.05–2.68 | 0.029 |
| Mother’s education level, N = 807 | |||||
| Middle school or more, n = 255 | 36.1 | 30.1–42.0 | Ref | ||
| Secondary or less, n = 552 | 38.8 | 34.7–42.8 | 1.28 | 0.78–2.11 | 0.328 |
| Crowding, N = 819 | |||||
| <3 people/room, n = 431 | 33.4 | 28.9–37.9 | Ref | ||
| ≥3 people/room, n = 388 | 42.0 | 37.1–46.9 | 1.69 | 1.07–2.67 | 0.024 |
CI = confidence interval; OR = odds ratio; Ref = reference group; SD = standard deviation.
Odds ratio. Logistic regression considering the correlation due to the presence of siblings in the sample. Univariate analysis.
Hemoglobin ≥11.5 g/dL in schoolchildren younger than 12 years or hemoglobin ≥12.0 g/dL in schoolchildren 12 years or older and ferritin ≥12 ng/mL.
Ferritin <12 ng/mL and/or hemoglobin <11.5 g/dL in schoolchildren younger than 12 years or hemoglobin <12.0 g/dL in schoolchildren 12 years or older.
WHO 2007 (17).
Changes in H pylori Infection Status
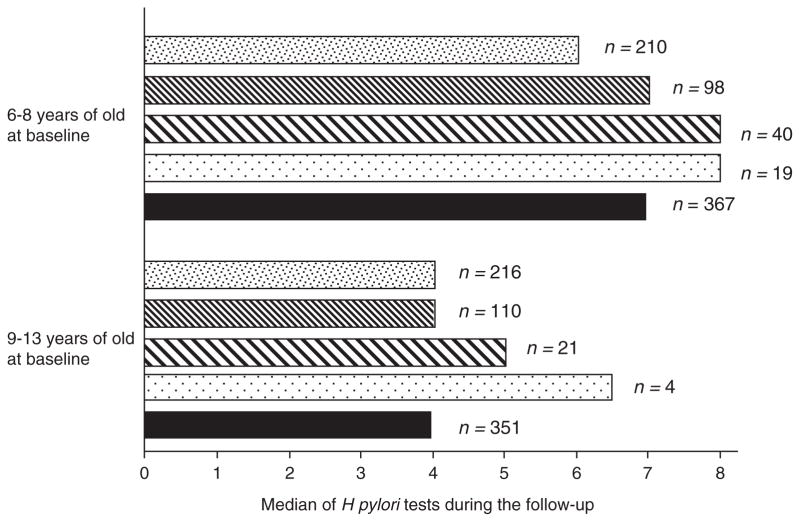
Changes in H pylori infection status were evaluated in 718 schoolchildren with a baseline measurement and at least 1 additional follow-up to H pylori detection. The median of measurements to H pylori detection was 5 (range 2–12). Twenty percent of children had 2 measurements to H pylori detection, 41% had between 3 and 5 measurements, 24% had between 6 and 8 measurements to H pylori detection, and 15% had ≥9 measurements. The time of follow-up of the schoolchildren and the number of tests for H pylori detection depended on age at the beginning of the study (participating schools are elementary school level). Children younger than 9 years at the beginning of the study had the largest number of measurements for H pylori infection, with a median of 7 (2–12); children ages 9 to 13 years at the beginning of the study had 4 measurements (2–11) (Fig. 1). Sixty-eight percent (n = 492) of participants had all of their measurements taken every 6 months until their follow-up ended, 15.6% (n = 112) had 1 missing measurement during the follow-up, 13.7% (n = 98) had 2 missing measurements, and 2.2% (n = 16) had 3 or 4 missing measurements.
FIGURE 1.
Median of test of Helicobacter pylori during the follow-up by H pylori status and group.
 Schoolchildren always negative to H pylori, ▨ schoolchildren always positive to H pylori,
Schoolchildren always negative to H pylori, ▨ schoolchildren always positive to H pylori,
 schoolchildren who presented acquisition of the infection,
schoolchildren who presented acquisition of the infection,
 schoolchildren who presented clearance of the infection, and ■ all of the schoolchildren.
schoolchildren who presented clearance of the infection, and ■ all of the schoolchildren.
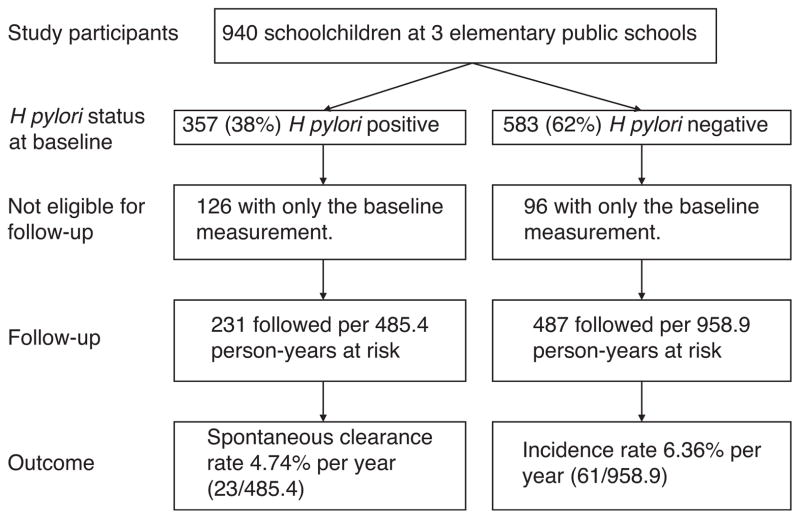
Almost three-fifths (59.3%) of schoolchildren remained always negative for infection, 29.0% remained always positive, and 11.7% showed changes in their infection status (acquisition or spontaneous clearance) (Fig. 2). Of the 84 children who showed changes in their H pylori infection status during follow-up, 61 (72.6%) correspond to the incident cases for the infection and 23 (27.4%) correspond to spontaneous clearance of the infection. Of the 61 incident cases, 18 (29.5%) correspond to persistent infection (at least 2 consecutive measurements with a positive breath test result), 24 (39.3%) correspond to transient infection (negative breath test result after the first positive result determination), and 19 cases (31.2%) showed infection incidence that could not be classified because their follow-up ended when incidence occurred. Of the 23 spontaneous cases of clearance, 7 (30.4%) showed persistent clearance of the infection (at least 2 consecutive negative breath test results), 6 (26.1%) showed transient clearance (positive breath test result after the first negative result), and in 10 (43.5%) of the cases, the clearance could not be classified because follow-up ended once the spontaneous clearance of the infection occurred.
FIGURE 2.
Helicobacter pylori status at baseline and during the follow-up.
Incidence of H pylori Infection During Follow-up
The incidence rate of H pylori infection was 6.36%/year; 61 schoolchildren of 487 followed per 958.9 person-years acquired the infection (Fig. 2). At baseline, 16.1% (n = 78) of the schoolchildren who were followed to infection acquisition had anemia or iron deficiency without anemia (7 and 71 children, respectively). After baseline, during all of the follow-ups, only in 4.9% of the 1763 visits with iron status determination, the children presented anemia (0.5%, n = 9) or iron deficiency without anemia (4.4%, n = 78).
In the univariate analysis on the effect of clinical and demographic variables on the incident rate of H pylori infection, children with anemia or iron deficiency had a higher rate of H pylori infection when compared with children with normal iron status (Table 2) (17–19). In the multivariate analysis, anemia and iron deficiency had higher effects on incidence rate. Children with anemia or with iron deficiency acquired the infection at a rate higher than children with normal iron status at the beginning of the study (HR 12.5, 95% CI 4.01%–39.12%, P = 0.001 for anemia, and HR 2.05, 95% CI 1.09%–3.87%, P = 0.027 for iron deficiency), after adjusting for age, sex, height for age, BMI, number of siblings, mother’s education level, and crowding.
TABLE 2.
Rates and hazard ratios of incidence of Helicobacter pylori infection: univariate analysis
| Baseline characteristics | Cases/person-years at risk | Incidence rate | HR (95% CI)* | P |
|---|---|---|---|---|
| Age, y | ||||
| 5–8 | 40/609.6 | 6.56 | Ref | |
| 9–13 | 21/349.3 | 6.01 | 0.96 (0.54–1.70) | 0.882 |
| Sex | ||||
| Male | 25/386.1 | 6.47 | Ref | |
| Female | 36/572.8 | 6.28 | 0.97 (0.58–1.62) | 0.898 |
| Iron status | ||||
| Normal† | 45/836.2 | 5.38 | Ref | |
| Anemia‡ | 3/9.4 | 31.72 | 6.76 (1.63–28.01) | 0.008 |
| Iron deficiency without anemia§ | 12/108.6 | 11.05 | 2.07 (1.10–3.91) | 0.025 |
| Height for age|| | ||||
| Normal | 48/726.9 | 6.60 | Ref | |
| ≤−1 SD | 13/222.5 | 5.84 | 0.87 (0.48–1.60) | 0.662 |
| Body mass index¶ | ||||
| Normal | 47/691.8 | 6.79 | Ref | |
| Overweight or obese | 14/257.1 | 5.44 | 0.80 (0.44–1.43) | 0.447 |
| No. siblings | ||||
| 0 or 1 | 33/529.4 | 6.23 | Ref | |
| ≥2 | 27/426.7 | 6.33 | 1.01 (0.60–1.70) | 0.964 |
| Mother’s education level | ||||
| Middle school or more | 12/291.1 | 4.12 | Ref | |
| Secondary or less | 47/634.5 | 7.41 | 1.75 (0.92–3.34) | 0.090 |
| Crowding | ||||
| <3 people/room | 32/530.1 | 6.04 | Ref | |
| ≥3 people/room | 28/420.5 | 6.66 | 1.07 (0.64–1.79) | 0.793 |
CI = confidence interval; HR = hazard ratio; SD = standard deviation.
Crude hazard ratios obtained through proportional hazard regression with robust standard error.
Hemoglobin ≥11.5 g/dL in schoolchildren younger than 12 years or hemoglobin ≥ 12.0 g/dL in schoolchildren 12 years or older and ferritin ≥ 12 ng/mL.
Hemoglobin <11.5 g/dL in schoolchildren younger than 12 years or hemoglobin <12.0 g/dL in schoolchildren 12 years or older.
Ferritin <12 ng/mL and hemoglobin ≥ 11.5 g/dL in schoolchildren younger than 12 years or hemoglobin ≥ 12.0 g/dL in schoolchildren 12 years or older.
Population of reference WHO 2007 (17).
Spontaneous Clearance of H pylori Infection
The spontaneous clearance rate was 4.74%/year; 23 schoolchildren of 231 followed per 485.4 person-years spontaneously eliminated the infection (Fig. 2). At baseline, 22.5% (n = 52) of children who were followed to spontaneous clearance of infection had iron deficiency without anemia. After baseline, during all of the follow-ups, only in 5.2% (n = 37) of 716 visits with iron status determination, the children presented iron deficiency without anemia.
The most important clinical and demographic factors regarding spontaneous clearance of the infection in the univariate analysis were iron nutritional status, number of siblings, and age (Table 3) (17–19). In the multivariate analysis, iron deficiency and number of siblings showed significant effect, after adjusting for age, sex, height for age, BMI, mother’s education level, and crowding. Schoolchildren with iron deficiency showed a higher spontaneous clearance rate of the infection than those with normal iron nutritional status, HR 5.02 (95% CI 1.33%–18.99%, P = 0.017); schoolchildren with ≥2 siblings had a lower spontaneous clearance rate compared with those with 1 or no siblings, HR 0.23 (95% CI 0.08%–0.63%, P = 0.004).
TABLE 3.
Rates and hazard ratios of spontaneous clearance of Helicobacter pylori infection: univariate analysis
| Baseline characteristics | Cases/person-years at risk | Spontaneous clearance rate | HR (95% CI)* | P |
|---|---|---|---|---|
| Age, y | ||||
| 5–8 | 19/303.0 | 6.27 | Ref | |
| 9–13 | 4/182.4 | 2.19 | 0.40 (0.12–1.34) | 0.138 |
| Sex | ||||
| Male | 11/179.5 | 6.12 | Ref | |
| Female | 12/305.9 | 3.92 | 0.61 (0.27–1.36) | 0.225 |
| Iron status | ||||
| Normal† | 16/443.1 | 3.61 | Ref | |
| Iron deficiency without anemia‡ | 7/42.2 | 16.58 | 6.24 (2.37–16.43) | <0.001 |
| Height for age§ | ||||
| Normal | 16/316.0 | 5.06 | Ref | |
| ≤−1 SD | 7/163.4 | 4.28 | 0.84 (0.35–2.02) | 0.705 |
| Body mass index|| | ||||
| Normal | 19/382.4 | 4.97 | Ref | |
| Overweight or obese | 4/96.9 | 4.13 | 0.81 (0.28–2.40) | 0.709 |
| No. siblings | ||||
| 0 or 1 | 18/219.3 | 8.21 | Ref | |
| ≥2 | 5/265.6 | 1.88 | 0.24 (0.09–0.64) | 0.005 |
| Mother’s education level | ||||
| Middle school or more | 8/146.8 | 5.45 | Ref | |
| Secondary or less | 15/335.2 | 4.47 | 0.85 (0.36–1.98) | 0.700 |
| Crowding | ||||
| <3 people/room | 12/228.4 | 5.25 | Ref | |
| ≥3 people/room | 11/255.6 | 4.30 | 0.79 (0.34–1.85) | 0.593 |
CI = confidence interval; HR = hazard ratio; SD = standard deviation.
Crude hazard ratios obtained through proportional hazard regression with robust standard error.
Hemoglobin ≥ 11.5 g/dL in schoolchildren younger than 12 years or hemoglobin ≥ 12.0 g/dL in schoolchildren 12 years or older and ferritin ≥ 12 ng/mL.
Ferritin<12 ng/mL and hemoglobin ≥ 11.5 g/dL in schoolchildren younger than 12 years or hemoglobin ≥ 12.0 g/dL in schoolchildren 12 years or older.
Population of reference WHO 2007 (17).
DISCUSSION
The estimated prevalence of H pylori infection in this population of low SES schoolchildren in Mexico City is high (38%), similar to that reported in other populations of developing countries (3) and in Mexican American populations of 6- to 19-year-olds in the United States (42%) (5); however, it is lower than that found in indigenous schoolchildren in Hidalgo state, Mexico, who also live in school shelters (53%) (25). These differences could be related to socioeconomic and demographic characteristics of these populations; the indigenous population frequently resides in rural and semirural areas, often under poor sanitary conditions at low socioeconomic and health levels, and in more crowded circumstances than urban populations (25–27).
The risk factors consistently reported for the presence of H pylori infection are related to indicators of low SES—little maternal education, crowding, low income, bad housing conditions, and poor sanitation—and the high possibility of infection transmission—crowding and presence of siblings close in age to the index child (3,6,10,28,29). Consistent with data reported in other studies, variables with the most effect on the prevalence of active H pylori infection in our study were age, low height for age, and crowding at home.
The incidence rate of 6.36%/year in our study population is high and similar to incidence rates reported in developing countries. It is between 3% and 18%/year (30,31) and 5.3% for Hispanics between 2 and 17 years old in San Francisco, CA (32). Comparable rates in developed countries range between 0.2% and 1.9%/year (1,30,31,33). In the Bogalusa Heart Study, children who were 1 to 3 years old in 1973 were followed until they were 18 to 23 years old. The frequency of infection by H pylori antibodies was 8.0% (13% in black vs 4% white participants) at ages 1 to 3 years. By ages 18 to 23 years, the prevalence of the infection was 24.5% (43% black vs 8% white participants). The crude incidence rate for H pylori was 1.4%/year, for the whole cohort, ranging from 2.1% at ages 4 to 5 years and 1.5% at ages 7 to 9 years to 0.3% at ages 21 to 23 years. The incidence was 2.3% for black children and 0.7% for white children. The estimated crude seroreversion rate was 1.1%/year (1.7% among white children and 0.8% among black children). The authors suggested that possible explanations for the higher rate of acquisition and the lower rate of loss of infection among black children include more intense exposure to higher infection rates among family members, differences in household hygiene, or differences in access to or use of health care facilities, although all of the participants were from a homogeneous, lower-middle socioeconomic class (1).
In our study, a higher infection incidence rate was estimated in children with anemia or iron deficiency without anemia (who received iron supplements) compared with schoolchildren with normal iron nutritional status. In fact, a trend toward a higher incidence rate in children having anemia (HR 12.52) or iron deficiency without anemia (HR 2.05) at baseline was observed compared with children with normal iron nutritional status. These results suggest that iron deficiency has an effect on the acquisition of H pylori infection and that its effect can be greater if the deficiency is chronic or more severe; however, children with iron deficiency without anemia and with H pylori infection at the beginning of the study (who received iron supplements) also showed higher spontaneous clearance of the H pylori infection rate. Clearance of the infection in these children could be related to iron deficiency that is less chronic or less severe—no children had anemia—linked to an acute effect of improving iron status by iron supplementation (34). More studies are needed to clarify these results.
Most studies have reported that H pylori infection is associated with iron deficiency and/or anemia due to the iron requirements of the bacteria and its ability to acquire iron (5,25,35,36). The mechanisms that explain this association have not been clearly established; however, (35,37) some authors have reported that the bacteria viability varies according to iron availability and that the need for iron depends on the growth stage of the bacterial colonies (38). Tan et al (39), using an experimental system, showed that the virulence bacterial factors CagA and VacA are important in iron acquisition by H pylori from the host epithelium during bacterial colonization.
In our study, spontaneous clearance of the infection rate is high, 4.74%/year. In the Bogalusa study, the spontaneous clearance rate of the infection for the entire cohort was 1.1%/year (1). The most important factors in this difference, besides higher exposure to the bacteria in developing countries, could be the difference in age in the studied groups (1–23 vs 5–13 years) and the type of test used for monitoring the infection (immunoglobulin G antibodies to H pylori vs the 13C-UBT). 13C-UBT is a convenient method for the noninvasive detection of H pylori infection in children in whom endoscopy is not required. 13C-UBT is noninvasive, valid, safe, and easy to use, and, unlike serology, it yields positive results for recently acquired infection and negative results for recent clearance of the infection (28). In a meta-analysis, 13C-UBT was found to be highly accurate for the diagnosis of H pylori infection in children older than 6 years (sensitivity 96.6% and specificity 97.7%) (13,28).
In our study, the clearance rate was lower in children with ≥2 siblings than in those with 1 or no siblings. This finding is consistent with other studies that have reported increases in H pylori prevalence and acquisition of transient or persistent H pylori infection according to the number of children in the family (40,41). A new infection generally occurs in early childhood, and person-to-person transmission remains the most likely transmission route; children with fewer siblings have lower intrafamily exposure to H pylori than do those with more siblings.
Higher incidence and clearance rates have been reported in infants and preschool children. In a group of children from the Mexico–US border, with follow-up to assess their infection status using a 13C-UBT every 6 months, the incidence of H pylori infection was 20.4%/year. In 80% of these children, clearance of the infection occurred during the 2 years of follow-up; however, 19% of them became reinfected (8,9). In a study in Peru in which 6- to 30-month-old infants were followed by a 13C-UBT, the infection was not persistent; there was a high acquisition rate and high spontaneous clearance rate (42). In a population on an Apache Indian reservation in the United States, newborns to 2-year-old children in one group and 2- to 11-year-old children in another group were studied. In the younger group, the incidence of infection was 54.4% during the first year of life; 15.9% was classified as transient infection due to seroreversion; and 38.5% was classified as persistent. In the 2- to 11-year-old group, 69.4% showed antibodies against H pylori at the beginning of the study; during 1 year of follow-up, 20.6% seroreverted and 33.3% seroconverted. By comparing spontaneous clearance of infection in these 2 groups, the authors found that the reversion phenomenon decreases with age (2). In the Stanford Infection and Family Transmission Study, conducted on children younger than 2 years living in San Francisco, Haggerty et al (43) reported a high transient infection rate and low persistent infection rate. The authors suggest that children, especially those living in crowded conditions, show frequent and recurrent exposure to H pylori that occasionally progresses to persistent infection. The differences in spontaneous clearance among the reported studies may reflect the different age groups and study environments.
The limitations of our study are related to the missing measurements during follow-up, in which we assumed that no change occurred in H pylori infection status. This assumption could lead to miscalculations of both the incidence and the spontaneous clearance rate; they could be higher. When we did the analyses including only the data until a missing measurement of H pylori occurred, however, we observed the same tendency to acquisition and spontaneous clearance of infection rates as in the effect of the variables on these events (data not shown).
In the present study, we did not record data about antimicrobial drug intake. Some authors have suggested that loss of infection may be related to the use of antimicrobial drugs for other common infections; however, we asked parents about the intake of antimicrobial drugs and only took samples 1 month after that treatment for other infections was finished. In a report about this topic, in a cohort of preschool children, it was found that exposure to antibiotics taken for other illnesses seems to explain only a small portion of all of the detected spontaneous clearance events (44).
In summary, although most of the schoolchildren in our study maintained their initial status of H pylori infection throughout the follow-up, 11.7% showed changes in their infection status. These results suggest that even for school-age children, H pylori infection status is dynamic. We observed persistent acquisition, spontaneous clearance, and transient events of acquisition and clearance. Variables related to health status and infection transmission, such as iron status and number of siblings, are shown to be important for the incidence of H pylori and for the spontaneous clearance of infection in school-age children.
Acknowledgments
The National Institute of Science and Technology (projects no. 2004-C01-74/A-1, 69667) and the Mexican Institute of Social Security, Coordination of Health Research (projects no. FP-2005/1/I/089 and 190) funded the present study.
Footnotes
The authors report no conflicts of interest.
References
- 1.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–5. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Pérez GI, Sack RB, Reid R, et al. Transient and persistent Helicobacter pylori colonization in Native American children. J Clin Microbiol. 2003;41:2401–7. doi: 10.1128/JCM.41.6.2401-2407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn MK, Friedman CR, Gold BD, et al. Seroincidence of Helicobacter pylori infection in a cohort of rural Bolivian children: acquisition and analysis of possible risk factors. Clin Infect Dis. 2002;35:1059–65. doi: 10.1086/342910. [DOI] [PubMed] [Google Scholar]
- 4.Goodman KJ, Cockburn M. The role of epidemiology in understanding the health effects of Helicobacter pylori. Epidemiology. 2001;12:266–71. doi: 10.1097/00001648-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas VM, Mulla ZD, Ortiz M, et al. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163:127–34. doi: 10.1093/aje/kwj018. [DOI] [PubMed] [Google Scholar]
- 6.Torres J, Leal-Herrera Y, Perez-Perez G, et al. A community-based seroepidemiologic study of Helicobacter pylori infection in Mexico. J Infect Dis. 1998;178:1089–94. doi: 10.1086/515663. [DOI] [PubMed] [Google Scholar]
- 7.Tindberg Y. Clinical Microbiology. Stockholm: Karolinska Institute; 2001. Helicobacter pylori infection among children in Sweden; p. 94. [Google Scholar]
- 8.Goodman KJ, O’Rourke K, Day RS, et al. Dynamics of Helicobacter pylori infection in a US-Mexico cohort during the first two years of life. Int J Epidemiol. 2005;34:1348–55. doi: 10.1093/ije/dyi152. [DOI] [PubMed] [Google Scholar]
- 9.Phillips CV, Goodman KJ. Interpreting data in the face of competing explanations: assessing the hypothesis that observed spontaneous clearance of Helicobacter pylori was all measurement error. Int J Epidemiol. 2009;38:1110–7. doi: 10.1093/ije/dyp006. [DOI] [PubMed] [Google Scholar]
- 10.Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11:1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 11.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 12.Hino B, Eliakim R, Levine A, et al. Comparison of invasive and non-invasive tests diagnosis and monitoring of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2004;39:519–23. doi: 10.1097/00005176-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Leal YA, Flores LL, Fuentes-Panana EM, et al. 13C-Urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327–37. doi: 10.1111/j.1523-5378.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.Rowland M, Daly L, Vaughan M, et al. Age-specific incidence of Helicobacter pylori. Gastroenterology. 2006;130:65–72. doi: 10.1053/j.gastro.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Bruce M, Parkinson A, Gessner B. Does delayed testing of urea breath test samples effect results? Digestion. 2005;71:261. doi: 10.1159/000087052. [DOI] [PubMed] [Google Scholar]
- 16.Deslandres C. 13C Urea breath testing to diagnose Helicobacter pylori infection in children. Can J Gastroenterol. 1999;13:567–70. doi: 10.1155/1999/328084. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. [Accessed June 26, 2012.];Growth Reference Data for School-aged Children and Adolescents of 5–19 Years. www.who.int/growthref/en.
- 18.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole TJ, Flegal KM, Nicholls D, et al. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194–7. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 21.UNICEF/UNU/WHO Consultation. Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Programme Managers. Geneva: World Health Organization; 2001. p. 114. [Google Scholar]
- 22.Koletzko S, Jones NL, Goodman KJ, et al. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230–43. doi: 10.1097/MPG.0b013e3182227e90. [DOI] [PubMed] [Google Scholar]
- 23.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2. College Station, TX: Stata Press; 2008. Dichotomous or binary responses; pp. 231–57. [Google Scholar]
- 24.Hosmer WD, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 25.Duque-Lopez MX, Moran S, Walter T, et al. Las múltiples facetas de la investigación en Salud 2: Proyectos estratégicos del Instituto Mexicano del Seguro Social Distrito Federal. 2002. Efecto de la infección por Helicobacter pylori sobre la respuesta a la suplementación semanal con hierro y vitaminas en usuarios de los albergues escolares del Instituto Nacional Indigenista en Huejutla, Hidalgo; pp. 99–114. [Google Scholar]
- 26.Olaiz-Fernandez G, Rivera-Dommarco J, Shamah-Levy T, et al. Encuesta Nacional de Salud y Nutrición. Cuernavaca, Mexico: Instituto Nacional de Salud Pública; 2006. [Google Scholar]
- 27.Vilchis J, Duque X, Mera R, et al. Association of Helicobacter pylori infection and height of Mexican children of low socioeconomic level attending boarding schools. Am J Trop Med Hyg. 2009;81:1091–6. doi: 10.4269/ajtmh.2009.09-0107. [DOI] [PubMed] [Google Scholar]
- 28.Malay MH, Logan ND, Graham DY, et al. Helicobacter pylori infection in preschool and school-aged minority children: effect of socioeconomic indicators and breast-feeding practices. Cin Infect Dis. 2001;32:1387–92. doi: 10.1086/320148. [DOI] [PubMed] [Google Scholar]
- 29.Ozen A, Ertem D, Pehlivanoglu E. Natural history and symptomatology of Helicobacter pylori in childhood and factors determining the epidemiology of infection. J Pediatr Gastroenterol Nutr. 2006;42:398–404. doi: 10.1097/01.mpg.0000215307.48169.7b. [DOI] [PubMed] [Google Scholar]
- 30.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 31.Gold BD, Colletti RB, Abbott M, et al. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:490–7. doi: 10.1097/00005176-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Perry S, de la Luz Sanchez M, Yang SHT, et al. Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerg Infect Dis. 2006;12:1701–8. doi: 10.3201/eid1211.060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malaty HM, Graham DY, Wattigney WA, et al. Natural history of Helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial community. Clin Infect Dis. 1999;28:279–82. doi: 10.1086/515105. [DOI] [PubMed] [Google Scholar]
- 34.Collins HL. The role of iron in infections with intracellular bacteria. Immunol Lett. 2003;85:193–5. doi: 10.1016/s0165-2478(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 35.Barabino A. Helicobacter pylori-related iron deficiency anemia: a review. Helicobacter. 2002;7:71–5. doi: 10.1046/j.1083-4389.2002.00073.x. [DOI] [PubMed] [Google Scholar]
- 36.Choe YH, Kim SK, Hong YC. Helicobacter pylori infection with iron deficiency anaemia and subnormal growth at puberty. Arch Dis Child. 2000;82:136–40. doi: 10.1136/adc.82.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokota S, Konno M, Mino E, et al. Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin Infect Dis. 2008;46:e31–3. doi: 10.1086/526784. [DOI] [PubMed] [Google Scholar]
- 38.Merrell DS, Thompson LJ, Kim CC, et al. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71:6510–25. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan S, Noto JM, Romero-Gallo J, et al. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervantes D, Fischbach LA, Goodman KJ, et al. Exposure to Helicobacter pylori-positive siblings and persistence of Helicobacter pylori infection in early childhood. J Pediatr Gastroenterol Nutr. 2010;50:481–5. doi: 10.1097/MPG.0b013e3181bab2ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodman KJ, Correa P, Tenganá Aux HJ, et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol. 1996;144:290–9. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- 42.Klein PD, Gilman RH, Leon-Barua R, et al. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol. 1994;89:2196–200. [PubMed] [Google Scholar]
- 43.Haggerty TD, Perry S, Sanchez L, et al. Significance of transiently positive enzyme-linked immunosorbent assay results in detection of Helicobacter pylori in stool samples from children. J Clin Microbiol. 2005;43:2220–3. doi: 10.1128/JCM.43.5.2220-2223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broussard Ch, Goodman KJ, Phillips CV, et al. Antibiotics taken for other illnesses and spontaneous clearance of Helicobacter pylori infection in children. Pharmacoepidemiol Drug Saf. 2009;18:722–9. doi: 10.1002/pds.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]