Abstract
Background
Strategies for successful implementation of hospitalwide glucose control efforts were addressed in a conceptual model for the development and implementation of an institutional inpatient glucose management program.
Conceptual Model Components
The Glucose Steering Committee incrementally developed and implemented hospitalwide glucose policies, coupled with targeted education and clinical decision support to facilitate policy acceptance and uptake by staff while incorporating process and outcome measures to objectively assess the effectiveness of quality improvement efforts. The model includes four components: (1) engaging staff and hospital executives in the importance of inpatient glycemic management, (2) educating staff involved in the care of patients with diabetes through structured knowledge dissemination, (3) executing evidence-based inpatient glucose management through development of policies and clinical decision aids, and (4) evaluating intervention effectiveness through assessing process measures, intermediary glucometric outcomes, and clinical and economic outcomes. An educational curriculum for nursing, provider, and pharmacist diabetes education programs and current glucometrics were also developed.
Outcomes
Overall the average patient-day–weighted mean blood glucose (PDWMBG) was below the currently recommended maximum of 180 mg/dL in patients with diabetes and hyperglycemia, with a significant decrease in PDWMBG of 7.8 mg/dL in patients with hyperglycemia. The program resulted in an 18.8% reduction in hypoglycemia event rates, which was sustained.
Conclusion
Inpatient glucose management remains an important area for patient safety, quality improvement, and clinical research, and the implementation model should guide other hospitals in their glucose management initiatives.
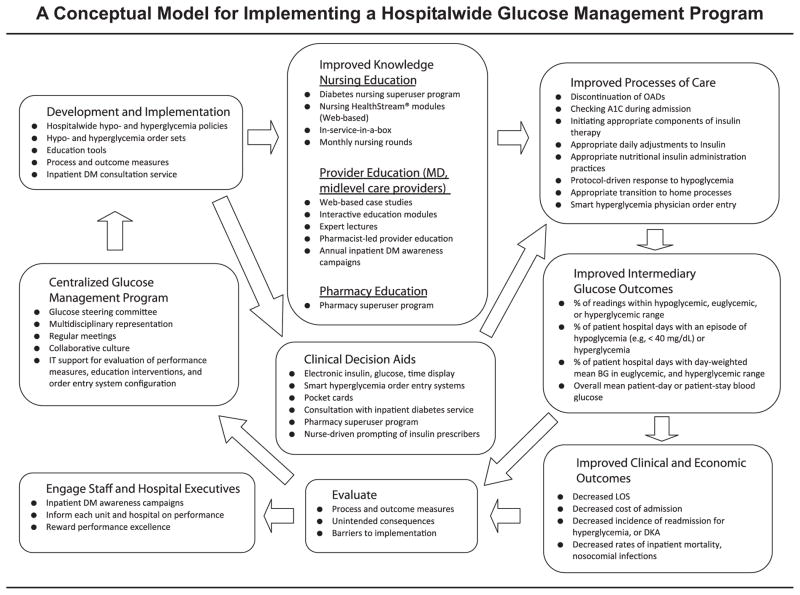
In 2006 the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) released a call to action in which they presented the case for improving care of hospitalized patients with diabetes and outlined overarching strategies to successfully implement hospitalwide glucose control efforts.1,2 The Joint Commission, in partnership with the ADA, has bolstered this national movement by establishing a set of expectations for the management of hospitalized patients with diabetes and by offering Advanced Certification in Inpatient Diabetes since 2006.3 We seek to expound on the strategies outlined by AACE/ADA through proposal of a conceptual model for the development and implementation of institutional inpatient glucose management programs on the basis of our experience at Johns Hopkins Hospital, a tertiary care teaching hospital (Figure 1, page 196). In the model, we outline and summarize what we believe are core principles to establishing a glucose management program; however, this paradigm has the flexibility to be adapted locally to meet the needs and context of individual hospitals. At the Johns Hopkins Hospital, the model includes a centralized glucose management program, which incrementally develops and implements hospitalwide glucose policies, coupled with targeted education and clinical decision support to facilitate policy acceptance and uptake by staff. It also incorporates process and outcome measures to objectively assess the effectiveness of quality improvement (QI) efforts. The model used in this program is based on a previously published model for putting research into practice, which entails summarizing evidence; identifying barriers to implementing the evidence; developing measures of performance; and then engaging, educating, executing, and evaluating to ensure that all patients receive the evidence-based practices.4
Figure 1.
The model, which outlines and summarizes the core principles for establishment of a glucose management program, can be adapted locally to meet the needs and context of individual institutions. OAD, oral antidiabetic; A1C, glycosylated hemoglobin; DM, diabetes mellitus; IT, information technology; BG, blood glucose; LOS, length of stay; DKA, diabetic ketoacidosis.
Centralized Glucose Management Program
In January 2006 a hospitalwide glucose control task force was commissioned at our hospital in response to a sentinel event related to hypoglycemia. We introduced a hospitalwide inpatient glucose management program to facilitate the development of uniform glucose management policies and staff education on the basis of current clinical practice guidelines. A spectrum of initiatives was implemented to improve hospitalwide glycemic control, including a hypoglycemia policy (July 2006), a diabetes nursing superuser program (January 2007), and a hyperglycemia policy and uniform subcutaneous (sub-Q) insulin order set (November 2007). Because of the ongoing need for diabetes policy development, education, and safety monitoring, the glucose control task force evolved into a standing Glucose Steering Committee (GSC) in July 2008.
The establishment of a centralized, multidisciplinary GSC is a core and critical element in the development of an inpatient glucose management program (Figure 1). The Johns Hopkins GSC, led by a diabetes clinician and researcher [S.H.G.], is multidisciplinary in representation and includes all departments involved in the care of hospitalized patients with hyperglycemia—physicians (for example, endocrinologists [S.H.G.], intensivist [S.M.B.], hospitalists, house staff), midlevel providers (for example, nurse practitioners [J.D., T.K.], physician’s assistants), nurse educators, pharmacists [L.E.], dietitians, and point-of-care testing (POCT)/laboratory medicine specialists. The GSC also includes key hospital administrative leaders—the nursing standards of care committee chairperson; the hospital’s chief medical information officer; the senior director of the Center for Innovation and Quality Patient Care; and the vice president of medical affairs, who also serves as the hospital’s patient safety officer. In addition to multidisciplinary representation, the GSC’s success is predicated, in part, on the degree of institutional support, as expressed by its commitment to a culture of safety, and the provision of financial and administrative resources to achieve and maintain the QI effort. At Johns Hopkins, institutional support for GSC initiatives has been championed by the vice president of medical affairs, who holds a position on the Johns Hopkins Hospital Medical Board—where all policies governing prescriber practices are approved.
Through regularly scheduled monthly meetings and a culture of collaboration and teamwork, the GSC guides the development and implementation of policies, protocols, order entry systems, education interventions, clinical decision aids, and performance measures, across the continuum of inpatient care.
Hospitalwide Policies Development and Implementation
Our institutional glucose management policies are constructed with the goals of establishing clear and uniform management guidelines while ensuring patient safety as a top priority. As shown in Table 1 (page 198), the key elements include definitions of hypoglycemia, hyperglycemia, and euglycemia for specific populations; responsibilities of authorized prescribers and nurses; specific procedures; reportable conditions; documentation requirements; and a list of policy education and communication channels. Before implementing new policies and proto- cols, we first established interdisciplinary consensus regarding glucose targets and diabetes care practices based on available evidence and society guidelines. We also performed a systematic review of the hospital’s current glucose policies, practices, and order sets, while identifying potential process-of-care and glucose outcome measures to assess the QI efforts. A baseline assessment of (1) providers’ knowledge of and attitudes regarding inpatient glucose management principles and (2) barriers to care is helpful in shaping policy and developing educational tools.
Table 1.
Key Elements to Include in Hospitalwide Glucose Management Policies*
| Hypoglycemia Policy | Hyperglycemia Policy (Subcutaneous [Sub-Q] Insulin) | IV Insulin Infusion Policy | External Sub-Q Insulin Pump Policy |
|---|---|---|---|
| Definition of hypoglycemia Emphasize nurse-driven assessment of conditions that increase risk for hypoglycemia Early recognition and prompt, standardized treatment of hypoglycemia Follow-up glucose monitoring strategy Early notification of prescribers to consider IV dextrose for persistent hypoglycemia Requirement of physicians to review and adjust insulin regimen or discontinue oral antidiabetic agents following a hypoglycemic episode Education of patients on hypoglycemic symptoms and how to prevent future episodes Provision of a snack at bed-time for glucose < 100 mg/dL, with a follow-up glucose check 2 hours later |
Definitions: Pre- and postmeal hyperglycemia, DKA, HHS, insulin- deficient patients, components of insulin therapy (for example, basal, nutritional, correction) Require order for A1C on admission if result within last 2–3 months unavailable Indications for IV insulin Fingerstick BG monitoring schedule according to patient’s nutritional status Assessment for signs and symptoms of hyperglycemia Indications for nutrition consultation Diet recommendations Indications for scheduled basal and nutritional insulin Guide to initiating insulin, and estimating total daily insulin requirements. Insulin administration schedule based on nutritional status Definition of appropriate use and schedule of correctional insulin Guidelines on insulin dose titration |
Options to choose protocols that target a tight or looser glucose range (for example, 100–140 mg/dL, 140–180 mg/dL) Guidelines for BG monitoring: frequency, site of testing, BG parameters necessitating a confirmatory serum BG Specific guidelines for initiating the drip via a nurse-driven protocol Specific titration parameters for adjusting drip rates for nursing staff Built-in safety guidelines for treatment of hypoglycemia Physician notification parameters relating to persistent hypo- or hyperglycemia, potassium status, and insulin infusion rates Protocol for transition to sub-Q insulin |
Requirement of patient to sign insulin pump self-management agreement Notification of physician, and/or legal department, if patient wishes to continue pump therapy but refuses to sign agreement Nurse review of contraindications to initial and continued use of insulin pump therapy Nurse to obtain order via an approved insulin pump order set Upon admission, insulin in the pump replaced with that supplied by the inpatient pharmacy Education of patient on pump self-management in the hospital setting Blood glucose monitoring plan Plan for documenting insulin dose administration by both patient and nurse Ongoing infusion and insertion site management Troubleshooting and managing pump disruptions Delineation of blood glucose criteria and special situations, which warrant discontinuation of insulin pump therapy and transition to sub-Q insulin |
IV, intravenous; DKA, diabetic ketoacidosis; HHS, hyperglycemic hyperosmolar state; BG, blood glucose; A1C, glycosylated hemoglobin.
We have found that hospitalwide glucose policy implementation is best facilitated by a targeted educational and clinical decision support infrastructure to facilitate staff’s policy acceptance and uptake. Multiple glycemic management educational interventions, including case-based learning modules and lectures for nursing and providers5–9 and dietitians, were implemented to improve insulin-prescribing practices and glycemic outcomes.
Educational Infrastructure
Nurses
Education was provided to nurses through (1) a diabetes nursing superuser program, (2) learning modules, (3) nursing rounds, (4) nursing in-service-in-a-box, and (5) nursing support for patient education.
1. Diabetes Nursing Superuser Program
Continuing nursing education can be effectively provided through the use of nurse educators, or “superusers,” who act as experts on institutional nursing policies and management principles and are tasked with the peer-to-peer education of their unit-specific nurse colleagues.10,11 We developed a diabetes superuser program to facilitate uptake and compliance with the hospital’s glucose management policies. Diabetes superusers are patient advocates committed to meeting the unique education and management needs of patients with diabetes through participation in interdisciplinary staff education and training, support of unit-based adherence to the hospital’s glucose management program, and development of unit-based approaches to coordinate safe discharge planning. In previous studies, incorporating a train-the-trainer approach for nursing staff into a multidisciplinary program reduced the median glucose and hyperglycemia frequency for diabetic inpatients8 and improved protocol compliance and incidence of hypoglycemia.12
The nurse champion [formerly J.D., now T.K.] of our diabetes superuser program is a nurse practitioner and certified diabetes educator. She coordinates regular rounds and superuser training workshops, develops case-based educational material for superusers to share with their units, updates superusers on current glucose management policies/procedures, supports unit projects, fields questions that arise—and attempts to make the superuser role a professionally rewarding experience.
In the glucose management program, after completing formalized training through online case-based modules and a hospitalwide diabetes workshop, diabetes superusers regularly update unit staff on current hypo- and hyperglycemia protocols and practices, support unit-based adherence to policies, and evaluate the effectiveness of staff education through administration of knowledge assessments. The glucose management topics covered by the diabetes education program are listed in Appendix 1 (available in online article). Nursing in-service sessions on institutional protocols have been shown to improve the percentage of time patients spent in the glucose target range without inducing severe hypoglycemic episodes.9
2. Learning Modules
Learning modules offer significant advantages over scheduled didactic sessions in allowing for flexible learning in unpredictable work shifts. We have found that they are most effective when tailored to the hospital’s or unit’s specific glucose policies and practices. They are periodically updated to incorporate new policies and practices and should ideally be case based, interactive, and relatively quick to complete. Modules can also serve a secondary function for the hospital by objectively demonstrating nursing competencies relating to inpatient glucose management principles (see topics in Appendix 1).
3. Nursing Rounds
At our hospital, the superuser nurse champions provide accredited continuing education (CE) monthly nursing rounds. These rounds consist of formal didactic sessions that highlight and reinforce hospital glucose management policies through a practical, case-based approach, while also providing opportunities to identify patient safety gaps or barriers to optimal care. Monthly topics (Appendix 1) correspond to the superuser curriculum and are carried forward from year to year.
4. Nursing In-Service-in-a-Box
Each month, diabetes superusers present one of the three cases presented in the larger diabetes nursing rounds session to their corresponding unit nurses. Newsletters posted on each floor summarize the monthly topics, which correspond to those of the nursing rounds. Like the nursing rounds program, these interactive in-services provide CE credits—and refreshments, particularly when the sessions occur during the lunch break.
5. Nursing Support for Patient Education
Diabetes self-management education is often delivered through a multidisciplinary approach, including teaching by physicians, nurses, and dietitians and through handouts provided at discharge. At our hospital, nurses are taught how to provide “survival skills” education to patients. The topics are as follows: (1) the importance, timing, and technique of self-monitoring of glucose and insulin administration; (2) recognition and treatment of hypo- and hyperglycemia, (3) sick-day guidelines; and (4) provision of emergency contact information.
Providers
We have disseminated provider education through learning modules, lectures, annual inpatient diabetes awareness campaigns, and establishment of a prescriber-based superuser program. Baseline information on prescriber practices and glucose metrics, along with assessment of their knowledge, perceptions, and attitudes toward institutional glucose management policies, has helped shape the content matter. At our hospital, provider education is primarily delivered through case-based expert lectures, but we plan on revising a set of online modules. To the extent possible, we have tried to have nurses and providers use the same learning modules for consistency of terminology and practice. The core learning objectives are summarized in Appendix 1.
In adopting these educational approaches, we drew on evidence that they can improve provider knowledge and inpatient glycemic outcomes. For example, Cook et al. found computer-based learning modules on inpatient management of diabetes and hyperglycemia to be an acceptable learning format for providers.13 In three studies, incorporation of provider-lecture seminars and case-based education sessions into a hospitalwide glycemic improvement program resulted in decreased use of sliding scale insulin,14,15 increased use of basal-bolus-correction insulin,14,15 greater modification of the glycemic regimen in response to severe hyperglycemia,16 and improved glycemic control,14–16 yet hypoglycemia rates were unchanged by these interventions.14–16
At our hospital, we also developed a pharmacist-led house staff education program to improve housestaff’s knowledge of and compliance with safe and effective insulin-prescribing practices. In previous studies, pharmacist oversight of insulin-prescribing practices as part of a multidisciplinary program improved hypo-17,18 and hyperglycemia8,17,18 and reduced prescribing of sliding scale insulin without use of basal insulin.17,18 Our program was initially designed as a stepwise privileging process for pharmacists (Figure 1).19,20 A pharmacy superuser—a clinical pharmacy specialist [L.E.] who actively participates in patient rounds and insulin management—first provided a one-hour accredited CE presentation to all point-of-care pharmacy specialists on four discrete topics covering basics of inpatient insulin management (Appendix 1). These core concepts were then highlighted and reinforced through 10 mock patient cases, in which the point-of-care pharmacy specialists involved in the privileging process devise and discuss an insulin management plan. After successfully completing the mock cases, each point-of-care pharmacy specialist was assigned to a clinical pharmacy specialist, with whom he or she meets at least three days a week to review glucose patterns, insulin regimens, and factors that may be contributing to dysglycemia in actual inpatients. Patients were identified through an electronically generated daily report of inpatients with two or more documented fingerstick glucose measures < 70 mg/dL or > 179 mg/dL in the previous 24 hours. The insulin management plan generated by the point-of-care pharmacy specialist was communicated to the patient’s providing team, either during work rounds or in the early afternoon, and was documented in the medical record. The point-of-care pharmacy specialist must complete a minimum of five notes, determined by the clinical pharmacy specialist to be adequate before independently making insulin recommendations. We are currently in the process of evaluating the effectiveness of this intervention on rates of hypo- and hyperglycemia and insulin-prescribing practices.
Dietitians
Education of hospital dietitians should emphasize the pivotal role of medical nutritional therapy (MNT) in achieving and maintaining acceptable inpatient glucose targets (Figure 1).21 At our hospital, core principles were established regarding the role of the dietitian or nutritional support provider in (1) constructing a consistent carbohydrate meal plan for both routine enteral feeds and diet progression, (2) communicating nutrition care recommendations to providers in an effective and timely manner, (3) providing individualized assessment and culturally sound nutritional counseling of patients with diabetes, (4) providing diabetes-specific enteral support formulas known to minimize wide glycemic excursions, and (5) conducting basic MNT self-management training as a component of discharge planning.2,22,23 Parenteral nutrition support members are additionally educated in the basic principles of insulin and glucose management and routinely make recommendations to providers on the initiation and titration of regular insulin, which is added as a component to the parenteral nutrition bags.
A fundamental, but often overlooked, component of MNT in hospitalized patients with diabetes involves ensuring the delivery of diet trays consistent with the carbohydrate-controlled diet order entered by the provider. Variability of carbohydrate intake from meal to meal due to poor appetite or nothing per os (NPO; nothing by mouth) status often contributes to dysglycemia in the inpatient setting.21,23 Quality control in meal preparation is hampered by the fact that dietary staff often work in entry-level positions with high turnover rates at our hospital. To address this issue, the GSC is collaborating with food service managers to implement a program of basic instruction and supervision to ensure accuracy in the carbohydrate content of diet trays.
Clinical Decision Support Infrastructure
A variety of clinical decision aids for providers were developed, including smart computerized prescriber order entry systems (CPOEs) and pocket cards.
Smart CPOE Systems
Glucose management programs have shown improved glycemic outcomes and insulin-prescribing practices through implementation of standardized, computerized order sets.9,24 Maynard and colleagues were the first to successfully implement an “indication-based” computerized insulin order set.17,25 In 2008, building on the indication-based order set, we developed a “smart” hyperglycemia order set that incorporates basic information entered on the patient’s weight, type of diabetes, total daily insulin dose, and nutritional status, and subsequently generates a set of tailored insulin order recommendations for use by providers. The next iteration of the hyper- glycemia order set will, ideally, seamlessly integrate patient data (for example, comorbidities, weight, insulin doses, blood glucose measures) with provider responses to embedded algorithms to guide prescribers through a logical decision-making process. The goal of such order sets is to generate a concise set of overridable sub-Q insulin order recommendations in keeping with the hospital’s glucose management policies, including actual basal and nutritional insulin doses, insulin-correctional scales based on the patient’s estimated insulin sensitivity, and advice on insulin-dose adjustments.
An electronic order set can also include a set of timely patient-safety prompts, such as reminders to discontinue a sulfonylurea in a patient made NPO, or the need to include orders for scheduled basal and nutritional sub-Q insulin in a patient with known insulin deficiency. The order set should be judged by its ability to improve processes of care, patient safety, and glucose outcomes, while preserving provider flexibility and efficiency in the process. Our hospital is currently engaged in efforts to monitor the perceived usefulness of, and adherence to, order set recommendations. In addition, order sets are modified periodically to incorporate new evidence-based practices or to improve usability or patient safety.
Pocket Cards
We have developed pocket cards to assist providers to act in accordance with recommended glucose management practices through provision of stepwise, algorithmic approaches to (1) insulin initiation and dose titration, (2) transitioning patients from intravenous (IV) to sub-Q insulin, (3) managing acute severe hyperglycemia (diabetic ketoacidosis [DKA], hyperglycemic hyperosmolar state [HHS], and non-DKA–related hyperglycemia), (4) management of insulin pump patients, and (5) transitioning patients to a home glucose management regimen. The pocket cards reflect a concise, action-oriented summary of the hospitalwide glucose policies. Distribution of pocket cards summarizing algorithms and protocols during implementation of hospitalwide glucose management programs have resulted in improved hyperglycemia,14,15, 26 increased use of scheduled as opposed to sliding scale insulin,14,15 improved physician adoption of practice guidelines,14 and more alternations in diabetes therapies before discharge.26 We are working on providing this support guide in multiple media formats (for example, laminated cards, downloadable PDF files, Web-based documents, or as a mobile device application tool) and have begun incorporating the key elements contained within the guide into the smart order set and provider education lectures.
Provider Prompts
We also plan to implement additional decision support tools, such as prescriber prompts, within the computerized order set to guide prescribers, with the impact determined by glucometrics analyses. Sources of patient-safety prompts might include pharmacy staff who oversee and approve prescriber orders, nurses who execute orders, and diabetes educators and endocrinologists who round with prescribers on medical units. Use of a real-time nursing intervention, coupled with an order set and glycemic-control protocol containing prompts, resulted in improved glycemic control and decreased use of sliding scale insulin alone.27 Protocol-based decision support with prompts has also been shown to reduce hypoglycemia and uncontrolled hyperglycemic days.24 Prompting can also be useful in mitigating prescriber inertia, which most often manifests as the failure to initiate or adjust the appropriate components of sub-Q insulin in the face of persistent hyperglycemia.28,29
Evaluation
Aiming for Improvement
The goal of the inpatient glucose management program, like any QI intervention, is the improvement of processes of care and outcomes—in this case, intermediary glucose outcomes and clinical, economic, and other outcomes, as shown in Figure 1.
Processes of Care
Processes of care data at our hospital are retrieved through (1) random selection and manual review of electronic or paper charts and (2) the use of pharmacy and administrative data queries. We are working on restructuring the current electronic medication administration record (eMAR) and order entry system to facilitate efficient retrieval and tracking of a variety of important process-of-care measures related to glucose management within and across hospital units.
Key processes of provider care include the following:
Documenting the patient’s diabetes type
Reviewing the patient’s outpatient glucose-lowering medications
Ordering a glycosylated hemoglobin (A1C) on admission if a recent result is unavailable
Ordering appropriate glucose monitoring frequency and initiation and titration of daily sub-Q insulin therapy components
Discontinuing oral antidiabetic agents in the presence of contraindications or poor glucose control
Having patients make appropriate transition from IV to sub-Q insulin
Transitioning patients to home processes
Key processes of nursing care include the following:
The nature and promptness of hypoglycemia treatments and adequacy of follow-up glucose monitoring
The notification of providers for persistent hypoglycemia
The timeliness of scheduled basal insulin administration
The appropriate withholding or administration of nutritional insulin coverage
The ascertainment as to whether the administered basal, nutritional, and correctional insulin doses accurately reflect the orders
Timeliness of blood glucose monitoring
The provision of diabetes self-management education to patients
Intermediary Glucose Outcomes
Focused efforts to improve processes of care can result in improved glucose control. Goldberg and colleagues, in 2006, were the first to establish a formal set of performance measures to evaluate glucose control, now widely referred to as “glucometrics,” to facilitate the cross-sectional and longitudinal assessment of inpatient glucose control within and across individual medical units and hospitals.30 Standardized, validated inpatient glucometrics serve an essential role in the assessment of QI efforts because changes in the processes of care are frequently targeted at improving these intermediary, patient-level glucose outcomes.
There are three commonly used models to evaluate repeated blood glucose measures, each of which has unique advantages and disadvantages, as summarized in Table 2 (above). These three models are commonly referred to as the population, patient-day, and patient-stay models. Because the patient-day model is thought to be more clinically relevant than the population model in the non–critical care setting and also appropriately accounts for differences in daily glucose testing frequency, we have adopted it as the preferred glucometric for our preliminary program evaluation, as described in the “Preliminary Evaluation” section, below.
Table 2.
Summary of Inpatient Glucometrics for Intensive Care Unit (ICU) and Non-ICU Settings*
| Glucometric Model | Numerator | Denominator | Interpretation | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Population | All glucose measurements over a given time period | None | Mean or median of all BGs % of all readings within a given glucose category |
|
|
| Patient-day | All glucose measurements over a given time period | Average number of blood glucose readings collected per patient-hospital day | Mean or median patient-day–weighted blood glucose % of patient-days with mean BG within a given glucose category % of patient-days with any episode of adverse glucose outcomes. |
|
Skewed somewhat toward patients with long hospital stays |
| Patient-stay | All glucose measurements over a given time period | Average number of blood glucose readings collected per patient-hospital stay | Mean patient-day– weighted BG for a patient’s hospital stay % of patient stays with day-weighted mean BG falling within a given glucose category. |
Best single measure of long-term glycemic control in a given patient |
|
BG, blood glucose; IV, intravenous.
In both the critical care and non–critical care literature, repeated glucose measures are conventionally translated into a mean or median blood glucose value, or otherwise characterized by rates of adverse glycemic events within specific glucose categories. However, the landscape of glucose metrics used in the critical care literature, as summarized in Table 3 (right), is more extensive than that of the non–critical care arena. Although many of these ICU metrics have demonstrated value in predicting short-term clinical outcomes,31–34 their relative predictive value over more traditional metrics is uncertain.
Table 3.
Summary of Suggested Inpatient Glucometrics for Intensive Care Unit (ICU) Settings*
| Time-Weighted Glucometrics |
| Area under the curve (AUC) glucose: Time spent within or outside a glucose range or target |
| Hyperglycemia Index, Glycemic Penalty Index†: Account for time spent outside a specified glucose target range |
| Glucometric Measures of Variability |
| Standard deviation of the sample mean31,32 |
| Mean absolute glucose change per hour34 |
| Mean amplitude of glycemic excursion (MAGE) |
| Percentage of coefficient of variation (% CV) |
Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: A retrospective study. Crit Care. 2004;8(3): R122–127 and Van Herpe T, et al. Glycemic penalty index for adequately assessing and comparing different blood glucose control algorithms. Crit Care. 2008;12(1):R24.
Improving Clinical, Economic, and Other Outcomes
The basis for establishing inpatient glucose control efforts involves prospective randomized data linking demonstration of improved glucose outcomes with improved clinical and economic outcomes in a variety of critically ill patients treated with intensive IV insulin protocols.35 Establishing causation is a difficult endeavor, but candidate outcomes include length of stay, cost of admission, readmission rates for DKA or hyperglycemia, inpatient mortality, and nosocomial infection rate (Figure 1). Other outcome measures that might be assessed include changes in provider understanding of and satisfaction with recommended practices and perceived barriers to compliance.
After QI efforts are implemented and process and outcome data are collected, the data should be systematically collated and analyzed through established QI methodologies, including use of process control charts when possible.36 The feasibility, timeliness, detail, and quality of abstraction of performance data is dependent on the nature of available health information system technologies and analytic support within a given institution.
Preliminary Evaluation
At Johns Hopkins Hospital, the metric evaluation process has, until recently, been slow to develop. The initial mandate of the GCS was limited to the development of safe and uniform hospitalwide glucose management policies, with no resource provision for metrics development or evaluation. In January 2010, after implementing the full ensemble of institutional glucose policies and education programs, the GSC then expanded its focus to metrics development and evaluation. Given limited resources, it then took approximately 18 months to perform an extensive review of the literature to identify suitable metrics to follow and to perform manual and electronic abstraction of glucose, process-of-care, and insulin-prescribing data. The data analysis process was further hindered by the need to internally develop statistical programs to translate raw glucose data into monthly unit- and specialty-specific glucometric reports. Despite these challenges, we have recently completed a preliminary evaluation of sequential changes in rates of hypo- and hyperglycemia occurrences and insulin-prescribing practices before and after each of the hospitalwide interventions. To inform future focused improvement efforts, our goals were to evaluate the impact of sequential inpatient glucose management interventions on hospitalwide glucometrics to determine which interventions were effective and which ones were not effective. Generation and analyses of our data set were approved by the Institutional Review Board of Johns Hopkins Medical Institutions.
Methods
We conducted a retrospective cohort study at Johns Hopkins Hospital. Data were drawn from the POCT database, which included fingerstick glucose measurements; and a subset of the data warehouse, Datamart, which included International Classification of Diseases, Ninth Revision (ICD-9) codes, demographics, data on hospital-acquired conditions, and hospital administrative data.
Study Population
All patients with POCT glucometer readings who were admitted to Johns Hopkins Hospital between January 2006 and December 2009 were assessed for eligibility. We included adult patients with (1) a diagnosis of diabetes based on ICD-9 codes or (2) hyperglycemia, defined as two POCT readings ≥ 180 mg/dL or ICD-9 codes for impaired fasting glucose, impaired glucose tolerance, hyperglycemia, or insulin use without ICD-9 codes for diabetes mellitus. Because our system interventions were targeted at adult, nonpregnant patients in non–critical care units, patients were excluded if they were in critical care units, pregnant, or admitted with diabetes ketoacidosis or hyperglycemic hyperosmolar state. Patients were also excluded if they had fewer than five POCT glucose readings during their entire hospital stay, which we considered the minimum number required to adequately characterize glycemic control.
Glucometrics Outcomes
We used a patient-day model, in which we determined the average glucose over time using the patient-day–weighted mean blood glucose (PDWMBG). We assessed hypoglycemia and hyperglycemia frequency by determining the percentage of patient-days with at least one hypoglycemic event < 70 mg/dL and the percentage of patient-days with PDWMBG ≥ 250 mg/dL within each admission, respectively.
Analysis Overview
We examined the change in the glucometric parameters over the course of each discrete intervention time period (Figure 2, above) and studied three subgroups of patients on the basis of their diabetes/hyperglycemia status—diabetes only, hyperglycemia only, and diabetes or hyperglycemia. We used multilevel mixed-effect linear regression to examine the change in the PDWMBG over the course of each intervention period. This approach allowed us to account for correlation among daily average blood glucose measures assessed within each admission and can be used to further account for potential correlations among admissions by the same patient. We used zero-inflated Poisson regression to estimate the event rates for hypoglycemia and hyperglycemia for each of the glucose intervention periods. This analytic approach accounts for right skewed data in which there are many zero events. Regression estimates were offset by the length of time in patient-days that a given patient was followed (exposure time) to derive rate ratios from the start to the end of each study time period. We used multivariable adjustment in both analyses to control for potential confounders. We excluded POCT readings obtained within one hour of the previous value to decrease the bias associated with repeat testing of extreme values.
Figure 2.
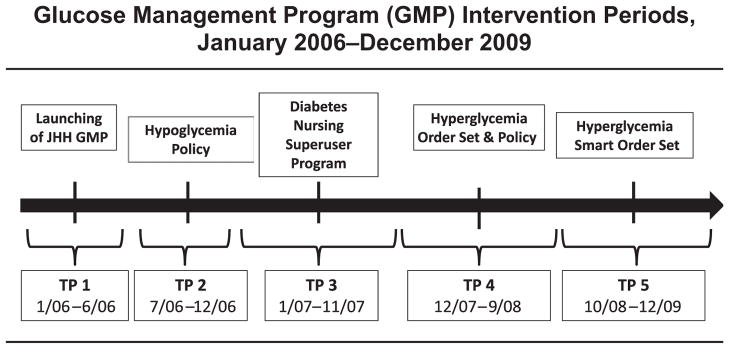
Changes in the glucometric parameters were examined over the course of each discrete intervention time period (TP). JHH, Johns Hopkins Hospital.
Results
Our final analyses included 25,160 admissions and 193,484 patient-days over the course of our four interventions. The data were fully adjusted for age, sex, race, severity of illness score, mortality risk, and hospital unit/department. Overall, we maintained the average glucose of our patients during admission below the currently recommended maximum of 180 mg/dL, with a range of 150 to 165 mg/dL in patients with diabetes or hyperglycemia. In the baseline period (January 2006–June 2006), there was a nonsignificant rise in PDWMBG of 2.4 mg/dL in patients with diabetes or hyperglycemia. During the intervention period (July 2006–December 2009), there was a nonsignificant decrease in PDWMBG of 1.1 mg/dL in patients with diabetes or hyperglycemia and 1.2 mg/dL in those with diabetes only, indicating that this measure remained stable. Among those with hyperglycemia only, however, there was a significant decrease in PDWMBG of 7.8 mg/dL during the intervention periods (p ≤ .001; Appendix 2, available in online article).
Incidence of Patient-Days with Hypoglycemia
Appendix 3 (available in online article) summarizes the trend in incidence of patient-days with hypoglycemia (any blood glucose < 70 mg/dL) associated with each intervention time period. In general, hypoglycemia rates were low, ranging from 3% to 10% throughout the intervention periods. Overall, there was a significant 18.8% reduction in hypoglycemia incidence rates during the intervention periods (July 2006–December 2009) in patients with diabetes or hyperglycemia. The implementation of the diabetes nursing superuser program (in January 2007) appeared to have the greatest impact on significantly reducing hypo -glycemia incidence rates (−24%; p ≤ .001), offsetting an increase in rates toward the end of the hypoglycemia policy intervention period (Appendix 3). Although there was no change in hypoglycemia incidence rates during the hyperglycemia policy and order set intervention period, there was an additional 22% decrease in incidence during the smart (CPOE) hyperglycemia order set intervention period (p < .05; Appendix 3). Results were similar in patients with diabetes only (data not shown).
Patient-Days with Extreme Hyperglycemia
In general, the percentage of patient-days with extreme hyperglycemia (PDWMBG ≥ 250 mg/dL) was low, ranging from 6% to 10% throughout the intervention periods (Appendix 4, available in online article). Overall, there was a 20% increase in the percentage of patient-days with hyperglycemia from January 2006 through December 2009 among patients with diabetes or hyperglycemia. This was likely related to a significant rise in hyperglycemia frequency before the uniform order set was implemented (24.4%; p ≤ .001). Implementation of the hypoglycemia policy (July 2006) and the hyperglycemia policy and orders set (November 2007) resulted in a nonsignificant 7.6% and 9.2% reduction, respectively, in the percentage of patient days with hyperglycemia. Results were similar among patients with diabetes only (data not shown).
Conclusions
We were able to maintain the mean glucose over time below the currently recommended target of 180 mg/dL, and in patients with new hyperglycemia (that is, hyperglycemia without previously diagnosed diabetes), there was a significant decrease in the mean glucose through the intervention periods. The interventions had the greatest impact on reducing the frequency of hypoglycemia. The diabetes nursing superuser program, which focused on teaching a nurse-driven hypoglycemia policy, had the most significant effect on hypoglycemia frequency. In contrast, the effect of the interventions on extreme hyperglycemia were less consistent. However, the implementation of the hyperglycemia policy and order set, which was also accompanied by an intense house staff educational program, resulted in a nonsignificant reduction in hyperglycemia.
Following Up on the Evaluation
To facilitate the process of metrics development and implementation going forward, in August 2011 we engaged the hospital’s newly developed QI Clinical Analytic Support Team. The hospital had also recently upgraded to a wireless glucometer system with accompanying software, which allows for generation of real-time glucometric reports within and across hospital units. This technological advancement allows for (1) timely evaluation of the effects of future interventions on glucometrics; (2) prompt identification of deviations from accepted glucose targets to help guide root cause investigations and development of targeted solutions; and (3) engagement of hospital staff by displaying monthly glucose reports throughout the hospital, coupled with a reward system for units consistently achieving glucose targets.37,38 To address the current obstacles in accurately and efficiently assessing processes of care relating to glucose management, the GSC is collaborating with the medical information technology department to reconfigure the CPOE and eMAR systems to expand the array and specificity of electronic processes of care documentation, while also facilitating data retrieval for ongoing analyses. In addition to evaluating performance metrics, glucose management programs must determine whether the recommendations are understood, supported, and adhered to, by all relevant members of the hospital care team. Unintended consequences, along with barriers to successful implementation often arise, necessitating additional tweaking of the processes. Equally important to our evaluation process has been review and follow-up on events reported to the Patient Safety Network, the hospital’s incident reporting system, relating to hypoglycemia or hyperglycemia; such cases often served as powerful impetuses for institutional policy revisions. We plan for the GSC, after data are analyzed, to summarize and discuss findings and generate new interventions through regularly scheduled cycles of performance improvement (for example, Plan-Do-Study-Act [PDSA]) to address ongoing areas of deficiency. As we finalize our glucometrics assessment, we will be poised to perform and evaluate future inpatient diabetes QI interventions more efficiently using PDSA cycles.
In addition to informing areas for future improvement, the evaluation process, including data collection, can be used to engage staff and hospital executives through inpatient diabetes awareness campaigns, regular provision of unit-specific and hospitalwide performance reports, and offering of reward compensations for performance excellence (Figure 1). It is essential for all staff to understand why glucose management is important, including the negative impact of poor glucose management, and the clinical benefits to patients when it is done well.
Discussion
We learned several key lessons in implementing the inpatient glucose management program. First, it is critical to have institutional champions, who, in our case, were supported by the GSC, which had the financial endorsement of the hospital leadership. The program’s institutional budget (approximately $350,000/ year) supports part of a physician champion (0.25 full-time equivalent [FTE]), two full-time diabetes nurse practitioners, a part-time diabetes nurse educator/QI nurse (0.5 FTE), and a full-time administrative staff member to assist with program coordination. Second, the leadership must also be engaged in supporting institutional acceptance of and adherence to policy. Third, in our program, the diabetes superuser model was critical to implementing the interventions. After the GSC advised on the policies and interventions, the superusers in the respective disciplines were then deployed to translate the interventions into daily practice, to provide feedback on program components that required revision, and to report ongoing safety gaps in the program—all key features in our program’s success.
The major weakness of the glucose management program was lack of data retrieval, management, and analysis through the departments of information technology and clinical analytics at the beginning of the development of the program to support real-time PDSA cycles. Although we are currently evaluating our specific major interventions retrospectively, it would have been ideal to perform them in real time. Recent changes, as described in the “Preliminary Evaluation” section, will allow us to move forward. On the basis of our experience, other institutions would be well advised to develop and incorporate an analytic strategy before program initiation.
Although the decrease in hypoglycemia frequency through December 2009 has since been sustained, the decrease in extreme hyperglycemia following implementation of the uniform sub-Q insulin order set and hyperglycemia policy has not. The hypoglycemia protocol treatment interventions are more conservative than they were before 2006 (for example, ½ ampule of 50% dextrose [D50] as opposed to 1 ampule of D50, or 4 ounces of orange juice as opposed to 8 ounces) and unlikely to be contributing to the observed hyperglycemia. We believe that the inability to produce sustained effects on hyperglycemia is related to the fact that while the hypoglycemia policy is implemented by the nursing staff, the hyperglycemia policy and order set are implemented by prescribers, primarily house staff. Because the house staff at the hospital turns over each year, it is imperative to orient them to our glucose management policies; however, there is not a centralized mechanism to do this across hospital departments. In contrast, the nursing staff is stable and is educated annually on all hospital policies through a centralized nursing education website. Recognizing this educational gap for the house staff, the GSC is working with the residency program directors to more consistently incorporate inpatient diabetes education into their curricula. On the basis of focus group feedback from our prescribers, we also plan to implement additional decision support tools within the computerized order set to guide prescribers, with the impact determined by performing glucometrics analyses.
Conclusion
Inpatient glucose management is an important area for patient safety, QI, and clinical research. We hope that the implementation model provided herein, on the basis of our experience at Johns Hopkins Hospital, will guide other hospitals and academic institutions in their own efforts to improve the quality and safety of inpatient diabetes care.
Acknowledgments
Dr. Munoz was supported by a training grant through the National Institute of Diabetes, Digestive, and Kidney Disease/NIH (T32 DK062707). This research was supported by the Johns Hopkins Prevention and Control Core of the Diabetes Research and Training Center from the National Institutes of Diabetes, Digestive, and Kidney Diseases grant number P60 DK079637.
The authors thank the Johns Hopkins Hospital for institutional support of our Glucose Steering Committee and Inpatient Diabetes Management Service. We are grateful for the support of Dr. Joseph Finkelstein for initial guidance on the approach to glucometrics analysis. We are grateful for the support of institutional leaders and Steering Committee members who have providing unwavering support of our implementation program: Mrs. Maria Cvach, Mrs. Kathy Kosmakos-Wagner, Mrs. Mikaela Olsen, Dr. Beryl Rosenstein, Dr. Redonda Miller, Dr. Peter S. Greene, Ms. Renee Demski, Ms. Nancy Yang, Mrs. LaTonya Jackson, Dr. Paul W. Ladenson, and Dr. Myron Weisfeldt.
Contributor Information
Miguel Munoz, Formerly Senior Endocrinology Fellow, Johns Hopkins University School of Medicine, Baltimore, is Staff, Endocrinology, Southern Endocrinology and Diabetes, Roswell, Georgia.
Peter Pronovost, Professor of Anesthesiology and Critical Care Medicine; Director, Armstrong Institute for Patient Safety and Quality; and Senior Vice President for Patient Safety and Quality, Johns Hopkins Hospital and Johns Hopkins University School of Medicine, Baltimore.
Joanne Dintzis, Formerly Inpatient Diabetes Nurse Practitioner, Johns Hopkins University School of Medicine, and Clinical Facilitator, Johns Hopkins Hospital Glucose Steering Committee, is Diabetes Nurse Practitioner, University of Maryland Center for Diabetes and Endocrinology at Upper Chesapeake Medical Center, Bel Air, Maryland.
Theresa Kemmerer, Inpatient Diabetes Nurse Practitioner, Johns Hopkins University School of Medicine; and Clinical Facilitator, Johns Hopkins Hospital Glucose Steering Committee.
Nae-Yuh Wang, Assistant Professor, Medicine and Biostatistics, Johns Hopkins University School of Medicine and Bloomberg School of Public Health, Baltimore; and Director, Biostatistics Core, Baltimore Diabetes Research and Training Center, Baltimore.
Yi-Ting Chang, Biostatistician, Departments of Medicine and Biostatistics, Johns Hopkins University School of Medicine and Bloomberg School of Public Health; and Research Assistant, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health.
Leigh Efird, Clinical Pharmacy Specialist, Department of Pharmacy, Johns Hopkins Hospital.
Sean M. Berenholtz, Associate Professor, Armstrong Institute for Patient Safety and Quality and Bloomberg School of Public Health; and Physician Director, Inpatient Quality and Safety, Armstrong Institute, Johns Hopkins University School of Medicine.
Sherita Hill Golden, Associate Professor of Medicine and Epidemiology, Johns Hopkins University School of Medicine and Bloomberg School of Public Health; Director, Inpatient Diabetes Management Service; and Chairperson, Johns Hopkins Hospital Glucose Steering Committee.
References
- 1.ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association Consensus statement on in-patient diabetes and glycemic control. Diabetes Care. 2006;29(8):1955–1962. doi: 10.2337/dc06-9913. [DOI] [PubMed] [Google Scholar]
- 2.Moghissi ES, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Joint Commission. [Accessed Mar 9, 2012];Advanced Certification in Inpatient Diabetes. http://www.jointcommission.org/certification/inpatient_diabetes.aspx.
- 4.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: A model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds LR, et al. An institutional process to improve inpatient glycemic control. Qual Manag Health Care. 2007;16(3):239–249. doi: 10.1097/01.QMH.0000281060.37979.83. [DOI] [PubMed] [Google Scholar]
- 6.Cook CB, et al. Diabetes and hyperglycemia quality improvement efforts in hospitals in the United States: Current status, practice variation, and barriers to implementation. Endocr Pract. 2010;16(2):219–230. doi: 10.4158/EP09234.OR. [DOI] [PubMed] [Google Scholar]
- 7.Korytkowski M, et al. Evolution of a diabetes inpatient safety committee. Endocr Pract. 2006;12 (Suppl 3):91–99. doi: 10.4158/EP.12.S3.91. [DOI] [PubMed] [Google Scholar]
- 8.Murphy DM, et al. Reducing hyperglycemia hospitalwide: The basal-bolus concept. Jt Comm J Qual Patient Saf. 2009;35(7):216–223. doi: 10.1016/s1553-7250(09)35029-1. [DOI] [PubMed] [Google Scholar]
- 9.Hermayer KL, et al. Impact of improvement efforts on glycemic control and hypoglycemia at a university medical center. J Hosp Med. 2009;4(6):331–339. doi: 10.1002/jhm.449. [DOI] [PubMed] [Google Scholar]
- 10.Boffa DP, Pawola LM. Identification and conceptualization of nurse super users. J Healthc Inf Manag. 2006;20(4):60–68. [PubMed] [Google Scholar]
- 11.McIntire S, Clark T. Essential steps in super user education for ambulatory clinic nurses. Urol Nurs. 2009;29(5):337–342. [PubMed] [Google Scholar]
- 12.Selig PM, Popek V, Peebles KM. Minimizing hypoglycemia in the wake of a tight glycemic control protocol in hospitalized patients. J Nurs Care Qual. 2010;25(3):255–260. doi: 10.1097/NCQ.0b013e3181d373e9. [DOI] [PubMed] [Google Scholar]
- 13.Cook CB, et al. Development of computer-based training to enhance resident physician management of inpatient diabetes. J Diabetes Sci Technol. 2009 Nov 1;3(6):1377–1387. doi: 10.1177/193229680900300618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ena J, et al. Long-term improvements in insulin prescribing habits and glycaemic control in medical inpatients associated with the introduction of a standardized educational approach. Diabetes Res Clin Pract. 2009;85(2):159–165. doi: 10.1016/j.diabres.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Schnipper JL, et al. Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: Results of a clinical trial. J Hosp Med. 2009;4(1):16–27. doi: 10.1002/jhm.385. [DOI] [PubMed] [Google Scholar]
- 16.Donihi AC, et al. Effect of a targeted glycemic management program on provider response to inpatient hyperglycemia. Endocr Pract. 2011;17(4):552–557. doi: 10.4158/EP10330.OR. [DOI] [PubMed] [Google Scholar]
- 17.Maynard G, et al. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: Effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15. doi: 10.1002/jhm.391. [DOI] [PubMed] [Google Scholar]
- 18.Kirk JK, Oldham EC. Hyperglycemia management using insulin in the acute care setting: Therapies and strategies for care in the non-critically ill patient. Ann Pharmacother. 2010;44(7–8):1222–1230. doi: 10.1345/aph.1M695. [DOI] [PubMed] [Google Scholar]
- 19.Galt KA. Credentialing and privileging for pharmacists. Am J Health Syst Pharm. 2004 Apr 1;61(7):661–670. doi: 10.1093/ajhp/61.7.661. [DOI] [PubMed] [Google Scholar]
- 20.Blair MM, et al. Pharmacist privileging in a health system: Report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007 Nov 15;64(22):2373–2381. doi: 10.2146/ajhp070149. [DOI] [PubMed] [Google Scholar]
- 21.Boucher JL, et al. Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional. J Am Diet Assoc. 2007;107(1):105–111. doi: 10.1016/j.jada.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Elia M, et al. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care. 2005;28(9):2267–2279. doi: 10.2337/diacare.28.9.2267. [DOI] [PubMed] [Google Scholar]
- 23.Swift CS, Boucher JL. Nutrition therapy for the hospitalized patient with diabetes. Endocr Pract. 2006;12 (Suppl 3):61–67. doi: 10.4158/EP.12.S3.61. [DOI] [PubMed] [Google Scholar]
- 24.Schnipper JL, et al. Effects of a computerized order set on the inpatient management of hyperglycemia: A cluster-randomized controlled trial. Endocr Pract. 2010;16(2):209–218. doi: 10.4158/EP09262.OR. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, et al. Indication-based ordering: a new paradigm or glycemic control in hospitalized inpatients. J Diabetes Sci Technol. 2008;2(3):349–356. doi: 10.1177/193229680800200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin D, et al. Eliminating inpatient sliding-scale insulin: A reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008–1011. doi: 10.2337/diacare.28.5.1008. [DOI] [PubMed] [Google Scholar]
- 27.Shabbir H, et al. A real-time nursing intervention reduces dysglycemia and improves best practices in noncritically ill hospitalized patients. J Hosp Med. 2010;5(1):E15–20. doi: 10.1002/jhm.590. [DOI] [PubMed] [Google Scholar]
- 28.Knecht LA, et al. Diabetes care in the hospital: Is there clinical inertia? J Hosp Med. 2006;1(3):151–160. doi: 10.1002/jhm.94. [DOI] [PubMed] [Google Scholar]
- 29.Cook CB, et al. Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211. doi: 10.1002/jhm.188. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg PA, et al. “Glucometrics”—Assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569. doi: 10.1089/dia.2006.8.560. [DOI] [PubMed] [Google Scholar]
- 31.Egi M, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Krinsley JS. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 33.Krinsley JS. Glycemic variability and mortality in critically ill patients: The impact of diabetes. J Diabetes Sci Technol. 2009 Nov 1;3(6):1292–1301. doi: 10.1177/193229680900300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermanides J, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38(3):838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 35.Newton CA, Young S. Financial implications of glycemic control: Results of an inpatient diabetes management program. Endocr Pract. 2006;12 (Suppl 3):43–48. doi: 10.4158/EP.12.S3.43. [DOI] [PubMed] [Google Scholar]
- 36.Eslami S, et al. Implementing glucose control in intensive care: A multicenter trial using statistical process control. Intensive Care Med. 2010;36(9):1556–1565. doi: 10.1007/s00134-010-1924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson M, Zito D, Kongable G. Benchmarking glucose results through automation: The 2009 Remote Automated Laboratory System Report. J Diabetes Sci Technol. 2010 Nov 1;4(6):1507–1513. doi: 10.1177/193229681000400627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook CB, et al. Inpatient point-of-care bedside glucose testing: Preliminary data on use of connectivity informatics to measure hospital glycemic control. Diabetes Technol Ther. 2007;9(6):493–500. doi: 10.1089/dia.2007.0232. [DOI] [PubMed] [Google Scholar]