Abstract
In Latin America, gastric cancer is a leading cancer, and countries in the region have some of the highest mortality rates worldwide, including Chile, Costa Rica, and Colombia. Geographic variation in mortality rates is observed both between neighboring countries and within nations. We discuss epidemiological observations suggesting an association between altitude and gastric cancer risk in Latin America. In the Americas, the burden of gastric cancer mortality is concentrated in the mountainous areas along the Pacific rim, following the geography of the Andes sierra, from Venezuela to Chile, and the Sierra Madre and Cordillera de Centroamérica, from southern Mexico to Costa Rica. Altitude is probably a surrogate for host genetic, bacterial, dietary, and environmental factors that may cluster in the mountainous regions. For example, H. pylori strains from patients of the Andean Nariño region of Colombia display European ancestral haplotypes, whereas strains from the Pacific coast are predominantly of African origin. The observation of higher gastric cancer rates in the mountainous areas is not universal: the association is absent in Chile, where risk is more strongly associated with the age of H. pylori acquisition and socio-economic determinants. The dramatic global and regional variations in gastric cancer incidence and mortality rates offer the opportunity for scientific discovery and focused prevention programs.
Keywords: Gastric cancer, Helicobacter pylori, Latin America, Andean countries, Altitude enigma
Introduction
Gastric cancer is the fourth most common cancer worldwide, and the second leading cause of cancer mortality, accounting for nearly one million incident cases and 737,000 deaths in 2008 [1, 2], and is projected to rise from fourteenth to eighth in all-cause mortality in the near term, due to the growing and aging populations in eastern Asia and Latin America [3]. As with other infection-related cancers, the incidence and mortality rates are higher in less developed countries and two-thirds of gastric cancer deaths occur outside of the high-income countries. Projections suggest that in high-income countries (HIC), gastric cancer deaths will decrease from 202,600 in 2010 to 127,700 in 2020, whereas in less developed areas, deaths will increase from 517,900 in 2010 to 573,300 in 2020. In Latin America, gastric cancer is one of the leading cancers, and countries of the region display some of the highest mortality rates worldwide. Estimated age-standardized mortality rates (ASMR) for males per 100,000 are elevated in Honduras (22.3), Costa Rica (16.8), Peru (18.2), Chile (15.0), and Ecuador (20.7), compared with lower rates observed in some regions of Latin America, such as Argentina (5.6) and Mexico (6.7) [1].
Gastric cancer is the leading global cause of infection-related cancer in men and the second in women after cervical cancer [4]. Helicobacter pylori colonizes the gastric mucosa of humans early in life establishing long-lasting chronic inflammation of the mucosa, the driving force for the development of pre-neoplastic lesions [5, 6]. In some instances, after decades of continuous bacteria-host dialogue, the balance is compromised, leading to peptic ulcer disease and gastric cancer. Bacterial virulence factors play a role in epithelial damage, via inflammatory and other pathways, and generate reactive oxygen species and nitroso-compounds, which also affect DNA stability [7]. The etiology of gastric cancer is multifactorial, wherein the genetics of both the host and the bacteria are important, with modulation by other environmental factors such as diet and smoking. These factors may contribute to regional variation from country to country, and between different racial and ethnic groups. Latin-American countries have among the highest rates of H. pylori infection worldwide [8] and constitute a mosaic of human groups with different genetic background and in consequence a mosaic of genetically diverse H. pylori strains.
The marked global variation in gastric cancer incidence and mortality rates is well described and offers the opportunity for accelerated scientific discovery and focused prevention programs. The variation is observed between regions, countries, and provinces within countries. It is not surprising to observe Latin-American countries with high mortality rates, such as Guatemala and Chile, contrasting with relatively low rates in neighboring countries like Mexico and Argentina, respectively [1, 9, 10]. This report aims to summarize the literature, which suggests an association of gastric cancer with altitude in the Latin-American countries with the highest mortality rates, primarily along the Pacific rim.
Is gastric cancer associated with altitude in Latin America?
In this section, we present epidemiological observations in several Latin-American countries that suggest a correlation between altitude and gastric cancer. These epidemiological data are described in national public health reports (accessed via the Ministry of Health web sites) and/or in the scientific literature. We present annual age and sex standardized rates, primarily as mortality rates in South America and incidence rates in Central America, based upon available sources.
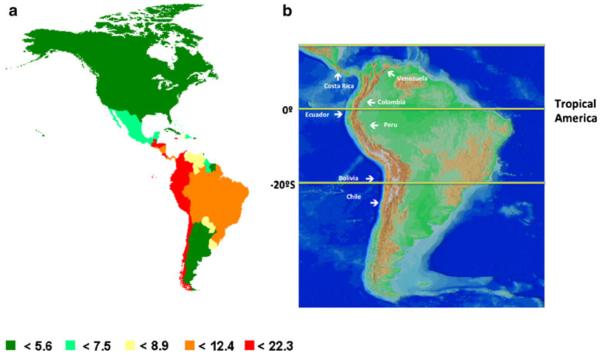
The distribution of gastric cancer mortality in the Americas [1] suggests that the burden of disease is concentrated in the nations along the Pacific rim (Fig. 1a, countries in red). The highest country mortality rates follow the mountainous geography of the Sierra Madre and Cordillera de Centroamérica of Mesoamerica, from southern Mexico to Costa Rica, and the Andes sierra, from Venezuela to Chile. (Fig. 1b). Furthermore, the highest mortality rates occur in communities in the mountains as compared with the coastal regions, as detailed in the preliminary correlation analyses summarized herein. [10–16].
Fig. 1.
a Estimated age-standardized gastric cancer mortality rate per 100,000 in the Americas [1]; b map showing that the countries with the higher GC mortality rates follow the Andes mountains and the Sierra Madre in Central America
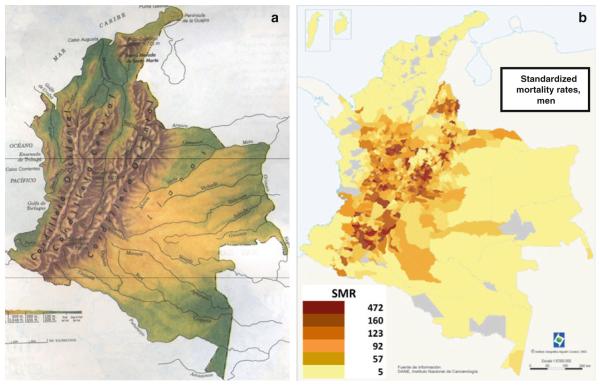
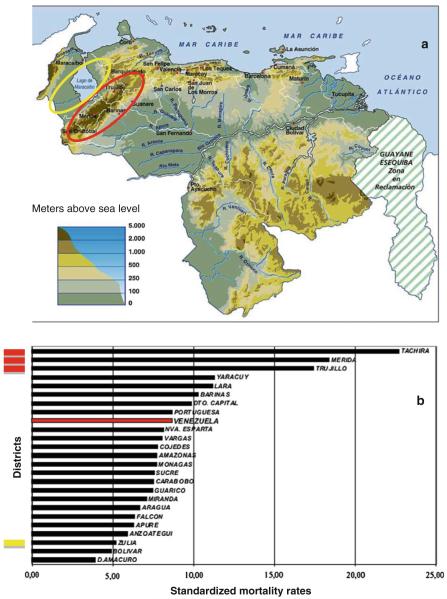
The first example is Colombia, as illustrated in Fig. 2, which presents a side-by-side comparison of the topography map (Fig 2a) and the distribution of gastric cancer mortality rates per district (Fig 2b) [11]. Also in Colombia, a series of investigations by Correa and colleagues have examined the contrasting gastric cancer rates in southern Colombia comparing the Andean Pasto region (age-adjusted incidence rate, ASIR, 150) and the coastal Tumaco area (ASIR of 6), only 100 miles apart and yet with a 25-fold difference in incidence rates [17]. The Andes Cordillera continues northeast into Venezuela in the states of Tachira (San Cristobal), Merida, and Trujillo (Fig. 3a), which have gastric cancer mortality rates 3–4 times higher than the neighboring state of Zulia at sea level (Fig. 3a), as well as the remainder of the country (Fig. 3b) [12, 13].
Fig. 2.
Maps of Colombia showing in a the distribution of the Andes Cordillera and in b the distribution of gastric cancer mortality rates in the different districts of the country [11]
Fig. 3.
In Venezuela, a the Andean districts with higher altitude, Tachira, Merida, and Trujillo (circle in red), and the neighbor coastal district Zulia (circle in yellow) b mortality rates in all districts of Venezuela, showing the higher gastric cancer mortality rates in the Andean districts and the very low rate in the coastal district of Zulia [12, 13]
Other Andean countries also have some of the highest mortality rates in the Americas [1]. In Ecuador, the ASMRs are 28.0 for males and 24.1 for females, and in Peru 22.6 for males and 19.5 for females. A study in Peru found that patients in La Oroya City, located at 3700 m in altitude, presented more severe gastric lesions, including atrophic gastritis and intestinal metaplasia in body and antrum than patients from Lima, at the sea level [18] further documenting higher risk for gastric cancer in mountainous regions. In Bolivia, the ASMRs are 15.1 for males and 13 for females, these lower rates potentially reflecting the transition to the Altiplano desert between Bolivia and Chile (Fig. 1b).
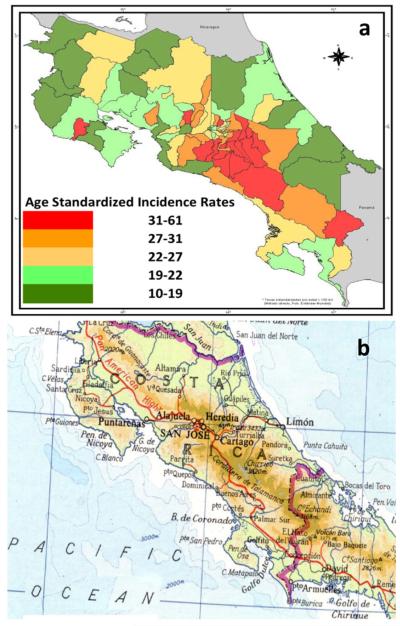
In Central America, a similar altitude phenomenon is observed, in spite of modest elevations in the mountainous regions. In Costa Rica, a comparative analysis of altitude and gastric cancer mortality demonstrates an important correlation with altitude (Fig. 4) [15]. Highest gastric cancer ASIRs (50 for males, in red in Fig. 4a) are observed in communities located in the “Cordillera Volcanica Central” mountains (Fig. 4b) versus the coastal regions (ASIRs for males, 10, in green in 4a). In the northern Central America, the central mountain range, Cordillera de Centroamérica, extends from Chiapas in southern Mexico, through Guatemala, Honduras, and El Salvador, to northern Nicaragua. A similar contrast in gastric cancer incidence is noted when comparing the mountainous and coastal regions. In mountainous western Honduras, the ASIRs are over 30 for males [19, 20], contrasting with nearby coastal Nicaragua, a distance of approximately 150 miles, with ASIRs less than 6.0 [14]. Lastly, a regional mountain versus valley phenomenon has been observed even in higher incidence regions, such as Colombia and Honduras. For example, in an analysis of incident cases from 1996 to 2005 in the 95 rural municipalities of western Honduras, with a range of elevations between approximately 300 and 1250 meters, a modest correlation was observed between gastric cancer incidence and municipality elevation (p = 0.028, F = 5.3, r = 0.36) [21].
Fig. 4.
In Costa Rica, a the incidence of gastric cancer is higher in b cantons on the mountains of the Cordillera Volcanica Central [15]
Possible causes for the observed altitude and gastric cancer association
It is unlikely that altitude per se is the central etiology driving a higher observed incidence in the mountainous regions, for example, via hypoxemia. Altitude is probably a surrogate for host genetic, bacterial, dietary, and environmental factors that may cluster in the mountainous regions. The clustering of multiple factors may differ among the mountainous regions in the Americas.
The role of host genetics and genotypes
In the original human migrations in South America, the geographic barriers such as the Andean highlands and the Amazon River [22] divided and isolated human populations, as reflected in the archeological records and distinctive genetic patterns [22]. This may explain the relative genetic homogeneity in certain mountain or inland areas of South America. A similar explanation has been given to the distribution of gallbladder cancer in Chile, which follows the distribution of gastric cancer, and has a marked preference for the Amerindian ancestry (e.g., Mapuche peoples) [23].
Host risk genotypes, such as proinflammatory cytokine polymorphisms, in concert with H. pylori infection, have been shown to correlate with gastric cancer risk in many populations [24–26]. The host genotypes, as well as H. pylori genotypes, may cluster more readily in somewhat isolate mountainous communities, as noted in western Honduras. In a sample of healthy, population-based Honduran controls of Hispanic mestizo origin, the IL-1B-511T+ genotype prevalence was 81 %, and the IL-10-1082A+ prevalence was 93 %, among the highest reported [19]. Host risk genotypes may also cluster by race and ethnicity. In Colombia, the Pacific coastal populations have a dominance of African ancestry, contrasting with those living in the Andean Nariño region which have a mix of Amerindian and European ancestry, such that host genetic differences may at least partially explain the difference in susceptibility [24].
The role of H. pylori genotypes and haplotypes
The prevalence of infection by H. pylori has been studied and whereas in general no differences in the frequency of infection was observed between high- and low-risk areas; infection with H. pylori type I cagA+ vacAs1m1 has been noted to be somewhat higher in isolates from the high-risk area [27, 28]. The ancestral origin of the H. pylori strains, as defined by phylogenetic haplotypes, may differ between the mountain and coastal areas. In a study of high-risk strains (cagA+ vacAs1m1+) in male patients with chronic gastritis in the Andean Pasto region of Colombia, it was found that all strains displayed European ancestry (100 % hpEurope haplotype), whereas in the neighboring Tumaco region on the Pacific coast, strains of African origin predominated (66 % hpAfrica1 haplotype). It was also observed that strains of European ancestry were associated with more severe gastritis and DNA damage than those of Africa ancestry [27]. Thus, the genetics of both humans and H. pylori may cluster in the mountainous regions and contribute to the observed differences in risk for gastric cancer.
The role of co-infection
The role of chronic helminthiasis in modulating the immune response has been suggested as one of the explanations for the “African enigma” [29, 30]. In the Colombia coastal Tumaco area (low risk), intestinal helminthiasis is very common, and subjects infected with H. pylori display a Th2 (“allergic”) type of immune response, as evidenced by their marked eosinophilic infiltrate in the gastric mucosa [31] and their elevated blood levels of IgE [32]. In contrast, the Th2 response and IgE levels were lower in subjects living in the Andean area (high risk) [32]. Epstein–Barr virus infection (EBV) is associated with 10 % of gastric adenocarcinomas worldwide, in both high- and low-incidence areas, and the percentage of H. pylori and EBV co-infections does not appear to be increased in the high incidence areas of Central America [33].
The role of nutrition
Dietary and environmental factors may be significantly different between the mountainous and coastal regions. In addition, access to foods such as antioxidant-rich fruits may be limited and/or seasonal in the more isolated mountainous regions. Selenium is one example and has been associated with gastric cancer risk [34]. The soil of volcanic regions and high altitude areas usually contain low amounts of selenium, as compared with soil in valleys or coastal regions. Inhabitants of these regions may not be selenium deficient, but usually have borderline or low normal range selenium levels, thereby potentially contributing to cancer risk. Selenium is essential for selenoprotein expression, particularly antioxidant enzymes such as glutathione peroxidases, thioredoxin reductase, and iodothyronine deiodinase, which may provide protection against the inflammatory effect of H. pylori leading to gastric cancer [34].
In Colombia, lower plasma selenium levels were observed in males in the mountain Nariño region when compared with the coastal Tumaco area; the levels of selenoprotein-P and glutathione peroxidase activity were not significantly different; the authors concluded that low, non-deficient, selenium levels may contribute, but not drive, the development of gastric cancer [35]. Similarly, in Honduras, in a study of incident gastric cancer cases and population-based controls, a significant difference was noted in plasma selenium mean values, among incident cancer cases versus controls: 86 mcg/L versus 117 mcg/L (p < 0.001); significant differences were also noted in selenium quartile levels between cases, 45–140 mcg/L versus controls, 95–170 mcg/L. The logistic regression showed that decreased selenium was a risk factor for gastric cancer: OR 0.90 (95 % CI, 0.85–0.96), when adjusted for age, sex, H. pylori status, and host IL-1β and IL-10 risk allele status [16]. Another possible factor is differences in the content of salt (NaCl) in the diet of people living in the mountains versus those living in the coast, since salt has been associated with gastric cancer risk in many countries [36].
Exceptions to the Altitude correlation
In Latin America, the contrasting gastric cancer rates in the mountain versus coastal areas are commonly observed, but not universal. The correlation is strongest in Central America and the majority of South America. Notable exceptions exist, as outlined below. In Costa Rica, one province (canton) on the Pacific coast is noted to have higher rates of gastric cancer [37]; historically, a significant percentage of the population has emigrated from mountainous Costa Rica, essentially “importing” their host and bacterial risk, which may offer an explanation.
The case of Chile
The association of gastric cancer mortality with altitude is not observed in Chile. As presented in Fig. 5, the population size and age-sex weighted stomach cancer mortality rates for each of the 333 counties relative to the national average [9] were correlated with the county's altitude. The highest mortality rates are noted in the districts in the South of the country (e.g., 40 in Valdivia), with a decreasing gradient in the high desert Altiplano to the north and to a lesser extent to the “extreme” South, but with no association with altitude (Fig. 5). The area with the highest rates is located south of Santiago, an area with higher Amerindian (Mapuche) presence than in other parts of Chile. The main difference between the districts with high risk was a significantly higher prevalence of the H. pylori infection, mainly observed at young ages [9] (Fig. 6). These observations would support the hypothesis that race and ethnicity are important, as well as the age of acquisition of the infection [38]. In addition to a higher prevalence of H. pylori infection, these areas are of lower socioeconomic conditions. According to the 2009 National Health Survey, these populations have lower intake of fruit and vegetables and higher consumption of salt, nutritional risk factors for gastric cancer [39]. Thus, in Chile, the risk of gastric cancer may be more related to race and ethnicity, age of H. pylori acquisition and socio-economic determinants.
Fig. 5.
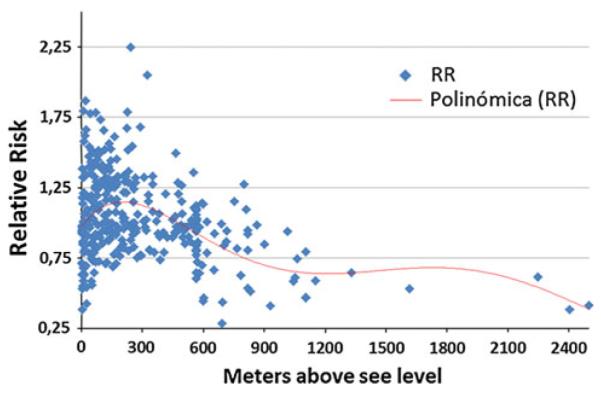
Relative risk of gastric cancer by altitude in Chilean counties, 1985–2002 adjusted by age, sex, and population size
Fig. 6.
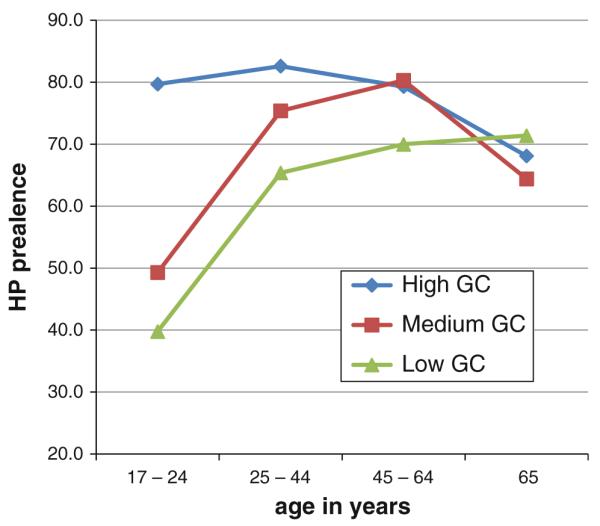
Seroprevalence of H. pylori in Chile is higher in regions with the higher risk for gastric cancer, particularly at young ages [9]
Conclusions
An association between altitude and gastric cancer incidence and mortality is observed in the countries of western Latin America along the Pacific rim. Altitude may be a surrogate for the clustering of host, bacterial, dietary, and environmental factors related to gastric cancer risk. The relation appears to be strongest in Central America and Andean South America. Exceptions are noted, such as in Mexico and Chile. The geographic variability offers the opportunity to better understand gastric cancer and focus prevention programs.
Acknowledgments
JT is a recipient of an exclusivity scholarship from Fundación IMSS, México. Portions of this study were grant supported in part by NIH CA1255884 (DRM).
References
- 1.Ferlay J, Shin HR, Bray F, Mathers C, Parkin DM. Globocan 2008 v1.2, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. International Agency for Research on Cancer; Lyon, France: 2010. http://globocan.iarc.fr. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5.Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, et al. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200. doi: 10.1097/CEJ.0b013e3282f0bff5. [DOI] [PubMed] [Google Scholar]
- 6.Ushijima T, Hattori N. Molecular pathways: involvement of H. pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res. 2012;18:923–929. doi: 10.1158/1078-0432.CCR-11-2011. [DOI] [PubMed] [Google Scholar]
- 7.Gobert AP, Asim M, Piazuelo MB, Verriere T, Scull BP, et al. Disruption of nitric oxide signaling by H. pylori results in enhanced inflammation by inhibition of heme oxygenase-1. J Immunol. 2011;187:5370–5379. doi: 10.4049/jimmunol.1102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro Lde P, Coelho LG. Helicobacter pylori in South America. Can J Gastroenterol. 1998;12:509–512. doi: 10.1155/1998/127352. [DOI] [PubMed] [Google Scholar]
- 9.Ferreccio C, Rollan A, Harris PR, Serrano C, Gederlini A, et al. Gastric cancer is related to early H. pylori infection in a high-prevalence country. Cancer Epidemiol Biomarkers Prev. 2007;16:662–667. doi: 10.1158/1055-9965.EPI-06-0514. [DOI] [PubMed] [Google Scholar]
- 10.Perfil Epidemiologico de los tumores malignos en Mexico. SINAIS/SINAVE/DGE/SALUD; Mexico, D.F., Mexico: 2011. [Google Scholar]
- 11.Piñeros M, Pardo C, Gamboa O, Hernandez G. Atlas de mortalidad por cancer en Colombia. 2nd edn. Instituto Geogragico Agustin Codazzi; Bogota: 2010. 2010. [Google Scholar]
- 12.Munoz N, Kato I, Peraza S, Lopez G, Carrillo E, et al. Prevalence of precancerous lesions of the stomach in Venezuela. Cancer Epidemiol Biomarkers Prev. 1996;5:41–46. [PubMed] [Google Scholar]
- 13.Centro de Control de Cancer Gastrointestinal Luis E Anderson 1980–2000. v.34 n.9 ed. INCI; Caracas, Venezuela: 2009. [Google Scholar]
- 14.Analysis of gastric cancer in Leon, Nicaragua 2006–2008. Nicaragua Ministry of Health; UNAN, Leon, HEODRA: 2009. [Google Scholar]
- 15.Registry CRNT, editor. Incidencia Cancer 1995–2005. San Jose, Costa Rica; 2006. http://www.ministeriodesalud.go.cr/index. php/component/ content/article/43pagina-inicio-ms/136-incidencias-cancer-2005-ms. [Google Scholar]
- 16.Morgan DR, Dominguez RL, Keku TO, et al. Gastric cancer and the synergy of selenium, host cytokine risk genotypes, and H. pylori infection in Central America. Gastroenterology. 2007;132(4, S2):A29. [Google Scholar]
- 17.Correa P, Cuello C, Duque E, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027–1035. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 18.Recavarren-Arce S, Ramirez-Ramos A, Gilman RH, Chinga-Alayo E, Watanabe-Yamamoto J, et al. Severe gastritis in the Peruvian Andes. Histopathology. 2005;46:374–379. doi: 10.1111/j.1365-2559.2005.02102.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan DR, Dominguez RL, Keku TO, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and H. pylori in Honduras. Clin Gastroenterol Hep. 2006;4:1103–1111. doi: 10.1016/j.cgh.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez R, Morgan DR. High incidence of gastric cancer in western Honduras. Fourth Internacional Gastric Cancer Congress; New York, NY: IGCA; 2001. p. A322. [Google Scholar]
- 21.Wilfley L, Dominguez R, Martin C, Heidt P, Morgan DR. The “Altitude Enigma” in gastric cancer inn Central America. In: Gastroenterology. 2007;132(4, S2):A345. [Google Scholar]
- 22.Rothammer F, Dillehay TD. The late pleistocene colonization ofnSouth America: an interdisciplinary perspective. Ann Hum Genet. 2009;73:540–549. doi: 10.1111/j.1469-1809.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 23.Andia ME, Hsing AW, Andreotti G, Ferreccio C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer. 2008;123:1411–1416. doi: 10.1002/ijc.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: a HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–270. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008;9:361–374. doi: 10.2174/138920208785699544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 27.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, et al. Phylogeographic origin of H. pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bravo LE, van Doom LJ, Realpe JL, Correa P. Virulence-associated genotypes of H. pylori: do they explain the African enigma? Am J Gastroenterol. 2002;97:2839–2842. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 29.Fox JG, Wang TC, Nagler-Anderson C. The African enigma: the parasite's perspective. Gut. 2001;49:156–157. doi: 10.1136/gut.49.1.156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal I, Ally R, Mitchell H. Gastric cancer in sub-Saharan Africa. Eur J Cancer Prev. 2001;10:479–482. doi: 10.1097/00008469-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Piazuelo MB, Camargo MC, Mera RM, Delgado AG, Peek RM, Jr, et al. Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol. 2008;39:1360–1369. doi: 10.1016/j.humpath.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whary MT, Sundina N, Bravo LE, Correa P, Quinones F, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to H. pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464–1469. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 33.Ryan JL, Morgan DR, Dominguez RL, Thorne LB, Elmore S, et al. High levels of epstein-barr virus DNA in latently infected gastric adenocarcinoma. Lab Invest. 2009;89:80–90. doi: 10.1038/labinvest.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabuto M, Imai H, Yonezawa C, Neriishi K, Akiba S, et al. Prediagnostic serum selenium and zinc levels and subsequent risk of lung and stomach cancer in Japan. Cancer Epidemiol Bio-markers Prev. 1994;3:465–469. [PubMed] [Google Scholar]
- 35.Camargo MC, Burk RF, Bravo LE, Piazuelo MB, Hill KE, et al. Plasma selenium measurements in subjects from areas with contrasting gastric cancer risks in Colombia. Arch Med Res. 2008;39:443–451. doi: 10.1016/j.arcmed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Vecchia C, Franceschi S. Nutrition and gastric cancer. Can J Gastroenterol. 2000;14(Suppl D):51D–54D. doi: 10.1155/2000/869862. [DOI] [PubMed] [Google Scholar]
- 37.Mora D. Evolucion de algunos aspectos epidemiologicos y ecologicos del cancer gastrico en Costa Rica. Rev Costarric Salud Publica. 2003;12(no. 21):7–17. [Google Scholar]
- 38.Camargo MC, Yepez MC, Ceron C, Guerrero N, Bravo LE, et al. Age at acquisition of H. pylori infection: comparison of two areas with contrasting risk of gastric cancer. Helicobacter. 2004;9:262–270. doi: 10.1111/j.1083-4389.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Russell RM. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66:237–249. doi: 10.1111/j.1753-4887.2008.00029.x. [DOI] [PubMed] [Google Scholar]