Abstract
Importance
The long-term effectiveness of Helicobacter pylori eradication programs for preventing gastric cancer will depend on recurrence risk and individual and community factors.
Objective
To estimate risk of H pylori recurrence and assess factors associated with successful eradication 1 year after treatment.
Design, Setting, and Participants
Cohort analysis of 1463 randomized trial participants aged 21 to 65 years from 7 Latin American communities, who were treated for H pylori and observed between September 2009 and July 2011.
Interventions
Randomization to 1 of 3 treatment groups: 14-day lansoprazole, amoxicillin, and clarithromycin (triple therapy); 5-day lansoprazole and amoxicillin followed by 5-day lansoprazole, clarithromycin, and metronidazole (sequential); or 5-day lansoprazole, amoxicillin, clarithromycin, and metronidazole (concomitant). Participants with a positive (13) C-urea breath test (UBT) 6 to 8 weeks posttreatment were offered voluntary re-treatment with 14-day bismuth-based quadruple therapy.
Measurements
Recurrent infection after a negative posttreatment UBT and factors associated with successful eradication at 1-year follow-up.
Results
Among participants with UBT-negative results who had a 1-year follow-up UBT (n=1091), 125 tested UBT positive, a recurrence risk of 11.5% (95% CI, 9.6%–13.5%). Recurrence was significantly associated with study site (P=.03), nonadherence to initial therapy (adjusted odds ratio [AOR], 2.94; 95% CI, 1.31–6.13; P=.01), and children in the household (AOR, 1.17; 95% CI, 1.01–1.35 per child; P=.03). Of the 281 with positive posttreatment UBT results, 138 completed re-treatment, of whom 93 tested UBT negative at 1 year. Among the 1340 who had a 1-year UBT, 80.4% (95% CI, 76.4%–83.9%), 79.8% (95% CI, 75.8%–83.5%), and 77.8% (95% CI, 73.6%–81.6%) had UBT-negative results in the triple, sequential, and concomitant groups, respectively (P=.61), with 79.3% overall effectiveness (95% CI, 77.1%–81.5%). In a single-treatment course analysis that ignored the effects of re-treatment, the percentage of UBT-negative results at 1 year was 72.4% (95% CI, 69.9%–74.8%) and was significantly associated with study site (P<.001), adherence to initial therapy (AOR, 0.26; 95% CI, 0.15–0.42; P<.001), male sex (AOR, 1.63; 95% CI, 1.25–2.13; P<.001), and age (AOR, 1.14; 95% CI, 1.02–1.27 per decade; P=.02). One-year effectiveness among all 1463 enrolled participants, considering all missing UBT results as positive, was 72.7% (95% CI, 70.3%–74.9%).
Conclusions and Relevance
One year after treatment for H pylori infection, recurrence occurred in 11.5% of participants who had negative posttreatment UBT results. Recurrence determinants (ie, nonadherence and demographics) may be as important as specific antibiotic regimen in determining the long-term success of H pylori eradication interventions. Study findings are relevant to the feasibility of programs for the primary prevention of gastric cancer in high-incidence regions of Latin America.
Trial Registration
clinicaltrials.gov Identifier: NCT01061437
Gastric adenocarcinoma is the second leading cause of cancer death worldwide.1 Although gastric cancer rates are declining in some areas, the number of deaths is expected to increase over the coming decades due to growing and aging populations in high-incidence regions such as Latin America and eastern Asia.2 Helicobacter pylori infects more than half of the world’s adult population, and chronic infection with this bacterium is the dominant risk factor for gastric cancer, accounting for an estimated two-thirds of all cases globally.3,4
In a randomized trial in Shandong, China, eradication of H pylori using amoxicillin and omeprazole reduced gastric cancer incidence by 39% over a 15-year period.5 If results of this and other trials are confirmed,6–9 focused community eradication programs may offer a promising approach for diminishing the enormous human and economic consequences of this cancer. The feasibility of large-scale programs is uncertain and success in specific populations will depend on the efficacy of the antibiotic regimen used and the risk of recurrent infection following eradication.10,11
We observed a cohort of patients enrolled in a randomized trial in 7 community populations in Latin America with moderate to high risk for gastric cancer to compare the short-term effectiveness of 3 regimens in eradicating H pylori.12,13 We previously reported the results of eradication therapy 6 to 8 weeks following randomization.14 One year after therapy, study participants were retested to determine risk of recurrent infection and to assess factors that influenced eradication effectiveness. We now present the key results of the 1-year follow-up.
METHODS
The trial sites and methods have been previously reported14 and were coordinated by SWOG, a federally funded cancer research cooperative group. In brief, men and women aged 21 to 65 years were recruited and screened for eligibility in 7 Latin American communities between September 2009 and June 2010. Potential participants were selected using a census of households (Colombia, Costa Rica, Nicaragua), a large public clinic registry (Chile), or household recruitment (Honduras and 2 sites in Mexico). Eligibility requirements included having no prior treatment for H pylori infection and no significant illness (eg, active cancer, other serious chronic illness).14 We explained the purpose and eligibility requirements of the study to potential participants and those who expressed an interest provided signed informed consent. The institutional review boards for each center and the SWOG statistical center approved the study protocol.14
H pylori infection was assessed using the (13) C-urea breath test (UBT) with a 75-mg oral dose of 13C-labeled urea, analyzed with infrared mass spectrometry (IRIS, Wagner Analysen Technik). A change in 13C carbon dioxide, relative to a baseline of 4.0% or greater, was considered positive. Serologic markers for the H pylori CagA protein (cytotoxin-associated gene A) were assessed by IgG antibodies in the study laboratory in Mexico (J.T.) by previously described methods.15 Standard instruments were used (including the Rome III Diagnostic Questionnaire for the assessment of baseline dyspepsia symptoms) to assess demographic factors, household conditions, and health history.16,17
Individuals who had positive UBT results and met other eligibility criteria were randomly assigned by a central computer to 1 of 3 treatment groups using a web-based dynamic randomization procedure that assured balance of sex, age, and study site across the 3 regimens. The treatments were: (1) triple therapy, given for 14 days of lansoprazole 30 mg, amoxicillin 1000 mg, and clarithromycin 500 mg; (2) sequential therapy, given for 5 days of lansoprazole 30 mg and amoxicillin 1000 mg, followed by 5 days of lansoprazole 30 mg, clarithromycin 500 mg, and metronidazole 500 mg; and (3) concomitant therapy, given for 5 days of lansoprazole 30 mg, amoxicillin 1000 mg, clarithromycin 500 mg, and metronidazole 500 mg.18,19 All medications were taken twice per day. The medications were generic and obtained from certified manufacturers. Treatment assignments were not blinded.
Participant follow-up was scheduled 6 to 8 weeks after randomization to include a UBT and assessment of adverse effects and adherence (defined as having taken ≥80% of each drug of the study regimen).14 Participants who had UBT-positive results at their follow-up visit were offered a voluntary 14-day retreatment regimen of standard quadruple therapy with twice-daily lansoprazole 30 mg, with tetracycline 500 mg, metronidazole 500 mg, and bismuth subsalicylate 524 mg (or bismuth subcitrate 420 mg), each taken 4 times per day.13,20 The protocol did not specify measures during re-treatment to encourage acceptance or adherence, or to assess adverse effects or re-treatment effectiveness. The 1-year follow-up examination, scheduled between 48 and 52 weeks following randomization for all participants, included a UBT and final questionnaire.
Statistical Considerations
The trial sample size of 1470 participants was chosen to provide a greater than 80% power to assess the first study aim—whether sequential therapy was superior to triple therapy and whether concomitant therapy was noninferior to triple therapy in terms of eradication success (UBT negativity) at the 6-to 8-week follow-up. This sample size was determined to be sufficient to address the 1-year study goals of estimating recurrence risk and eradication success. Specifically, we assumed a recurrence risk of as much as 10% based on prior studies in Latin America,21,22 and with projected sample sizes of 1000 and 1400 participants, the estimated probabilities of recurrence and overall effectiveness would have standard errors of 0.95% and 0.80%, respectively. Eradication success or failure was determined by UBT results. The term infection recurrence was used to identify participants who had UBT-negative results at the 6- to 8-week visit, but UBT-positive results at the 1-year visit; H pylori strain data were not available for differentiating recrudescence (same strain) and true reinfection (new strain).
Statistical analyses considered all participants as belonging to the treatment group to which they were assigned, regardless of adherence to their assigned regimens. Analyses of recurrence risk and treatment outcomes were based on participants who had a conclusive (definite) UBT result at the 1-year visit. Treatment outcome results are also presented using 2 additional approaches: (1) a 1-year intent-to-treat analysis in which participants without a follow-up UBT were considered as treatment failures (UBT positive); and (2) a single-treatment course analysis in which the effects of retreatment were ignored, ie, participants who had UBT-positive results at 6 to 8 weeks that became UBT negative at 1 year were considered still to be UBT positive. A strategy of retesting all participants and re-treating those with positive results shortly after initial eradication therapy may not be a cost-effective cancer prevention strategy,23 and the single-treatment course analysis represents the 1-year outcome of a strategy without re-treatment.
The 95% CIs for estimates of recurrence risk and treatment success were calculated using the binomial exact method, and P values for comparisons among the 3 treatment groups for these outcomes were based on the likelihood ratio χ2 test for independence. Univariate and multivariate logistic regression analyses were used to explore associations between participant characteristics and recurrence risk, initial treatment success, and 1-year treatment success. Multivariate models were adjusted for the effects of age, sex, and study center. P values for the standard logistic regression models were based on the Wald χ2 test statistic and were 2-sided without adjustment for multiplicity, and values less than .05 were considered statistically significant. Analyses were conducted using SAS version 9.2 and R version 2.12.
RESULTS
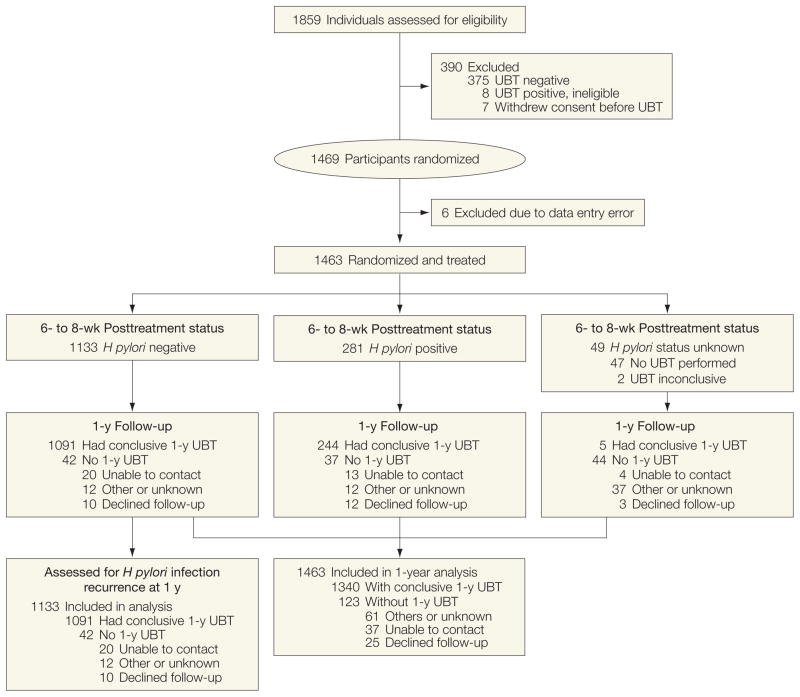
We identified 1859 adults who agreed to participate, of whom 1844 were potentially eligible with positive UBT results (Figure). Exclusions included 375 individuals (20.3%) because of negative UBT results, 7 withdrew consent, and 8 were ineligible on subsequent interviews. Six individuals with negative UBT results were incorrectly randomized due to data entry error and immediately withdrawn, leaving 1463 participants randomized to receive 1 of the 3 antibiotic regimens.
Figure.
Latin America Helicobacter pylori Eradication Trial Profile
Table 1 shows participants’ characteristics according to their treatment assignment and follow-up status: 59% were women, 55% older than 40 years, 84% were H pylori CagA positive, and 26% had chronic dyspepsia symptoms. Reported use of alcohol (8%, ≥1 drink/week) and tobacco (16%, ≥1 cigarette/d) was relatively infrequent. We obtained a conclusive UBT result at the posttreatment (6- to 8-week) visit from 1414 participants (96.7%) and from 1340 (91.6%) at the 1-year follow-up visit.
Table 1.
Participant Characteristics at Enrollment, Posttreatment, and 1-Year Follow-up
| UBT Status, No. (%)
|
|||||||
|---|---|---|---|---|---|---|---|
| Total | Posttreatment (6–8 wk)a
|
1 Year
|
|||||
| Negative | Positive | Unknown or Inconclusive | Negative | Positive | Unknown or Inconclusive | ||
| Total, No. | 1463 | 1133 | 281 | 49 | 1063 | 277 | 123 |
|
| |||||||
| Treatment regimen | |||||||
| 14-d Triple | 488 (33.4) | 401 (35.4) | 74 (26.3) | 13 (26.5) | 364 (34.2) | 89 (32.1) | 35 (28.5) |
|
| |||||||
| 10-d Sequential | 486 (33.2) | 372 (32.8) | 96 (34.2) | 18 (36.7) | 356 (33.5) | 90 (32.5) | 40 (32.5) |
|
| |||||||
| 5-d Concomitant | 489 (33.4) | 360 (31.8) | 111 (39.5) | 18 (36.7) | 343 (32.3) | 98 (35.4) | 48 (39.0) |
|
| |||||||
| Study site | |||||||
| Santiago, Chile | 209 (14.3) | 164 (14.5) | 31 (11) | 14 (28.6) | 145 (13.6) | 34 (12.3) | 30 (24.4) |
|
| |||||||
| Túquerres, Colombia | 212 (14.5) | 170 (15.0) | 41 (14.6) | 1 (2.0) | 146 (13.7) | 59 (21.3) | 7 (5.7) |
|
| |||||||
| Guanacaste, Costa Rica | 210 (14.4) | 178 (15.7) | 29 (10.3) | 3 (6.1) | 182 (17.1) | 21 (7.6) | 7 (5.7) |
|
| |||||||
| Copán, Honduras | 213 (14.6) | 186 (16.4) | 25 (8.9) | 2 (4.1) | 178 (16.7) | 26 (9.4) | 9 (7.3) |
|
| |||||||
| Obregón, México | 210 (14.4) | 148 (13.1) | 55 (19.6) | 7 (14.3) | 139 (13.1) | 52 (18.8) | 19 (15.4) |
|
| |||||||
| Tápachula, México | 210 (14.4) | 152 (13.4) | 55 (19.6) | 3 (6.1) | 147 (13.8) | 47 (17.0) | 16 (13.0) |
|
| |||||||
| León, Nicaragua | 199 (13.6) | 135 (11.9) | 45 (16.0) | 19 (38.8) | 126 (11.9) | 38 (13.7) | 35 (28.5) |
|
| |||||||
| Sex | |||||||
| Women | 861 (58.9) | 655 (57.8) | 190 (67.6) | 16 (32.7) | 614 (57.8) | 189 (68.2) | 58 (47.2) |
|
| |||||||
| Men | 602 (41.1) | 478 (42.2) | 91 (32.4) | 33 (67.3) | 449 (42.2) | 88 (31.8) | 65 (52.8) |
|
| |||||||
| Age, y | |||||||
| 21–29 | 246 (16.8) | 183 (16.2) | 52 (18.5) | 11 (22.4) | 166 (15.6) | 54 (19.5) | 26 (21.1) |
|
| |||||||
| 30–39 | 417 (28.5) | 323 (28.5) | 89 (31.7) | 5 (10.2) | 296 (27.8) | 96 (34.7) | 25 (20.3) |
|
| |||||||
| 40–49 | 377 (25.8) | 284 (25.1) | 80 (28.5) | 13 (26.5) | 278 (26.2) | 66 (23.8) | 33 (26.8) |
|
| |||||||
| ≥50 | 423 (28.9) | 343 (30.3) | 60 (21.4) | 20 (40.8) | 323 (30.4) | 61 (22.0) | 39 (31.7) |
|
| |||||||
| Adherence to initial therapy, pills returnedb | |||||||
|
| |||||||
| < 20% | 1319 (90.2) | 1081 (95.4) | 233 (82.9) | 5 (10.2) | 1017 (95.7) | 232 (83.8) | 70 (56.9) |
|
| |||||||
| ≥20% | 98 (6.7) | 39 (3.4) | 43 (15.3) | 16 (32.7) | 34 (3.2) | 41 (14.8) | 23 (18.7) |
|
| |||||||
| Baseline dyspepsia symptomsc | |||||||
| Present | 373 (25.5) | 278 (24.5) | 86 (30.6) | 9 (18.4) | 269 (25.3) | 85 (30.7) | 19 (15.4) |
|
| |||||||
| Absent | 1090 (74.5) | 855 (75.5) | 195 (69.4) | 40 (81.6) | 794 (74.7) | 192 (69.3) | 104 (84.6) |
|
| |||||||
| Posttreatment (6–8-wk) UBT status | |||||||
| Negative | 1133 (77.4) | 966 (90.9) | 125 (45.1) | 42 (34.1) | |||
|
| |||||||
| Positive | 281 (19.2) | 93 (8.7) | 151 (54.5) | 37 (30.1) | |||
|
| |||||||
| Unknown or inconclusive | 49 (3.3) | 4 (0.4) | 1 (0.4) | 44 (35.8) | |||
Abbreviation: (13) UBT, C-urea breath test.
The posttreatment UBT was obtained 6 to 8 weeks following treatment initiation.
Adherence was defined as having taken at least 80% of each drug of the study regimen. Adherence was not reported for 46 participants.
Dyspepsia symptoms persisting for at least 6 months as determined by the ROME III questionnaire and accompanying scoring algorithm. The SAS program used to score the questionnaires was obtained from http://www.romecriteria.org/rome_iii_sas/.
Infection Recurrence
Of the 1133 participants who were UBT negative following initial treatment, 1091 had a 1-year UBT result, of whom 125 had become UBT positive, a recurrence risk of 11.5% (95% CI, 9.6%–13.5%). The recurrence risk ranged from 6.8% in Costa Rica to 18.1% in Colombia. Recurrence at 1 year was significantly associated with study site (P =.03), number of children in the household (odds ratio [OR], 1.17; (95% CI, 1.01–1.35 per child; P = .03), and nonadherence to therapy (OR, 2.94; 95% CI, 1.31–6.13; P =.01), but not with treatment assignment (P =.63) (Table 2).
Table 2.
Helicobacter pylori Recurrence at 1 Year by Participant Characteristics and Treatment Regimen
| Positive, No./Total No. (%) | Adjusted | ||||
|---|---|---|---|---|---|
| OR (95% CI)a | P Valuea | ||||
| 1-year UBT status | 125/1091 (11.5) | ||||
| Treatment regimen 14-d Triple |
47/389 (12.1) | 1 [Reference] |
|
.63 | |
| 10-d Sequential | 36/356 (10.1) | 0.82 (0.51–1.30) | |||
| 5-d Concomitant | 42/346 (12.1) | 1.01 (0.64–1.58) | |||
| Study site Santiago, Chile |
21/154 (13.6) | 1.91 (0.95–3.92) |
|
.03 | |
| Túquerres, Colombia | 30/166 (18.1) | 2.45 (1.29–4.80) | |||
| Guanacaste, Costa Rica | 12/176 (6.8) | 0.83 (0.37.1.81) | |||
| Copán, Honduras | 16/181 (8.8) | 1 [Reference] | |||
| Obregón, México | 19/143 (13.3) | 1.56 (0.77–3.21) | |||
| Tápachula, México | 14/147 (9.5) | 1.13 (0.52–2.40) | |||
| León, Nicaragua | 13/124 (10.5) | 1.44 (0.65–3.15) | |||
| Sex Women |
81/633 (12.8) | 1 [Reference] |
|
.08 | |
| Men | 44/458 (9.6) | 0.70 (0.46–1.04) | |||
| Age, yb 21–29 |
22/174 (12.6) |
|
0.88 (0.74–1.04) | .13b | |
| 30–39 | 43/310 (13.9) | ||||
| 40–49 | 26/287 (9.4) | ||||
| ≥50 | 34/329 (10.3) | ||||
| Children in the householdb 0 |
34/332 (10.2) |
|
1.17 (1.01–1.35) | .03b | |
| 1–2 | 64/566 (11.3) | ||||
| ≥3 | 27/184 (14.7) | ||||
| Adherence, pills returnedc <20% |
114/1041 (11) | 1 [Reference] |
|
.01 | |
| ≥20% | 10/39 (25.6) | 2.94 (1.31–6.13) | |||
Abbreviations: OR, odds ratio; UBT, (13) C-urea breath test.
Statistic estimated from a logistic regression model that accounts for age (continuous), sex, and study center. CIs are based on the profile likelihood method. P values are based on the Wald χ2 test statistic. Statistics do not include missing values for children in the household (n=9) and adherence (n=11).
P value corresponds to a logistic regression model in which the association of interest was considered as a continuous variable (for age, per 10 years).
Adherence was defined as having taken at least 80% of each drug of the study regimen.
1-Year Outcomes
In the primary analysis of treatment effectiveness based on the 1340 participants with definitive 1-year UBT results, the estimated 1-year eradication success rate was 80.4% (95% CI, 76.4%–83.9%) for triple therapy, 79.8% (95% CI, 75.8%–83.5%) for sequential therapy, and 77.8% (73.6%–81.6%) for concomitant therapy (P =.61). Overall effectiveness was 79.3% (95% CI, 77.1%–81.5%; Table 3). Outcome of treatment effectiveness among study sites ranged from a higher level (87%–90%) in Costa Rica and Honduras to a lower level (71%–76%) in Colombia, Nicaragua, and in Obregón and Tapachula, Mexico. Women 21 to 44 years of age were significantly less likely to have eradication success at 1 year (72.3%; 95% CI, 68.0%–76.2%) when compared with women 45 to 65 years of age (82.8%; 95% CI, 78.2%–86.8%), and when compared with men who were both younger (82.1%; 95% CI, 77.3%–86.2%) and older (85.6%; 95% CI, 80.5%–89.9%).
Table 3.
Helicobacter pylori Eradication Success at 1 Year by Treatment Regimen and Analytic Approach
| Analytic Approach
|
||||||
|---|---|---|---|---|---|---|
| Definitive UBT (n = 1340)a
|
Single-Treatment Course (n = 1340)b
|
1-y ITT (n = 1463)c
|
||||
| Negative No./Total No. | % (95% CI)d | Negative No./Total No. | % (95% CI)d | Negative No./Total No. | % (95% CI)d | |
| Treatment regimen | ||||||
| 14-d Triple | 364/453 | 80.4 (76.4–83.9) | 342/453 | 75.5 (71.3–79.4) | 364/488 | 74.6 (70.5–78.4) |
|
| ||||||
| 10-d Sequential | 356/446 | 79.8 (75.8–83.5) | 323/446 | 72.4 (68.0–76.5) | 356/486 | 73.3 (69.1–77.1) |
|
| ||||||
| 5-d Concomitant | 343/441 | 77.8 (73.6–81.6) | 305/441 | 69.2 (64.6–73.4) | 343/489 | 70.1 (65.9–74.2) |
|
| ||||||
| Overall | 1063/1340 | 79.3 (77.1–81.5) | 970/1340 | 72.4 (69.9–74.8) | 1063/1463 | 72.7 (70.3–74.9) |
Abbreviations: UBT, (13) C-urea breath test; ITT, intention to treat.
Analysis includes all participants with a conclusive UBT result at the 1-year visit. P value=.61 based on a χ2 test.
Analysis includes all participants with a conclusive 1-year UBT result and assumes that those with UBT-positive results at 6 to 8 weeks were still positive at 1 year, statistically eliminating the effect of retreatment. P value=.11 based on a χ2 test.
Analysis includes all 1463 randomized participants and assumes that those lost to follow-up and without a 1-year UBT result are UBT positive. P value=.28 based on a χ2 test.
Eradication success is expressed as percent (95% CI). All 95% CIs are exact binomial.
Of participants with positive post-treatment UBT results, 244 of 281 returned for a 1-year examination. Of those who returned, 198 (81%) had accepted a prescription for re-treatment quadruple therapy but only 138 (57%) reported that they had completed the regimen; 37 (15%) refused re-treatment. The UBT result had become negative for 38% overall (93/244) and also for 54% of those who reported completing re-treatment (74/138). Of the individuals with UBT-positive results who declined retreatment, 4 of 46 had UBT-negative results at 1 year.
In a 1-year analysis that included all 1463 randomized participants, and that considered as treatment failures (UBT positive) the 123 individuals (8.4%) without a UBT result, treatment effectiveness estimates were 74.6% (95% CI, 70.5%–78.4%), 73.3% (95% CI, 69.1%–77.1%), and 70.1% (95% CI, 65.9%–74.2%) for the triple, sequential, and concomitant treatment groups, respectively, or about 7% lower than those in the analysis mentioned previously. (Table 3).
To explore the possible outcomes of a program without the retest and retreatment component at 6 to 8 weeks, we conducted the single-treatment course analysis that considered as treatment failures the 93 participants whose negative 1-year UBT had been preceded by a positive UBT result at 6 to 8 weeks. Results of this analysis showed an overall effectiveness of 72.4% (95% CI, 69.9%–74.8%; Table 3). Voluntary re-treatment tended to dilute differences in effectiveness among the treatment groups, and removing these effects yielded estimates of 75.5% (95% CI, 71.3%–79.4%), 72.4% (95% CI, 68.0%–76.5%), and 69.2% (95% CI, 64.6%–73.4%) for the triple, sequential, and concomitant therapy groups, respectively (P =.11).
Predictors of Treatment Success
In the logistic regression models of the posttreatment (6–8 weeks) and the 1-year (single-treatment course) outcomes (Table 4), significant associations were observed with study site, male sex, older age, and adherence to initial therapy. Having fewer children in the household was associated with the 1-year outcomes but not with 6- to 8-week outcome, while treatment assignment was significantly associated with 6- to 8-week outcome,14 but not with 1-year outcome. Other factors such as cigarette smoking, alcohol use, water source, sanitation, and baseline chronic dyspepsia were unrelated to outcome (eTable, available at http://www.jama.com).
Table 4.
Characteristics Associated With Helicobacter pylori Eradication Success Posttreatment and at 1 Year by Single-Treatment Analysis
| Posttreatment (6–8 Week) Success (n = 1414)a
|
1-Year Success Single-Treatment Course Analysis (n = 1340)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative No./Total No. (%) | Adjusted
|
Negative No./Total No. (%) | Adjusted
|
|||||||
| OR (95% CI)c | P Valuec | OR (95% CI)c | P Valuec | |||||||
| Treatment regimen | ||||||||||
| 14-d Triple | 401/475 (84.4) | 1 [Reference] |
|
.005 | 342/453 (75.5) | 1 [Reference] |
|
.09 | ||
|
|
|
|||||||||
| 10-d Sequential | 372/468 (79.5) | 0.70 (0.50–0.98) | 323/441 (72.4) | 0.84 (0.62–1.14) | ||||||
|
|
|
|||||||||
| 5-d Concomitant | 360/471 (76.4) | 0.58 (0.41–0.80) | 305/446 (69.2) | 0.71 (0.53–0.96) | ||||||
|
| ||||||||||
| Study site | ||||||||||
| Santiago, Chile | 164/195 (84.1) | 0.60 (0.33–1.07) |
|
<.001 | 134/179 (74.9) | 0.59 (0.36–0.97) |
|
<.001 | ||
|
|
|
|||||||||
| Túquerres, Colombia | 170/211 (80.6) | 0.51 (0.29–0.87) | 136/205 (66.3) | 0.42 (0.26–0.66) | ||||||
|
|
|
|||||||||
| Guanacaste, Costa Rica | 178/207 (86.0) | 0.75 (0.42–1.33) | 166/203 (81.8) | 0.96 (0.58–1.60) | ||||||
|
|
|
|||||||||
| Copán, Honduras | 186/211 (88.2) | 1 [Reference] | 165/204 (80.9) | 1 [Reference] | ||||||
|
|
|
|||||||||
| Obregón, México | 148/203 (72.9) | 0.37 (0.22–0.62) | 124/191 (64.9) | 0.45 (0.28–0.71) | ||||||
|
|
|
|||||||||
| Tápachula, México | 152/207 (73.4) | 0.36 (0.21–0.60) | 133/194 (68.6) | 0.50 (0.31–0.79) | ||||||
|
|
|
|||||||||
| León, Nicaragua | 135/180 (75.0) | 0.33 (0.19–0.57) | 112/164 (68.3) | 0.41 (0.25–0.67) | ||||||
|
| ||||||||||
| Sex | ||||||||||
| Women | 655/845 (77.5) | 1 [Reference] |
|
.004 | 553/803 (68.9) | 1 [Reference] |
|
.003 | ||
|
|
|
|||||||||
| Men | 478/569 (84.0) | 1.53 (1.14–2.05) | 417/537 (77.7) | 1.63 (1.25–2.13) | ||||||
|
| ||||||||||
| Age, y | ||||||||||
| 21–29 | 183/235 (77.9) |
|
1.14 (1.02–1.29) | .03d | 155/220 (70.5) |
|
1.14 (1.02–1.27) | .02d | ||
|
|
|
|||||||||
| 30–39 | 323/412 (78.4) | 268/392 (68.4) | ||||||||
|
|
|
|||||||||
| 40–49 | 284/364 (78.0) | 252/344 (73.3) | ||||||||
|
|
|
|||||||||
| ≥50 | 343/403 (85.1) | 295/384 (76.8) | ||||||||
|
| ||||||||||
| Children in the household | ||||||||||
| 0 | 343/411 (83.5) |
|
0.99 (0.89–1.11) | .87d | 298/390 (76.4) |
|
0.91 (0.82–1.00) | .05d | ||
|
|
|
|||||||||
| 1–2 | 586/745 (78.7) | 506/707 (71.6) | ||||||||
|
|
|
|||||||||
| ≥3 | 194/246 (78.9) | 157/232 (67.7) | ||||||||
|
| ||||||||||
| Adherence, pills returned | ||||||||||
| <20% | 1081/1314 (82.3) | 1 [Reference] |
|
<.001 | 929/1249 (70.4) | 1 [Reference] |
|
<.001 | ||
|
|
|
|||||||||
| ≥20% | 39/82 (47.6) | 0.23 (0.14–0.37) | 30/75 (30.6) | 0.26 (0.15–0.42) | ||||||
Abbreviations: OR, odds ratio; UBT, (13) C-urea breath test.
Analysis only includes participants with a conclusive UBT result at the 6- to 8-week visit after treatment initiation.
Analysis includes all participants with 1-year UBT results and assumes that those who were UBT positive at 6 to 8 weeks are still positive at 1 year, statistically eliminating the effect of retreatment.
Statistic estimated from a logistic regression model that accounts for age (continuous), sex, and study center. CIs are based on the profile likelihood method. P values are based on the Wald χ2 test statistic. Statistics do not include missing values for children in the household (n=12 at 6 to 8 weeks and n=11 at 1 year) and adherence (n=18 at 6 to 8 weeks and n=16 at 1 year).
In the regression models, the variables age (per 10 y) and number of children in the household were considered as continuous variables.
COMMENT
In our 1-year follow-up study from this randomized trial in 7 community populations in Latin America, the risk of recurrent H pylori infection following apparently successful eradication was 11.5%. Although triple therapy in our initial analyses had appeared to be superior to sequential and concomitant therapies at 6 to 8 weeks, there were only modest and nonsignificant differences in 1-year outcomes among the 3 treatment groups. Triple therapy succeeded in eradicating H pylori infection in 84.4% of participants who had a UBT 6 to 8 weeks posttreatment14 but its observed efficacy at 1 year was 80.4%, and its success was estimated to be 75.5% if the effects of re-treating participants whose initial treatment had failed were ignored. Significant predictors of successful eradication of H pylori infection at 1 year were study site, male sex, older age, and adherence to initial therapy.
Recurrence of Infection
The 11.5% 1-year recurrence risk observed in our trial is consistent with prior reports from Latin America and other low- and middle-income regions.21,24,25 In a review by Gisbert21 of more than 100 studies, the overall annual recurrence risk ranged from 3.4% (95% CI, 3.1%–3.7%) in high-income countries to 8.7% (95% CI, 8.8%–9.6%) in lower-income countries. In studies from Latin America with at least 50 person-years of follow-up,22,26–29 the 1-year recurrence risk ranged from 0% to 17.3%.28,29 In the largest prior study in Latin America, conducted in Colombia, the mean annual recurrence rate over 6 years of cohort follow-up was 5.4%.22 In our trial, the Colombia site had the highest recurrence risk (18.1%), and notably, the participants were recruited from the same region as in the aforementioned study. A high rate of recurrent infection was also seen in the eradication trial from Shandong,5 wherein UBT negativity in the group treated with amoxicillin-based treatment declined from 74% at 1 year to 47% by the seventh year.30 Nonetheless, participants randomized to eradication therapy in the Shandong trial had a statistically significant 39% decrease in gastric cancer incidence over a 14.7-year period of follow-up.5
H pylori recurrence in the first year following eradication seems likely to represent a mixture of recrudescent infection and reinfection, whereas reinfection dominates in subsequent years and the overall annual risk of recurrence tends to diminish.22,24,26 We found an association between recurrence with both medication nonadherence and study site (a possible marker of regional antibiotic resistance), suggesting that recrudescence was an important component of 1-year recurrence in the populations in this study.31 The borderline association between number of children in the household and recurrence suggests that an element of reinfection also occurred during the first year, consistent with previous reports wherein number of children was a risk factor for infection.32,33 Our finding that women who were between 21 and 44 years old were less likely to have successful 1-year eradication is also consistent with a risk of reinfection mediated through contact with young children. Differences in generic medications were unlikely to explain site differences because Honduras and Nicaragua used drugs from the same batch and manufacturer in Central America, yet they had disparate 1-year outcomes. Continued cohort follow-up should provide important insights.
Implications of 1-Year Outcomes
The observed 1-year outcomes of our study represent a mixture of the effects of initial eradication therapy, retreatment, recurrence, and participant and community characteristics. Although H pylori eradication programs may be cost effective, particularly in high-incidence areas,34–36 retesting and re-treating individuals shortly after initial eradication therapy may not be cost effective, especially when the probability of successful eradication with initial therapy is relatively high and the efficacy of re-treatment is modest.21 To simulate a program that did not include an early retest and retreatment stage, we conducted the single-treatment course analysis, which ignored the effects of voluntary retreatment. Our estimated 75.5% success rate for triple therapy in this analysis was not remarkably better than that for the other 2 regimens tested and the success of all 3 regimens without retreatment was comparable to what has been reported from prior eradication trials.5–7,10 Thus, while our data underscore the continued use of 14-day triple therapy in Latin America, from a program perspective they also point to the possible acceptability of a lower-cost regimen (eg, sequential therapy). Assessment of program effectiveness must also consider potential adverse outcomes such as adverse effects of treatment, rare serious events,37 and the potential contribution to community antimicrobial resistance.38 In low- and middle-income nations, the incremental effects of an eradication program on resistance are difficult to gauge given the prevalence of unsanitary conditions that facilitate spread of resistant bacteria and the common practice of self-prescription with over-the-counter antibiotics.39
In our current study, adherence, study site, sex, and age were significantly associated with the probability of a successful 1-year outcome. From the public health perspective, a “one size fits all” intervention strategy may not be optimal. For example, the fact that age-specific rates of gastric cancer incidence in women lag those of men by 10 to 15 years,1 coupled with the higher risk of recurrent infection in younger women, suggests that an eradication program could enroll men beginning at age 30 years but delay enrolling women until they reach 40 or 50 years of age. In general, programs will be more effective if tailored to the demographics and community ecology of their target populations.40
The feasibility and success of an H pylori eradication strategy for preventing gastric cancer focused on specific populations in high-risk regions will depend on the cancer risk in the target population, the prevalence of virulent H pylori strains, the probability of success of initial treatment, the risk of recurrent infection, and the per-person program cost of screening and eradication. Eradication programs seem likely to be cost effective if they prevent at least 10% of cancer deaths,41,42 a threshold that was exceeded in the Shangdong Intervention Trial5 and in a trial from Japan that randomized patients with resected gastric cancer to antibiotics or placebo and observed a statistically significant 64% decrease in risk of metachronous cancers over 3 years of follow-up.9 A combined analysis of the effects of 5 randomized trials of H pylori eradiation on gastric cancer incidence5 reported a pooled relative risk of 0.58 (95% CI, 0.42–0.81).5
Study Strengths and Limitations
Our trial was designed as a public health intervention with a vision toward future programs of H pylori eradication in high-risk areas of Latin America. The trial incorporated data from 7 heterogeneous community populations in 6 countries, noninvasive H pylori testing with UBT, generic medications purchased locally, and standard antimicrobial regimens. However, this public health approach had inherent limitations. Participants were recruited from the community and the results may not be generalizable to symptomatic patients requiring clinical evaluation. We also did not assess antibiotic resistance or gastric histology. Additionally, re-treatment of UBT-positive participants was voluntary, and thus, efficacy estimates with quadruple therapy are qualified.
CONCLUSIONS
In this large study in diverse community populations in Latin America, our results indicate that geographic site, demographic factors, adherence to initial therapy, and infection recurrence may be as important as the choice of antibiotic regimen in H pylori eradication interventions. Ongoing research initiatives are needed, given the expected increase in the gastric cancer burden in Latin America over the next 2 decades, evidence that H pylori infection is the dominant risk factor, and evidence that eradication reduces gastric cancer risk.
Acknowledgments
Funding/Support: The Bill & Melinda Gates Foundation (#43930) provided financial support for the trial, and the National Institutes of Health (NIH) (CA037429) supported the SWOG (Southwest Oncology Group) administrative and statistical infrastructure.
Role of the Sponsor: The Bill & Melinda Gates Foundation conducted an external scientific review of the proposal before approving it, and the investigators later incorporated some of the reviewers’ suggestions regarding the design of the study. The NIH was not directly involved in the study design, protocol execution, or analysis and reporting of data. The SWOG data coordinating center (CRAB) housed all data during the study and performed the data analyses. The decision to publish was made by the study investigators.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Contributions: We thank the trial participants for making this study possible and recognize the many contributions of the investigative team members.
Online-Only Material: The eTable is available at http://www.jama.com.
Author Contributions: Ms Sexton had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Morgan and Torres were both lead authors and contributed equally to this article.
Study concept and design: Morgan, Torres, Herrero, Greenberg, Ferreccio, Chey, Anderson, Baker.
Acquisition of data: Herrero, Salazar-Martínez, Bravo, Dominguez, Ferreccio, Lazcano-Ponce, Meza-Montenegro, E. Peña, R. Peña, Correa.
Analysis and interpretation of data: Morgan, Torres, Sexton, Herrero, Salazar-Martínez, Greenberg, Chey, Anderson, Crowley, Baker.
Drafting of the manuscript: Morgan, Torres, Greenberg, Anderson.
Critical revision of the manuscript for important intellectual content: Morgan, Torres, Sexton, Herrero, Salazar-Martínez, Greenberg, Bravo, Dominguez, Ferreccio, Lazcano-Ponce, Meza-Montenegro, E. Peña, R. Peña, Correa, Martínez, Chey, Valdivieso, Anderson, Goodman, Crowley, Baker.
Statistical analysis: Sexton, Anderson, Crowley.
Obtained funding: Baker, Crowley.
Administrative, technical, or material support: Morgan, Herrero, Greenberg, Dominguez, Lazcano-Ponce, Meza-Montenegro, E. Peña, Martínez, Valdivieso.
Study supervision: Greenberg.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Marugame T, Dongmei Q. Comparison of time trends in stomach cancer incidence (1973–1997) in East Asia, Europe and USA, from Cancer Incidence in Five Continents Vol. IV-VIII. Jpn J Clin Oncol. 2007;37(3):242–243. doi: 10.1093/jjco/hym014. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104(6):488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of anti-oxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92(23):1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 7.Wong BC, Lam SK, Wong WM, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291(2):187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 8.Saito D, Boku N, Fujioka T, et al. Impact of H pylori eradication on gastric cancer prevention: endoscopic results of the Japanese intervention trial ( JITHP study), a randomized multi-center trial [abstract 23] Gastroenterology. 2005;128(4 suppl 2):A4. doi: 10.1053/j.gastro.2005.04.003. [DOI] [Google Scholar]
- 9.Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 10.Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 11.Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005;54(6):735–738. doi: 10.1136/gut.2004.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 13.Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378(9790):507–514. doi: 10.1016/S0140-6736(11)60825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camorlinga-Ponce M, Torres J, Perez-Perez G, et al. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am J Gastroenterol. 1998;93(8):1264–1270. doi: 10.1111/j.1572-0241.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Morgan D, Squella F, Pena E, et al. Multinational validation of the Spanish Rome III questionnaire: comparable sensitivity and specificity to the English instrument [abstract M1350] Gastroenterology. 2010;138(5 suppl 1):S-386. doi: 10.1016/S0016-5085(10)61774-X. [DOI] [Google Scholar]
- 18.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104(12):3069–3079. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 19.Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14(2):109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105(1):65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert JP. The recurrence of Helicobacter pylori infection: incidence and variables influencing it: a critical review. Am J Gastroenterol. 2005;100(9):2083–2099. doi: 10.1111/j.1572-0241.2005.50043.x. [DOI] [PubMed] [Google Scholar]
- 22.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54(11):1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fendrick AM, Chernew ME, Hirth RA, Bloom BS, Bandekar RR, Scheiman JM. Clinical and economic effects of population-based Helicobacter pylori screening to prevent gastric cancer. Arch Intern Med. 1999;159(2):142–148. doi: 10.1001/archinte.159.2.142. [DOI] [PubMed] [Google Scholar]
- 24.Niv Y, Hazazi R. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter. 2008;13(1):56–61. doi: 10.1111/j.1523-5378.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 25.Parsonnet J. What is the Helicobacter pylori global reinfection rate? Can J Gastroenterol. 2003;17 (suppl B):46B–48B. doi: 10.1155/2003/567816. [DOI] [PubMed] [Google Scholar]
- 26.Rollan A, Giancaspero R, Fuster F, et al. The long-term reinfection rate and the course of duodenal ulcer disease after eradication of Helicobacter pylori in a developing country. Am J Gastroenterol. 2000;95(1):50–56. doi: 10.1111/j.1572-0241.2000.01700.x. [DOI] [PubMed] [Google Scholar]
- 27.Leal-Herrera Y, Torres J, Monath TP, et al. High rates of recurrence and of transient reinfections of Helicobacter pylori in a population with high prevalence of infection. Am J Gastroenterol. 2003;98 (11):2395–2402. doi: 10.1111/j.1572-0241.2003.07708.x. [DOI] [PubMed] [Google Scholar]
- 28.Silva FM, Navarro-Rodriguez T, Barbuti RC, Mattar R, Hashimoto CL, Eisig JN. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter. 2010;15(1):46–52. doi: 10.1111/j.1523-5378.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 29.Soto G, Bautista CT, Roth DE, et al. Gastrointestinal Physiology Working Group in Peru. Helicobacter pylori reinfection is common in Peruvian adults after antibiotic eradication therapy. J Infect Dis. 2003;188(9):1263–1275. doi: 10.1086/379046. [DOI] [PubMed] [Google Scholar]
- 30.Gail MH, Pfeiffer RM, Brown LM, et al. Garlic, vitamin, and antibiotic treatment for Helicobacter pylori: a randomized factorial controlled trial. Helicobacter. 2007;12(5):575–578. doi: 10.1111/j.1523-5378.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 31.Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther. 2006;23(2):275–280. doi: 10.1111/j.1365-2036.2006.02741.x. [DOI] [PubMed] [Google Scholar]
- 32.McCallion WA, Murray LJ, Bailie AG, Dalzell AM, O’Reilly DP, Bamford KB. Helicobacter pylori infection in children: relation with current household living conditions. Gut. 1996;39(1):18–21. doi: 10.1136/gut.39.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halitim F, Vincent P, Michaud L, et al. High rate of Helicobacter pylori reinfection in children and adolescents. Helicobacter. 2006;11(3):168–172. doi: 10.1111/j.1523-5378.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(5):875–885. doi: 10.1158/1055-9965.EPI-06-0758. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenberg A, Inadomi JM. Review article: medical decision models of Helicobacter pylori therapy to prevent gastric cancer. Aliment Pharmacol Ther. 1998;12(suppl 1):111–121. doi: 10.1111/j.1365-2036.1998.00001.x. [DOI] [PubMed] [Google Scholar]
- 36.Yeh JM, Kuntz KM, Ezzati M, Goldie SJ. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer. 2009;124(1):157–166. doi: 10.1002/ijc.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nawaz A, Mohammed I, Ahsan K, Karakurum A, Hadjiyane C, Pellecchia C. Clostridium difficile colitis associated with treatment of Helicobacter pylori infection. Am J Gastroenterol. 1998;93(7):1175–1176. doi: 10.1111/j.1572-0241.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- 38.Guzmán-Blanco M, Casellas JM, Sader HS. Bacterial resistance to antimicrobial agents in Latin America: the giant is awakening. Infect Dis Clin North Am. 2000;14(1):67–81. viii. doi: 10.1016/s0891-5520(05)70218-x. [DOI] [PubMed] [Google Scholar]
- 39.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5(1):18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):32–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 41.Mason J, Axon AT, Forman D, et al. Leeds HELP Study Group. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16(3):559–568. doi: 10.1046/j.1365-2036.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 42.Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348(9021):150–154. doi: 10.1016/s0140-6736(96)01501-2. [DOI] [PubMed] [Google Scholar]