Abstract
Desiccation-tolerant plants are able to withstand dehydration and resume normal metabolic functions upon rehydration. These plants can be dehydrated until their cytoplasm enters a ‘glassy state’ in which molecular mobility is severely reduced. In desiccation-tolerant seeds, longevity can be enhanced by drying and lowering storage temperature. In these conditions, they still deteriorate slowly, but it is not known if deteriorative processes include enzyme activity. The storage stability of photosynthetic organisms is less studied, and no reports are available on the glassy state in photosynthetic tissues. Here, the desiccation-tolerant moss Syntrichia ruralis was dehydrated at either 75% or <5% relative humidity, resulting in slow (SD) or rapid desiccation (RD), respectively, and different residual water content of the desiccated tissues. The molecular mobility within dry mosses was assessed through dynamic mechanical thermal analysis, showing that at room temperature only rapidly desiccated samples entered the glassy state, whereas slowly desiccated samples were in a ‘rubbery’ state. Violaxanthin cycle activity, accumulation of plastoglobules, and reorganization of thylakoids were observed upon SD, but not upon RD. Violaxanthin cycle activity critically depends on the activity of violaxanthin de-epoxidase (VDE). Hence, it is proposed that enzymatic activity occurred in the rubbery state (after SD), and that in the glassy state (after RD) no VDE activity was possible. Furthermore, evidence is provided that zeaxanthin has some role in recovery apparently independent of its role in non-photochemical quenching of chlorophyll fluorescence.
Key words: Darkness, desiccation tolerance, glassy state, long-term desiccation, molecular mobility, rapid desiccation, slow desiccation, Tortula, xanthophyll cycle.
Introduction
Desiccation-tolerant (DT) organisms are able to dry to relative water contents (RWCs) as low as 5% without dying and to resume normal metabolic functions upon rehydration (Gaff, 1997). In plants, this strategy is abundantly found in pollen, spores, and seeds in many algae and lichens. Desiccation tolerance also occurs in bryophytes and some pteridophytes, but in only very few angiosperms (Alpert, 2000; Oliver et al., 2000a). Cell viscosity drastically increases with falling WC, and at ~0.8–0.1g H2O g–1 dry weight (DW), tissues dried at room temperature enter the so-called glassy state. A glass is a highly viscous liquid that resembles a solid but maintains the disorder and physical properties of the liquid state (Franks et al., 1991) in which the molecular diffusion and the probability of chemical reactions are severely decreased. Glass formation depends on WC, temperature, and chemical composition, and has been thoroughly studied in seeds. As yet, no direct evidence for glass formation has been obtained in photosynthetic tissues (Buitink et al., 2002; Buitink and Leprince, 2008).
Molecular mobility is a key factor that determines tissue longevity in the dry state, but is experimentally difficult to assess in plants. Measurement of molecular mobility, mainly using seeds and pollen grains, has only been attempted using indirect methods, such as differential scanning calorimetry (DSC), electron paramagnetic resonance (EPR) spectroscopy, and Fourier transform infrared spectroscopy (FTIR); the limitations of these and other techniques are discussed by Leprince and Buitink (2007). However, Ballesteros and Walters (2011) have recently demonstrated that dynamic mechanical thermal analysis (DMTA) is a very accurate technique for directly measuring molecular mobility in dry seeds. DMTA assesses the structure and properties of solids and viscoelastic liquids via their dynamic and damping moduli. The DMTA imposes a sinusoidal stress on a sample and determines the sample modules G’, G’’, and tanδ as a function of temperature and/or frequency. Maximum loss in the scan is observed when the frequency of the motional process coincides with the impressed frequency. With an increase in measurement frequency the loss process is found at higher temperatures, where molecular motion is faster. Changes in motion occur in discrete steps called molecular relaxations.
Desiccation tolerance relies on the protection of cellular integrity during desiccation and on the repair of induced cellular damage upon rehydration. Responses of photosynthetic DT organisms triggered by water loss involve the down-regulation of photosynthesis-related genes (Bockel et al., 1998), the synthesis of desiccation-induced proteins (Alamillo and Bartels, 2001), and the up-regulation of antioxidant protection (Kranner, 2002). Among the photoprotective mechanisms, the xanthophyll cycle, or violaxanthin (V) cycle, is one of the best known. In the V-cycle, V is de-epoxidized to antheraxanthin (A) and then zeaxanthin (Z). The V-cycle generally represents one of the most important photoprotective mechanisms in terrestrial plants and in green and brown algae (Demmig-Adams, 1990), whereby excess light energy is dissipated as heat (thermal dissipation), which protects the photosynthetic apparatus from photodamage (Demmig-Adams and Adams, 1996). In addition, Z is thought to increase the efficiency of chlorophyll triplet quenching (Betterle et al., 2010) and to decrease the diffusion of molecular oxygen into domains containing photoactive pigments (Ruban and Johnson, 2010), with the consequent prevention of reactive oxygen species (ROS) production. Furthermore, Z can directly scavenge ROS (Havaux and Niyogi, 1999; Johnson et al., 2007; Dall’Osto et al., 2010), contributing to the stabilization of thylakoid membranes (Havaux, 1998; Kostecka-Gugala et al., 2003) under environmental stresses.
V-cycle activity is regularly observed in DT plants when the process of desiccation occurs in the light, and under light/dark cycles (Harker et al., 1999; Augusti et al., 2001; Kranner et al., 2002, 2003; Heber et al., 2006; Hooijmaijers, 2008). More recently it has been described that this process also occurs in the dark, activated solely by desiccation (Fernández-Marín et al., 2009, 2010, 2011). However, V de-epoxidation was only observed upon slow desiccation (SD), while rapid desiccation (RD) failed to activate the process (Fernández-Marín et al., 2010, 2011). Upon rewatering, the Z formed upon SD enhances thermal energy dissipation, estimated as non-photochemical quenching of chlorophyll fluorescence (NPQ) (Fernández-Marín et al., 2010), thereby contributing to photoprotection on rehydration.
The rate of desiccation that DT organisms can tolerate varies between species. However, fast drying is often more deleterious than slow drying, because some of the cellular and molecular responses necessary to maintain cell functionality need a threshold time to be developed (Oliver et al., 2000a; Gasulla et al., 2009; Pressel and Ducket, 2010; Fernández-Marín et al., 2011). Also, the length of time spent in the desiccated state determines the capacity to recover physiological functions (Kranner et al., 2002; Gasulla et al., 2009) and, importantly, the final WC affects the molecular mobility and the functional preservation of dry tissues.
In its natural environment, the DT moss Syntrichia ruralis is continuously subjected to desiccation–rehydration cycles, being able to survive following a wide range of desiccation rates (Platt et al., 1994; Fernández-Marín et al., 2011). Syntrichia ruralis possesses both a constitutive protection system and an efficient recovery mechanism to deal with the risks of dehydration (Wood et al., 1999; Oliver et al., 2000b). Being the model DT bryophyte (Oliver et al., 2000a; Dinakar et al., 2012), its responses to desiccation have been extensively studied at the molecular (Wood and Oliver, 1999; Zeng et al., 2002; Oliver et al., 2009) and physiological level (Tuba et al., 1996).
The main objectives of this work were: (i) to provide new insights into the molecular mobility in DT photosynthetic tissues under different regimes of desiccation; (ii) to analyse the effects of desiccation rate, final WC, and length of desiccation on V-cycle activity of S. ruralis; and (iii) to investigate further the role of Z formed in darkness on the recovery of photosynthetic activity during the rehydration.
Materials and methods
Plant material
Syntrichia ruralis Weber et D. Mohr (synonymous: Tortula ruralis) samples were collected in the field on grassland above lime stone in Alava (Basque Country). After collection, samples were kept in the dark at 100% relative humidity (RH) for 12h to allow tissues to regain full turgor and xanthophyll cycle pigments to be completely re-epoxidized to V prior to experimentation. The apical part of each caulid (shoot) containing photosynthetically active phyllids (leaves) was removed from the shoot immediately before experimentation (old phyllids become brown and photosynthetically inactive with age) and used for the analyses.
Desiccation methods
Desiccation was performed in hermetically closed chambers at two RHs. Low RH (<5%) was achieved by equilibrating samples over silica gel, resulting in RD. High RH (75%) was generated by equilibrating samples over a saturated solution of NaCl, leading to SD. The RWC of samples was estimated as follows:
| RWC (%)=(FWi–DW)/(TW–DW) ×100 |
where FWi is the weight at times (i) of desiccation; DW is the weight after drying for 60h in an oven at 80 ºC; and TW (turgor weight) is the weight of completely hydrated samples.
Rehydrated and control samples were kept at 100% RH in hermetically closed chambers above deionized water. Samples were weighed using a Perkin Elmer AD-4 Autobalance (USA) with 0.01mg accuracy.
Experimental design
Three different experiments were performed as follows.
Experiment A: short-term desiccation
To analyse the effects of short-term desiccation regimes on the physiology, ultrastructure, and molecular mobility of S. ruralis (objectives i and ii), moss samples were subjected to SD or RD in darkness for 24h. At time intervals of desiccation, RWC, F v/F m, V-cycle pigments, and tocopherol content were monitored (results shown in Figs 1 and 2). After 24h of desiccation, samples were also withdrawn for DMTA measurements and transmission electron microscopy (TEM) analyses (results shown in Figs 3 and 4). To test the influence of WC versus temperature on V-cycle activity of dry phyllids, two sets of samples were rapidly desiccated in darkness at either 30 ºC or 40 ºC, for 24h (inset, Fig. 3).
Fig. 1.
Changes in the RWC and F v/F m of S. ruralis during 24h of desiccation at two different rates: slow desiccation (SD) (filled circles) and rapid desiccation (RD) (open circles). Each point represents the mean ±SE (n=5). Asterisks above symbols represent significant differences between treatments (P < 0.05)
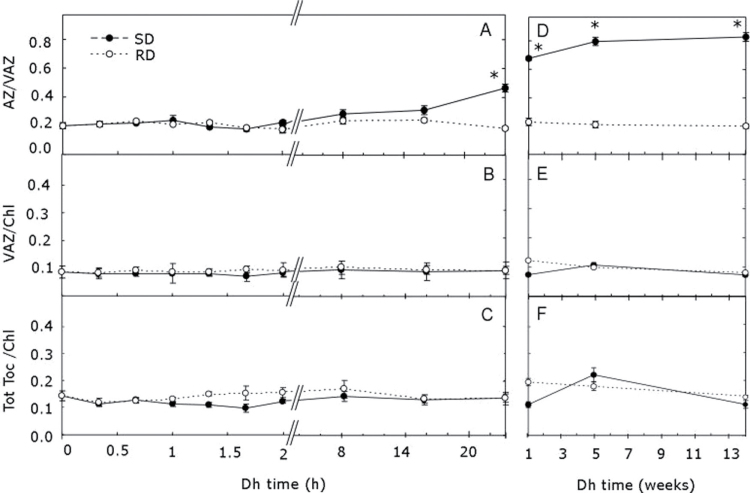
Fig. 2.
Variations in the V-cycle de-epoxidation ratio, total V-cycle pigments, and tocopherol content during slow (SD) (filled circles) and rapid desiccation (RD) (open circles). Samples were monitored during the first 24h of dehydration (A, B, and C) and in a separate experiment during 14 weeks (D, E, and F). No significant differences were detected at t 0 between samples from the two experiments in any of the parameters (data not shown). Each point represents the mean ±SE (n=5). Asterisks above symbols represent significant differences between treatments (P < 0.05)
Fig. 3.
Dynamic mechanical thermal analysis (DMTA) scans of S. ruralis phyllids after 24h of slow desiccation (SD) (black) or rapid desiccation (RD) (grey). Grey rectangles highlight the glass transitions (α-relaxation). Greek symbols denote α- and β-relaxations. Water content was 0.03±0.01 and 0.18±0.00g H2O g–1 DW in RD and SD samples, respectively. The inset depicts the AZ/VAZ content of control hydrated samples (t 0, white column), and of RD samples (grey columns) dehydrated at temperatures around the glass transition (30 ºC and 40 ºC).
Fig. 4.
Chloroplast ultrastructure and changes in thylakoid organization in S. ruralis leaves upon desiccation treatments. (A, B) Ultrastructure and detail of plastoglobules in hydrated samples (A) and after 24h of slow desiccation (B). (C, D) Details of chloroplast ultrastructure showing the stacking of thylakoids in hydrated samples (C), and after 24h of SD (D). (E) Number of plastoglobules per chloroplast of hydrated samples (white bars), after SD (black bars) and after RD (grey bars). (E, F, G) Stacking of thylakoids of hydrated samples (white bars), after SD (black bars) and after RD (grey bars). The numbers of stacks in which thylakoids were stacked in groups of two (E), three (F), or more than three (G) are shown. Each bar represents the mean ±SE (n ≥12 different cells). Letters above bars represent significant differences between treatments (P < 0.05)
Experiment B: illumination treatment prior to RD
To test the influence of the presence of Z synthesis upon rehydration (objective iii), a set of samples was illuminated at 400 μmol m–2 s–1 for 15min before desiccation to induce Z formation. Afterwards, these Z-containing samples were rapidly desiccated in darkness together with samples that had been kept in darkness throughout (and consequently lacked Z). Before desiccation, light-pre-treated samples were collected, and immediately frozen for pigment analysis. After 24h of desiccation, all other samples were rehydrated and F v/F m was monitored 10min, 30min, and 1h after the onset of rehydration (Fig. 5).
Fig. 5.
Effects of Z synthesis prior to desiccation on the recovery of F v/F m during rehydration of S. ruralis. Prior to desiccation, half of the samples were exposed to 400 μmol m–2 s–1 for 15min (white bars) and the other half were kept in darkness as a control (black bars). After that, all samples were rapidly desiccated in darkness. The F v/F m ratio was monitored 10min, 30min, and 1h after the start of rehydration. Bars represent the means ±SE (n=10). Letters indicate significant differences (P < 0.05). The inset shows changes of lipophilic antioxidants induced by short light pre-treatment: β-carotene, α-tocopherol, and AZ/VAZ. The asterisk indicates significant differences between light and dark treatments (P < 0.05)
Experiment C: long-term desiccation
To investigate the effects of long-term desiccation on V-cycle activity and survival (objective ii), samples from both SD and RD treatments were stored for 1, 5, or 14 weeks in darkness. V-cycle pigments and tocopherols were analysed after each period of desiccation (Fig. 2). The F v/F m ratio was monitored during rehydration in the dark (5min, 1, 24, and 96h after rehydration commenced). After 24h, samples were exposed to a day (using dim light of <10 μmol m–2 s–1)/night cycle (12/12h) for the next 3 d to allow recovery of metabolic function (results shown in Fig. 6).
Fig. 6.
Recovery of F v/F m during rehydration of S. ruralis phyllids following long-term desiccation. Samples were desiccated in darkness for 1, 5, or 14 weeks (A, B, and C, respectively) either slowly (SD; black bars) or rapidly (RD; white bars). Rehydration was performed in the dark for 24h. Thereafter, samples were exposed to dim light (<10 μmol m–2 s–1) under a day/night cycle (12/12h) for the next 3 d to allow metabolic functions to recover. Bars represents the means ±SE (n=5). Letters indicate significant differences between desiccation treatments and time points (P < 0.05)
Dynamic mechanical thermal analysis
Mechanical properties of S. ruralis phyllids were measured using a DMA/SDTA861e mechanical thermal analyser (Mettler Toledo, Switzerland) in the shear mode. Shear tests required the production of circular samples ≤2mm thick and 13mm in diameter. For that purpose, the samples were compressed in a hydraulic press using a pressure of 10 t. All tests were carried out in the dynamic mode, from –100 °C to 150 ºC at a heating rate of 2 ºC min–1. The frequencies used were 1, 3, and 10 Hz (results at 1 Hz only are shown), the deformation was 50 μm, and the maximal applied strength was 1 N. Each sample was scanned twice. Shear storage modulus (G’), shear loss modulus (G’’), and the loss tangent (tanδ = G’’/G’) were calculated using the Mettler Toledo STARTe software during DMTA scans.
Transmission electron microscopy analyses
TEM was used to compare the ultrastructure of hydrated mosses (as controls) with that of mosses after SD or RD in darkness for 24h. After desiccation, phyllids from samples subjected to SD or RD were allowed to rehydrate for 5min. Phyllids from the three treatments were pre-fixed with glutaraldehyde (3%) (Fluka/Sigma-Aldrich, Madrid, Spain), fixed with osmium tetroxide (1% OsO4) (Sigma-Aldrich, Madrid, Spain), and dehydrated with acetone. To enhance contrast, uranyl acetate (2%) was used. Later, tissues were embedded in Durcupan ACM resin (Fluka/Sigma-Aldrich). Semi-thin sections (3–5 μm) were sliced using a Leica ULTRACUT UCT ultra-microtome, stained with toluidine blue, and viewed with a Zeiss Axioskop light microscope. Pictures were captured with an Olympus OLY-220 camera. Ultra-thin sections (30–50nm) were cut with a Leica ULTRACUT UCT ultra-microtome and observed with a PHILIPS EM208S transmission electron microscope with a Morada digital camera. TEM images were analysed with ImageJ software. For thylakoid analysis, the middle sector of each chloroplast was considered. A straight line perpendicular to the thylakoid orientation was drawn in the centre of chloroplasts over the original photograph. Thylakoids on this virtual line were considered in the analysis.
HPLC analyses
For analyses of chlorophylls, carotenoids, and tocopherol, ~5mg per replicate of plant material was frozen in liquid N2 and stored at –80 ºC until use. Frozen samples were homogenized with a Tissue Tearor homogenizer (Model 395, Dremel, Mexico) in 0.5ml of pure acetone solution buffered with CaCO3. The extracts were centrifuged at 16 100 g for 20min, and supernatants were filtered through 0.2 μm PTFE filters (Teknokroma, Barcelona, Spain). The pigments were separated by HPLC on a reversed-phase C18 column (Waters Spherisorb ODS1, 4.6×250mm, Milford, MA, USA) and detected with a photodiode array detector, following the method of García-Plazaola and Becerril (1999, 2001). Tocopherol detection and quantification was conducted with a scanning fluorescence detector (SRD) Waters 474 operating in series with a photodiode array detector following García-Plazaola and Becerril (1999, 2001). The relative de-epoxidation state of the V-cycle pigments was estimated by the ratio (A+Z)/(V+A+Z), abbreviated AZ/VAZ.
Chlorophyll fluorescence
Chlorophyll a fluorescence was measured using a portable modulated fluorometer (OS 5-FL, Optisciences, Tyngsboro, MA, USA). The minimum chlorophyll fluorescence (F 0) was determined in dark-adapted mosses. The maximum chlorophyll fluorescence (F m) was induced with a saturating pulse. The variable chlorophyll fluorescence (F v) was calculated as F m–F 0. The ratio F v/F m represents the maximum photochemical efficiency of photosystem II (PSII).
Statistics
One-way analysis of variance (ANOVA) was used to test for differences among treatments in RWC, F v/F m, pigments, and tocopherol content after using the Levene test to check for homogeneity of variances. The Duncan post-hoc test was used to discriminate among different treatments. The Mann–Whitney U-test was used for non-normal data. All analyses were performed using the SPSS 17.0 statistical package.
Results
Physiological changes during desiccation
Changes in RWC, F v/F m, V-cycle pigments, and tocopherol contents during SD or RD are shown in Figs 1 and 2. The loss of water occurred significantly earlier and faster during RD than in SD samples, and the final RWC (%) was lower (1.4±0.4 versus 9.2±0.3) (Fig. 1A). Concomitantly, F v/F m values decreased with RWC, dropping faster in RD samples (Fig. 1B). The final F v/F m after 24h of desiccation was close to zero, with no significant differences between SD and RD. Conversely, the de-epoxidation ratio of xanthophyll cycle pigments (expressed as AZ/VAZ) remained constant for most of the experiment (Fig. 2A), but after 24h increased only upon SD, reaching values significantly higher (AZ/VAZ=0.47±0.03) than upon RD. Interestingly, this activation of the V-cycle in RD samples occurred several hours after the minimum RWC was reached (Fig. 1A) and increased until the fifth week of incubation, reaching values of 0.85±0.02 (three times higher than before the start of desiccation) (Fig. 2D). Neither VAZ content nor tocopherol showed any significant change during the RD or SD durng the 14 weeks of incubation (Fig. 2B–F).
Analysis of molecular mobility in desiccated samples
To study the transitions (or molecular relaxations) obtained in the mechanical dynamic behaviour of samples desiccated for 24h, the variation of tanδ was plotted versus temperature (Fig. 3). Tanδ scans showed two main transitions for both treatments (β- and α-relaxations, Fig. 3). The first (at lower temperature) corresponds to a β-transition associated with the motion of short chains and pendant chains in the macromolecular structure, whereas the α-relaxation is attributed to glass transition and corresponds to the activation of relatively long-range motions involved in glass transition and, therefore, to the alteration of the system from a rigid into a fluid state. α-Transition occurred at a lower temperature range after SD at 75% RH compared with RD at 5% RH (α-transition took place at 10–30 ºC upon SD and at 20–50 ºC upon RD). Thus, at room temperature (20 ºC), SD samples were in the rubbery state. In contrast, after RD, samples at room temperature were in the glassy state. To study which of the main factors involved in glass formation (WC or temperature) was impeding the enzymatic conversion of V into Z upon RD (Fig. 2A, D), S. ruralis samples were rapidly desiccated in the dark at either 30 ºC or 40 ºC (inset of Fig. 3). DMTA scans revealed that rapidly desiccated samples were in a glassy state and no change in AZ/VAZ was observed (inset of Fig. 3). This indicates that the lack of water was limiting enzymatic reactions in the samples, which could not be induced by higher temperatures that would increase the speed of chemical reactions generally.
Ultrastructural changes in chloroplasts
TEM analysis showed significant changes in the ultrastructure of chloroplasts after SD only (Fig. 4). SD samples showed an increase in the number of plastoglobules per chloroplast (Fig. 4A, B, E). Similarly, changes in the stacking of thylakoids were also observed after SD (Fig. 4C, D, F–H). Stacking of thylakoids in groups of two was significantly more frequent after SD than in either the hydrated samples or in samples after RD (Fig. 4F). Stacking in groups of more than three thylakoids was significantly less frequent (Fig. 4H).
Effects of the presence of zeaxanthin upon recovery
To analyse the effect of Z on the recovery of physiological function upon rehydration, S. ruralis samples were rapidly desiccated in the dark; half of them were briefly illuminated to induce Z synthesis, while the other half of samples were kept in darkness (Fig. 5). Before desiccation, the effects of the short illumination pre-treatment on the main lipophilic photoprotective and antioxidant molecules present in thylakoid membranes were tested to discard any effect on the subsequent recovery of S. ruralis due to their synthesis: α-tocopherol, β-carotene, and lutein (inset of Fig. 5). The only observed effect of light pre-treatment was the de-epoxidation of V with concomitant formation of A+Z (Fig. 5 inset). After RD in darkness (24h), F v/F m was monitored at different times during rehydration also in darkness (Fig. 5). Interestingly, from the first 10min of rehydration, pre-illuminated samples (which had more A+Z available during desiccation) showed significantly higher F v/F m values than dark-pre-treated mosses. Furthermore, F v/F m rose significantly during the first hour of rehydration in light-pre-treated samples, whereas it remained constant in the dark-pre-treated samples. Thus, the recovery of photosynthetic performance was faster and better in light-pre-treated samples in which Z was present during desiccation, confirming a protective role for Z independent of the thermal energy dissipation.
Long-term desiccation and Z formation effects on survival
The effect of both SD and RD in combination with longer time periods spent in the desiccated state on S. ruralis survival was also studied (Fig. 6). The recovery of maximal photochemical efficiency (F v/F m) upon rehydration was analysed to assess the survival capability of S. ruralis after desiccation periods of 1, 5, and 14 weeks (Fig. 6A, B, and C, respectively). The longer the storage of samples in the dry state, the worse was the recovery of F v/F m. Maximum F v/F m reached values >0.7 after 96h of rehydration in samples dried for 1 week (Fig. 6B), whereas it remained below 0.5 in samples dried for 14 weeks (Fig. 6D). The two desiccation treatments also affected the recovery. Although no differences were observed after 1 week (Fig. 6B), samples recovered faster from RD than from SD (Fig. 6C, D) after longer periods in the dry state.
Discussion
V-cycle activity, molecular mobility, and ultrastructural changes upon desiccation
As reviewed by Proctor et al. (2007), dehydration and rehydration events in DT plants involve a highly organized series of cellular changes. Whilst tissue WC will critically influence the speed of chemical reactions, it is not understood at which WC enzymatic activity ceases. Seeds also deteriorate when kept in the glassy state at subzero temperatures, and it is unclear which chemical reactions contribute to viability loss. Transcription has been reported to occur in dry seeds (Bove et al., 2005; Leubner-Metzger, 2005; Leymarie et al., 2007), potentially facilitated by the existence of localized regions of higher WC. While several investigations have characterized the molecular mobility in dry seeds, this study is the first to report on the molecular mobility in dry leaves together with ultrastructural changes and V-cycle activity. The V-cycle activity was chosen for this study, whereby the conversion from V to Z cannot occur randomly, but depends upon the activity of violaxanthin de-epoxidase (VDE), and the findings could have potential relevance for stress tolerance mechanisms in DT organisms.
In the woody DT angiosperm Myrothamnus flabellifolia, V-cycle activity was observed during prolonged desiccation by withholding water under a day/night cycle, and most V was converted at leaf water potentials between –5MPa and –18MPa (Kranner et al., 2002). In another DT angiosperm, Ramonda serbica, Augusti et al. (2001) also observed a sharp increase in the AZ/VAZ upon desiccation, followed by a subsequent decrease at very low RWC. In the present study, in accordance with previous work (Fernández-Marín et al., 2009, 2010, 2011), the AZ/VAZ ratio in S. ruralis increased only after SD that resulted in a higher final RWC of 9.2% compared with RD that led to faster dehydration with a final RWC of 1.4%. The possibility of de novo Z synthesis under these conditions (instead of conversion of V to Z) was rejected in previous studies (Fernández-Marín et al., 2009, 2010, 2011) and confirmed here by the lack of variation in the total VAZ pool (Fig. 2B, E).
The key factors which trigger the metabolic changes that prepare DT plants to survive in the desiccated state are still unknown. Early changes in cell turgor, cell wall tension, and solute concentration are thought to be involved (Bartels et al., 2007). If this were true, a fast decrease of RWC upon RD could lead to an early de-epoxidation of V to A and then to Z. However, in S. ruralis, RD did not trigger V-cycle activity at all, whereas SD did (Fig. 2A). Intermediate speeds of dehydration in darkness at 55% RH induce an increase in AZ/VAZ lower than that of SD (Fernández-Marín et al., 2009). Hence, both the speed and extent of drying apparently influence the activity of VDE. It is proposed that upon RD, the rapid transition into the glassy state limited VDE activity. No change in the AZ/VAZ was observed upon and following RD when the mosses were kept at a RWC of 1.4% for 14 weeks (Fig. 2D). Upon SD (that led to 9.2% RWC), V de-epoxidation, and thus enzyme activity, was observed (Fig. 1A), and this enzymatic activity was maintained for 5 weeks in the dry state at 20 ºC (Fig. 2D).
Thermal analysis with DMTA showed that at room temperature (20 ºC) rapidly desiccated moss tissues were in the glassy state, and slowly desiccated tissues were not. Between temperatures of 20 ºC and 50 ºC, α-relaxation occurred, which is characteristic of the transition from a glass into a liquid (Fig. 3). When S. ruralis samples were subjected to RD at 30 °C or 40 ºC, no V-cycle activity was observed (Fig. 3, inset), indicating that the low WC (0.03g H2O g–1 DW) was still too low for enzyme activity to occur, and that increasing the speed of chemical reactions at higher temperatures did not suffice to re-instate enzyme activity. Furthermore, additional DMTA scans revealed that RD samples dehydrated only for 40min (at which point their WC was similar to that of SD at 24h of dehydration, Fig. 1A) had already reached the glassy state (Supplementary Fig. S1 available at JXB online). This reinforces the view that the speed of dehydration has a profound impact on the physiological processes associated with the loss of water. The glass transition temperature was lower in mosses subjected to SD, with a final WC of 0.18g H2O g–1 DW, probably due to a plasticizing effect, whereby water molecules are placed among macromolecular chains, leading to an increase in the free volume. Such a plasticizing effect of water has been reported in many studies on the thermo-mechanical properties of biopolymer-based films (Slade and Levine, 1991; Kalichevsky et al., 1992; Gontard and Ring, 1996; Biliaderis et al., 1999; Kristo and Biliaderis, 2007). Thus, the scans obtained from SD samples were consistent with a less viscous (rubbery) state at room temperature (Fig. 3) in which enzymatic reactions are still possible at slow rates (Fig. 2A).
Ultrastructural changes were also observed upon SD of S. ruralis, including more plastoglobules and less stacking of thylakoids (Fig. 4), indicating regulated ultrastructural changes inside the chloroplasts in the rubbery state. Increased numbers of plastoglobules have been related to various stress factors such as excess light, drought, senescence, and nutrient deficiency (Bréhélin et al., 2007), and unstacking of thylakoids has been observed under light stress (Sharma et al., 1996). In a very recent work, Krupijnska et al. (2012) have related an increased number of single or pairwise thylakoids (occurring in parallel with the reduction in large grana stacks) to a reduced risk of photoinhibition during the ageing of barley chloroplasts. Plastoglobules are also involved in the synthesis, the dismantling, and the repair of thylakoid membranes (Smith et al., 2000; Bréhélin et al., 2007). Of the very rare reports on plastoglobules in DT plants, accumulation of plastoglobules upon desiccation has been described in DT aeroterrestrial algae (Holzinger et al., 2011), moss protonemata (Pressel and Duckett, 2010), and tracheophytes (Tuba et al., 1993). Tuba et al. (1993) also found an inverse relationship between thylakoid stacking and the number of plastoglobules during desiccation and rehydration cycles. In S. ruralis, reorganization of thylakoids and concomitant accumulation of plastoglobules were only observed upon SD, again indicating a capability for programmed re-structuring and adjustment of the photosynthetic apparatus, which failed upon RD.
Putative functions of Z in desiccated S. ruralis
With duration of time spent in the desiccated state, viability is gradually lost, and this coincides with the failure of the antioxidant system (Kranner et al., 2002; Varghese et al., 2011). Accordingly, following long-term storage in the desiccated state, the ability of S. ruralis upon rehydration to recover its maximal photochemical efficiency of PSII progressively decreased (Fig. 6A–C). This decrease occurred in both SD and RD samples, despite the higher content of Z retained in the SD samples (Fig. 1D). However, in DT plants, the Z accumulated during desiccation correlates with the improvement of photosynthetic performance upon rehydration through the enhancement of non-photochemical energy dissipation (Fernández-Marín et al., 2010). Furthermore, Z seems to play a fundamental role in the photoprotection of hydrated DT mosses (Yamakawa et al., 2012).
To elucidate the roles of Z further, two sets of rapidly desiccated samples were prepared that differed in their Z content (induced by brief illumination prior to desiccation) (Fig. 5) but not in the content of the other main lipophilic antioxidants (α-tocopherol, β-carotene, and lutein; inset of Fig. 5). The Z-containing samples recovered photochemical efficiency significantly faster and more completely (Fig. 5). As desiccation and recovery took place in darkness, the well-known role of Z in photoprotection as an NPQ modulator (Demmig-Adams and Adams, 1996) cannot be responsible for the better recovery. Other functions of Z include a role as an antioxidant (Havaux et al., 2007; Dall’Osto et al., 2010), and in the preservation of thylakoid structure (Sarry et al., 1994; Havaux, 1998; Kostecka-Gugala et al., 2003). Importantly, during desiccation of DT plants, movement of a part of the antenna complexes from PSII to PSI has been reported (Georgieva et al., 2009). Furthermore, state transitions have been proposed to play a role during desiccation (Chakir and Jensen, 1999). Clearly, the capability to tolerate stressful conditions requires structural reorganization of the photosynthetic membranes, also involving movements of the light-harvesting complex II (LHCII) inside thylakoids, which are dissociated from PSII and aggregated (Johnson et al., 2011). Very recently it has been shown that these protective structural changes are enhanced by the de-epoxidation of V to Z (Johnson et al., 2011). Hence, in DT plants, Z could play a fundamental role in the reorganization of the photosynthetic apparatus upon desiccation and rehydration.
In conclusion, this study shows that the enzymatic activity of VDE is impeded in the glassy state. In addition, both WC and speed of dehydration contribute to the ability of S. ruralis to cope with drying. Concomitantly, reorganization of the photosynthetic apparatus also failed in the glassy state. In contrast, enzyme activity and reorganization of the photosynthetic apparatus was possible in the rubbery state. Furthermore, the results provide evidence for a role for Z independent of light.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Dynamic mechanical thermal analysis (DMTA) scans of S. ruralis phyllids after 40min of rapid desiccation (RD).
Acknowledgements
We would like to thank Dr Javier Martinez-Abaigar for help regarding bryological questions, Dr Jorge Lago for useful comments, and John Adams (RBG Kew) for technical assistance. Technical and human support provided by SGIker (UPV/EHU, MICINN, GV/EJ, ESF) is gratefully acknowledged. FM received a fellowship from the Basque Government. BFM received a fellowship from the Basque Government, and two post-doctoral fellowships from the Research Vicerrectorate of the UPV/EHU (Grant for recent PhDs and Grant for specialization of researchers). The work was also supported by the Spanish Ministry of Education and Science [BFU 2010–15021] and by the Basque Government [UPV/EHU-GV IT-299-07]. The Royal Botanic Gardens, Kew, receive grant-in-aid from DEFRA.
Glossary
Abbreviations:
- A
anteraxanthin
- DT
desiccation-tolerant
- DMTA
dynamic mechanical thermal analysis
- DSC
differential scanning calorimetry
- DW
dry weight
- EPR
electron paramagnetic resonance
- F0
minimum chlorophyll fluorescence yield
- Fm
maximum chlorophyll fluorescence
- FTIR
Fourier transform infrarred spectro scopy
- Fv
variable chlorophyll fluorescence
- Fv/Fm
maximal photochemical efficiency of PSII
- FW
fresh weight
- R
rehydration
- RD
rapid desiccation
- RH
relative humidity
- RWC
relative water content
- SD
slow desiccation
- TEM
transmission electron microscopy
- TW
turgor weight
- V
viola-xanthin
- VAZ
violaxanthin+antheraxanthin+zeaxanthin
- VDE
violaxanthin de-epoxidase
- Z
zeaxanthin
- ZE
zeaxanthin epoxidase.
References
- Alamillo JM, Bartels D. 2001. Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantantagineum. Plant Science 160, 1161–1170. [DOI] [PubMed] [Google Scholar]
- Augusti A, Scartazza A, Navari-Izzo F, Sgherri CLM, Stevanovic B, Brugnoli E. 2001. Photosystem II photochemical efficiency, zeaxanthin and antioxidant contents in the poikilohydric Ramonda serbica during dehydration and rehydration. Photosynthesis Research 67, 79–88. [DOI] [PubMed] [Google Scholar]
- Alpert P. 2000. The discovery, scope, and puzzle of desiccation-tolerance in plants. Plant Ecology 151, 5–17. [Google Scholar]
- Ballesteros D, Walters C. 2011. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. The Plant Journal 68, 607–619. [DOI] [PubMed] [Google Scholar]
- Bartels D, Phillips J, Chandler J. 2007. Desiccation tolerance: gene expression, pathways, and regulation of gene expression. In: Jenks MA, Wood AJ, eds. Plant desiccation tolerance. Iowa: Blackwell Publishing, 115–150. [Google Scholar]
- Betterle N, Ballottari M, Hienerwadel R, Dall’Osto L, Bassi R. 2010. Dynamics of zeaxanthin binding to the Photosystem II monomeric antenna protein Lhcb6 (CP24) and modulation of its photoprotection properties. Archives of Biochemistry and Biophysics 504, 67–77. [DOI] [PubMed] [Google Scholar]
- Biliaderis CG, Lazaridou A, Arvanitoyannis I. 1999. Glass transition and physical properties of polyol-plasticized pullulan–starch blends at low moisture. Carbohydrate Polymers 40, 29–47. [Google Scholar]
- Bockel C, Salamini F, Bartels D. 1998. Isolation and characterization of genes expressed during early events of the dehydration process in the resurrection plant Craterostigma plantagineum. Journal of Plant Physiology 152, 158–166. [Google Scholar]
- Bove J, Lucas P, Godin B, Oge L, Jullien M, Grappin P. 2005. Gene expression analysis by cDNA-AFLP highlights a set of new signaling networks and translational control during seed dormancy breaking in Nicotiana plumbaginifolia. Plant Molecular Biology 57, 593–612. [DOI] [PubMed] [Google Scholar]
- Bréhélin C, Kessler F, Wijk KJ. 2007. Plastoglobules: versatile lipoprotein particles in plastids. Trends in Plant Science 12, 260–266. [DOI] [PubMed] [Google Scholar]
- Buitink J, Hoekstra FA, Leprince O. 2002. Biochemistry and biophysics of tolerance systems. In: Black M, Pritchard HW, eds. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing, 293–318. [Google Scholar]
- Buitink J, Leprince O. 2008. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biologies 331, 788–795. [DOI] [PubMed] [Google Scholar]
- Chakir S, Jensen M. 1999. How does Lobaria pulmonaria regulate photosystem II during progressive desiccation and osmotic water stress? A chlorophyll fluorescence study at room temperature and at 77 K. Physiologia Plantarum 105, 257–265. [Google Scholar]
- Dall’Osto L, Cazzaniga S, Havaux M, Bassi R. 2010. Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Molecular Plant 3, 576–593. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. 1990. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochimica et Biophysica Acta 1020, 1–24. [Google Scholar]
- Demmig-Adams B, Adams WW., III 1996. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science 1, 21–26. [Google Scholar]
- Dinakar C, Djilianov D, Bartels D. 2012. Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defence. Plant Science 182, 29–41. [DOI] [PubMed] [Google Scholar]
- Fernández-Marín B, Balaguer L, Esteban R, Becerril JM, García-Plazaola JI. 2009. Dark induction of the photoprotective xanthophyll cycle in response to dehydration. Journal of Plant Physiology 166, 1734–1744. [DOI] [PubMed] [Google Scholar]
- Fernández-Marín B, Becerril JM, García-Plazaola JI. 2010. Unravelling the roles of desiccation-induced xanthophyll cycle activity in darkness: a case study in Lobaria pulmonaria. Planta 231, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Fernández-Marín B, Míguez F, Becerril JM, García-Plazaola JI. 2011. Dehydration-mediated activation of the xanthophyll cycle in darkness: is it related to desiccation tolerance? Planta 243, 579–588. [DOI] [PubMed] [Google Scholar]
- Franks F, Hatley RHM, Mathias SF. 1991. Materials science and the production of shelf-stable biologicals. Biopharm—the Technology and Business of Biopharmaceuticals 4, 38–42. [Google Scholar]
- Gaff DF. 1997. Mechanisms of environmental stress resistance in resurrection vascular plants. In: Basra AS, Basra RK, eds. Mechanisms of environmental stress resistance in plants. Harwood Academic Publishers, 43–58. [Google Scholar]
- García-Plazaola JI, Becerril JM. 1999. A rapid HPLC method to measure lipophilic antioxidants in stressed plants: simultaneous determination of carotenoids and tocopherols. Phytochemical Analysis 10, 307–313. [Google Scholar]
- García-Plazaola JI, Becerril JM. 2001. Seasonal changes in photosynthetic pigments and antioxidants in beech (Fagus sylvatica) in a Mediterranean climate: implications for tree decline diagnosis. Australian Journal of Plant Physiology 28, 225–232. [Google Scholar]
- Gasulla F, Gómez de Nova P, Esteban-Carrasco A, Zapata JM, Barreno E, Guéra A. 2009. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: alternative and classical protective mechanisms. Planta 231, 195–208. [DOI] [PubMed] [Google Scholar]
- Georgieva K, Roding A, Buchel C. 2009. Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. Journal of Plant Physiology 166, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Gontard N, Ring S. 1996. Edible wheat gluten film: influence of water content on glass transition temperature. Journal of Agricultural and Food Chemistry 44, 3474–3478. [Google Scholar]
- Harker M, Berkaloff C, Lemoine Y, Britton G, Young AJ, Duval JC, Rmiki NE, Rousseau B. 1999. Effects of high light and desiccation on the operation of the xanthophyll cycle in two marine brown algae. European Journal of Phycology 34, 35–42. [Google Scholar]
- Havaux M. 1998. Carotenoids as membrane stabilizers in chloroplasts. Trends in Plant Science 3, 147–151. [Google Scholar]
- Havaux M, Dall’Osto L, Bassi R. 2007. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PS II antennae. Plant Physiology 145, 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. 1999. The violaxanthin cycle protects plants from oxidative damage by more than one mechanism. Proceedings of the National Academy of Sciences, USA 96, 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Wolfgang B, Shuvalov VA. 2006. Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photo-oxidation. Journal of Experimental Botany 57, 2993–3006. [DOI] [PubMed] [Google Scholar]
- Holzinger A, Lütz C. 2011. Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. Journal of Phycology 47, 591–602. [DOI] [PubMed] [Google Scholar]
- Hooijmaijers C. 2008. Membrane integrity, oxidative damage and chlorophyll fluorescence during dehydration of the thalloid liverwort Monoclea forsteri Hook. Journal of Bryology 30, 217–222. [Google Scholar]
- Johnson MP, Goral TK, Duffy CDP, Brain APR, Mullineaux CW, Ruban AV. 2011. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. The Plant Cell 23, 1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Havaux M, Triantaphyllides B, Kass B, Pascal AA, Robert B, Davidson PA, Ruban AV, Horton P. 2007. Elevated zeaxanthin bound to oligomeric LHC II enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. Journal of Biological Chemistry 282, 22605–22618. [DOI] [PubMed] [Google Scholar]
- Kalichevsky MT, Jaroszkiewicz EM, Ablett S, Blanshard JMV, Lillford PJ. 1992. The glass-transition of amylopectin measured by DSC, DMTA and NMR. Carbohydrate Polymers 18, 77–88. [Google Scholar]
- Kostecka-Gugala A, Latowski D, Strzalka K. 2003. Thermotropic phase behaviour of alpha-dipalmitoylphosphatidylcholine multibilayers is influenced to various extents by carotenoids containing different structural features—evidence from differential scanning calorimetry. Biochimica et Biophysica Acta 1609, 193–202. [DOI] [PubMed] [Google Scholar]
- Kranner I. 2002. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytologist 154, 451–460. [DOI] [PubMed] [Google Scholar]
- Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW. 2002. Revival of a resurrection plant correlates with its antioxidant status. The Plant Journal 31, 13–24. [DOI] [PubMed] [Google Scholar]
- Kranner I, Zorn M, Turk B, Wornik S, Backett RP, Patic F. 2003. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytologist 160, 167–176. [DOI] [PubMed] [Google Scholar]
- Kristo E, Biliaderis CG. 2007. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydrate Polymers 68, 146–158. [Google Scholar]
- Krupinska K, Mulisch M, Hollmann J, Tokarz K, Zschiesche W, Kage H, Humbeck K, Bilger W. 2012. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Plant Physiology 144, 189–200. [DOI] [PubMed] [Google Scholar]
- Leprince O, Buitink J. 2007. The glassy state in dry seeds and pollen. In: Jenks MA, Wood AJ, eds. Plant desiccation tolerance. Iowa: Blackwell Publishing, 193–214. [Google Scholar]
- Leubner-Metzger G. 2005. Beta-1,3-glucanase gene expression in low-hydrated seeds as a mechanism for dormancy release during tobacco after-ripening. The Plant Journal 41, 133–145. [DOI] [PubMed] [Google Scholar]
- Leymarie J, Bruneaux E, Gibot-Leclerc S, Corbineau F. 2007. Identification of transcripts potentially involved in barley seed germination and dormancy using cDNA-AFLP. Journal of Experimental Botany 58, 425–437. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Hudgeons J, Dowd SE, Payton PNR. 2009. A combined subtractive suppression hybridization and expression profiling strategy to identify novel desiccation response transcripts from Tortula ruralis gametophytes. Physiologia Plantarum 136, 437–460. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Tuba Z, Mishler BD. 2000a. The evolution of vegetative desiccation tolerance in land plants. Plant Ecology 151, 85–100. [Google Scholar]
- Oliver MJ, Velten J, Wood AJ. 2000b. Bryophytes as experimental models to study the environmental stress tolerance: Tortula ruralis and desiccation-tolerance in mosses. Plant Ecology 151, 73–84. [Google Scholar]
- Piller LE, Abraham M, Dörmann P, Kessler F, Besagni C. 2012. Plastid lipid droplets at the crossroads of prenylquinone metabolism. Journal of Experimental Botany 63, 1609–1618. [DOI] [PubMed] [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW. 1994. Membranes and organelles of dehydrated Selaginella and Tortula retain their normal configuration and structural integrity. Protoplasma 178, 57–65. [Google Scholar]
- Pressel S, Duckett JG. 2010. Cytological insights into the desiccation biology of a model system: moss protonemata. New Phytologist 185, 944–963. [DOI] [PubMed] [Google Scholar]
- Proctor CF, Ligrone R, Duckett JG. 2007. Desiccation tolerance in the moss Polytrichum formossum: physiological and fine-structural changes during desiccation and recovery. Annals of Botany 99, 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP. 2010. Xanthophylls as modulators of membrane protein function. Archives of Biochemistry and Biophysics 504, 78–85. [DOI] [PubMed] [Google Scholar]
- Sarry JE, Montillet JL, Sauvaire Y, Havaux M. 1994. The protective function of the xanthophyll cycle in photosynthesis. FEBS Letters 353, 147–150. [DOI] [PubMed] [Google Scholar]
- Sharma M, Bhardwaj U, Bhardwaj R. 1996. High light-induced unstacking of thylakoids and randomization of pigment–protein complexes of chloroplast. Journal of Plant Biochemistry and Biotechnology 5, 131–133. [Google Scholar]
- Shu S, Wang Y, Chen W, Wang Z. 2009. Influence of dehydration on the desert moss in molecular mobility and membrane fluidity monitored by spin label. Biochemical Systematics and Ecology 36, 935–937. [Google Scholar]
- Slade L, Levine H. 1991. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Critical Reviews in Food Science and Nutrition 30, 115–360. [DOI] [PubMed] [Google Scholar]
- Smith MD, Licatalosi DD, Thompson JE. 2000. Co-association of cytrochrome f catabolites and plastid-lipid-associated protein with chloroplast lipid particles. Plant Physiology 124, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuba Z, Csintalan Z, Proctor MCF. 1996. Photosynthetic responses of a moss, Tortula ruralis ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation: a baseline study at present-day CO2 concentration. New Phytologist 133, 353–361. [DOI] [PubMed] [Google Scholar]
- Tuba Z, Lichtenthaler HK, Maroti I, Csintalan Z. 1993. Resynthesis of thylakoids and functional chloroplasts in the desiccated leaves of the poikilochlorophyllous plant Xerophyta scabrida upon rehydration. Journal of Plant Physiology 142, 742–748. [Google Scholar]
- Varghese B, Sershen, Berjak P, Varghese D, Pammenter NW. 2011. Differential drying rates of recalcitrant Trichilia degeana embronic axes: a study of survival and oxidative stress metabolism. Physiologia Plantarum 142, 326–338. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Duff RJ, Oliver MJ. 1999. Expressed sequence tags (ESTs) from desiccated Tortula ruralis identify a large number of novel plant genes. Plant and Cell Physiology 40, 361–368. [DOI] [PubMed] [Google Scholar]
- Wood A, Oliver M. 1999. Translational control in plant stress: the formation of messenger ribonucleoprotein particles (Mrnps) in response to desiccation of Tortula ruralis gametophytes. The Plant Journal 18, 359–370. [Google Scholar]
- Yamakawa H, Fukushima Y, Itoh S, Heber U. 2012. Three different mechanisms of energy dissipation of a desiccation-tolerant moss serve one common purpose: to protect reaction centres against photo-oxidation. Journal of Experimental Botany 63, 3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng O, Chen XB, Wood AJ. 2002. Two early light-indudible protein (ELIP) cDNAs from the resurrection plant Tortula ruralis are differentially expressed in response to desiccation, rehydration, salinity, and high light. Journal of Experimental Botany 371, 1197–1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.