Abstract
In Arabidopsis thaliana, the phytohormone auxin is an important patterning agent during embryogenesis and post-embryonic development, exerting effects through transcriptional regulation. The main determinants of the transcriptional auxin response machinery are AUXIN RESPONSE FACTOR (ARF) transcription factors and AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) inhibitors. Although members of these two protein families are major developmental regulators, the transcriptional regulation of the genes encoding them has not been well explored. For example, apart from auxin-linked regulatory inputs, factors regulating the expression of the AUX/IAA BODENLOS (BDL)/IAA12 are not known. Here, it was shown that the HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIP) transcription factor HOMEOBOX PROTEIN 5 (HB5) negatively regulates BDL expression, which may contribute to the spatial control of BDL expression. As such, HB5 and probably other class I HD-ZIP proteins, appear to modulate BDL-dependent auxin response.
Key words: Arabidopsis, auxin, BODENLOS (BDL), embryo, HOMEOBOX PROTEIN 5 (HB5), transcriptional regulation.
Introduction
The proper distribution of auxin as well as the adequate translation of its accumulation into developmental outputs is crucial for normal plant development. During early embryogenesis, auxin is transported from the basal to the apical cell(s) where it induces embryo proper development. Later in embryogenesis, the auxin flux is reversed and auxin accumulates in the hypophysis, triggering root meristem initiation. In addition, cotyledon initiation, which establishes the bilaterally symmetric apical part of the embryo, has also been shown to depend on auxin transport and/or response (Vanneste and Friml, 2009; Lau et al., 2012).
Generally, the transcriptional auxin response is controlled by AUXIN RESPONSE FACTOR (ARF) transcription factors and AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) proteins. The latter interact with ARFs and inhibit transcriptional induction by (activating) ARFs. This inhibition is relieved by the auxin-facilitated degradation of AUX/IAAs by the 26S proteasome via interaction with the ubiquitin-ligating SCFTIR1/AFB complex (Lau et al., 2008; Chapman and Estelle, 2009). For example, BODENLOS (BDL)/IAA12 and its interacting ARF partner MONOPTEROS (MP)/ARF5 play a pivotal role during the earliest stages of embryonic development. Most prominently, both stabilizing bdl and loss-of-function mp mutants lack a seedling root and frequently display cotyledon defects (Berleth and Jürgens, 1993; Hardtke and Berleth, 1998; Hamann et al., 1999; Hamann et al., 2002).
AUX/IAAs, as negative regulators of (activating) ARFs, are probably not only subject to auxin-mediated degradation for proper plant development but also to spatio-temporal control of expression. The latter is reflected in the restricted expression domain of, for example, IAA18 (Ploense et al., 2009), SOLITARY ROOT (SLR)/IAA14 (Fukaki et al., 2002; Vanneste et al., 2005), IAA28 (De Rybel et al., 2010) or BDL (Hamann et al., 2002). While BDL and MP seem to be expressed in the whole embryo proper early on, their expression is restricted during further embryo development (Hamann et al., 2002). How this is brought about is not fully known. We recently showed that MP itself is an important regulator of BDL expression (Lau et al., 2011), and therefore BDL expression might mainly follow MP expression. However, MP is expressed more broadly than BDL. This is most apparent at later embryonic stages and also during post-embryonic development when MP is expressed at the basal pole of the embryo and in the root columella cells, respectively, while BDL is not expressed there (Hamann et al., 2002; Weijers et al., 2006), implying that there are factors besides MP that regulate BDL expression.
The HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIP)-encoding superfamily of homeobox genes is unique to plants and consists of more than 40 members in Arabidopsis, which have been divided into four subfamilies (Ruberti et al., 1991; Schena and Davis, 1992; Ariel et al., 2007). HD-ZIP family members are involved in the regulation of meristem activity and patterning, and also in various physiological responses (Harris et al., 2011; Zúñiga-Mayo et al., 2012). In these situations, links between plant hormones and HD-ZIPs have been reported, but these connections are often in the context of drought stress or shade avoidance (Himmelbach et al., 2002; Sorin et al., 2009; Son et al., 2010; Harris et al., 2011).
In this study, we investigate the transcriptional control of the well-characterized AUX/IAA gene BDL. We identify HOMEOBOX PROTEIN 5 (HB5)/ARABIDOPSIS THALIANA HOMEOBOX PROTEIN 5 (ATHB5), a member of the HD-ZIP I subfamily, as a negative regulator of BDL expression. HB5 might as such contribute to the spatial regulation of BDL expression, although there appear to be additional negative regulatory influences.
Materials and methods
Plant material and growth conditions
Plants were grown under long-day conditions (16h light, 8h dark) at 22 to 24 °C. Seedlings used for expression or phenotypic analyses were grown from surface-sterilized seeds on vertical half-strength MS agar plates containing 10g l–1 of sucrose. Arabidopsis thaliana ecotype Columbia, Landsberg erecta or Wassilewskija was used. The HB5 allele hb5-1 as well as transgenic lines pBDL::bdl:GUS, pBDL::NLS:3×GFP, LTP::LhG4, and UAS::bdl have been described previously (Baroux et al., 2001; Johannesson et al., 2003; Dharmasiri et al., 2005; Weijers et al., 2006; De Smet et al., 2010).
Transient activity assays
Transient activity assays were performed as described previously (Lau et al., 2011).
Cloning and constructs
pGreenII/BAR pBDL 921 ::bdl:GUS, pGreenII/BAR pBDL 245 ::bdl: GUS, and pGreenII/BAR pBDL 195 ::bdl:GUS were constructed by replacing the BDL promoter of pGreenII/BAR pBDL::bdl: GUS (Dharmasiri et al., 2005) by EcoRI/PstI-cut fragments amplified with primers 5′-CCGAATTCAACATAATGATGAATA TCTCATCACC-3′ and 5′-GGCTGCAGACAACAAGAAGAGA AAGAG-3′ (for pGreenII/BAR pBDL 921 ::bdl:GUS), 5′-CCGAATT CGCCATTACAAGACATATGGG-3′ and 5′-GGCTGCAGACAA CAAGAAGAGAAAGAG-3′ (for pGreenII/BAR pBDL 245 ::bdl: GUS), and 5′-CCGAATTCAAATCCTCCTCTCTCTCTC-3′ and 5′-GGCTGCAGACAACAAGAAGAGAAAGAG-3′ (for pGreenII/BAR pBDL 195 ::bdl:GUS).
To clone GIIK p3×PF:m35S::NLS:3×GFP, first GIIK m35S:: NLS:3×GFP was generated by amplifying m35S from pGreen II 35Smini::NLS:3×EGFP::nost (Takada and Jürgens, 2007) with primers 5′-TTTCTGCAGCTTCGCAAGACCCTTCCTCTATATA AG-3′ and 5′-AAGGATCCATCCCCCGTGTTCTCTCCAAATGA AATG-3′, cutting the amplified m35S with PstI and BamHI, and inserting the PstI/BamHI-cut m35S fragment into PstI/BamHI-cut pGreenII KAN NLS:3×GFP (Schlereth et al., 2010). Then 3×PF was amplified from ligated annealed linkers 5′-AATTCGCCATTACAAGACAT ATGGGTCCCAATTCTCATCACTCTCTCCACCACCG-3′ and 5′-AATTCGGTGGTGGAGAGAGTGATGAGAATTGGG ACCCATATGTCTTGTAATGGCG-3′ with primers 5′-CGATAAG CTTGATATCCTCGAGGCCATTACAAGAC-3′ and 5′-CTTTAT TATTTTAATTAAATACTGCAGGGTGGTGGAG AG-3′, cut with XhoI and PstI, and inserted into XhoI/PstI-cut GIIK m35S::NLS:3×GFP.
LucTrap p3×PF:m35S was generated by amplifying the 3×PF fragment from ligated annealed linkers 5′-AATTCGCCATTACA AGACATATGGGTCCCAATTCTCATCACTCTCTCCACCA CCG-3′ and 5′-AATTCGGTGGTGGAGAGAGTGATGAGAATT GGGACCCATATGTCTTGTAATGGCG-3′ with primers 5′-CGA TAAGCTTGATATCGGATCCGCCATTACAAGAC-3′ and 5′-CT TTATTATTTTAATTAAATAGAGCTCGGTGGTGGAGAG-3′, cutting the amplified fragment with BamHI and SacI, and inserting it into BamHI/SacI-cut LucTrap m35S (Lau et al., 2011).
LucTrap 4×PF 36bp :m35S was generated by amplifying 4×PF 36bp from ligated annealed linkers 5′-GGCCCGCCATTACAAGACATA TGGGTCCCAATTCTCATCACG-3′ and 5′-GGCCCGTGATGA GAATTGGGACCCATATGTCTTGTAATGGCG-3′ with primers 5′-GTCGACCTCGAGGGGGGATCCGCCATTACAAGACA-3′ and 5′-AGGGCGAATTGGGTACCGAGCTCGTGATGAGAAT TG-3′, cutting this fragment with BamHI and SacI, and inserting it into BamHI/SacI-cut LucTrap m35S (Lau et al., 2011).
pHB5::HB5:3×GFP was cloned by first inserting EcoRI/ PstI-digested pHB5, which was amplified with primers 5′-CC GAATTCAGCATTGGATAAAGGTGTTTGG-3′ and 5′-CCCTG CAGCTTGTTTGGTCGGAACA-3′, into EcoRI/PstI-cut pGreenIIKAN 3×GFP (Schlereth et al., 2010), to yield GIIK pHB5::3×GFP, and then inserting a PstI/BamHI-cut genomic HB5 fragment from the start codon to the last codon before the stop codon, which was amplified with primers 5′-CCCTGCAGATGAAGAGATCACGTGGAA-3′ and 5′-CCGGATCCCGAATTCCACTGATCGGAG-3′, into PstI/ BamHI-cut GIIK pHB5::3×GFP.
To generate GIIK pRPS5A::HB5, JIT60 HB5, and pRSET A HB5, HB5 was amplified from cDNA with primers 5′-CC CTGCAGATGAAGAGATCACGTGGAA-3′ and 5′-CCGGATCC TTACGAATTCCACTGATCGGAG-3′, cut with PstI and BamHI, and inserted into either PstI/BamHI-cut GIIK RPS5A-tNOS (Weijers et al., 2006), PstI/BamHI-cut pJIT60 (Schwechheimer et al., 1998) or pRSET A (Invitrogen), in which the multiple cloning site had been modified to allow insertion of PstI/BamHI-cut HB5.
JIT60 HB6 was generated by amplifying HB6 from cDNA with primers 5′-AAAGTCGACATGATGAAGAGATTAAGTAGT TCAGATTCAGTG-3′ and 5′-TTGGATCCTCAATTCCAATGAT CAACGGTGGAGTAC-3′ and inserting the SalI/BamHI-digested frag ment into SalI/BamHI-digested pJIT60 (Schwechheimer et al., 1998).
pBluescript 2×PF 36bp was generated by inserting two copies of annealed linkers 5′-GGCCCGCCATTACAAGACATATGGGTC CCAATTCTCATCACG-3′ and 5′-GGCCCGTGATGAGAATTG GGACCCATATGTCTTGTAATGGCG-3′ into Bsp120I-cut pBluescript, and pBluescript 4×mPF 36bp by inserting four copies of annealed linkers 5′-GGCCCGCCCTCACAAGACATCTGGGTCC TAGTGCTCATCACG-3′ and 5′-GGCCCGTGATGAGCACTAG GACCCAGATGTCTTGTGAGGGCG-3′ into Bsp120I-cut pBluescript.
pHIS3NX PF was created by inserting annealed linkers 5′-AA TTCGCCATTACAAGACATATGGGTCCCAATTCTCA TCACTCTCTCCACCACCG-3′ and 5′-AATTCGGTGGTGGAG AGAGTGATGAGAATTGGGACCCATATGTCTTGTA ATGGCG-3′ into EcoRI-cut pHIS3NX (Ouwerkerk and Meijer, 2001). To create pINT1 PF HIS3, PF HIS3 was cut out from pHIS3NX PF with NotI and XbaI, and inserted into NotI/XbaI-cut pINT1 (Ouwerkerk and Meijer, 2001). JIT60 MP and LucTrap pBDL have been described previously (Lau et al., 2011).
Plant transformation
Plants were transformed by the floral dip method (Clough and Bent, 1998). Primary transformants were selected on half-strength MS agar plates containing either 15mg l–1 of phosphinothricin or 62.5mg l–1 of kanamycin.
Electrophoretic mobility shift assay (EMSA)
Recombinant HB5 was obtained by expressing pRSET A HB5 in Escherichia coli BL21(DE3)pLysS; the recombinant HB5 was purified. The PF 36bp and mPF 36bp probes for the EMSA were cut out from pBluescript 2×PF 36bp and pBluescript 4×mPF 36bp, respectively, with Bsp120I and purified; overhangs were filled with radiolabelled dNTPs by the Klenow fragment. Binding reactions were performed in a total volume of 10 μl, combining 10 000 cpm of radiolabelled probe, 1 μl of binding buffer [250mM HEPES (pH 7.6), 10mM EDTA pH 8, 50 % (w/v) glycerol, 10mM KCl] and 200ng poly(dI-dC) in the presence or absence of recombinant HB5. Samples were incubated for 30min at room temperature and loaded on a pre-run polyacrylamide gel.
Yeast one-hybrid screen
A yeast one-hybrid screen was performed as described previously (Ouwerkerk and Meijer, 2001). The PF sequence was used as the bait sequence in pINT1 PF HIS3.
Expression analysis
For β-glucuronidase (GUS) staining, seedlings were fixed in 90% acetone for 20min at –20 °C and then washed twice for 10min in washing buffer [0.1M phosphate (pH 7.0), 10mM EDTA, 2mM K3Fe(CN)6]. Subsequently, seedlings were incubated in staining buffer [0.1M phosphate (pH 7.0), 10mM EDTA, 1mM K3Fe(CN)6, 1mM K4Fe(CN)6.3H2O, 1mg ml–1 5-bromo-4-chloro-3-indolyl glucuronide] in a desiccator for 10min at room temperature, and then overnight at 37 °C. GUS-stained seedlings were fixed in a 3:1 mixture of ethanol and acetic acid for 1h, and then washed in 75, 50, and 25% ethanol for 10min each. For microscopy, seedlings were mounted in chloral hydrate solution (8:3:1 mixture of chloral hydrate, water, and glycerol). GFP fluorescence was analysed in whole-mount preparations of embryos or seedlings. Propidium iodide was used at a concentration of 10 μg ml–1.
Image acquisition
Images were acquired using a confocal laser-scanning microscope (TCS-SP2; Leica), a Zeiss Axiophot microscope or a digital camera (Coolpix 990; Nikon). Images were processed using Adobe Photoshop software.
Data mining
Protein and genomic sequence information used in this study were retrieved from the Arabidopsis Information Resource (http://www.arabidopsis.org), Joint Genome Insitute (http://genome.jgi-psf.org), Brassica Genome Gateway (http://brassica.bbsrc.ac.uk), Plant Transcription Factor Database (http://plntfdb.bio.uni-potsdam.de) and NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence details are given in Supplementary List S1 at JXB online.
Phylogenetic analysis
Protein sequences were aligned using CLC DNA Workbench 5.7.1 with the following settings: gap open cost: 10, gap extension cost: 1, end gap cost: as any other, alignment: very accurate. The phylogenetic tree was created using the unweighted pair group method with arithmetic mean (UPGMA) algorithm and a bootstrap analysis with 1000 replicates within CLC DNA Workbench 5.7.1.
Analysis of 5′-upstream sequences
A 1kb 5′-upstream region was used for each gene of interest and uploaded into mVISTA (http://genome.lbl.gov/vista/mvista/submit.shtml). Analyses were performed using the standard settings (using MLAGAN alignment). Subsequently, individual comparisons against the 1kb 5′-upstream region of IAA12/BDL from A. thaliana were optimized for each sequence by adjusting the conservation parameters (‘0’ for minimum y-value on the VISTA plot, ‘50’ for minimum length for a conserved non-coding region, and a minimum conservation identity as indicated for the respective comparison as a percentage). A sequence logo was made from manually optimized 50bp promoter sequence alignments (generated by CLC DNA Workbench 5.7.1) using WebLogo 3 (http://weblogo.threeplusone.com/) (Schneider and Stephens, 1990; Crooks et al., 2004).
Statistics
Details of the statistical tests used are given in Supplementary Table S1 at JXB online.
Results
Conserved regulatory fragment important for BDL expression
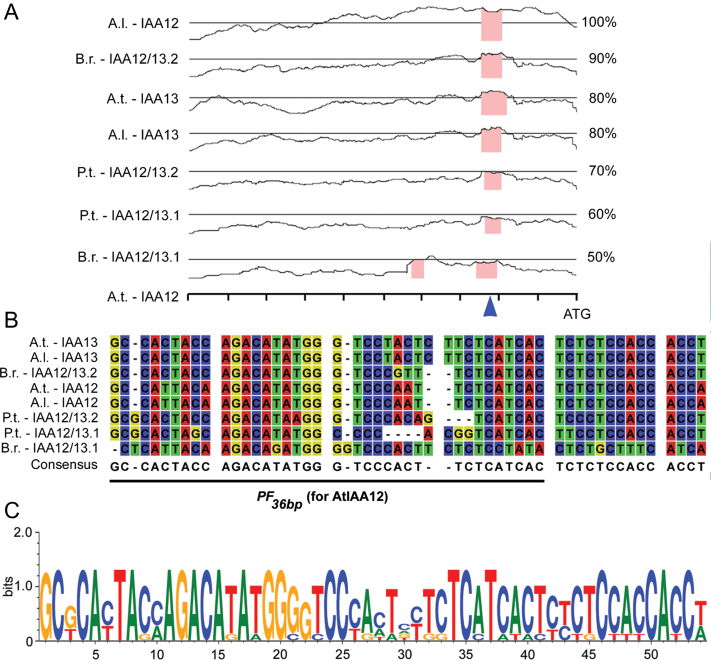
To gain insight into the transcriptional control of BDL expression, in addition to the positive control exerted by auxin and MP (Abel et al., 1995;; Tian et al., 2002; Lau et al., 2011), we analysed the BDL upstream regulatory region. As BDL is involved in crucial developmental processes during embryogenesis and later stages of development, we reasoned that the regulation of its expression is probably evolutionarily conserved. Hence, assuming this conserved regulatory mechanism, the BDL promoter would be suitable for a phylogenetic shadowing analysis (Boffelli et al., 2003; Yamaguchi et al., 2013). In order to delineate the relevant conserved regulatory regions within the BDL promoter, we compared promoter sequences across different species. We chose to analyse orthologues of BDL and its similarly expressed paralogue IAA13 (Weijers et al., 2005) in Arabidopsis lyrata and Brassica rapa, two Brassicaceae species closely related to A. thaliana, and in the more distant species Populus trichocarpa belonging to the Salicaceae; the orthologues were identified by database mining and subsequent phylogenetic analyses (Fig. S1 at JXB online). In A. thaliana, BDL is expressed in the apical cell lineage during embryogenesis, and its expression is restricted to the stele in the main root (Hamann et al., 2002). A promoter-deletion series for pBDL::bdl:GUS revealed that an ~1kb promoter fragment was sufficient to mimic the BDL expression pattern in the root tip (Fig. S2A at JXB online). Therefore, we focused our analysis on the 5′ region 1kb upstream of the start codon. Using the mVISTA tool for comparative genomics (Mayor et al., 2000; Frazer et al., 2004), we identified a conserved 50bp region in the different 5′-upstream sequences of the homologues from A. thaliana, A. lyrata, B. rapa, and P. trichocarpa (Fig. 1A). Sequence alignment of this 50bp fragment showed its high level of conservation (Fig. 1B, C).
Fig. 1.
Identification of a conserved regulatory element in the 5′ region of BDL/IAA12. (A) mVISTA analysis of 1kb upstream regulatory regions of the indicated genes with reference to the AtIAA12 (BDL) sequence. Blue arrowhead/pink block, region of conservation. A.l., Arabidopsis lyrata; A.t., Arabidopsis thaliana; B.r., Brassica rapa; P.t., Populus trichocarpa. (B) Sequence alignment of a 50bp stretch of the respective 5′ regulatory regions indicated by the blue arrowhead in (A) (corresponding to 245–196bp upstream of the start codon in BDL/AtIAA12). PF 36bp indicates the fragment used for EMSA (see Fig. 3). (C) Sequence logo of the conserved 50bp stretch.
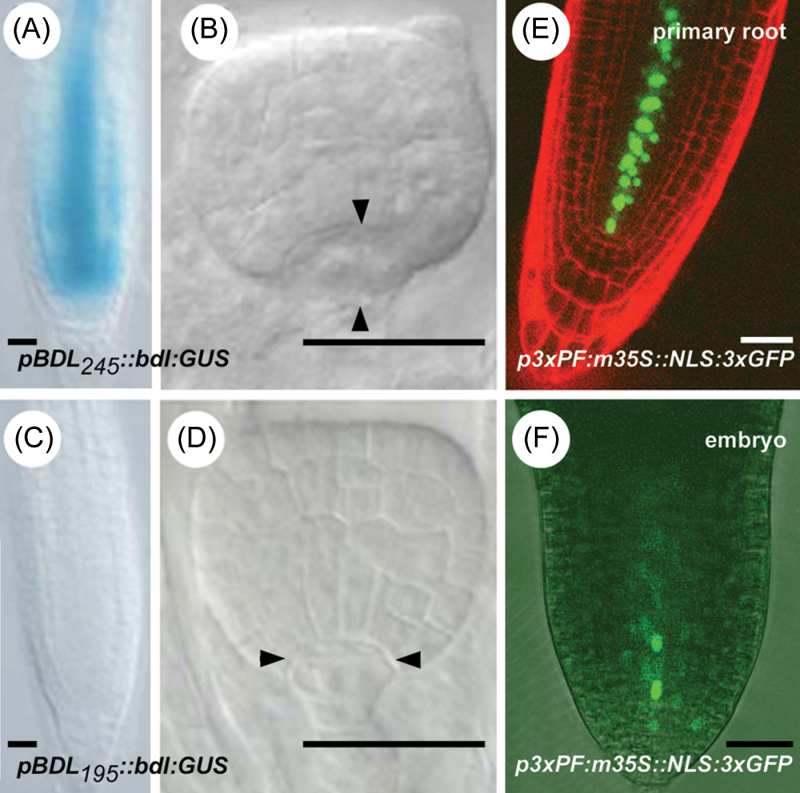
The next step was the functional analysis of this in silico-identified conserved region with respect to BDL expression in A. thaliana. Therefore, we generated specific deletions of the A. thaliana BDL promoter. Driving the expression of bdl:GUS, a promoter fragment starting 245bp upstream of the start codon and containing the conserved 50bp at its 5′ end still resulted in normal BDL expression and yielded bdl mutant phenotypes (pBDL 245 ::bdl:GUS) (Fig. 2A, B). However, by deleting the conserved 50bp from this promoter fragment, BDL expression was reduced and characteristic bdl phenotypes were not observed (pBDL 195 ::bdl:GUS) (Fig. 2C, D). Therefore, the highly conserved region that was identified using an in silico approach (hereafter referred to as Promoter Fragment or PF), was also relevant in planta for BDL expression. To check if PF was by itself sufficient for normal BDL expression, we fused three copies of this element to m35S::NLS:3×GFP (p3×PF:m35S::NLS:3×GFP). p3×PF:m35S::NLS:3×GFP mimicked the BDL expression pattern as visualized by pBDL::NLS:3×GFP (Fig. 2E, F and Fig. S2B). However, with neither constructs was it possible to visualize BDL expression at the very early stages of embryogenesis. These analyses demonstrated that a fragment of 50bp is relevant for BDL expression and is sufficient to mimic the BDL expression pattern, at least in the later stages of embryo development.
Fig. 2.
pBDL deletion study. (A) GUS-stained pBDL 245 ::bdl:GUS seedling root tip. (B) Hypophysis division defect (vertical instead of horizontal division) in pBDL 245 ::bdl:GUS embryos. (C) GUS-stained pBDL 195 ::bdl:GUS seedling root tip. (D) Normal hypophysis division in pBDL 195 ::bdl:GUS embryos. (E, F) p3×PF:m35S::NLS:3×GFP expression in a seedling root counterstained with propidium iodide (E) or in a torpedo-stage embryo (basal part shown) (F). Bars, 25 μm.
HB5 interacts with PF of the BDL promoter
To identify proteins interacting with PF, we performed a yeast one-hybrid screen and isolated several transcription factors as putative PF interactors (data not shown). One of these, HB5 (Johannesson et al., 2001, 2003), was chosen for a more detailed analysis because other members of the HD-ZIP family, such as MERISTEM LAYER 1 (ATML1), PROTODERMAL FACTOR 2 (PDF2) and GLABRA 2 (GL2), play important roles in plant development (Ariel et al., 2007).
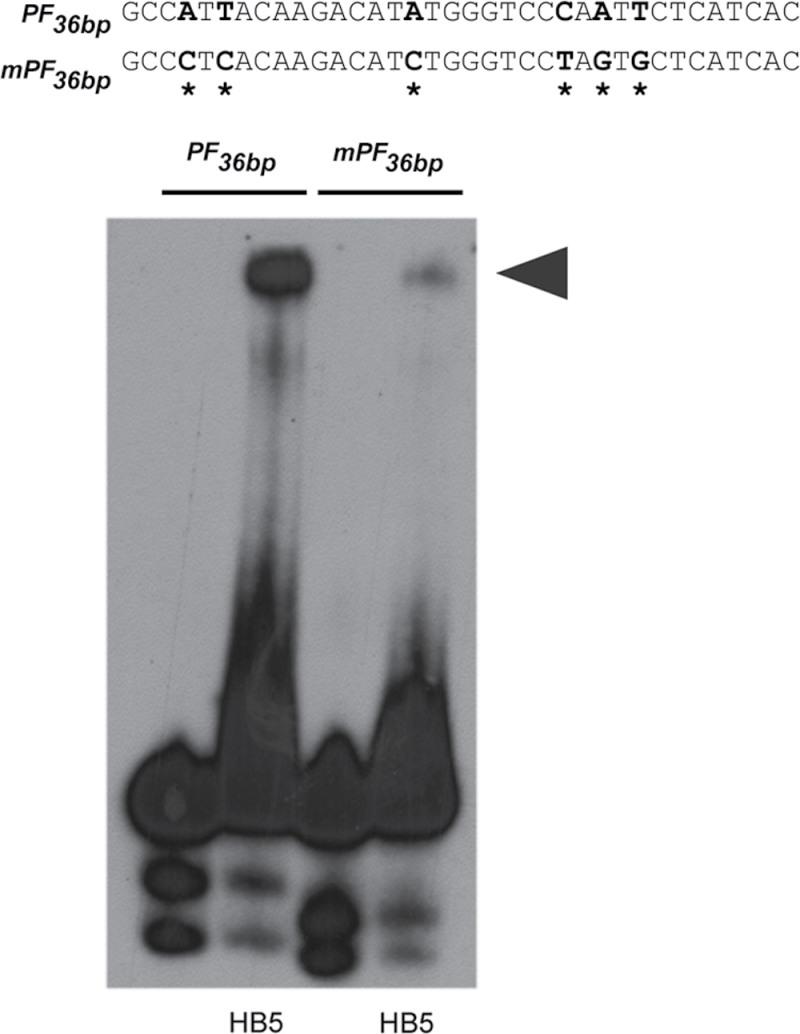
We confirmed the yeast one-hybrid data by demonstrating that HB5 bound to a 36bp subfragment of the PF element (hereafter referred to as PF 36bp) in vitro (Fig. 1B and Fig. 3). An EMSA with recombinant HB5 protein revealed a shift of the radiolabelled wild-type probe, and this shift was almost abolished when six mutations were introduced into PF 36bp (Fig. 3). Thus, we concluded that HB5 can bind directly to this 36bp BDL promoter fragment.
Fig. 3.
Direct binding of HB5 to a BDL promoter fragment (see Fig. 1). Results of an EMSA with PF 36bp and mPF 36bp in the absence or presence of HB5. Mutations are indicated by asterisks and the shifted band by an arrowhead.
HB5 accumulates outside the BDL expression domain
To assess the biological relevance of this interaction, we monitored HB5 expression with a pHB5::HB5:3×GFP reporter gene. HB5:3×GFP accumulated outside the BDL expression domain in the protoderm of the embryo (Fig. 4A, B) and in the epidermis and cortex of the main root tip (Fig. 4C). Thus, the inner cells in which BDL is normally expressed in the embryo and in the seedling root (Hamann et al., 2002; Dharmasiri et al., 2005; Weijers et al., 2006) are outside the domain where HB5:3×GFP was detected. This suggested that HB5 is not a positive, but rather is a negative regulator of BDL expression.
Fig. 4.
HB5 expression. (A–C) pHB5::HB5:3×GFP expression (green) in a globular-stage embryo (A), heart-stage embryo (B), and seedling root counterstained with propidium iodide (red) (C). Bars, 50 μm.
Expression of bdl in the epidermis impairs cotyledon development
Auxin response in the (globular) embryo is important for cotyledon initiation and development (Hamann et al., 1999; Benková et al., 2003; Ploense et al., 2009). Therefore, it could be significant that BDL expression gets restricted to the inner cells of the embryo (Hamann et al., 2002), which coincides with the detectable onset of HB5 expression in protodermal cells at the globular stage during embryogenesis (Fig. 4A). Because in the hb5-1 knockout mutant (Johannesson et al., 2003) cotyledon formation and embryogenesis in general are not obviously impaired, HB5 is either not the only factor involved in excluding BDL expression from the protoderm and/or the effect of possible misexpression of BDL in the hb5-1 mutant is not strong enough to affect cotyledon development. The latter is in agreement with the absence of obvious phenotypes when altering the levels of wild type AUX/IAAs—despite higher BDL transcript levels, BDL protein levels might actually not increase due to auxin-facilitated BDL degradation.
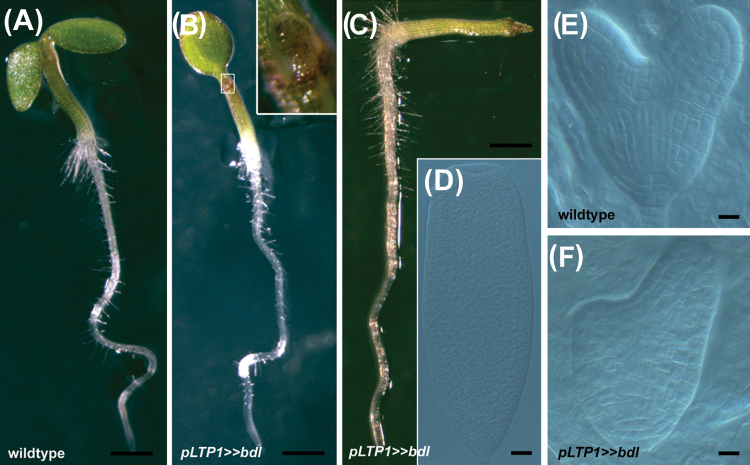
To circumvent these problems, we expressed stabilized BDL (bdl), which is not prone to auxin-facilitated degradation, ectopically in the developing epidermis, using the protoderm-specific driver line pLTP1 for transactivation of bdl (pLTP1>>bdl) (Baroux et al., 2001; Weijers et al., 2006). This resulted in mild effects on cotyledon development in F1 LTP1>>bdl seedlings (n= 22). For example, seedlings with no cotyledons (1%), with only one fully developed cotyledon (16%), or with cotyledons of different size (9%) were observed, in contrast to the wild-type control (Fig. 5A–C). These defects had their origin in embryogenesis, with embryos not developing two equal-sized cotyledons or appearing cup-shaped, which contrasted to wild-type embryos with two normally developing cotyledons (Fig. 5D–F). These results suggested that HB5 might contribute to the repression of BDL expression in the protodermal layer.
Fig. 5.
Transactivation of bdl by pLTP1 results in cotyledon defects. (A–C) Seedlings with cotyledon defects caused by protoderm-specific transactivation of bdl expression (B, C) compared with the wild-type (A). Bar, 1mm. (D–F) Cotyledon development is already impaired in embryogenesis in pLTP1>>bdl (compare panels D and F with panel E). Bars, 10 μm.
Ectopic expression of HB5 rescues the bdl rootless phenotype
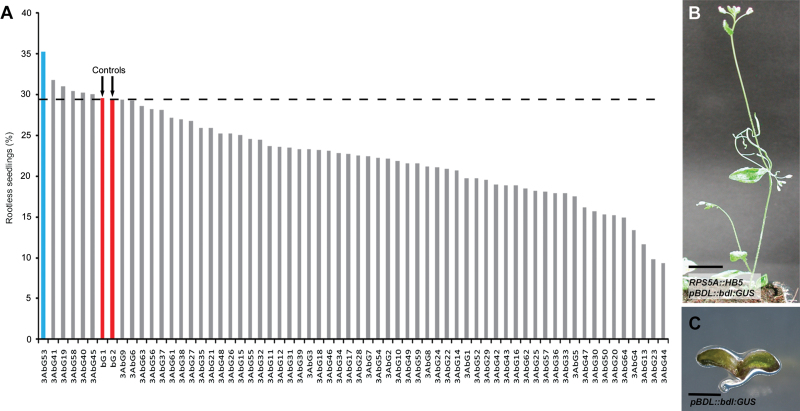
To assess the repressive effect of HB5 on BDL expression when expressing HB5 in the normal BDL expression domain, we used a transgenic line expressing stabilized BDL (bdl) from its endogenous promoter (pBDL::bdl:GUS). The pBDL::bdl:GUS line resembles the originally identified bdl line and gives rise to ~29% rootless seedlings (Dharmasiri et al., 2005) (Fig. 6A, red bars). HB5 was ectopically expressed in this pBDL::bdl:GUS line via the strong embryo promoter pRPS5A (Weijers et al., 2001) (pRPS5A::HB5) to determine if the rootless seedling phenotype resulting from the non-degradation of bdl could be suppressed. Indeed, ectopic expression of HB5 in the pBDL::bdl:GUS transgenic background reduced the proportion of characteristic bdl rootless seedlings in multiple independent transgenic lines variably to a minimum of 9% (Fig. 6A). We observed rescued plants that were homozygous for pBDL::bdl:GUS (Fig. 6B, C). One T1 plant carrying two pRPS5A::HB5 transgenes was homozygous for pBDL::bdl:GUS and segregated only about 35% rootless seedlings (Fig. 6A, blue bar). Taken together, these data suggested that, in planta, HB5 plays a negative regulatory role in controlling the expression of BDL.
Fig. 6.
Rescue of the bdl rootless phenotype by HB5 overexpression. (A) Segregation analysis of independent pRPS5A::HB5 transgenic lines in the pBDL::bdl:GUS background (T2 seedlings were counted). Bars for the pBDL::bdl:GUS controls are shown in red and the bar for the homozygous pBDL::bdl:GUS line with two pRPS5A::HB5 transgenes in blue. (B) Five-week-old plant homozygous for pBDL::bdl:GUS ‘rescued’ by pRPS5A::HB5. Bar, 1cm. (C) One-week-old rootless pBDL::bdl:GUS plant. Bar, 1mm.
HB5 represses BDL expression in protoplasts
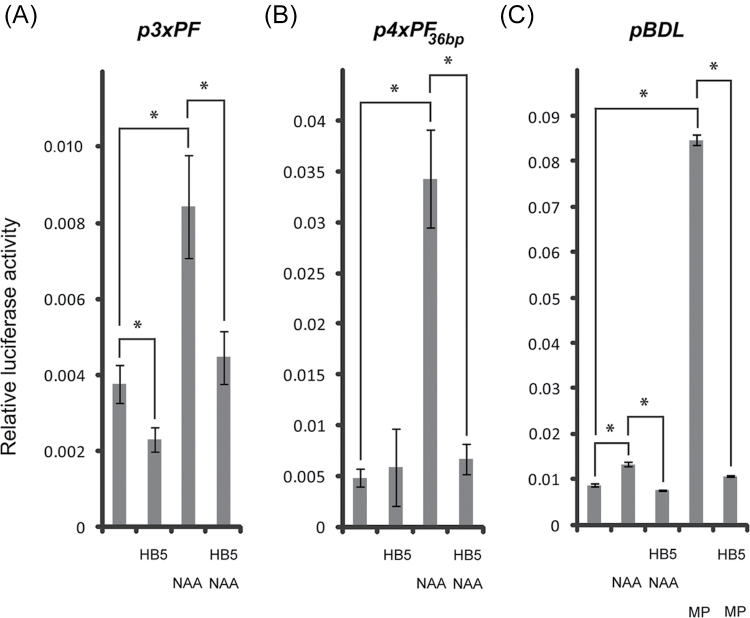
To further support the results on the negative effect of HB5 on BDL expression, we investigated this relationship quantitatively, using a well-established luciferase reporter system in protoplasts (Lau et al., 2011; Niu and Sheen, 2012). To examine HB5 for BDL-repressing activity, we made use of the auxin inducibility of BDL expression (Abel et al., 1995; Tian et al., 2002; Lau et al., 2011). Auxin inducibility of BDL could be mimicked in protoplasts by p3×PF:m35S::LUC and p4×PF 36bp :m35S::LUC (Fig. 7A, B), where copies of these PFs were fused in tandem to a minimal cauliflower mosaic virus 35S promoter to drive expression of firefly LUCIFERASE (LUC). This auxin-mediated induction was repressed by HB5 (Fig. 7A, B). To rule out non-specific trans-effects of the HB5 effector construct, we also co-transfected the empty effector vector, which had no comparable effects (Fig. S3A at JXB online). Furthermore, HB5 repressed auxin-induced expression of the full-length BDL promoter as well as its recently shown stronger induction by MP (Lau et al., 2011) (Fig. 7C). Taken together, these results demonstrated that HB5 functions as a negative regulator of BDL expression in vivo.
Fig. 7.
Effect of HB5 on the expression of different pBDL reporter constructs. (A–C) Transient activity assays using p3×PF::LUC (A), p4×PF 36bp ::LUC (B), or pBDL::LUC (C) as reporter constructs in the presence or absence of HB5, MP, and 1-naphthaleneacetic acid (NAA), as indicated in the respective panels. Values represent mean ±standard error. Statistically significant differences (P <0.05) are indicated by asterisk (Student’s t-test; for statistical details see Table S1).
To get an idea of whether HBs other than HB5 might negatively regulate BDL expression, we analysed whether its close homologue, HB6 (Henriksson et al., 2005; Ariel et al., 2007), would also be able to repress auxin- or MP-mediated induction of BDL expression. Indeed, in transient activity assays, HB6 repressed the induction of pBDL::LUC by auxin or MP essentially as efficiently as HB5 (Fig. S3B). Given that in silico analyses using CORNET (De Bodt et al., 2012) suggested co-expression between HB5 and HB6 (data not shown), there is probably functional redundancy among HB5-related transcription factors regulating BDL expression.
Discussion
Auxin plays a major role in plant development. Auxin response relies on AUX/IAA degradation to release ARFs from inhibition (De Smet and Jürgens, 2007; Lau et al., 2008; Vanneste and Friml, 2009). Next to their auxin-mediated degradation, AUX/IAAs exhibit distinct expression patterns (Fukaki et al., 2002; Hamann et al., 2002; Vanneste et al., 2005; Ploense et al., 2009; De Rybel et al., 2010). While the spatio-temporal control of AUX/IAA expression is likely to be relevant for the proper execution of developmental processes, very little is known about their transcriptional regulation. To address this problem, we focused on BDL, an AUX/IAA involved in embryonic and post-embryonic processes (Hamann et al., 1999; Hamann et al., 2002; De Smet et al., 2010). Previously, we showed that MP activates the expression of its AUX/IAA inhibitor BDL, with auxin being able to act as a threshold-specific trigger by promoting the degradation of the inhibitor (Lau et al., 2011). Here, we explored whether HB5 might be an additional transcriptional regulator contributing to the control of BDL expression.
Many animal and plant homeodomain proteins play a critical role in diverse developmental processes, including pattern formation and specification of cell fates of many tissues (Gehring et al., 1994; Hake et al., 2004; Ariel et al., 2007). HB5 has been described as a potential regulator of abscisic acid (ABA) responsiveness, but has not been implicated in auxin response (Johannesson et al., 2003). HD-ZIPs function as transcriptional regulators that are characterized by an evolutionarily conserved HD responsible for DNA binding and a leucine zipper motif adjacent to the HD, which facilitates homo- and heterodimerization of these transcriptional regulators (Gehring et al., 1994; Johannesson et al., 2001; Ariel et al., 2007). Members of the HD-ZIP I and II families form homo- and heterodimers exclusively with other members of their own family as a prerequisite to DNA binding, and target similar cis elements under in vitro conditions (Harris et al., 2011). For example, HB16 regulates leaf development and flowering time, and has been demonstrated to heterodimerize with HB5 in vitro (Johannesson et al., 2001; Wang et al., 2003). In vitro DNA-binding assays have shown that HB5 preferentially interacts with the pseudopalindromic binding site CAATNATTG (Johannesson et al., 2001). At least half of such a site is present in the BDL promoter, namely in the PF 36bp element to which HB5 binds in vitro. This supported the view that HB5 interacts with the BDL promoter but did not reveal the regulatory effect of HB5.
HD-ZIPs can act as positive and negative regulators (Harris et al., 2011). HD-ZIP Is, including HB5, have been described to be able to induce transcription (Henriksson et al., 2005); HB7 and HB12, also members of HD-ZIP Is, have been reported to act as both transcriptional activators and repressors (Valdés et al., 2012); and HB2 has been described to negatively regulate gene expression (Steindler et al., 1999; Ohgishi et al., 2001). Within the clade containing HB5, the ABA-inducible HB6 positively regulates gene expression in protoplasts, and overall represents a negative regulator of the ABA signalling pathway downstream of ABI1 (Himmelbach et al., 2002). Here, we demonstrated that HB5 acts as a negative regulator of BDL expression, and thus might contribute to the exclusion of BDL from the epidermis and cortex. The transcriptional regulation of BDL by HB5, HB6 and potentially other HD-ZIPs, might thus represent another means of auxin-response control—in addition to the auxin-inducible degradation of BDL.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Phylogenetic relationship of IAA12 and IAA13 homologues and AtIAA3, AtIAA9, AtIAA10, AtIAA11 and AtIAA14.
Supplementary Fig. S2. (A) pBDL 921bp ::bdl:GUS expression in the seedling root tip. (B) pBDL::NLS:3×GFP expression in a torpedo-stage embryo.
Supplementary Fig. S3. (A) Empty vector (pJIT60) does not significantly repress p3×PF::LUC expression. (B) HB6 represses auxin- or MP-induced expression of pBDL::LUC.
Supplementary Table S1. Statistical details.
Supplementary List S1. Sequences used for the phylogenetic and the VISTA analysis.
Acknowledgements
We thank Johan Memelink and Pieter Ouwerkerk for the yeast one-hybrid system and the cDNA library, Melina Zourelidou for instructions on protein expression in E. coli as well as EMSAs, Rada Kancheva and Ender Özdemir for help with plant work, Ole Herud for contributions to microscopy, and Peter Engström and Claus Schwechheimer for sharing materials. This work was supported by the Max Planck Society, a grant from the Deutsche Forschungsgemeinschaft (SFB 446) to G.J., and long-term post-doctoral fellowships from the European Molecular Biology Organization (ALTF 582–2001 to D.W.; ALTF 108–2006 to I.D.S.) and the Marie Curie Intra-European Fellowship Scheme (FP6 MEIF-CT-2007–041375 to I.D.S.).
Glossary
Abbreviations:
- ABA
abscisic acid
- EMSA
electrophoretic mobility shift assay
- GUS
β-glucuronidase.
References
- Abel S, Nguyen MD, Theologis A. 1995. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana . Journal of Molecular Biology 251, 533–549. [DOI] [PubMed] [Google Scholar]
- Ariel FD, Manavella PA, Dezar CA, Chan RL. 2007. The true story of the HD-Zip family. Trends in Plant Science 12, 419–426. [DOI] [PubMed] [Google Scholar]
- Baroux C, Blanvillain R, Moore IR, Gallois P. 2001. Transactivation of BARNASE under the AtLTP1 promoter affects the basal pole of the embryo and shoot development of the adult plant in Arabidopsis . The Plant Journal 28, 503–515. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118, 575–587. [Google Scholar]
- Boffelli D, McAuliffe J, Ovcharenko D, Lewis KD, Ovcharenko I, Pachter L, Rubin EM. 2003. Phylogenetic shadowing of primate sequences to find functional regions of the human genome. Science 299, 1391–1394. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M. 2009. Mechanism of auxin-regulated gene expression in plants. Annual Review of Genetics 43, 265–285. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Research 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bodt S, Hollunder J, Nelissen H, Meulemeester N, Inzé D. 2012. CORNET 2.0: integrating plant coexpression, protein–protein interactions, regulatory interactions, gene associations and functional annotations. New Phytologist 195, 707–720. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, et al. 2010. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Current Biology 20, 1697–1706. [DOI] [PubMed] [Google Scholar]
- De Smet I, Jürgens G. 2007. Patterning the axis in plants—auxin in control. Current Opinion in Genetics & Development 17, 337–343. [DOI] [PubMed] [Google Scholar]
- De Smet I, Lau S, Voβ U, et al. 2010. Bimodular auxin response controls organogenesis in Arabidopsis . Proceedings of the National Academy of Sciences, USA 107, 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. 2005. Plant development is regulated by a family of auxin receptor F box proteins. Developmental Cell 9, 109–119. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Research 32, W273–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. 2002. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis . The Plant Journal 29, 153–168. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Affolter M, Bürglin T. 1994. Homeodomain proteins. Annual Review of Biochemistry 63, 487–526. [DOI] [PubMed] [Google Scholar]
- Hake S, Smith HMS, Holtan H, Magnani E, Mele G, Ramirez J. 2004. The role of knox genes in plant development. Annual Review of Cell and Developmental Biology 20, 125–151. [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. 2002. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes & Development 16, 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jürgens G. 1999. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO Journal 17, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JC, Hrmova M, Lopato S, Langridge P. 2011. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytologist 190, 823–837. [DOI] [PubMed] [Google Scholar]
- Henriksson E, Olsson ASB, Johannesson H, Johansson H, Hanson J, Engström P, Söderman E. 2005. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiology 139, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Hoffmann T, Leube M, Höhener B, Grill E. 2002. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis . EMBO Journal 21, 3029–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson H, Wang Y, Engström P. 2001. DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro . Plant Molecular Biology 45, 63–73. [DOI] [PubMed] [Google Scholar]
- Johannesson H, Wang Y, Hanson J, Engström P. 2003. The Arabidopsis thaliana homeobox gene ATHB5 is a potential regulator of abscisic acid responsiveness in developing seedlings. Plant Molecular Biology 51, 719–729. [DOI] [PubMed] [Google Scholar]
- Lau S, De Smet I, Kolb M, Meinhardt H, Jürgens G. 2011. Auxin triggers a genetic switch. Nature Cell Biology 13, 611–615. [DOI] [PubMed] [Google Scholar]
- Lau S, Jürgens G, De Smet I. 2008. The evolving complexity of the auxin pathway. Plant Cell 20, 1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Slane D, Herud O, Kong J, Jürgens G. 2012. Early embryogenesis in flowering plants: setting up the basic body pattern. Annual Review of Plant Biology 63, 483–506. [DOI] [PubMed] [Google Scholar]
- Mayor C, Brudno M, Schwartz JR, Poliakov A, Rubin EM, Frazer KA, Pachter LS, Dubchak I. 2000. VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 16, 1046–1047. [DOI] [PubMed] [Google Scholar]
- Niu Y, Sheen J. 2012. Transient expression assays for quantifying signaling output. Methods in Molecular Biology 876, 195–206. [DOI] [PubMed] [Google Scholar]
- Ohgishi M, Oka A, Morelli G, Ruberti I, Aoyama T. 2001. Negative autoregulation of the Arabidopsis homeobox gene ATHB-2 . The Plant Journal 25, 389–398. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk PBF, Meijer AH. 2001. Yeast one-hybrid screening for DNA–protein interactions. Current Protocols in Molecular BiologyUnit 12.12 [DOI] [PubMed] [Google Scholar]
- Ploense SE, Wu MF, Nagpal P, Reed JW. 2009. A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 136, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti I, Sessa G, Lucchetti S, Morelli G. 1991. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO Journal 10, 1787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M, Davis RW. 1992. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proceedings of the National Academy of Sciences, USA 89, 3894–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464, 913–916. [DOI] [PubMed] [Google Scholar]
- Schneider TD, Stephens RM. 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Research 18, 6097–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Smith C, Bevan MW. 1998. The activities of acidic and glutamine-rich transcriptional activation domains in plant cells: design of modular transcription factors for high-level expression. Plant Molecular Biology 36, 195–204. [DOI] [PubMed] [Google Scholar]
- Son O, Hur YS, Kim YK, et al. 2010. ATHB12, an ABA-inducible homeodomain-leucine zipper (HD-Zip) protein of Arabidopsis, negatively regulates the growth of the inflorescence stem by decreasing the expression of a gibberellin 20-oxidase gene. Plant and Cell Physiology 51, 1537–1547. [DOI] [PubMed] [Google Scholar]
- Sorin C, Salla-Martret M, Bou-Torrent J, Roig-Villanova I, Martínez-García JF. 2009. ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. The Plant Journal 59, 266–277. [DOI] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. 1999. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126, 4235–4245. [DOI] [PubMed] [Google Scholar]
- Takada S, Jürgens G. 2007. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development 134, 1141–1150. [DOI] [PubMed] [Google Scholar]
- Tian Q, Uhlir NJ, Reed JW. 2002. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14, 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés AE, Övernäs E, Johansson H, Rada-Iglesias A, Engström P. 2012. The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant Molecular Biology 80, 405–418. [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GT, et al. 2005. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana . Plant Cell 17, 3035–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Wang Y, Henriksson E, Söderman E, Henriksson KN, Sundberg E, Engström P. 2003. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis . Developmental Biology 264, 228–239. [DOI] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G. 2005. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO Journal 24, 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Franke-van Dijk M, Vencken RJ, Quint A, Hooykaas P, Offringa R. 2001. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128, 4289–4299. [DOI] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. 2006. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Developmental Cell 10, 265–270. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Wu MF, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA, Wagner D. 2013. A molecular framework for auxin-mediated initiation of flower primordia. Developmental Cell 24, 271–282. [DOI] [PubMed] [Google Scholar]
- Zúñiga-Mayo VM, Marsch-Martínez N, de Folter S. 2012. JAIBA, a class-II HD-ZIP transcription factor involved in the regulation of meristematic activity, and important for correct gynoecium and fruit development in Arabidopsis . The Plant Journal 71, 314–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.