Abstract
Zn deficiency is a widespread problem in rice (Oryza sativa L.) grown under flooded conditions, limiting growth and grain Zn accumulation. Genotypes with Zn deficiency tolerance or high grain Zn have been identified in breeding programmes, but little is known about the physiological mechanisms conferring these traits. A protocol was developed for growing rice to maturity in agar nutrient solution (ANS), with optimum Zn-sufficient growth achieved at 1.5 μM ZnSO4.7H2O. The redox potential in ANS showed a decrease from +350 mV to −200 mV, mimicking the reduced conditions of flooded paddy soils. In subsequent experiments, rice genotypes contrasting for Zn deficiency tolerance and grain Zn were grown in ANS with sufficient and deficient Zn to assess differences in root uptake of Zn, root-to-shoot Zn translocation, and in the predominant sources of Zn accumulation in the grain. Zn efficiency of a genotype was highly influenced by root-to-shoot translocation of Zn and total Zn uptake. Translocation of Zn from root to shoot was more limiting at later growth stages than at the vegetative stage. Under Zn-sufficient conditions, continued root uptake during the grain-filling stage was the predominant source of grain Zn loading in rice, whereas, under Zn-deficient conditions, some genotypes demonstrated remobilization of Zn from shoot and root to grain in addition to root uptake. Understanding the mechanisms of grain Zn loading in rice is crucial in selecting high grain Zn donors for target-specific breeding and also to establish fertilizer and water management strategies for achieving high grain Zn.
Key words: Agar nutrient solution, grain Zn, grain Zn loading, remobilization, rice, Zn deficiency tolerance, Zn efficiency, Zn translocation, Zn uptake.
Introduction
Zn deficiency is a global threat that affects both crop production and human nutrition (Quijano-Guerta et al., 2002; Hotz and Brown, 2004). In humans, Zn deficiency-induced malnutrition adversely affects overall growth, leading to stunting in children, susceptibility to infectious diseases, iron deficiency anaemia, and poor birth outcome in pregnant women (Prasad, 2009; Graham et al., 2012). The lack of diversity in the diet and poor-quality foods with routine consumption of cereal-based staples are the main causes of Zn deficiency in humans (Pfeiffer and McClafferty, 2007). In rice, low plant-available Zn in soil causes leaf bronzing and poor tillering at the early growth stages, leading to delayed maturity and significant yield loss (Neue et al., 1998; Dobermann and Fairhurst, 2000). The main cause of deficiency of plant-available Zn in soil is the precipitation or adsorption of Zn with various soil components, depending on the pH and redox potential (Impa and Johnson-Beebout, 2012). One of the interventions that have been proposed to overcome Zn deficiency in humans is the biofortification of staple foods with Zn during their natural growth cycle, through either agronomic practices or genetic manipulations (Cakmak, 2008; White and Broadly, 2009).
Zn efficiency, which is used synonymously with Zn deficiency tolerance, reflects the ability of a plant to grow and yield well under Zn-deficient conditions. The proposed physiological mechanisms for Zn efficiency in rice at the early vegetative growth stage can be grouped into two categories: those related to increased root uptake of Zn and those related to internal Zn distribution. Mechanisms for increased root uptake in rice include proliferation of crown root growth, exudation of organic acids or phytosiderophores, and increased tolerance of radical oxygen stress (Frei et al., 2010; Widodo et al., 2010). The proposed mechanisms for improved internal Zn distribution in crop plants are increased phloem mobility and root-to-shoot Zn translocation, subcellular Zn compartmentation, and biochemical Zn use (Hacisalihoglu and Kochian, 2003; White and Broadly 2011). However, in rice, a consistent association of any of the above-mentioned processes with Zn efficiency over a large number of genotypes is still lacking.
Grain Zn accumulation mechanisms in rice plants can be grouped into two categories according to the predominant sources of Zn loading: as continued root uptake during the grain-filling stage (Jiang et al., 2007) and remobilization of Zn from shoots or roots (Wu et al., 2010). Two published studies on grain Zn loading mechanisms in rice using Zn-sufficient or excess conditions reported contradictory results, with Jiang et al. (2007) observing continued root uptake as the predominant source of grain Zn loading and Wu et al. (2010) observing remobilization from source tissues to grain as an important source of grain Zn in some genotypes. Based on observations from other crop plants such as wheat, the relative importance of these two sources could change under Zn-deficient conditions (Kutman et al., 2012). The variations in predominant grain Zn loading sources in rice genotypes that have been selected for high grain Zn concentration and the factors influencing these sources are still unknown.
Because of the difficulty in controlling the timing and severity of Zn deficiency in the field, it is important to develop a solution culture method that can be used to compare Zn uptake in contrasting Zn supply conditions and genotypes, at both the seedling stage and maturity. Agar nutrient solution (ANS), also called stagnant solution, is known to induce reduced rhizosphere conditions due to the restricted convection or slower gas diffusion (Wiengweera et al., 1997). Low convection in ANS inhibits the free movement of gases and solutes, thus mimicking the reduced conditions of flooded soils. ANS has been used as a growth medium for rice at the early vegetative stage to screen genotypes for Zn deficiency tolerance, to study the radial oxygen loss from roots, and to study root morphology (Colmer, 2003; Wang et al., 2008). To grow rice to maturity in ANS, which usually takes 4–5 months instead of only a few weeks for early vegetative phase studies, requires optimization of nutrient supply throughout the growth cycle, which is especially challenging under Zn-deficient conditions. To the authors’ knowledge, this is the first study that uses ANS to grow rice to maturity for the purpose of understanding the effects of variable Zn concentrations on grain Zn accumulation.
The overall aim of the study is to understand the effect of contrasting solution Zn concentrations on Zn uptake by roots, Zn translocation within the plant, and Zn loading into grains of selected rice genotypes. The specific objectives are (i) to establish Zn-sufficient and Zn-deficient conditions for the growth of rice in ANS until maturity; (ii) to identify the physiological traits that influence Zn deficiency tolerance; and (iii) to unravel the predominant sources of grain Zn loading in rice. Related to these objectives, the following hypotheses were tested. (H1) With low redox potential induced in ANS, increased Zn supply beyond the optimum concentration would not benefit the plants. (H2) Zn-efficient genotypes would have a higher total Zn uptake and root-to-shoot Zn translocation during the vegetative growth phase compared with Zn-inefficient genotypes. (H3) Grain Zn loading occurs primarily through continued root uptake under Zn-sufficient conditions, whereas, in Zn-deficient conditions, grain Zn is predominantly remobilized from source tissues such as leaves, stems, and roots.
Materials and methods
Three separate experiments were conducted in a greenhouse using ANS. The first was designed to optimize Zn concentrations of ANS for Zn-sufficient and Zn-deficient conditions for growth of rice and used only one genotype (IR74). In the second and third experiments, 10 and four genotypes, respectively, were used to compare the physiological traits influencing Zn uptake and transport under Zn-deficient and sufficient conditions (Table 1).
Table 1.
Initial seed Zn concentration, days to flowering, and descriptions of the rice genotypes used in Experiments 2 and 3.
| Rice genotypes | Referred to in text as | Seed Zn concentration before sowing (mg kg–1) | Days to 50% flowering (DAP) (Zn- sufficient/Zn- deficient conditions) | Days to maturity (DAP) | Description |
|---|---|---|---|---|---|
| IR74 | IR74 | 14.5 | 84/83 | 114/114 | Irrigated lowland indica variety, susceptible to Zn deficiency at vegetative stage (Wissuwa et al., 2006) |
| Jalmagna | Jalmagna | 10.7 | Did not flower | NA | Deepwater cultivar from Northern India, tolerant of Zn deficiency at vegetative stage (Wissuwa et al., 2006) |
| RIL-46 (Jalmagna×IR74) | RIL-46 | 18.5 | 74/74 | 110/110 | Recombinant inbred line known to have tolerance alleles for Zn deficiency at all major QTLs (Wissuwa et al., 2006) |
| IR64 | IR64 | 17.0 | 54/61 | 84/91 | Lowland-adapted indica variety with moderate grain Zn (Wu et al., 2010) |
| Joryoongbyeo | Joryoongbyeo | 21.0 | 44/44 | 85/85 | Temperate japonica, Zn biofortification donor |
| SWHOO | SWHOO | 14.0 | 44/45 | 84/84 | Temperate japonica, Zn biofortification donor |
| IR69428-6-1-1-3-3 (IR68510× IR65600-1-3-2) | IR69428 | 30.0 (Exp 2), 23.0 (Exp 3) | 92/94, 102/105 | 128/128, 155/155 | Tropical japonica, Zn biofortification breeding line |
| IR75862-206-2-8-3-B-B-B (IR75083× IR65600-81-5-3-2) | IR75862 | 29.0 | 98/98 | 128/128 | Tropical japonica, Zn biofortification breeding line |
| IR68144-2B-2-2-3-1-127 (IR72× Zawa Bonday) | IR68144 | 14.0 | 62/62 | 93/93 | Zn biofortification breeding line |
| IR82247-5-3-3-2 (Jalmagna×Zuchein) | IR82247 | 15.0 | 89/89 | 114/114 | Zn biofortification breeding line |
| A69-1 (BG94-1×Pokkali) | A69-1 | 14.0 | 92/102 | 139/150 | Zn-efficient line |
| IR55179-3B-11–3 (IR4630-22-2-5-1-3× Nona Bokra) | IR55179 | 18.0 | 92/98 | 142/151 | Zn-efficient line |
| Kinandang Patong | KP | 20.0 | 102/NA | 149/NA | Upland japonica, Zn deficiency-susceptible line |
Seed includes hull and brown rice.
Parents of breeding lines are given in parentheses.
NA, not available. DAP, days after planting.
Experiment 1
Plant husbandry
The experiment was carried out in the greenhouse facility of the International Rice Research Institute (IRRI), using IR74, a lowland Zn deficiency-sensitive rice variety. All the plasticware and glassware were thoroughly washed with soap solution, later soaked in 3 N HCl for 30min, and then rinsed twice with de-ionized water to minimize Zn contamination. Seed dormancy was broken by incubation at 50 °C for 3 d, followed by germination in moist aerobic conditions in the dark at 25 °C for 4 d. The sprouted seeds were floated on 0.5mM CaCl2 solution with 10 μM FeNaEDTA (ferric sodium ethylenediaminetetra-acetic acid) for 1 week. The seedlings were then transferred to 3.5 litre pots filled with half-strength modified Yoshida nutrient solution (YNS) without Zn for 2 weeks. The composition of modified YNS at full strength is as follows: 1.77mM NH4NO3, 0.32mM NaH2PO4·2H2O, 0.5mM K2SO4, 1mM CaCl2·2H2O, 1mM MgSO4·7H2O, 9 μM MnCl2·4H2O, 0.5 μM (NH4)6Mo7O24·4H2O, 18.5 μM H3BO3, 0.16 μM CuSO4·5H2O, 36 μM FeNaEDTA. The first day of transferring plants in YNS is considered as day 0 [0 days after planting (DAP)] for all the reported data. The pots were replenished with fresh half-strength YNS once every 3 d. The 3-week-old plants were then transferred to ANS, which contained 0.1% agar in modified full-strength YNS with Zn at five different concentrations ranging from 0.005 μM to 6.5 μM ZnSO4·7H2O. The pH of the solution was adjusted to 8 with NaOH. The pots were arranged in a randomized complete block design with three replicates, and ANS in these pots was replenished once every 14 d. The 3.5 litre plastic pots were fitted with styrofoam lids with six openings for the plant growth.
Sampling and measurements
Portable data loggers (HOBOware Pro, Version 2.2.1) were placed at ~15cm above the plant canopy in the greenhouse to record the temperature and relative humidity at 1h intervals. The pH and redox potential of ANS were monitored once every 3 d, using a pH meter with an internal Ag/Ag2Cl2 reference electrode (Oakton Portable pH/mV/Temperature Meter, Eutech Instruments, Malaysia) to measure pH, and by using the same meter set on mV mode to measure the redox potential in conjunction with platinum electrodes that had been permanently installed in both the rhizosphere and toward the outer edge of the pot (bulk solution). The temperature of the ANS in the pots was also recorded once every 3 d at ~09:00h using the same meter set on temperature mode. Leaf deficiency symptoms were scored every other day starting from 23 to 35 DAP and given scores from 0 to 9, with 0 for no symptoms and 9 for the most severe symptoms, according to IRRI’s standard evaluation system (IRRI, 1996). The ANS was sampled initially for every batch and also once from each pot after 14 d, immediately prior to replenishment, and analysed for Zn concentration using flame atomic absorption spectrometry (Analyst 200 AAS, Perkin-Elmer, USA).
Destructive sampling was done at three different growth stages: early vegetative stage (15 d after exposure to Zn treatment in ANS), maximum tillering stage (40 d after exposure to Zn treatments in ANS), and at maturity. Maximum root length, from the bottom-most node of the stem to the tip of the longest root, was recorded at each growth stage. To estimate the dry weight and Zn concentration of plant tissues, the harvested plants were thoroughly washed with de-ionized water, separated into different tissues, namely stem (including leaf sheaths), leaf blades, roots, and panicles (at later growth stages), and dried in an oven at 80 °C for at least 3 d until constant weight was obtained. Dried samples were ground to a fine powder using a Wiley mill (Wiley® Mini Mill, Thomas Scientific, USA). The powdered samples were sent to IRRI’s analytical service laboratory (ASL, IRRI, http://asl.irri.org/lims/; last accessed on 21st April 2013) for quantification of Zn concentration using acid digestion in 1% nitric acid (HNO3) and 2.8% perchloric acid (HClO4), followed by inductively coupled plasma–optical emission spectrometry (ICP-OES) analysis (Perkin Elmer Inc.; Optima5300DV, USA).
Experiments 2 and 3
These two experiments were set up in the same location as Experiment 1. Crop growth conditions, management, and experimental design were similar to those in Experiment 1, with the following modifications: (i) instead of growing plants continuously for 2 weeks in half-strength YNS without Zn, the plants were grown for 1 week in half-strength YNS without Zn and 1 week in half-strength YNS with the respective Zn-deficient and sufficient treatments; (ii) Zn was supplied in two concentrations, 0.005 μM (Zn-deficient) and 1.5 μM ZnSO4·7H2O (Zn-sufficient), with five replications each. All the parameters measured were similar to those in Experiment 1, except that the three different growth stages sampled were early vegetative (15 d after exposure to Zn treatment in ANS), 50% flowering, and maturity. The exact timing of the latter two growth stages varied by genotype and Zn treatment, as is listed in Table 1.
Calculations and statistics
For the nutrient solution measurements, redox potential was corrected for temperature variation and expressed in reference to a standard hydrogen electrode by using the following equation (PCRA, 2007):
| Redox potential corrected=Redox potential measured+{200−[0.65×(measured temperature °C−25 °C)]} |
For the plant measurements, total dry matter (TDM) accumulated per plant was derived by summing the dry weights of roots, stems, leaves, and panicles (if any). Zn efficiency was expressed as the ratio of shoot dry weight under Zn-deficient conditions to the shoot dry weight under Zn-sufficient conditions (Rengel and Graham, 1996). Total Zn content in a specific plant tissue was calculated as the product of the tissue’s Zn concentration and dry weight. The root-to-shoot Zn translocation index was calculated as the ratio of total shoot Zn content to total Zn content per plant (Rengel and Graham, 1996). Analysis of variance (ANOVA) was performed using R/aov, and correlation and regression analysis were performed using R/corr [R version 2.11.0 (2010-04-22)]. The predominant sources of grain Zn loading in each genotype were derived by a mass-balance technique, by calculating the difference in root, stem+sheath, leaf blade, and panicle Zn content between 50% flowering and maturity. The results are interpreted with the assumption that grains are the major sinks in plants during the grain-filling period and Zn would mostly remobilize from stems, leaves, and roots into grain. There could also be some remobilization of Zn from leaves/stems to roots, especially in Zn-deficient conditions, but this is considered to be negligible during the grain-filling period.
Results
Across the three experiments, the average day and night mean temperatures in the greenhouse ranged from 33 to 36 °C and from 26 to 28 °C, respectively, whereas average day and night relative humidity ranged from 54% to 63% and from 75% to 83 %, respectively. The ANS average temperature was ~30 °C across the three experiments.
Redox potential of ANS
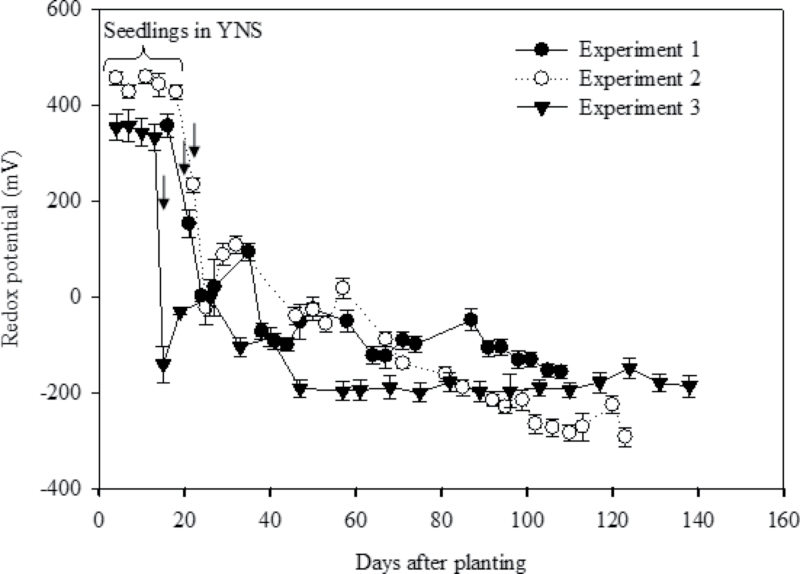
The redox potential of YNS remained more or less constant, whereas the redox potential of ANS showed a gradual decrease over time in all the experiments, with a sharp reduction during the first 3 d following transfer in the ANS (Fig. 1). On average, the redox potential of ANS decreased from +350 mV to –200 mV during the experimental period. There was no significant difference between the redox potential in the bulk agar and the rhizosphere; hence, the average of the rhizosphere and bulk agar redox potential is presented (Fig. 1).
Fig. 1.
Redox potential of Yoshida nutrient solution (YNS) and Agar nutrient solution (ANS) over time in both the experiments. Arrows near each line indicate the time when plants were transferred from YNS to ANS. Values are means ±SE (n=8 for Experiment 1, n=39 for Experiment 2, and n=15 for Experiment 3).
Experiment 1
Treatment verification and Zn deficiency-induced leaf symptoms
Zn deficiency-induced leaf symptom development and severity were closely related to the Zn supply in ANS, with treatments of 0.005, 0.15, 1.5, 3.5, and 6.5 μM ZnSO4·7H2O recording Zn deficiency-induced symptom scores of 7, 2.3, 0, 0.32, and 0.67, respectively. Within a week after beginning the Zn treatments, brownish lesions started to appear in the lower mature leaves at 0.005 μM and 0.15 μM ZnSO4·7H2O, which later enlarged and covered the whole leaf. Subsequently, the symptoms started to develop on the upper young leaves too. The higher Zn supply treatments (3.5 μM and 6.5 μM ZnSO4·7H2O) showed burning symptoms at the tip of the older leaves and rolling of leaves, which might be due to Zn toxicity. Zn deficiency symptoms on the newly developed leaves disappeared as the plants entered the recovery phase at ~45 DAP. The plants that survive Zn deficiency usually enter the recovery phase starting at ~10–12 weeks after transplanting rice in flooded conditions, during which the newly developed leaves did not show Zn deficiency symptoms (Wissuwa et al., 2006).
Plant growth, Zn uptake, and translocation
Plants grown in 0.005 μM Zn recorded significantly lower total dry matter (TDM) than all four higher solution Zn concentrations at the early vegetative and maximum tillering stage, whereas, at maturity, both 0.005 μM and 0.15 μM Zn supply treatments showed a significantly lower TDM than the other three higher solution Zn concentrations (Table 2). There was no increase in TDM beyond 1.5 μM Zn supply. Total Zn content per plant at different growth stages increased with increasing Zn supply. Moreover, the root-to-shoot Zn translocation index was significantly higher at high Zn supply (≥1.5 μM ZnSO4·7H2O) than at low Zn supply at the early vegetative stage. In contrast, during later growth stages, the Zn translocation index was significantly lower at high Zn supply than at low Zn supply.
Table 2.
Biomass accumulation, total Zn content, and root-to-shoot Zn translocation index in IR74 grown under different Zn concentrations in Experiment 1.
| Growth stages | Trait | ZnSO4·7H2O concentrations (μM) | ||||
|---|---|---|---|---|---|---|
| 0.005 | 0.15 | 1.5 | 3.5 | 6.5 | ||
| Early vegetative (14 d in ANS) | Shoot dry weight (g plant–1) | 0.37±0.02 b | 0.54±0.04 a | 0.49±0.03 a | 0.47±0.01 a | 0.48±0.02 a |
| Root dry weight (g plant–1) | 0.16±0.01 b | 0.26±0.03 a | 0.23±0.02 a | 0.24±0.01 a | 0.22±0.01 a | |
| Total dry matter (g plant–1) | 0.53±0.03 b | 0.80±0.06 a | 0.72±0.04 a | 0.71±0.02 a | 0.70±0.03 a | |
| Total Zn content (μg plant–1) | 9±1 d | 12±2 d | 21±0.6 c | 39±0.8 b | 47±4 a | |
| Root-to-shoot Zn translocation index (%) | 50±2 b | 50±3 b | 69±1 a | 67±2.5 a | 66±3 a | |
| Maximum tillering (40 d in ANS) | Shoot dry weight (g plant–1) | 1.3±0.03 b | 1.6±0.09 a | 1.8±0.1 a | 1.7±0.09 a | 1.7±0.08 a |
| Root dry weight (g plant–1) | 0.73±0.06 b | 0.87±0.02 ab | 0.96±0.03 a | 0.89±0.06 a | 0.87±0.002 ab | |
| Total dry matter (g plant–1) | 1.6±0.06 b | 1.9±0.07 a | 2.1±0.1 a | 2.0±0.1 a | 2.0±0.05 a | |
| Total Zn content (μg plant–1) | 26±4 d | 33±2 d | 81±0.8 c | 111±7 b | 160±5 a | |
| Root-to-shoot Zn translocation index (%) | 51±0.6 a | 52±3 a | 45±2 b | 48±1 ab | 41±3 c | |
| Maturity | Shoot dry weight (g plant–1) | 3.1±0.8 b | 3.5±0.2 b | 6.4±0.9 a | 6.1±0.2 a | 6.1±0.2 a |
| Root dry weight (g plant–1) | 0.8±0.1 c | 1.3±0.02 b | 1.6±0.2 ab | 1.8±0.08 a | 1.8±0.06 a | |
| Total dry matter (g plant–1) | 4.0±0.9 b | 4.8±0.2 b | 8.3±1 a | 8.2±0.2 a | 8.3±0.2 a | |
| Total Zn content (μg plant–1) | 56±2 d | 89±3.6 d | 195±17 c | 322±18.7 b | 595±36.4 a | |
| Root-to-shoot Zn translocation index (%) | 58±4 a | 52±3 ab | 44±3 bc | 34±3.8 c | 35±4.3 c | |
Values within a row with different letters are significantly different between different Zn treatments at 5% LSD. The values given are means ±SE (n=3).
Experiments 2 and 3
Although the ANS conditions were designed to be similar in Experiments 2 and 3, more severe Zn deficiency symptoms were observed in Experiment 3. Measurements of the initial Zn concentration in ANS showed that differences of at least 0.1mg kg–1 between Zn-deficient and Zn-sufficient conditions were maintained for both Experiment 2 (Zn deficiency, 0.08±0.06mg kg–1; and Zn suffficiency, 0.24±0.06mg kg–1) and Experiment 3 (Zn deficiency, 0.023±0.004mg kg–1; and Zn sufficiency 0.15±0.06mg kg–1). The lower Zn concentrations in Experiment 3 were attributed to cleaner ANS prior to Zn addition, perhaps due to a change in the batch of agar v or to greater water purity. The average depletion of Zn in ANS over 14 d was ~0.15mg kg–1 under Zn-sufficient conditions in both the experiments and at Zn-deficient conditions it was 0.03mg kg–1 and 0.014mg kg–1 in Experiment 2 and 3, respectively.
Plant growth, Zn uptake, and translocation
Genotypes differed significantly in Zn efficiency at the early vegetative stage (Table 3), with IR74 showing the lowest and IR75862 the highest Zn efficiency in Experiment 2, respectively, and with KP showing significantly lower Zn efficiency than IR69428 and IR55179 in Experiment 3. Under the Zn-deficient conditions of Experiment 2, SWHOO, Joryoongbyeo, RIL46, and Jalmagna had lower leaf deficiency symptom scores (ranging from 0 to 0.6), while IR82247, IR64, and IR74 had the highest leaf deficiency symptom scores (ranging from 5 to 6.3) at the early vegetative stage (Table 3). In contrast, in Experiment 3, A69-1 showed significantly lower Zn deficiency symptoms than the other three lines. There was no significant correlation between leaf symptom scores and Zn efficiency of genotypes in either experiment; however, Zn efficiency showed a significant positive correlation with the root-to-shoot Zn translocation index (Experiment 2, r=0.5, P=0.01, n=25; Experiment 3, r=0.6, P=0.027, n=12) and total Zn content per plant (Experiment 3, r=0.78, P=0.01, n=13) at the early vegetative stage (Supplementary Table S1 available at JXB online).
Table 3.
Zn efficiency and Zn deficiency leaf symptom scores of rice genotypes under Zn-deficient conditions at early vegetative stage in Experiments 2 and 3.
| Experiment | Genotype | Zn efficiency (%) | Zn deficiency symptom score |
|---|---|---|---|
| Experiment 2 | IR82247 | 103 ab | 6.3 a |
| IR64 | 90 abc | 5.0 ab | |
| IR74 | 49 c | 5.0 ab | |
| IR75862 | 135 a | 3.4 abc | |
| IR68144 | 58 bc | 3.0 bcd | |
| IR69428 | 93 abc | 2.2 bcd | |
| Joryoongbyeo | 88 abc | 0.6 cd | |
| Jalmagna | 68 bc | 0.3 cd | |
| RIL-46 | 88 abc | 0.3 cd | |
| SWHOO | 98 abc | 0 d | |
| Experiment 3 | IR55179 | 83 a | 4.3 a |
| IR69428 | 66 b | 4.3 a | |
| KPatong | 50 c | 3 a | |
| A69-1 | 62 bc | 0.6 b |
Within each experiment, values with different letters represent significant differences between genotypes for Zn efficiency at 5% HSD in Experiment 2 and 5% LSD in Experiment 3.
In Experiment 2, at early vegetative stage, shoot Zn concentration among the genotypes ranged from 12.7mg kg–1 to 36mg kg–1 under Zn-sufficient conditions and from 5.6mg kg–1 to 12.7mg kg–1 under Zn-deficient conditions (Supplementary Table S2 at JXB online). On the other hand, the root Zn concentration among the genotypes ranged from 13.5mg kg–1 to 39.5mg kg–1 under Zn-sufficient and from 11mg kg–1 to 14.5mg kg–1 under Zn-deficient conditions. Total Zn content per plant and root-to-shoot Zn translocation varied significantly between genotypes and Zn treatments in the early vegetative stage. Similarly, in Experiment 3, leaf blade, root, and stem+sheath Zn concentration varied significantly between the genotypes and Zn treatments at the early vegetative stage (Supplementary Table S3). Zn deficiency significantly reduced the total Zn content per plant and the root-to-shoot Zn translocation index compared with Zn-sufficient condition in both experiments. At the early vegetative stage, the Zn concentration was substantially higher in stems (+leaf sheaths) under Zn-sufficient conditions, suggesting that they probably act as reservoirs for excess Zn when Zn supply is not limiting.
Panicle and brown rice Zn
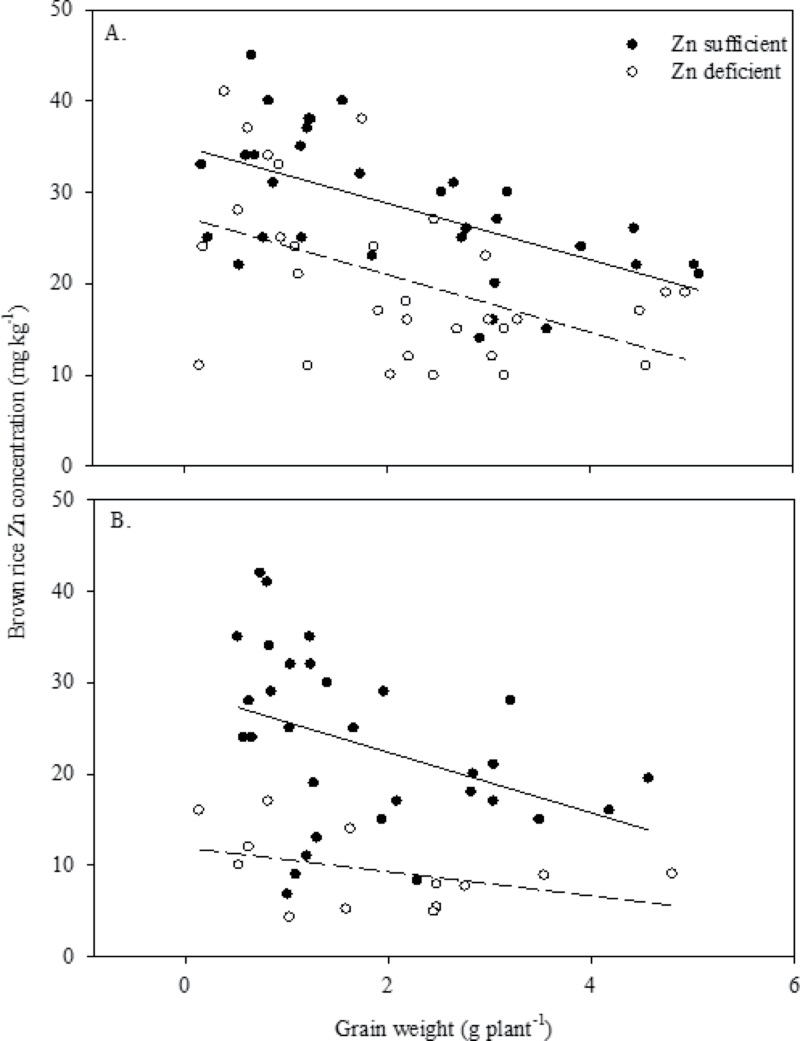
Panicle Zn concentration at 50% flowering and brown rice Zn concentration at maturity varied significantly among the genotypes and between Zn treatments in both Experiments 2 and 3; however, there was no significant difference between the Zn treatments for panicle weight or grain weight in Experiment 2 or 3 (Table 4). In Zn-sufficient conditions, a higher panicle and brown rice Zn concentration of >30 ppm was noticed in Joryoongbyeo, IR69428, and SWHOO in Experiment 2, and in A69-1 and IR69428 in Experiment 3. There was a significant positive correlation between panicle Zn concentration at 50% flowering and brown rice Zn concentration at maturity in both Zn-deficient (r=0.86, P ≤ 0.001, n=12) and Zn-sufficient (r=75, P ≤ 0.01, n=13) conditions (Table 5). Brown rice Zn concentration showed a significant positive correlation with Zn concentrations of rachis and hull under both Zn-deficient and Zn-sufficient conditions. A significant negative relationship was noticed between grain weight (g plant–1) and brown rice Zn concentration (mg kg–1) in both Zn-sufficient and Zn-deficient conditions in Experiment 2, and in Zn-sufficient condition in Experiment 3 (Fig. 2). The low genotypic variation for brown rice Zn concentration due to the greater severity of Zn deficiency in Experiment 3 resulted in a poor relationship between brown rice Zn concentration and grain weight under Zn-deficient conditions.
Table 4.
Panicle Zn concentration and weight at 50% flowering and brown rice Zn concentration and grain weight at maturity among rice genotypes grown in ANS with Zn-sufficient and Zn-deficient conditions.
| Genotype | 50% flowering | Maturity | ||||||
|---|---|---|---|---|---|---|---|---|
| Panicle Zn concentration (mg kg–1) | Panicle weight (g plant–1) | Brown rice Zn concentration (mg kg–1) | Grain weight (g plant–1) | |||||
| Zn-sufficient | Zn-deficient | Zn-sufficient | Zn-deficient | Zn-sufficient | Zn-deficient | Zn-sufficient | Zn-deficient | |
| Experiment 2 | ||||||||
| IR64 | 39 | 35 | 0.60 | 0.42 | 26 | 17 | 3.2 | 2.5 |
| IR68144 | 50 | 31 | 0.31 | 0.52 | 24 | 12 | 1.5 | 1.8 |
| IR74 | 34 | 30 | 0.71 | 1.15 | 21 | 16 | 4.5 | 4.2 |
| IR82247 | 47 | 42 | 0.79 | 0.93 | 18 | 16 | 3.2 | 2.6 |
| IR69428 | 62 | 49 | 0.32 | 0.29 | 35 | 23 | 0.7 | 1.4 |
| IR75862 | 49 | 18 | 0.40 | 0.85 | 29 | 18 | 0.2 | 0.2 |
| Joryoongbyeo | 75 | 57 | 0.54 | 0.37 | 33 | 35 | 1.1 | 0.6 |
| RIL-46 | 49 | 30 | 1.04 | 1.08 | 26 | 20 | 3.4 | 3.0 |
| SWHOO | 55 | 50 | 0.83 | 0.44 | 38 | 32 | 1.2 | 1.2 |
| 5% HSD (G) | 12*** | 0.40*** | 7.7*** | 1.0* | ||||
| 5% LSD (Zn) | 3*** | 0.12NS | 2.0*** | 0.3NS | ||||
| 5% HSD (G×Zn) | 19*** | 0.72* | 12.6* | 1.6NS | ||||
| Experiment 3 | ||||||||
| A69-1 | 35 | 9 | 1.04 | 0.54 | 32 | 10 | 1.0 | 1.3 |
| IR55179 | 29 | 8 | 1.04 | 0.98 | 20 | 7 | 3.3 | 2.9 |
| IR69428 | 46 | 19 | 0.28 | 0.28 | 30 | 18 | 1.0 | 0.5 |
| KP | 27 | – | 0.22 | – | 12 | – | 1.4 | – |
| 5% LSD(G) | 5.4*** | 0.30*** | 4*** | 0.5* | ||||
| 5% LSD (Zn) | 3.9*** | 0.20NS | 3*** | 0.4NS | ||||
| 5% HSD (G×Zn) | 11NS | 0.57NS | 8*** | 1.1NS | ||||
Values given are means (n=5). Data for Jalmagna are not given as it did not flower.
****, **, * indicate significant difference at P ≤ 0.001, 0.01, and 0.05, respectively. NS, non-significant.
G, genotype, Zn, Zn treatment. Grain weight includes hull and brown rice weight.
Table 5.
Correlation coefficients for the association between brown rice Zn concentration and other plant tissue Zn concentrations at 50% flowering and maturity in Zn-deficient and sufficient conditions.
| Zn concentration (mg kg–1 of brown rice) compared with | 50% flowering | Maturity | ||
|---|---|---|---|---|
| Zn-deficient | Zn-sufficient | Zn-deficient | Zn-sufficient | |
| Root | 0.33NS | -0.14NS | 0.32NS | –0.04NS |
| Leaf | 0.48NS | 0.34NS | 0.04NS | –0.49NS |
| Stem+sheath | 0.56NS | 0.53NS | 0.36NS | –0.23NS |
| Panicle | 0.86*** | 0.75** | – | – |
| Rachis | – | – | 0.76** | 0.55* |
| Hull | – | – | 0.93*** | 0.71** |
***, **, and * indicate significant correlations at P ≤ 0.001, 0.01, and 0.05, respectively. NS, non-significant.
n=12 and 13 for Zn-deficient and sufficient conditions, respectively, at both growth stages.
Fig. 2.
Relationship between grain weight (g plant–1) and brown rice Zn concentration (mg kg–1) under both Zn-deficient and Zn-sufficient conditions in Experiment 2 (A) (Zn sufficient, y= –3.0935x+35.005, R 2=0.33, P=0.005; Zn deficient, y= –3.1552x+27.299, R 2=0.23, P=0.006) and Experiment 3 (B) (Zn sufficient, y= –3.3119x+29.01, R 2=0.153, P=0.029; Zn deficient, y= –1.3221x+11.942, R 2=0.175, P=0.15; non-significant).
Zn allocation in different plant parts at maturity
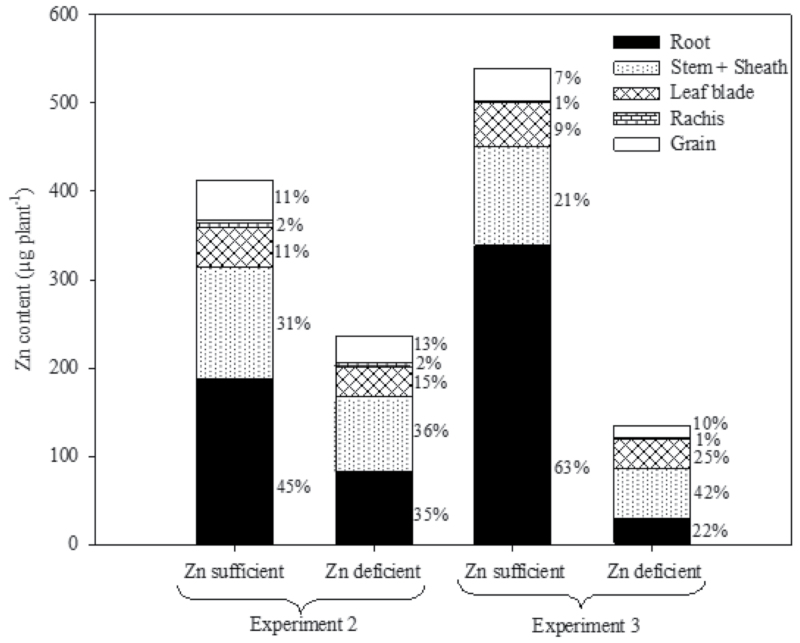
Zn deficiency significantly reduced the Zn concentration of all the plant parts at 50% flowering and maturity compared with Zn-sufficient conditions (Table 6; Supplementary Tables S4, S5 at JXB online). At maturity, higher Zn concentrations were noticed in roots than in other plant parts, irrespective of the Zn treatments and experiments. Among the above-ground plant parts, the brown rice Zn concentration was higher, while hull Zn concentration was lower than in other plant parts, irrespective of the Zn treatments and experiments. Zn deficiency drastically reduced the total Zn content per plant compared with Zn-sufficient conditions. The percentage Zn allocation to roots in Zn-sufficient conditions was higher than in Zn-deficient conditions, which contrasts with the increased allocation to stem+sheath under Zn-deficient conditions (Fig. 3). Under Zn-sufficient conditions, the percentage Zn allocation was higher in roots than in other plant tissues, whereas, under Zn-deficient conditions, the percentage Zn allocation in roots was either similar to or lower than that of stem+sheath. The percentage Zn allocation to other plant parts such as the leaf blade, rachis, and grain remained similar between the treatments (Fig. 3).
Table 6.
Average Zn concentration in different plant tissues under Zn-deficient and Zn-sufficient conditions in both Experiments 2 and 3 at maturity. Values given are averages of eight and four genotypes in Experiments 2 and 3, respectively.
| Zn concentration (mg kg–1) | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Zn-sufficient | Zn-deficient | Pr(>F) | Zn-sufficient | Zn-deficient | Pr(>F) | |
| Root | 105.0 | 58.0 | *** | 357 | 35.2 | *** |
| Stem+sheath | 23.2 | 16.0 | *** | 22.7 | 11.0 | *** |
| Leaf blade | 21.8 | 18.0 | *** | 21.3 | 14.0 | *** |
| Rachis | 22.3 | 12.6 | *** | 22.9 | 10.4 | *** |
| Hull | 20.9 | 14.7 | *** | 14.3 | 7.6 | *** |
| Brown rice | 27.7 | 20.9 | *** | 23.5 | 12.6 | *** |
*** indicates significant difference between the treatments at P ≤ 0.001.
Fig. 3.
Zn allocation to different plant tissues of rice genotypes at maturity under Zn-sufficient and Zn-deficient conditions in Experiments 2 and 3. % values indicate the percent Zn allocation to each tissue within a bar. Values given are average of eight and four genotypes in Experiments 2 and 3, respectively. Grain=brown rice+hull.
Sources of grain Zn loading
Total plant Zn at maturity increased compared with that at flowering in most cases, indicating continuing uptake of Zn from the nutrient solution during the grain-filling period (Table 7). The difference in panicle Zn content between maturity and 50% flowering was always positive, indicating net movement of Zn into the panicle. Under Zn-sufficient conditions, six of the genotypes showed net movement of Zn into all the parts of the plant, whereas the other six genotypes showed net remobilization of Zn out of at least one plant part. Under Zn-deficient conditions, three of the genotypes (IR69428, A69-1, and IR55179) still showed net movement of Zn into all plant parts. Three of the genotypes (IR68144, IR64, and Joryoongbyeo) showed net movement of Zn into roots under Zn-deficient conditions, presumably through continued root uptake, while also showing net remobilization of Zn out of the above-ground vegetative tissues, presumably into the panicle. Five of the genotypes showed net remobilization of Zn out of both the roots and above-ground vegetative tissues under Zn deficiency, presumably into the panicle in all cases except IR82247, which lost more Zn from the roots than could be accounted for by uptake into the rest of the plant, apparently indicating root leakage.
Table 7.
Mass balance comparisons of net Zn movement in various plant tissues between 50% flowering and maturity.
| Treatment | Genotype | Changes in total plant Zn content | Differences in Zn content of individual plant parts: maturity–50% flowering (μg plant–1) | Predominant sources of grain Zn loading | |||
|---|---|---|---|---|---|---|---|
| Root | Leaf blade | Stem and sheath | Panicle | Continued root uptake versus remobilization | |||
| Zn-sufficient | IR64 | 228±23 | 98±7 | 10±6 | 25±16 | 98±9 | Continued root uptake |
| IR82247 | 255±38 | 153±17 | 11±6 | 52±35 | 67±6 | Continued root uptake | |
| IR69428 (Exp 2) | 151±29 | 78±17 | 6±3 | 38±12 | 34±6 | Continued root uptake | |
| IR69428 (Exp 3) | 329±33 | 242±32 | 29±6 | 79±11 | 21±2 | Continued root uptake | |
| RIL-46 | 264±50 | 143±25 | 13±5 | 54±26 | 84±17 | Continued root uptake | |
| A69-1 | 368±22 | 312±7 | 10±5 | 56±14 | 26±4 | Continued root uptake | |
| IR55179 | 267±35 | 180±30 | 32±13 | 23±6 | 37±7 | Continued root uptake | |
| KP | 57±2 | –6±2 | 24±4 | 13±6 | 11±0.04 | Continued root uptake | |
| IR74 | 280±40 | 95±30 | –15±5 | –20±11 | 170±30 | Both | |
| SWHOO | 167±50 | 122±15 | –17±5 | –49±7 | 49±6 | Remobilization from shoot | |
| IR68144 | 71±34 | 53±4 | –7±5 | –11±10 | 50±14 | Both | |
| Joryoongbyeo | 139±29 | 105±30 | 0.6±1.7 | –14±4 | 36±12 | Both | |
| IR75862 | No dataa | 45±20 | –6±2 | –6±6 | No dataa | ND | |
| Zn-deficient | IR64 | 90±31 | 44±14 | –8±3 | –38±9 | 50±11 | Both |
| IR82247 | –115±37 | –114±11 | 9±4 | –16±6 | 46±16 | Remobilization from roots | |
| IR69428 (Exp 2) | 165±49 | 70±28 | 14±3 | 35±23 | 47±12 | Continued root uptake | |
| IR69428 (Exp 3) | 141±22 | 22±3 | 42±18 | 35±12 | 50±15 | Continued root uptake | |
| RIL-46 | –25±14 | –49±21 | –6±3 | –36±14 | 49±11 | Remobilization from root and shoot | |
| A69-1 | 42±15 | 7±4 | 3.4±2.6 | 25±12 | 9±3 | Continued root uptake | |
| IR55179 | 31±8 | 6±3 | 4±3 | 8±3 | 16±5 | Continued root uptake | |
| IR74 | 97±24 | –5±2 | –6±4 | –40±16 | 110±35 | Both | |
| SWHOO | 6±3 | –9±8 | –7±2 | –39±9 | 38±18 | Remobilization from root and shoot | |
| IR68144 | 13±5 | 7±3 | –9±2 | –13±3 | 18±5 | Remobilization from shoot | |
| Joryoongbyeo | 70±30 | 52±12 | –4±2 | –20±7 | 23±4 | Remobilization from shoot | |
| IR75862 | No dataa | –36±28 | –2±1 | –35±4 | No dataa | ND | |
Panicle includes rachis, hull, and brown rice. Values given are means ±SE (n=5).
a IR75862 had insufficient amount of ground hull samples for Zn measurement through ICP-MS at maturity. KP did not produce grains under Zn-deficient conditions, ND = not determined.
Discussion
Objective 1: Optimization of ANS protocol
The development of reduced conditions using ANS (Fig. 1) showed that this method mimics the reducing environment in flooded soils. Maintaining the required concentration of Zn in ANS solution was challenging due to Zn contamination from sources such as plastic pots, glassware, stock nutrient solution chemicals, and the water used in the experiment. A significantly higher bronzing of leaves and lower biomass production at the vegetative stage (Table 2) at the lower solution Zn concentrations in IR74 indicate the successful induction of Zn deficiency. The Zn-deficient conditions that were developed at 0.005 μM Zn supply were appropriate for Zn deficiency that allowed growth of all the genotypes until maturity, except the most susceptible genotype, KP. The faster formation of a chemically reduced rhizosphere and the faster induction of Zn deficiency in ANS suggest that the 0.005 μM Zn supply can be used successfully for physiological screening of rice genotypes for Zn deficiency tolerance under low redox conditions.
The consistent increase in total Zn uptake with increasing Zn supply in Experiment 1 (Table 2) did not result in a similar increase in TDM, which suggests that plants require a certain level of Zn for normal growth and development (Dobermann and Fairhurst, 2000), beyond which any increase in Zn supply would not result in an additional benefits to the plants, thus confirming the first hypothesis (H1). Moreover, at maximum tillering and maturity, most of the Zn taken up by the plants at high Zn supply accumulated in the roots rather than being translocated to upper plant parts. Hence, low leaf deficiency symptoms accompanied by high biomass accumulation at 1.5 μM Zn supply indicate that this is an optimum concentration for growing rice plants in ANS through maturity.
Objective 2: Physiological traits that influence Zn deficiency tolerance
In this study, the initial seed Zn concentration did not correlate with leaf deficiency symptoms (r=0.16NS, n=13) or Zn efficiency at the early vegetative stage (Supplementary Table S1 at JXB online), indicating that the genotypic differences observed in Zn efficiency were not influenced by initial seed Zn concentration. This poor correlation between initial seed Zn concentration and Zn efficiency could be due to the poor translocation of Zn from grain to growing plant parts (Cakmak et al., 1999).
The shoot Zn concentration under Zn deficiency at the early vegetative stage was well below the range of 15–20 ppm (Supplementary Tables S5, S6 at JXB online), considered critical for lowland rice (Dobermann and Fairhurst, 2000). Among the different traits that may be responsible for inducing Zn deficiency tolerance, root-to-shoot Zn translocation emerged as the most important trait contributing to genotypic variation in Zn efficiency under the moderate Zn deficiency of Experiment 2, whereas in severe Zn deficiency conditions of Experiment 3, both total Zn uptake and root-to-shoot Zn translocation are probably important (Supplementary Table S1). This confirms the second hypothesis (H2). Other traits such as root length and leaf and stem Zn concentrations did not correlate with Zn efficiency.
Objective 3: Physiological processes that influence grain Zn accumulation
The significant genotypic variation noticed for brown rice Zn concentration was highly influenced by variation in grain weight per plant (Fig. 2). Grain weight and grain micronutrient concentration are often negatively correlated (Mc Donald et al., 2008). Enhanced growth or an increase in yield generally reduces the concentration of a micronutrient even though the total uptake increases (Marschner, 1995), which is often termed the ‘dilution effect’, but which may be more usefully thought of as a ‘concentration effect’ that occurs when grain yield is lower than desired. In other words, a genotype with a higher harvest index would show a lower grain Zn concentration than a genotype with a lower harvest index. Therefore, while screening genotypes for high grain Zn, grain yield must be closely monitored in order to select the genotypes that have inherent capacity for increased grain Zn accumulation. However, in the present study, the harvest index varied significantly but it did not show significant correlations with brown rice Zn concentration in either of the Zn treatments (r= –0.49NS, n=12 in Zn-deficient and r= –0.47NS, n=13 in Zn-sufficient conditions).
Internal distribution and retention of Zn in different plant parts play a key role in determining grain Zn accumulation (Jiang et al., 2007). In this study, a significantly higher root Zn concentration compared with upper plant parts was observed under both Zn treatments at maturity (Table 6), and 45–63% of total Zn taken up by the plant accumulated in roots rather than being transported to upper plant parts under Zn-sufficient conditions at maturity (Fig. 3), which indicates a possible threshold level for Zn translocation to the shoots. The fact that this high Zn concentration in roots was seen only during the reproductive stage (Table 6), but not at the early vegetative stage (Supplementary Tables S2, S3 at JXB online), may suggest that translocation from root to shoot probably slowed down during later stages, but this awaits further confirmation.
Under Zn-sufficient conditions in almost all the genotypes, continued root uptake of Zn during grain filling was apparently the predominant source of grain Zn loading (Table 7), except in SWHOO, IR68144, and Joroyoongbyeo, in which most of the Zn taken up by roots accumulated in roots, rather than being transported to grain, and net remobilization of Zn from shoots also contributed to increased grain Zn. In this study, four genotypes, namely SWHOO, Joryoongbyeo, IR69428, and A69-1, showed a brown rice Zn concentration of ≥30mg kg–1 in Zn-sufficient conditions. Out of these four high grain Zn genotypes, in IR69428 and A69-1, continued root uptake was the predominant source of grain Zn loading irrespective of Zn treatments. In contrast, in SWHOO and Joryoongbyeo, net remobilization was the predominant source of grain Zn loading under both the Zn treatments and these genotypes also showed a brown rice Zn concentration of ≥30mg kg–1 under Zn-deficient conditions, unlike IR69428 and A69-1. This suggests that genotypes with a high remobilization capacity benefit more in terms of grain Zn loading under Zn-deficient conditions. Apart from Zn availability in the rhizosphere, the other factors that could determine the mode of grain Zn loading are root activity during the grain-filling period and the rate of senescence. In this study, genotypes such as IR69428, A69-1, IR55179, and KP that matured late showed continued root uptake as the predominant source of grain Zn loading, whereas, in SWHOO and Joryoongbyeo that matured earlier, net remobilization of Zn from shoot and root to grain was predominant.
The first part of the third hypothesis (H3) was confirmed by the observations that continued root uptake was the most important source of grain loading in almost all of the genotypes at Zn sufficient condition. In contrast, under Zn-deficient conditions, genotypes varied considerably in their predominant sources of grain Zn loading, which disagrees with the latter part of the third hypothesis (H3), suggesting a greater genotypic variability for this trait. In Zn-deficient or especially in flooded soils, which tend to develop Zn deficiency with a progressive decrease in their redox potential, any small amount of available Zn, particularly in the aerated layers of the soil, will be taken up by the plants early in the season, so plants would have to depend on remobilization for grain Zn accumulation. In contrast, in the case of lowlands that are flood prone during the pre-rice fallow period or during the early part of the rice cropping season, which makes Zn less available, genotypes with strong root uptake capacity may be able to achieve high grain Zn during the normal terminal drainage period before harvest. For Zn-sufficient conditions, genotypes with both continued root uptake and efficient remobilization would have an advantage in terms of Zn uptake and grain Zn accumulation.
Conclusions
In this study (i) a protocol for growing rice plants in Zn-sufficient and Zn-deficient conditions in ANS through maturity was established. Moreover, the induction of reduced conditions and faster Zn deficiency in ANS suggests the suitability of this system to screen rice genotypes for Zn deficiency tolerance and higher grain Zn under reduced conditions; (ii) higher root-to-shoot translocation of Zn and total Zn uptake were the two most important factors responsible for the expression of high Zn efficiency in rice genotypes grown under reduced conditions; and (iii) under Zn-sufficient conditions, continued root uptake of Zn during grain filling is the predominant source of grain Zn loading in rice, whereas in Zn-deficient conditions, wide variation was observed among genotypes in their predominant sources of grain Zn loading. For some genotypes, continued root uptake dominated even under Zn-deficient conditions. The results of this study provide useful guidance for breeding and to unravel the mechanisms associated with Zn uptake and loading into grains. The study also provided an impetus for further investigations using a larger and more diverse set of genotypes to establish the most important traits likely to be effective in breeding Zn-efficient genotypes.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Correlation coefficients for the association of Zn efficiency with different traits in both Experiments 2 and 3 at early vegetative stage.
Table S2. Shoot and root Zn concentration, total Zn content per plant, and root-to-shoot Zn translocation index at early vegetative stage under Zn-sufficient and Zn-deficient conditions in Experiment 2.
Table S3. Different plant tissue Zn concentration, total Zn content per plant, and root-to-shoot Zn translocation index at early vegetative stage under Zn-sufficient and Zn-deficient conditions in Experiment 3.
Table S4. Different plant tissue Zn concentration at 50% flowering under Zn-sufficient and Zn-deficient conditions.
Table S5. Different plant tissue Zn concentration at maturity under Zn-sufficient and Zn-deficient conditions.
Acknowledgements
The authors acknowledge the financial support and the post-doctoral fellowship of SMI provided by the Swiss Agency for Development and Cooperation (SDC) through its Research Fellow Partnership Programme. The authors thank Mr Randell Eusebio for technical assistance, Drs Glenn Gregorio and Parminder Virk for providing the Zn-deficiency-tolerant and high grain Zn lines, Violeta Bartolome for providing guidance on statistical analysis and Bill Hardy for editing the manuscript.
Glossary
Abbreviations:
- ANS
agar nutrient solution
- DAP
days after planting
- TDM
total dry matter
- YNS
Yoshida nutrient solution.
References
- Cakmak I. 2008. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and Soil 302, 1–17. [Google Scholar]
- Cakmak I, Tolay I, Ozdemir A, Ozkan H, Ozturk L, Kling CI. 1999. Differences in zinc efficiency among and within diploid, tetraploid and hexaploid wheats. Annals of Botany 84, 163–171. [Google Scholar]
- Colmer TD. 2003. Arenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Annals of Botany 91, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobermann A, Fairhurst T. 2000. Rice: nutrient disorders and nutrient management. Potash and Phosphate Institute, Potash and Phosphate Institute of Canada, and International Rice Research Institute. [Google Scholar]
- Frei M, Wang YX, Ismail AM, Wissuwa M. 2010. Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Functional Plant Biology 37, 74–84. [Google Scholar]
- Graham RD, Knez M, Welch RM. 2012. How much nutritional iron deficiency in humans globally is due to an underlying zinc deficiency? Advances in Agronomy 115, 1–40. [Google Scholar]
- Hacisalihoglu G, Kochian LV. 2003. How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytologist 159, 341–350. [DOI] [PubMed] [Google Scholar]
- Hotz C, Brown KH. 2004. International Zinc Nutrition Consultative Group (IZiNCG). Technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25, S91–S203. [PubMed] [Google Scholar]
- Impa SM, Johnson-Beebout SE. 2012. Mitigating zinc deficiency and achieving high grain Zn in rice through integration of soil chemistry and plant physiology research. Plant and Soil 361, 3–41. [Google Scholar]
- IRRI. 1996. Standard evaluation system for rice. Manila: International Rice Research Institute (IRRI). [Google Scholar]
- Jiang W, Struik PC, Lingna J, van Keulen H, Ming Z, Stomph TJ. 2007. Uptake and distribution of root-applied or foliar-applied 65Zn after flowering in aerobic rice. Annals of Applied Biology 150, 383–391. [Google Scholar]
- Kutman BU, Kutman BY, Ceylan Y, Ova EA, Cakmak I. 2012. Contributions of root uptake and remobilization to grain zinc accumulation in wheat depending on post-anthesis zinc availability and nitrogen nutrition. Plant and Soil 361, 177–187. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants 2nd edn. London: Academic Press. [Google Scholar]
- McDonald GK, Genc Y, Graham RD. 2008. A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant and Soil 306, 49–55. [Google Scholar]
- Neue HU, Quijano C, Senadhira D, Setter T. 1998. Strategies for dealing with micronutrient disorders and salinity in lowland rice systems. Field Crops Research 56, 139–155. [Google Scholar]
- PCRA (Protection cathodique et revêtements associés). 2007. Recommendations PCRA 005, rev 1. Recommendations for the verification of reference electrodes. Centre Français de l’Anticorrosion: Committee for Cathodic Protection and Associated Coatings; 1–11. [Google Scholar]
- Pfeiffer WH, McClafferty B. 2007. Harvestplus: breeding crops for better nutrition. Crop Science 47, S88–S105. [Google Scholar]
- Prasad AS. 2009. Impact of the discovery of human zinc deficiency on health. Journal of American College of Nutrition 28, 257–265. [DOI] [PubMed] [Google Scholar]
- Quijano-Guerta C, Kirk GJD, Portugal AM, Bartolome VI, McLaren GC. 2002. Tolerance of rice germplasm to zinc deficiency. Field Crops Research 76, 123–130. [Google Scholar]
- Rengel Z, Graham RD. 1996. Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. Journal of Experimental Botany 47, 217–226. [Google Scholar]
- Wang Y, Frei M, Wissuwa M. 2008. An agar nutrient solution technique as a screening tool for tolerance to zinc deficiency and iron toxicity in rice. Soil Science and Plant Nutrition 54, 744–750. [Google Scholar]
- White PJ, Broadley MR. 2009. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182, 49–84. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. 2011. Physiological limits to Zn biofortification of edible crops. Frontiers in Plant Science 2, 80 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. 1997. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80, 115–123. [Google Scholar]
- Widodo BMR, Rose T, Frei M, et al. 2010. Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytologist 186, 400–414. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Ismail AM, Yanagihara S. 2006. Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiology 142, 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lu L, Yang X, Feng Y, Wei Y, Hao H, Stoffella PJ, He Z. 2010. Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. Journal of Agricultural and Food Chemistry 58, 6767–6773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.