Abstract
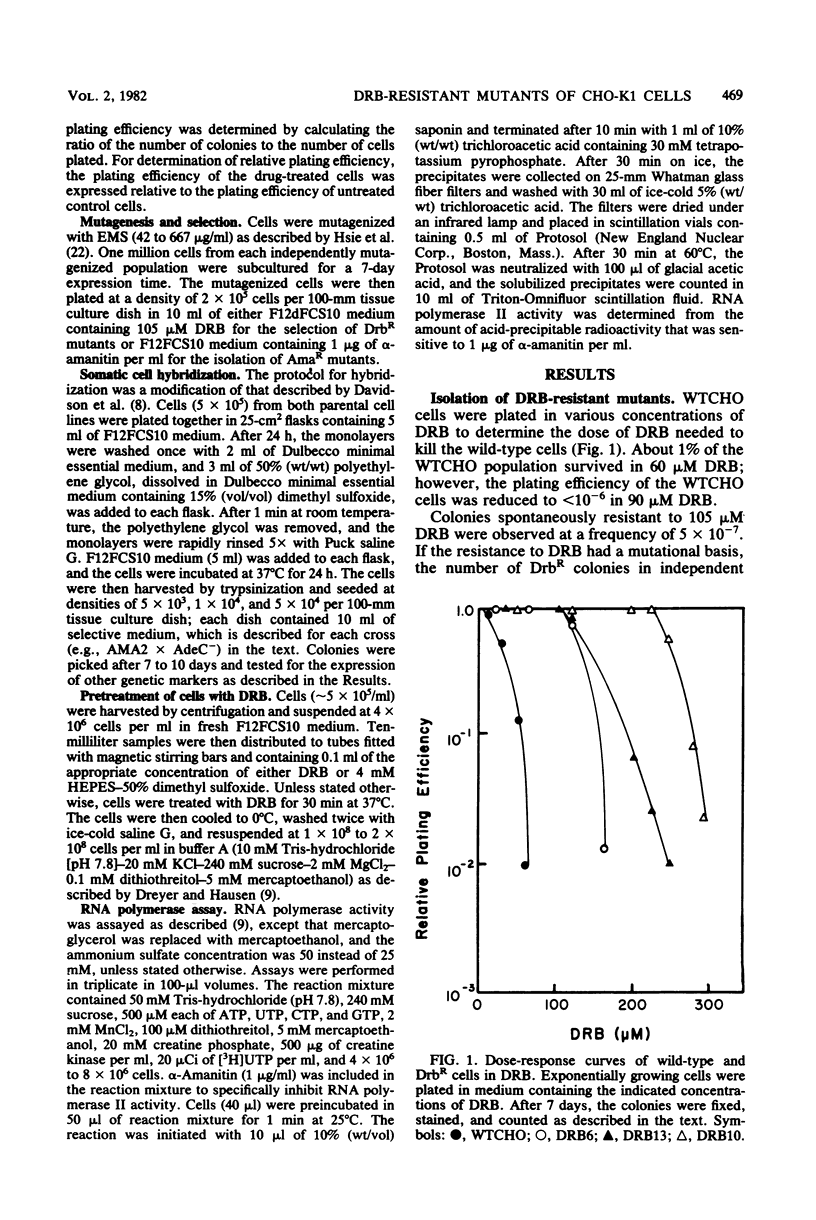
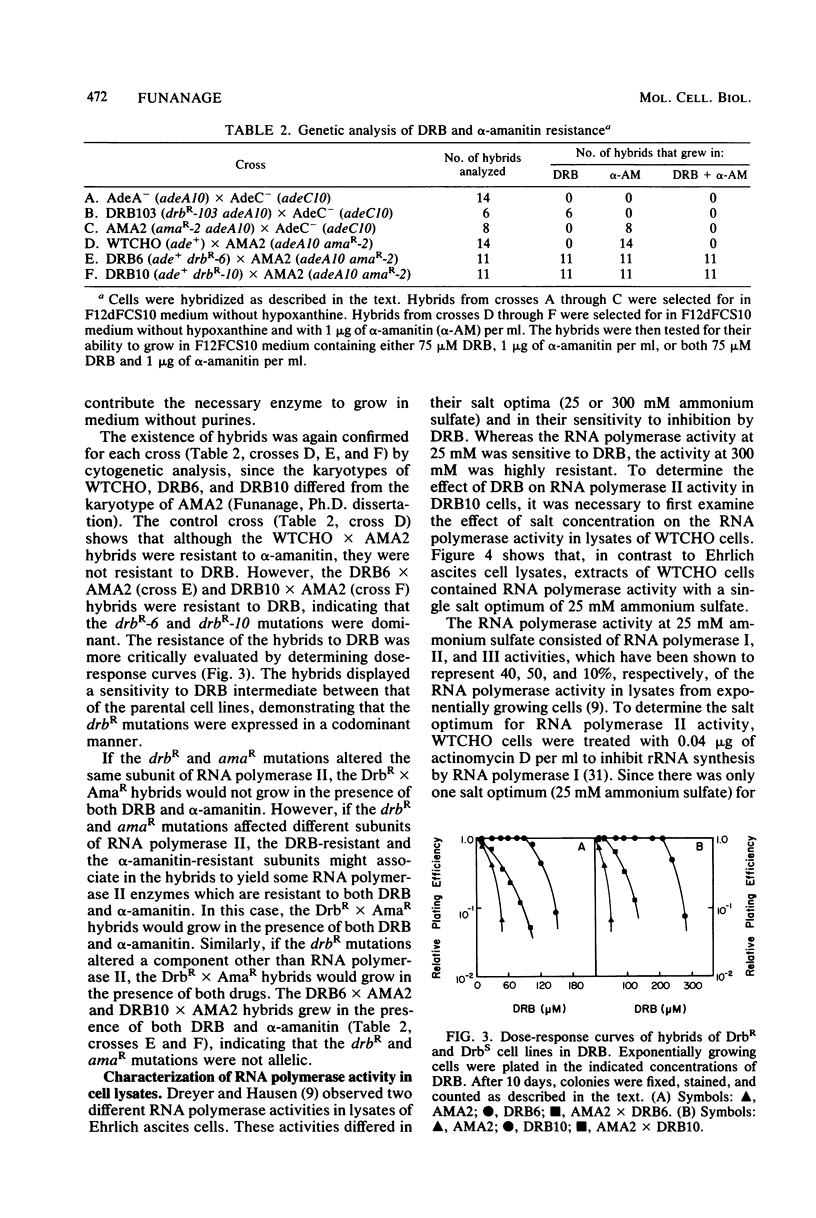
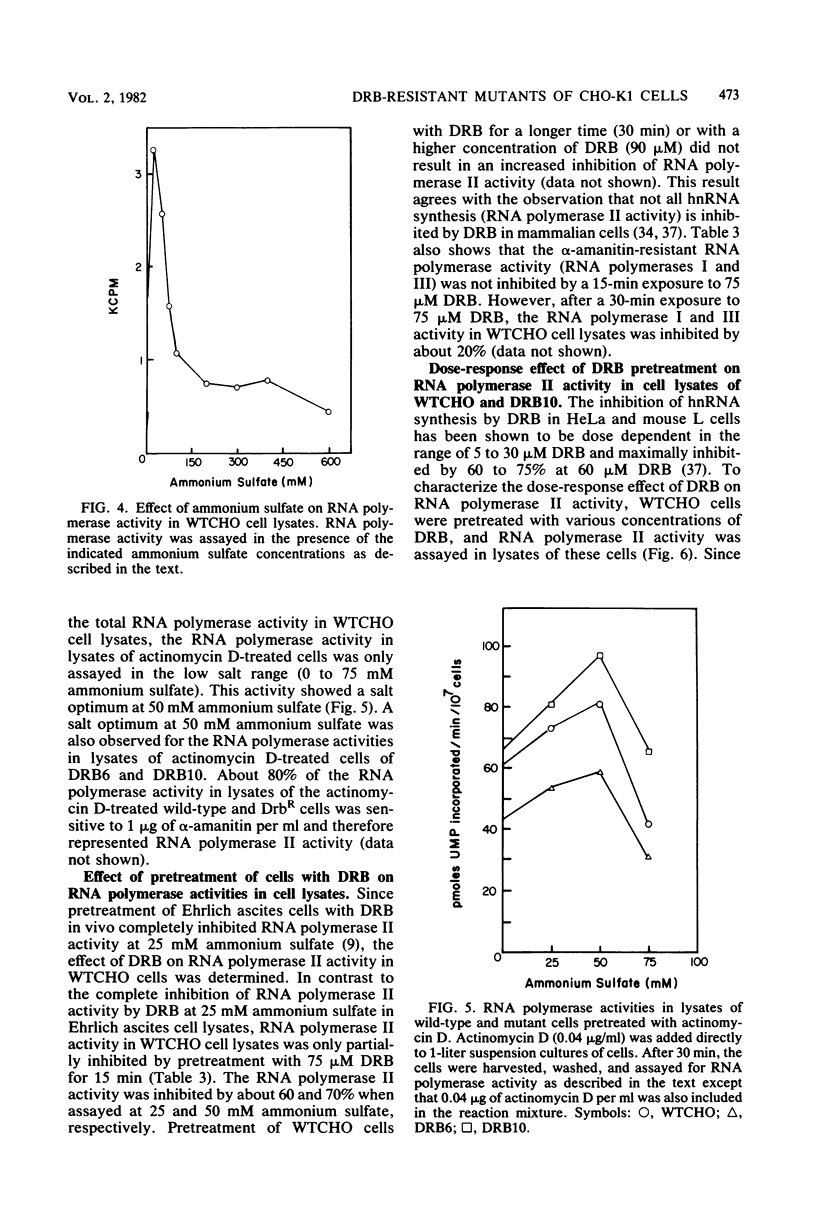
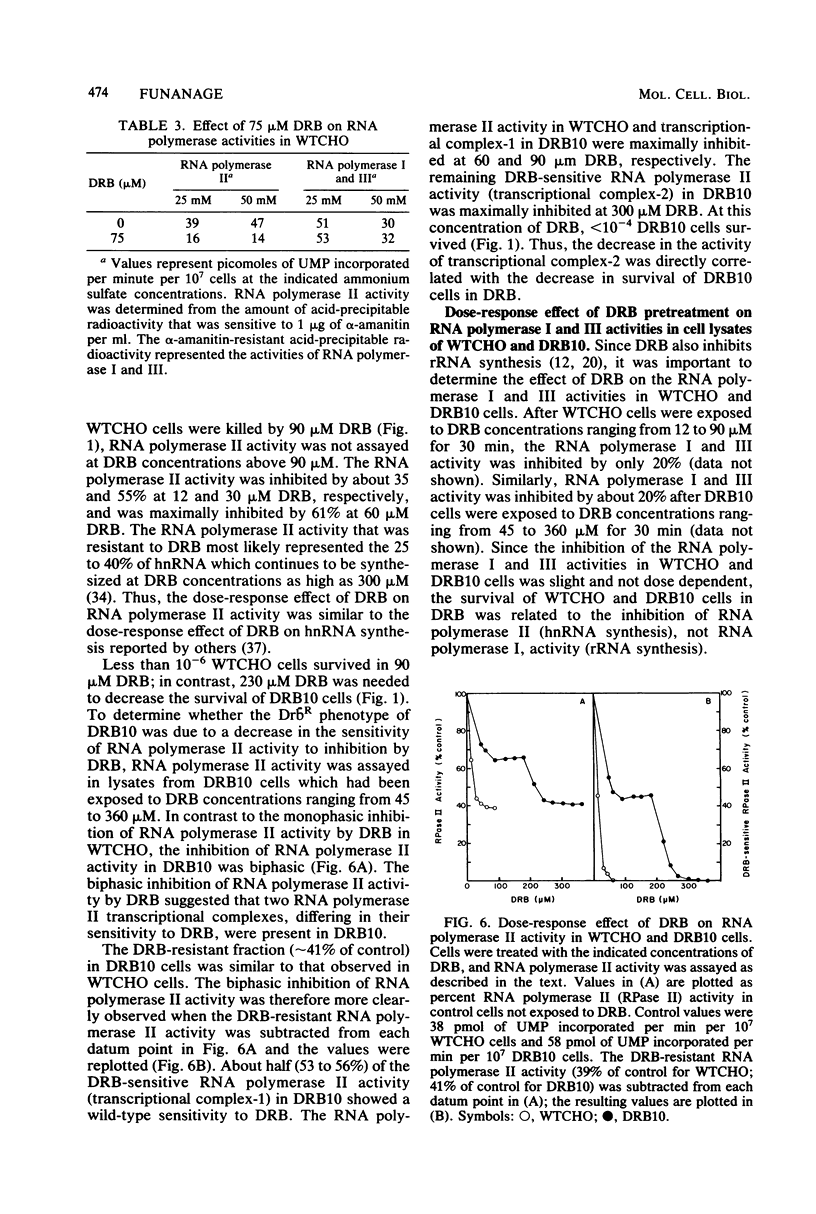
Mutants resistant to the RNA synthesis inhibitor 5,6-dichloro-1-beta-D-ribofurano-sylbenzimidazole (DRB) have been isolated in the Chinese hamster ovary cell line CHO-K1. Three independently isolated mutants, DRB6 DRB10, and DRB13, were 3-, 5-, and 3.5-fold, respectively, more resistant to DRB than the parental cell line WTCHO. The DRB-resistant mutations were expressed codominantly in somatic cell hybrids of DRB-resistant and DRB-sensitive cell lines. In vivo treatment of CHO-K1 cells with DRB resulted in specific inhibition of endogenous RNA polymerase II activity in cell lysates. Whereas DRB inhibited RNA polymerase II activity in WTCHO cells by a maximum of 60% at concentrations as low as 60 microM, 300 microM DRB was required to inhibit 60% of the RNA polymerase II activity in DRB10 cells. However, the inhibition of the DRB-sensitive RNA polymerase II activity in DRB10 was biphasic. About half (53 to 56%) of this activity was inhibited by 90 microM DRB and thus showed a DRB sensitivity similar to the wild-type RNA polymerase II activity; the remaining DRB-sensitive RNA polymerase II activity was maximally inhibited by 300 microM DRB. These results indicated that there were two copies of the drbR locus (drb+ and drbR-10) in DRB10 and confirmed that the drbR-10 mutation was expressed codominantly. Somatic cell hybrids of DRB-resistant and alpha-amanitin-resistant cell lines grew in medium containing both DRB and alpha-amanitin, demonstrating that the drbR and amaR mutations were not in the same gene. Thus, the drbR mutations may define an additional component of the RNA polymerase II transcriptional complex in mammalian cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billen D., Olson A. C. The use of Tween-80-permeabilized mammalian cells in studies of nucleic acid metabolism. Methods Cell Biol. 1978;20:315–324. [PubMed] [Google Scholar]
- Brodner O. G., Wieland T. Identification of the amatoxin-binding subunit of RNA polymerase B by affinity labeling experiments. Subunit B3-the true amatoxin receptor protein of multiple RNA polymerase B. Biochemistry. 1976 Aug 10;15(16):3480–3484. doi: 10.1021/bi00661a013. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Ingles C. J. Human diploid fibroblast mutants with altered RNA polymerase II. Somatic Cell Genet. 1976 May;2(3):225–233. doi: 10.1007/BF01538961. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Chan V. L., Whitmore G. F., Siminovitch L. Mammalian cells with altered forms of RNA polymerase II. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3119–3123. doi: 10.1073/pnas.69.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cohen A., Ullman B., Martin D. W., Jr Characterization of a mutant mouse lymphoma cell with deficient transport of purine and pyrimidine nucleosides. J Biol Chem. 1979 Jan 10;254(1):112–116. [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Dreyer C., Hausen P. Inhibition of mammalian RNA polymerase by 5,6-dichlororibofuranosylbenzimidazole (DRB) and DRB triphosphate. Nucleic Acids Res. 1978 Sep;5(9):3325–3335. doi: 10.1093/nar/5.9.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer C., Hausen P. On the activity of RNA polymerase B in lysates from Ehrlich ascites cells. Eur J Biochem. 1978 May;86(1):241–253. doi: 10.1111/j.1432-1033.1978.tb12305.x. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Spindler S. M., Siminovitch L. Genetic characterization of methotrexate-resistant chinese hamster ovary cells. In Vitro. 1976 Nov;12(11):749–757. doi: 10.1007/BF02835450. [DOI] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. I. Formation of necklaces by dichlororibobenzimidazole and other adenosine analogues that decrease RNA synthesis and degrade preribosomes. J Cell Biol. 1975 May;65(2):398–417. doi: 10.1083/jcb.65.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. II. Microscope observations of the effect of adenosine analogues on nucleolar necklace formation. J Cell Biol. 1975 May;65(2):418–427. doi: 10.1083/jcb.65.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L., Borsett L. M., Jiamachello P. F., Coulter D. E. Alpha-amanitin-resistant D. melanogaster with an altered RNA polymerase II. Cell. 1979 Nov;18(3):613–622. doi: 10.1016/0092-8674(79)90116-8. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Chan D. Y., Siminovitch L. Evidence for variation in the number of functional gene copies at the AmaR locus in Chinese hamster cell lines. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):461–467. doi: 10.1002/jcp.1040970321. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. DRB resistance in Chinese hamster and human cells: genetic and biochemical characteristics of the selection system. Somatic Cell Genet. 1980 Mar;6(2):151–169. doi: 10.1007/BF01538793. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical characterization of mutants of CHO cells resistant to the protein synthesis inhibitor trichodermin. Somatic Cell Genet. 1978 May;4(3):355–374. doi: 10.1007/BF01542848. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical studies with the adenosine analogs toyocamycin and tubercidin: mutation at the adenosine kinase locus in Chinese hamster cells. Somatic Cell Genet. 1978 Nov;4(6):715–735. doi: 10.1007/BF01543160. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Harlow P., Molloy G. Effect of 5,6-dichloro-1-beta-D-ribofuranosyl benzimidazole on ribonucleotide metabolism and accumulation of mitochondrial RNA and low-molecular-weight cytoplasmic RNA in HeLa cells. Arch Biochem Biophys. 1980 Sep;203(2):764–773. doi: 10.1016/0003-9861(80)90237-4. [DOI] [PubMed] [Google Scholar]
- Harris J. F., Whitmore G. F. Chinese hamster cells exhibiting a temperature dependent alteration in purine transport. J Cell Physiol. 1974 Feb;83(1):43–51. doi: 10.1002/jcp.1040830107. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Brimer P. A., Mitchell T. J., Gosslee D. G. The dose-response relationship for ethyl methanesulfonate-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in Chinese hamster ovary cells. Somatic Cell Genet. 1975 Jul;1(3):247–261. doi: 10.1007/BF01538449. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Guialis A., Lam J., Siminovitch L. Alpha-Amanitin resistance of RNA polymerase II in mutant Chinese hamster ovary cell lines. J Biol Chem. 1976 May 10;251(9):2729–2734. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse D. S., Roeder R. G. Accurate transcription initiation on a purified mouse beta-globin DNA fragment in a cell-free system. Cell. 1980 Jul;20(3):691–699. doi: 10.1016/0092-8674(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Matsui T., Segall J., Weil P. A., Roeder R. G. Multiple factors required for accurate initiation of transcription by purified RNA polymerase II. J Biol Chem. 1980 Dec 25;255(24):11992–11996. [PubMed] [Google Scholar]
- Moehring T. J., Danley D. E., Moehring J. M. Codominant translational mutants of Chinese hamster ovary cells selected with diphtheria toxin. Somatic Cell Genet. 1979 Jul;5(4):469–480. doi: 10.1007/BF01538881. [DOI] [PubMed] [Google Scholar]
- Oates D. C., Patterson D. Biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism: characterization of Chinese hamster cell mutants defective in phosphoribosylpyrophosphate amidotransferase and phosphoribosylglycinamide synthetase and an examination of alternatives to the first step of purine biosynthesis. Somatic Cell Genet. 1977 Nov;3(6):561–577. doi: 10.1007/BF01539066. [DOI] [PubMed] [Google Scholar]
- Patterson D., Kao F. T., Puck T. T. Genetics of somatic mammalian cells: biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism. Proc Natl Acad Sci U S A. 1974 May;71(5):2057–2061. doi: 10.1073/pnas.71.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehm H., Biedler J. L. Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res. 1972 Jun;32(6):1195–1200. [PubMed] [Google Scholar]
- SZYBALSKI W. Genetics of human cell lines. II. Method for determination of mutation rates to drug resistance. Exp Cell Res. 1959 Nov;18:588–591. doi: 10.1016/0014-4827(59)90327-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Darnell J. E., Jr, Tamm I. The inhibition by DRB (5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976 Nov;9(3):473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Tamm I. Definition of subclasses of nucleoplasmic RNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5011–5015. doi: 10.1073/pnas.74.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T., Darnell J. E., Jr, Salditt-Georgieff M. Short capped hnRNA precursor chains in HeLa cells: continued synthesis in the presence of 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Biochemistry. 1980 Jun 10;19(12):2743–2748. doi: 10.1021/bi00553a032. [DOI] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T. Early termination of heterogeneous nuclear RNA transcripts in mammalian cells: accentuation by 5,6-dichloro 1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5750–5754. doi: 10.1073/pnas.76.11.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]



