Abstract
Caspase-2 (casp-2) is the most conserved caspase across species, and is one of the initiator caspases activated by various stimuli. The casp-2 gene produces several alternative splicing isoforms. It is believed that the long isoform, casp-2L, promotes apoptosis, whereas the short isoform, casp-2S, inhibits apoptosis. The actual effect of casp-2S on apoptosis is still controversial, however, and the underlying mechanism for casp-2S-mediated apoptosis inhibition is unclear. Here, we analyzed the effects of casp-2S on DNA damage induced apoptosis through “gain-of-function” and “loss-of-function” strategies in ovarian cancer cell lines. We clearly demonstrated that the over-expression of casp-2S inhibited, and the knockdown of casp-2S promoted, the cisplatin-induced apoptosis of ovarian cancer cells. To explore the mechanism by which casp-2S mediates apoptosis inhibition, we analyzed the proteins which interact with casp-2S in cells by using immunoprecipitation (IP) and mass spectrometry. We have identified two cytoskeleton proteins, Fodrin and α-Actinin 4, which interact with FLAG-tagged casp-2S in HeLa cells and confirmed this interaction through reciprocal IP. We further demonstrated that casp-2S (i) is responsible for inhibiting DNA damage-induced cytoplasmic Fodrin cleavage independent of cellular p53 status, and (ii) prevents cisplatin-induced membrane blebbing. Taken together, our data suggests that casp-2S affects cellular apoptosis through its interaction with membrane-associated cytoskeletal Fodrin protein.
Introduction
Apoptosis is a highly conserved mechanism which plays an important role in normal development and tissue homeostasis [1]. Apoptosis is also one of the cell death mechanisms that can be triggered in cancer cells by various cancer treatment schemes, e.g., chemotherapy, radiotherapy, immunotherapy or targeted therapy [2], [3], [4], [5]. Apoptosis usually requires the activation of a series of cysteine aspartate-specific proteases referred to as caspases [6]. Caspases, whose activation is a hallmark of apoptosis, are a family of proteins that are one of the main effectors of apoptosis. To date, about 14 mammalian caspases have been identified, and can be classified into three groups based on their function: inflammatory caspases, apoptotic initiator caspases, and apoptotic effector caspases (Reviewed in [7]).
Capase-2 is the most conserved caspase across species [8], [9]. Despite its early discovery, caspase-2's physiological function has long remained an enigma [10]. The difficulty in determining its function is due to the existence of two caspase-2 isoforms, each serving opposing functions in apoptosis. The caspase-2 gene produces several alternative splicing isoforms. The inclusion of exon 9 incorporates an in-frame stop codon in the casp-2 short isoform (casp-2S) mRNA, producing a truncated protein that inhibits cell death. The exclusion of exon 9 results in the casp-2 long isoform (casp-2L) mRNA, whose protein product induces cell death [8], [11]. However, further characterization of casp-2S isoform (Nedd2S) indicated that casp-2S did not act as a general inhibitor of apoptosis in all cell types and it did not exert its effect by directly competing with casp-2L [12]. The levels of casp-2L and casp-2S are governed by alternative promoters and splicing [13]. The average casp-2L/2S mRNA ratio is always high, and is often above 100-fold in several cell lines including leukemia (U937), carcinoma (HeLa, HCT116, HepG2, HT29), and immortalized (293T) [14] cells.
The subtle phenotype of casp-2 knockout mice does not clarify the biological role of this protein because both casp-2L and casp-2S are deficient in the mice (Reviewed in [15]). Similarly, confounding data was also generated in siRNA-based studies. Casp-2 downregulation by siRNA was initially reported to strongly inhibit etoposide-induced cell death [16], but doubts have been raised regarding the specificity of the siRNA used in these experiments [17]. The lack of siRNA specific to the distinct isoforms of casp-2 also makes this data open to questioning. Other researchers observed weak to no protection against etoposide-induced apoptosis, using a variety of techniques to abolish casp-2 expression in a number of cell types [10], [18], [19], [20], [21].
Apoptosis is characterized by cell membrane blebbing, cell shrinkage, chromatin condensation, and DNA fragmentation [22]. Using casp-2S overexpression, Droin et al [23] demonstrated that casp-2S selectively inhibits chromatin condensation, apoptotic body formation, membrane blebbing, and phosphatidylserine externalization following etoposide treatment in the human leukemic cell line U937. Regarding the mechanisms underlying the anti-apoptotic role of Casp-2S, it has been reported that casp-2S antagonizes apoptosis by inhibiting the activation of casp-2L and caspase-3, preventing ROCK-1-mediated apoptotic blebbing and body formation [24], [25]. However, simple overexpression in cells is not optimal for studying the physiological function of this protein. By using casp-2S specific siRNA, we have recently demonstrated that the downregulation of casp-2S enhanced the cleavage of PARP, the activation of caspase-9 and caspase-6, and the phosphatidylserine externalization in XPC-deficient human fibroblasts [21].
Fodrin (αII-Spectrin) and the Fodrin-based cytoskeleton confer resiliency and durability to integral membrane proteins, and are believed to participate in the formation and maintenance of specialized membrane subdomains [26]. The cleavage of Fodrin leads to the disruption of the actin filament network. This disruption may specifically contribute to the loss of overall cell shape and detachment from the matrix during apoptosis [27]. Fodrin cleavage is thought to be involved in the membrane blebbing observed during apoptosis [28]. Fodrin is susceptible to cleavage by both calpain and caspases. Fodrin cleavage by calpain is thought to be involved in membrane remodeling, necrosis, and apoptosis. The cleavage of Fodrin induced by caspases appears to occur only during apoptosis [29].
In this study, we assessed the role of casp-2S in cancer cells during apoptosis through gain-of-function and loss-of-function strategies. Our studies using casp-2S-specific siRNA clearly demonstrate the anti-apoptotic role of casp-2S. In addition, we have found that casp-2S interacts with cytoplasmic Fodrin and inhibits its cleavage during apoptosis. As a result, casp-2S selectively inhibits the membrane blebbing and phosphatidylserine externalization that are characteristics of apoptosis.
Materials and Methods
Cell Culture and treatment
The human ovarian cancer cell line A2780 and its resistant subline CP70 [30] were kindly provided by Dr. Paul Modrich (Duke University). Another A2780-derivative resistant subline, CDDP [31], was kindly provided by Dr. Karuppaiyah Selvendirany (The Ohio State University). The A2780-derivative cisplatin-resistant cell lines were produced by exposing the sensitive parental line to intermittent and incrementally increasing concentrations of cisplatin [30], [31]. SKOV-3, PEO1 and PEO4 ovarian cancer cell lines were kindly provided by Dr. Thomas C. Hamilton (Fox Chase Cancer Center) and have been described previously [32]. Ovarian cancer cell line 2008 and its resistant cell line 2008C13 were kindly provided by Dr. Francois X. Claret (University of Texas - M. D. Anderson Cancer Center), and have been characterized [33], [31], [34]. A549, H460 and H1299 cell lines (originally purchased from ATCC) were kindly provided by Dr. Wenrui. Duan (The Ohio State University). HeLa cells with overexpression of casp-2S (HeLa-Casp-2S) were established in our own lab. These cell lines were maintained in RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 units/ml penicillin. HCT116(p53−/−) and HCT116(p53+/+) colorectal carcinoma cells were kindly provided by Dr. B. Vogelstein (Johns Hopkins University) [35], and were cultured in McCoy's 5A medium (Life Technologies) with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 units/ml penicillin. The glioblastoma cell line Gli36 [36] was provided by Dr. Balveen Kaur (The Ohio State University), and T98G and U87MG (originally purchased from ATCC) were provided by Dr. Arnab Chakravarti (The Ohio State University). These cells were cultured in MEM medium supplied with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 units/ml penicillin. All cells were grown at 37°C in humidified atmosphere of 5% CO2 in air.
For cisplatin treatment, cells were maintained in medium with the desired doses of cisplatin (Sigma, St. Louis, MO) for 1 h, washed with PBS, and incubated in fresh cisplatin-free medium for varying times post-treatment. For UV exposure, the cultures were washed with PBS, irradiated with UV at 20 J/m2, and then incubated for varying times. UV-C light (254 nm) was delivered from a germicidal lamp at a dose rate of 0.5 J/m2/s, as measured by a UVX digital radiometer connected to a UVX-31 sensor (UVP, Inc., Upland, CA).
Plasmid and siRNA transfection
The plasmid encoding the full-length human casp-2S (p3XFLAG-CMV-14-casp-2S) has been described previously [21]. siRNA directed against casp-2S (5′- GAA UAC UAC UGG UAA ACU AUU -3′) (5′UTR), and a scramble non-targeting siRNA (5′- UUC UCC GAA CGU GUC ACG UdTdT -3′), were synthesized by Dharmacon Inc. (Lafayette, CO). The knockdown efficiency of casp-2S siRNA has been validated previously [21]. 100 nM siRNA was transfected into cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacture's instruction.
Apoptosis Analysis by Annexin V staining
Phosphatidylserine exposure on the outer leaflet of the plasma membrane was detected using the Annexin V-GFP apoptosis detection kit II (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. Briefly, 1 X 106 cells were pelleted following treatment and washed in PBS. The cells were then resuspended in 500 µl of binding buffer, mixed with Annexin V-GFP and propidium iodide (PI) and incubated at room temperature (22°C) for 5–10 min in the dark. The Annexin V-positive cells were analyzed by flow cytometry.
Purification of casp-2S and mass spectrometric analysis
1 X 108 HeLa cells stably transfected with FLAG-tagged casp-2S (HeLa-Casp-2S cells) and mock-transfected HeLa cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, protease inhibitor cocktail) for 30 min at 4°C. After centrifugation at 14,000 rpm for 10 min, supernatant was collected for immunoprecipitation (IP). Three milligrams of total proteins were incubated with anti-FLAG affinity gel (Sigma) for 2 h at 4°C. The resin was washed 4 times with lysis buffer and eluted with 400 µl of 3 X FLAG peptide (200 µg/ml final). The FLAG peptide elutes were precipitated with 10% TCA, washed twice with cold acetone, and dissolved in 30 µl of 6 M urea. The total protein was resolved in 8–16% gel and stained with Coomassie bright blue. The differential bands between HeLa and HeLa-Casp-2S samples were cut and subjected to in-gel protease digestion and analyzed by nano LC-MS/MS on a LTQ mass spectrometer (Mass Spectrometry & Proteomics Facility, Ohio State University, Columbus, OH). The resulting files were searched in the SwissProt 2011-06 database using Mascot Daemon.
IP and immunoblotting
Whole cell lysates were prepared from HeLa-Casp-2S and CDDP cells using RIPA buffer. One milligram of total protein was incubated with 2 µg of rabbit anti-Fodrin antibody (Cell Signaling Technology, Danvers, MA) or normal rabbit IgG overnight at 4°C with continuous rotation. The immunoprecipitates were recovered following 2 h incubation with 30 µl of Protein G plus/Protein A agarose beads (Calbiochem, Gibbstown, NJ) and centrifugation. Western blot analysis was carried out to visualize the presence of Fodrin and casp-2S.
For immunoblotting analysis, whole cell lysates were prepared by boiling cell pellets for 10 min in SDS lysis buffer [2% SDS, 10% Glycerol, 62 mM Tris-HCl, pH 6.8 and a complete mini-protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)]. After protein quantification with Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Hercules, CA), equal amounts of proteins were loaded, separated on a polyacrylamide gel, and transferred to a nitrocellulose membrane. Protein bands were immuno-detected with appropriate antibodies, e.g., anti-casp-2 (551093, BD Bioscience, recognizes both long and short isoforms of casp-2), anti-Fodrin (Cell Signaling Technology, Danvers, MA), anti-FLAG (Sigma, St. Louis, MO), anti-Tubulin (Santa Cruz Biotechnology, Dallas, TX), anti-cleaved PARP and anti-cleaved caspase-3 (Cell Signaling Technologies).
Subcellular fractionation
Cells were harvested and resuspended in 200 µl of lysis buffer (5 mM Tris-HCl, pH 7.4, 50 mM KCl, 5 mM MgCl2, 1 mM EGTA, protease inhibitors) and permeabilized with NP-40 (0.1%) for 10 min at 4°C. The cytosol and nuclei were separated by centrifuging the lysate at 14,000 rpm for 10 min. The nuclei pellets were further lysed in SDS lysis buffer. Each protein fraction, corresponding to an equivalent cell number, was loaded for SDS-PAGE and analyzed by immunoblotting with the indicated antibodies.
Membrane blebbing detection
Membrane blebbing was detected by using the Apoptotic Blebs Assay Kit (Cayman Chemical, Ann Arbor, MI). Briefly, both floating and attached cells were harvested after treatment with cisplatin, washed with assay buffer, and incubated with scFv Fusion Protein Solution, followed by incubation with Fluorescein-labeled Rabbit IgG Solution. After adding the PI Solution, cells were observed under a fluorescent microscope. Dead cells stained with PI and showed red, while apoptotic blebs stained with Fluorescein-labeled Rabbit IgG, and showed green.
Statistical Analysis
Evaluation of statistical significance for data from flow cytometry and membrane blebbing analysis was assessed using 2-Sample t-test. Differences were considered to be statistically significance at a value of P<0.05.
Results
The expression of casp-2 isoforms in various cancer cell lines
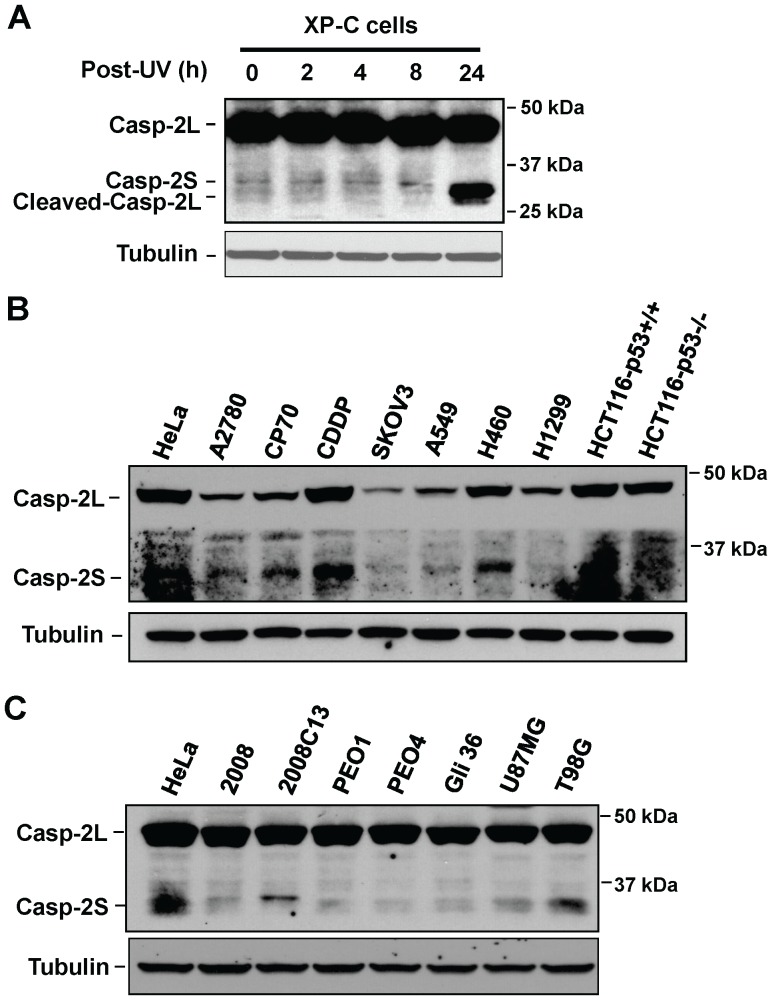
Although the protein expression of casp-2L has been detected in many cell lines [37], the protein level of casp-2S has seldom been revealed, and whether the casp-2S isoform exists as a protein in human cells remains a controversial issue [14]. We and others have successfully detected casp-2S in human XP-C fibroblasts and U937 monocytes [21], [38]. To further validate the specificity of this antibody and ensure the specific band detected around 33 kDa is casp-2S but not cleaved casp-2L, we UV irradiated XP-C cells and further cultured these cells for various time periods. As shown in Fig. 1A, cleaved casp-2L (30 kDa), which migrates faster than casp-2S (33 kDa), is only visible after 24 h of UV irradiation, whereas casp-2S can be clearly detected in other samples without the interference of cleaved casp-2L. Thus, the specific band around 33 kDa detected by anti-casp-2 antibody, especially in those cells without cellular stress induction, is casp-2S.
Figure 1. The expression of caspase-2 in various cancer cell lines.
A, Human XP-C cells were UV irradiated at 10 J/m2, and further cultured for the indicated time periods. Whole cell lysates were prepared and subjected to Western blot to detect casp-2L, casp-2S and cleaved casp-2L simultaneously with the anti-casp-2 antibody. B, C. The expression of casp-2L and casp-2S was determined by Western blot analysis of protein extracts of the cervical cancer cell line (HeLa), ovarian cancer cell lines (A2780, CP70, CDDP, 2008, 2008C13, PEO1, PEO4 and SKOV3), lung cancer cell lines (A549, H460, and H1299), colorectal cancer cell lines (HCT116p53+/+ and HCT116p53−/−), and glioblastoma cell lines (Gli36, U87MG, and T98G) with the anti-casp-2 antibody used in A. Tubulin was detected to serve as loading control.
To test for the presence of casp-2S protein in human cancer cells, we determined the protein expression levels of casp-2L and casp-2S in various cancer cell lines by using this anti-casp-2 antibody [21]. As shown in Fig. 1B and C, casp-2L was detectable, with varying levels of expression, in all tested cell lines. We also detected casp-2S in various cancer cell lines, e.g., HeLa, CP70, CDDP, H460, 2008, 2008C13, PEO1 and PEO4. In contrast, casp-2S was undetectable in the SKOV3, H1299 and HCT116 p53−/− cells.
It was reported that casp-2S mRNA is expressed predominantly in the heart, brain, and skeletal muscle with the highest expression found in embryonic brain [11]. However, we did not find a high casp-2S protein expression in three glioblastoma cell lines (Fig. 1C). In contrast, the highest casp-2S protein expression was found in cisplatin-resistant ovarian cancer cell lines, CDDP and 2008C13.
Casp-2S inhibits cisplatin-induced apoptosis
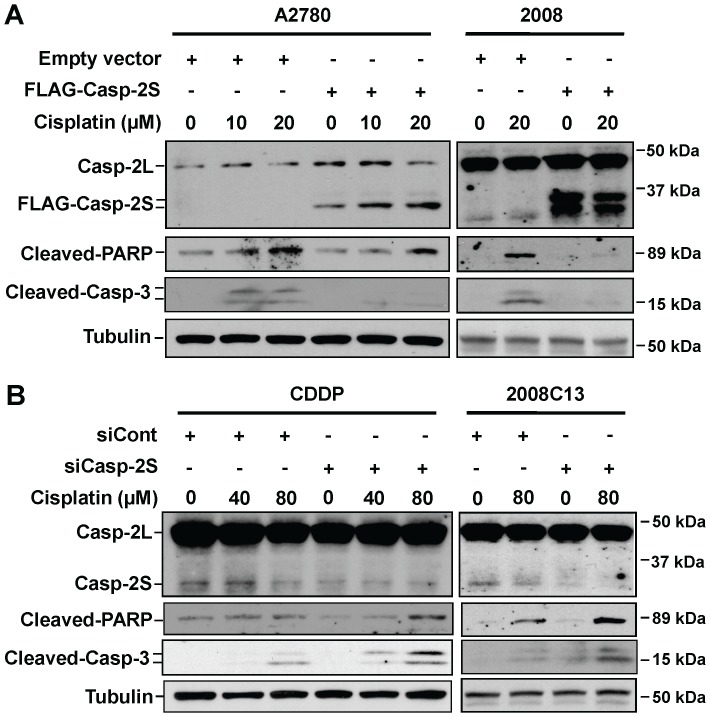
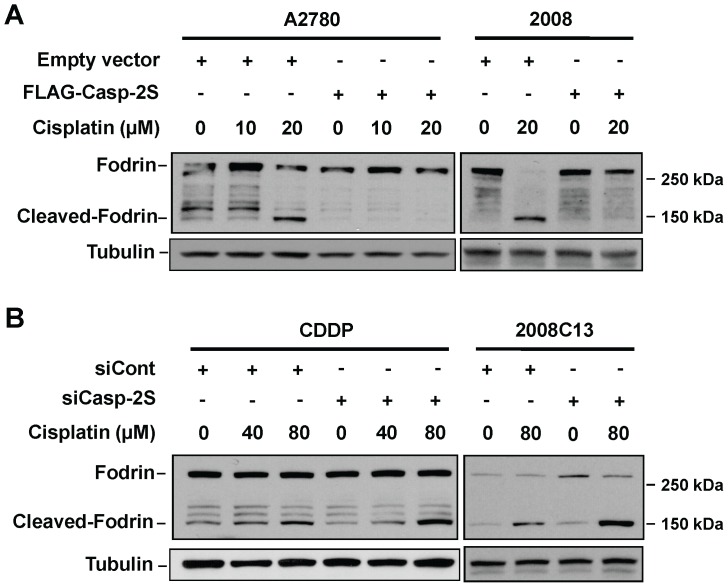
It has been reported that overexpression of casp-2S selectively prevented some phenotypic aspects of apoptosis, including chromatin condensation, apoptotic body formation and membrane blebbing in etoposide treated human leukemic U937 cells following treatment with etoposide [23]. To confirm the anti-apoptotic role of casp-2S, we either overexpressed or downregulated casp-2S in ovarian cancer cell lines and detected their effect on cisplatin-induced apoptosis. As shown in Fig. 1, A2780 and 2008 cells have an extremely low level of casp-2S, while CDDP and 2008C13 cells display a relatively high level of casp-2S. Thus, we overexpressed casp-2S in A2780 and 2008 cells (Fig. 2A), and knocked down the expression of casp-2S in CDDP and 2008C13 cells (Fig. 2B). Since there are two in-frame ATG initiation codons in casp-2S cDNA, both functioning as protein initiation sites for casp-2S synthesis [13], two bands were detected in the A2780 and 2008 cells transfected with FLAG-tagged casp-2S (Fig. 2A). Using a siRNA that only targets casp-2S [21], we were able to down-regulate the expression of casp-2S in both CDDP and 2008C13 cells without affecting casp-2L expression (Fig. 2B). Apoptosis analysis showed that over-expression of casp-2S in both A2780 and 2008 cells attenuated PARP cleavage and caspase-3 cleavage following cisplatin treatment (Fig. 2A), while down-regulation of casp-2S in CDDP and 2008C13 cells enhanced cisplatin-induced PARP cleavage and caspase-3 cleavage (Fig. 2B), indicating that casp-2S is an anti-apoptotic factor.
Figure 2. Casp-2S inhibits cisplatin-induced apoptosis.
Casp-2S was either over-expressed in A2780 and 2008 cells (A), or knocked down in CDDP cells and 2008C13 cells (B) for 48 h. Cells were treated with cisplatin for another 48 h, the cleaved PARP and cleaved casp-3 were detected in these cells by using Western blotting with anti-cleaved PARP and anti-cleaved casp-3 antibodies. Meanwhile, the expression of casp-2L and casp-2S was also detected. Tubulin was blotted to serve as a loading control.
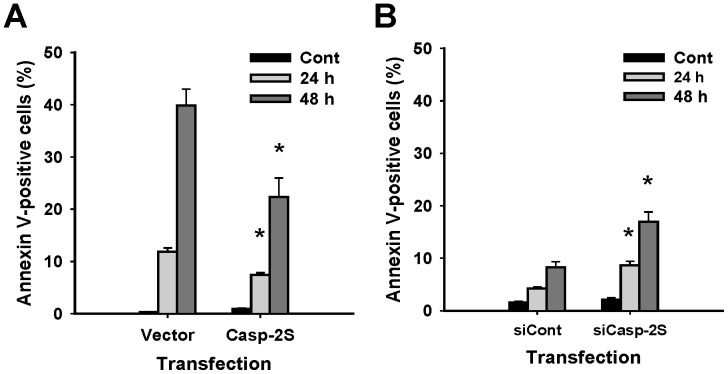
We also analyzed phosphatidylserine externalization in A2780 cells over-expressing casp-2S, and CDDP cells with down-regulation of casp-2S following cisplatin treatment. Similar to the etoposide-treated U937 cells [23], over-expression of casp-2S also reduced phosphatidylserine externalization in A2780 cells following cisplatin treatment, as reflected by a decrease of Annexin V-positive cells (Fig. 3A). In addition, knockdown of casp-2S in CDDP cells enhanced cisplatin-induced phosphatidylserine externalization (Fig. 3B), further confirming the inhibitory effect of casp-2S on apoptosis.
Figure 3. Casp-2S inhibits cisplatin-induced phosphatidylserine externalization.
Casp-2S was over-expressed in A2780 cells (A), or knocked down in CDDP cells (B) for 48 h. Cells were then treated with cisplatin for another 24 and 48 h, and the phosphatidylserine externalization was detected with Annexin V staining by using Flow cytometry. n = 3, bar: SD, *: p<0.05 compared to the cisplatin-treated vector or control siRNA transfected cells at the same time point.
Casp-2S interacts with cytoskeleton proteins
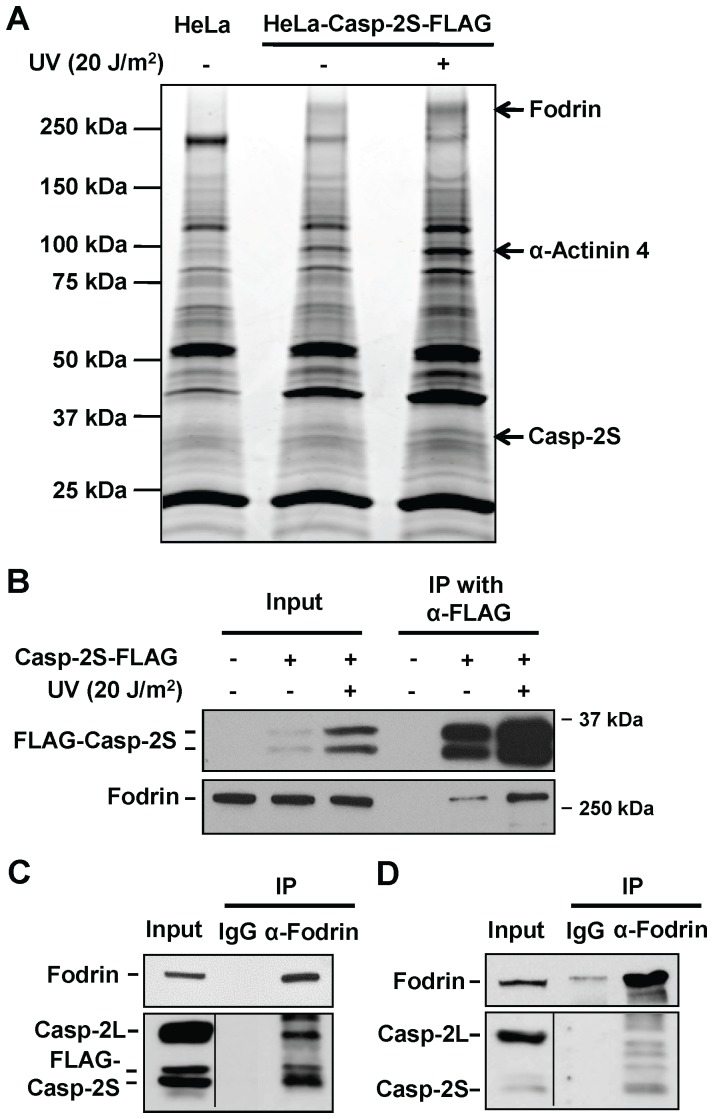
To explore the molecular roles of casp-2S in cells, we purified casp-2S as multimeric complexes and analyzed the co-purified proteins using mass spectrometry. Casp-2S was stably expressed as FLAG-epitope fusions in HeLa cells. FLAG-tagged casp-2S was purified from whole cell lysates using IP with anti-FLAG affinity gel. The purified complexes were further separated on an 8–16% SDS-PAGE gel. As a control, we performed a mock purification from untransfected HeLa cells. Coomassie blue staining of the protein bands is shown in Fig. 4A. We found three differentially displayed bands in the HeLa-Casp-2S cells in comparison to the untransfected HeLa cells. Using mass spectrometric analysis, Fodrin was detected in the 270 kDa band, α-Actinin 4 was detected in the 100 kDa band, and casp-2S was detected in 36 kDa band. We further confirmed the interaction between casp-2S and Fodrin through use of reciprocal IP and immunoblotting. As shown in Fig. 4B and 4C, Fodrin was detectable in the complex immunoprecipitated with the anti-FLAG antibody, and FLAG-tagged casp-2S was detectable in the complex immunoprecipitated with the anti-Fodrin antibody in HeLa-Casp-2S-FLAG cells. We further attempted to investigate the interaction between Fodrin and endogenous casp-2S in CDDP cells. As shown in Fig. 4D, the anti-Fodrin antibody is able to pull down endogenous casp-2S but not casp-2L. Thus, our data suggests that casp-2S forms a complex with cytoskeleton proteins.
Figure 4. Casp-2S interacts with cytoskeleton proteins.
A, Casp-2S was purified from HeLa cells stably transfected with the FLAG-tagged casp-2S using an anti-FLAG affinity gel. Purified proteins were separated by SDS-PAGE and stained with Coomassie blue. The differentially expressed bands in the HeLa-casp-2S cells were identified by mass-spectrometry. B, FLAG-tagged casp-2S was immunoprecipitated from the HeLa-Casp-2S cells and Fodrin was detected using Western blotting with the anti-Fodrin antibody. C, D, Fodrin was immunoprecipitated from the HeLa-Casp-2S cells (C) or CDDP cells (D) with an anti-Fodrin antibody, and the existence of casp-2S was detected with anti-casp-2S antibody.
Casp-2S inhibits cytoplasmic Fodrin cleavage during apoptosis
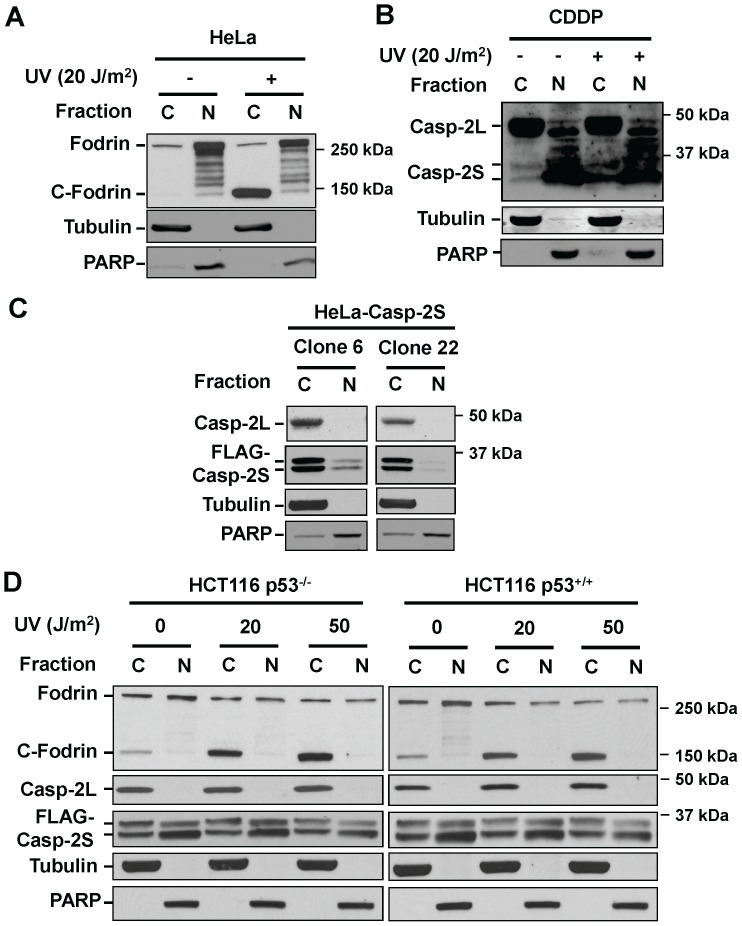
The cleavage of Fodrin is an early event in apoptosis [28]. Since we have found that casp-2S interacts with Fodrin, we sought to know whether this interaction protects Fodrin from being cleaved during apoptosis. Again, FLAG-tagged casp-2S was over-expressed in A2780 and 2008 cells, and the endogenous casp-2S in CDDP and 2008C13 cells was specifically knocked down. These cells were treated with cisplatin, and the cleavage of Fodrin was determined using immunoblotting with the anti-Fodrin antibody. As shown in Fig. 5, when the cells were treated with cisplatin, Fodrin cleavage was reduced in the A2780 and 2008 cells over-expressing casp-2S (Fig. 5A), while cleavage was enhanced in CDDP and 2008C13 cells with casp-2S down-regulation (Fig. 5B). Given casp-2 and Fodrin are present in both the cytosol and nucleus [39], [40], [41], we determined the location where Fodrin is cleaved during apoptosis. HeLa cells were UV irradiated to induce apoptosis, and fractionated into the cytosolic and nuclear components. Both the existence and cleavage of Fodrin were detected in these two fractions. The results indicate that UV-induced Fodrin cleavage occurs exclusively within the cytoplasm (Fig. 6A).
Figure 5. Casp-2S inhibits cisplatin-induced Fodrin cleavage.
Casp-2S was either over-expressed in A2780 cells and 2008 cells (A), or knocked down in CDDP cells and 2008C13 cells (B) for 48 h as in Fig. 2. Cells were treated with cisplatin for another 48 h, and the cleavage of Fodrin was detected with the anti-Fodrin antibody. Tubulin was detected as a loading control.
Figure 6. Casp-2S and cleavage of Fodrin mainly occur in cytoplasm.
A, HeLa cells were UV irradiated at 20 J/m2, and further cultured for 4 h. Cytoplasm (C) and nuclei (N) were separated and the Fodrin cleavage in protein extracts was detected with an anti-Fodrin antibody. Tubulin and PARP were used to indicate the cytoplasm and nuclei fraction, respectively. B. CDDP cells were fractionated into cytoplasm and nuclei, the endogenous casp-2L and casp-2S were detected simultaneously with the anti-casp-2 antibody. C, Two clones of casp-2S stably expressed HeLa cells were fractionated to the cytoplasm and nuclei, casp-2L and FLAG-tagged casp-2S were detected with the anti-casp-2 and the anti-FLAG antibodies, respectively. D, HCT116 p53−/− and HCT116 p53+/+ cells were transiently transfected with FLAG-tagged casp-2S, UV irradiated at various doses, and further cultured for 4 h. The cytoplasm and nuclei were separated, the Fodrin cleavage, casp-2L, and FLAG-tagged casp-2S were detected with anti-Fodrin, anti-casp-2 and anti-FLAG antibodies, respectively.
We then attempted to detect the subcellular localization of endogenous casp-2L and casp-2S in CDDP cells. The cytoplasm and nucleus were fractionated, and the presence of casp-2L and casp-2S were detected simultaneously with the anti-casp-2 antibody. As shown in Fig. 6B, both casp-2L and casp-2S can be detected in the cytoplasm and nucleus with casp-2L predominantly residing in the cytoplasm while casp-2S mainly localizing in the nucleus. UV irradiation did not change the localization of casp-2L, but increased the amount of cytoplasmic casp-2S.
To easily detect the subcellular localization of casp-2S, HeLa cells were stably transfected with FLAG-tagged casp-2S. The cytoplasm and nucleus were fractionated in two clones of FLAG-tagged casp-2S over-expressed HeLa cells. The presence of casp-2L and casp-2S were detected with anti-casp-2 and anti-FLAG antibodies, respectively. As shown in Fig. 6C, casp-2L was detected exclusively in the cytosol, but not in the nucleus. Ectopically expressed casp-2S, however, was detectable in both the cytosol and nucleus, and found to exist predominantly in the cytosol. We then analyzed the subcellular distribution of casp-2L and ectopically expressed FLAG-tagged casp-2S, as well as cleavage of Fodrin in HCT116 cells with different p53 status. As shown in Fig. 6D, UV-induced Fodrin cleavage occurs exclusively in the cytoplasm and is independent of p53 function. Again, casp-2L was only detected in the cytoplasm, but not in the nucleus, whereas transiently transfected FLAG-tagged casp-2S was equally detected in both the cytoplasm and nucleus. In addition, the distribution of casp-2L and FLAG-tagged casp-2S was not affected by the status of p53. This data indicates that the subcellular localization of casp-2L and casp-2S is cell type-dependent, and it is the cytoplasmic casp-2S that protects Fodrin cleavage during apoptosis.
Casp-2S inhibits apoptotic blebbing
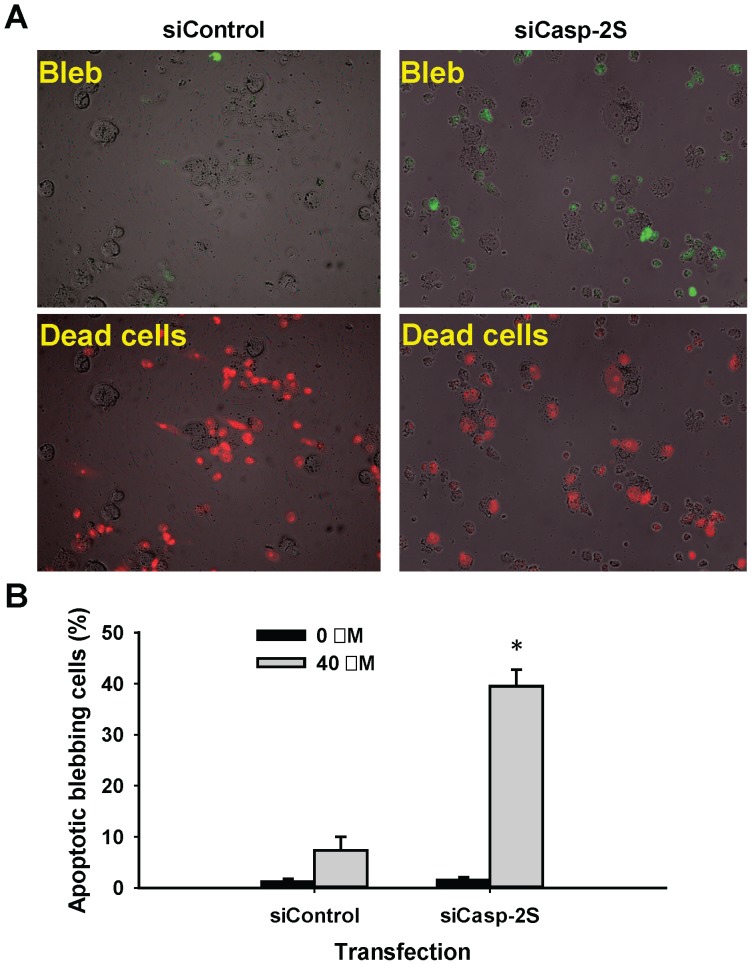
The Fodrin-actin scaffold underlying the lipid bilayer is considered to participate in cell-shape stabilization. Proteolysis of Fodrin, which occurs during apoptosis, leads to the destabilization of the scaffold, and produces membrane blebbing [28]. It has been reported that casp-2S over-expression prevents membrane blebbing in human leukemia cell line U937 and human B lymphoma Namalwa cells following VP-16 treatment [23], [25]. To further evaluate the effect of casp-2S on apoptotic blebbing under physiological conditions, we used casp-2S siRNA to knock down casp-2S expression in the human ovarian cancer cell line CDDP, which expresses a high level of casp-2S, and analyzed membrane blebbing after cisplatin treatment. We found that down-regulation of casp-2S significantly increased apoptotic blebbing in CDDP cells (Fig. 7A, B). Given our finding that casp-2S prevents Fodrin cleavage during apoptosis, we reasoned that casp-2S inhibits apoptotic blebbing through stabilization of the Fodrin-related cytoskeleton scaffold.
Figure 7. Caps-2S inhibits cisplatin-induced membrane blebbing.
A, CDDP cells were transfected with casp-2S siRNA or control siRNA for 48 h, and treated with cisplatin for 48 h. The apoptotic bleb and dead cells were stained separately as described in Materials and Methods. B, The number of apoptotic blebbing cells were counted and plotted. n = 50, bar: SD, *: p<0.05 compared to siControl.
Discussion
A major contributing factor for the confounding results describing the role of casp-2 in apoptosis is the existence and differential expression of casp-2L and casp-2S, which exhibit opposing functions in apoptosis [11]. Casp-2L (always referred to as casp-2) mRNA expression was observed to be dominant over casp-2S. This is most likely due to a stronger promoter [13] and the instability of casp-2S mRNA [14]. The casp-2L protein was abundantly expressed in numerous human and mouse cell lines [37]. The protein expression of casp-2S, in contrast, was seldom detected because its predicted mass is very close to that of the intermediate cleavage product of casp-2L. Furthermore, the casp-2S mRNA was shown to be very short-lived and thus may normally be expressed at very low levels. Nevertheless, we have successfully detected the casp-2S protein in human XP-C cells, as well as in various cancer cell lines in this study, confirming that casp-2S protein does exist in cells and its expression level depends on cell type.
One remarkable feature of casp-2 is its nuclear localization. Different from all other caspases, casp-2 has been widely detected within the nucleus [37], [42], [40], [43], [44], [45], [46], [47]. However, the subcellular localization of casp-2 continues to be debated. Although overwhelming evidence demonstrates the predominant casp-2 localization in the nucleus, this has been contradicted by others showing that the majority of casp-2 is detected in the cytoplasm by using both fractionation and microscopic analysis [44], [39], [48], [49]. Thus, further studies are necessary to unambiguously address this issue, especially the distinct localization of casp-2L and casp-2S. Our ability to successfully detect the individual isoforms of casp-2 protein expression enabled us to detect the subcellular localization of both casp-2L and casp-2S. Our subcellular fractionation analysis revealed that casp-2L was predominantly detected in the cytosol with a relatively small amount in nucleus. Casp-2S, however, was detected in both the cytosol and nucleus, with the predominant subcellular localization of casp-2S being dependent on the cell type. It was proposed that the accumulation of casp-2 (primarily casp-2L) in the cytosolic compartment observed after subcellular fractionation might be attributed to the dissociation of casp-2 from the nucleus as a result of the experimental procedure [50]. Although we failed to detect the existence of casp-2L in the nucleus of HeLa and HCT116 cells, we did detect casp-2S and Fodrin in the nuclear compartment of the same sample. This indicates that the absence of nuclear casp-2L in HeLa and HCT116 cells does not stem from an error in the fractionation procedure. Another speculation is that redistribution of nuclear casp-2 may occur upon cell lysis; pre-treatment of cells with N-ethylmaleimide (NEM) for 10 min increased the amount of casp-2 in the nuclear fraction, suggesting that NEM treatment can completely prevent redistribution of casp-2 from the nucleus to the extra-nuclear fraction when cells are lysed [51]. However, it is also possible that NEM treatment itself can induce the translocation of casp-2 to the nucleus. In addition, many immunofluorescence analyses, which do not lyse the cell, also support the predominant cytosolic localization of casp-2 [39], [48]. Thus, this apparent discrepancy in the subcellular localization of casp-2 is likely due to differences among different cell types.
Casp-2S has been shown to selectively inhibit apoptotic-blebbing and -body formation during apoptosis. It is well known that membrane blebbing and apoptotic body formation require important remodeling of the cytoskeleton [52], [53], which is normally maintained by the Spectrin-Actin scaffold. Fodrin is a major substrate for proteases during apoptosis. Proteolysis of Fodrin leads to the destabilization of the Spectrin network and the loss of Actin filament crosslinks [28], [54], [55], which is thought to be involved in apoptotic membrane blebbing [28]. During apoptosis, casp-3 appears to be the major executioner caspase of Spectrin [56]. However, several lines of evidence suggest a casp-3 independent cleavage [57], [58], and that Fodrin can be cleaved by casp-2, casp-7 and calpain. It has been reported that Fodrin cleavage by casp-2, 3 and 7 is inhibited by the binding of calmodulin to Fodrin [59]. By using ectopic expression of FLAG-tagged casp-2S and mass spectrometric analysis, we revealed that the cytoskeleton protein Fodrin and α-actinin 4 bind to casp-2S under the physiological conditions, and this binding protects the cleavage of Fodrin and prevents the formation of membrane blebbing during apoptosis. In addition, our data also showed that casp-2L interacts with Fodrin in casp-2S overexpressing cells. Thus, it is also possible that casp-2S may bind to and inhibit casp-2L-mediated cleavage of Fodrin and apoptosis.
There is no doubt that apoptosis has dramatic effects on the cytoskeleton. However, disruption of the cytoskeleton by itself can induce or accelerate apoptosis [60], [61], [62]. Given the role of casp-2S in protecting cytoskeleton protein Fodrin from being cleaved, it is also possible that casp-2S may inhibit cytoskeleton damage induced apoptosis.
In non-erythroid cells, Fodrin is not only distributed at the plasma membrane but is also present in the nucleus [41]. Our subcellular fractionation analysis clearly demonstrated that the cleavage of Fodrin during apoptosis occurs exclusively in the cytoplasm. Thus, it is the cytosolic casp-2S, not the nuclear casp-2S, which interacts with Fodrin and protects its cleavage. While not involved in protection of Fodrin cleavage, the function of nuclear casp-2S is still unclear. It has been suggested that nuclear casp-2 might play a role in tumor suppression by functioning in DNA damage signaling [63]. However, whether this role is mediated by casp-2L or casp-2S is unknown and warrants further study.
Casp-2S has been shown to interact with various proteins. By using yeast two-hybrid screening, ISBP was identified as possessing an interaction with casp-2S. This interaction has been proposed as a means to mediate the anti-apoptotic function of casp-2S by preventing procaspase-2L processing and activation [24]. Casp-2S was also found to interact with caspase-3, inhibiting procaspase-3 processing and activation, thus preventing ROCK-1 activation [25]. Activated ROCK-1 induces membrane blebbing through enhanced myosin light chain phosphorylation, actin filamentous structures stabilization, and promotion of actin-myosin contractile force [64]. Casp-2S-mediated ROCK-1 inactivation was proposed to contribute to the inhibition of apoptotic blebbing by casp-2S. Our finding that casp-2S binds to Fodrin and inhibits its cleavage provides another mechanism underlying the anti-apoptotic function of casp-2S, and further supports the survival role of casp-2S.
In summary, we have identified a novel mechanism for the inhibition of apoptosis and apoptotic bleb formation by casp-2S. Given casp-2S executes its inhibitory role in preventing Fodrin cleavage in the cytoplasm, the function of nucleus-localized casp-2S remains to be elucidated.
Acknowledgments
We greatly acknowledge Drs. Bert Vogelstein for the HCT116 cell lines; Paul Modrich for the A2780 and CP70 cell lines; Karuppaiyah Selvendiran for CDDP cells, Thomas Hamilton for the PEO1, PEO4 and SKOV3 cell lines; Francois Claret for the 2008 and 2008C13 cell lines; Balveen Kaur for the Gli36 cell line; Arnab Chakravarti for the T98G and U87MG cell lines; Wenrui Duan for the H460, H1299 and A549 cell lines. We also thank the members of the Wani laboratory for their valuable input. Due to space limitations, we regret our inability to cite all the related papers in this manuscript.
Funding Statement
Funding came from a National Institute of Health grant CA151248 (QEW), and CA093413 (AAW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Prindull G (1995) Apoptosis in the embryo and tumorigenesis. Eur J Cancer 31A: 116–123. [DOI] [PubMed] [Google Scholar]
- 2. Kaufmann SH (1989) Induction of endonucleolytic DNA cleavage in human acute myelogenous lukemia cells by etopside, campothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res 49: 5870–5878. [PubMed] [Google Scholar]
- 3. Reed JC (1997) Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 34: 9–19. [PubMed] [Google Scholar]
- 4. Makin G, Dive C (2001) Apoptosis and cancer chemotherapy. Trends Cell Biol 11: S22–S26. [DOI] [PubMed] [Google Scholar]
- 5. Fulda S, Debatin KM (2004) Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets 4: 569–576. [DOI] [PubMed] [Google Scholar]
- 6. Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, et al. (1996) Human ICE/CED-3 protease nomenclature. Cell 87: 171. [DOI] [PubMed] [Google Scholar]
- 7. Jin Z, El-Deiry WS (2005) Overview of cell death signaling pathways. Cancer Biol Ther 4: 139–163. [DOI] [PubMed] [Google Scholar]
- 8. Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA (1994) Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1β- converting enzyme. Genes Dev 8: 1613–1626. [DOI] [PubMed] [Google Scholar]
- 9. Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR (1993) The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75: 641–652. [DOI] [PubMed] [Google Scholar]
- 10. Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, et al. (2009) Caspase-2 activation in the absence of PIDDosome formation. J Cell Biol 185: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Miura M, Bergeron L, Zhu H, Yuan J (1994) Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell 78: 739–750. [DOI] [PubMed] [Google Scholar]
- 12. Kumar S (1997) The Bcl-2 family of proteins and activation of the ICE-CED-3 family of proteases: a balancing act in apoptosis? Cell Death Differ 4: 2–3. [DOI] [PubMed] [Google Scholar]
- 13. Logette E, Wotawa A, Solier S, Desoche L, Solary E, et al. (2003) The human caspase-2 gene: alternative promoters, pre-mRNA splicing and AUG usage direct isoform-specific expression. Oncogene 22: 935–946. [DOI] [PubMed] [Google Scholar]
- 14. Solier S, Logette E, Desoche L, Solary E, Corcos L (2005) Nonsense-mediated mRNA decay among human caspases: the caspase-2S putative protein is encoded by an extremely short-lived mRNA. Cell Death Differ 12: 687–689. [DOI] [PubMed] [Google Scholar]
- 15. Kitevska T, Spencer DM, Hawkins CJ (2009) Caspase-2: controversial killer or checkpoint controller? Apoptosis 14: 829–848. [DOI] [PubMed] [Google Scholar]
- 16. Lassus P, Opitz-Araya X, Lazebnik Y (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297: 1352–1354. [DOI] [PubMed] [Google Scholar]
- 17. Lassus P, Optiz-Araya X, Lazenbik Y (2004) Corrections and clarifications. Science 306: 1683.15576593 [Google Scholar]
- 18. Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem 277: 29803–29809. [DOI] [PubMed] [Google Scholar]
- 19. Marsden VS, Ekert PG, Van DM, Vaux DL, Adams JM, et al. (2004) Bcl-2-regulated apoptosis and cytochrome c release can occur independently of both caspase-2 and caspase-9. J Cell Biol 165: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner KW, Engels IH, Deveraux QL (2004) Caspase-2 can function upstream of bid cleavage in the TRAIL apoptosis pathway. J Biol Chem 279: 35047–35052. [DOI] [PubMed] [Google Scholar]
- 21. Wang QE, Han C, Zhang B, Sabapathy K, Wani AA (2012) Nucleotide excision repair factor XPC enhances DNA damage-induced apoptosis by downregulating the antiapoptotic short isoform of caspase-2. Cancer Res 72: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savill J, Fadok V (2000) Corpse clearance defines the meaning of cell death. Nature 407: 784–788. [DOI] [PubMed] [Google Scholar]
- 23. Droin N, Rebe C, Bichat F, Hammann A, Bertrand R, et al. (2001) Modulation of apoptosis by procaspase-2 short isoform: selective inhibition of chromatin condensation, apoptotic body formation and phosphatidylserine externalization. Oncogene 20: 260–269. [DOI] [PubMed] [Google Scholar]
- 24. Ito A, Uehara T, Nomura Y (2000) Isolation of Ich-1S (caspase-2S)-binding protein that partially inhibits caspase activity. FEBS Lett 470: 360–364. [DOI] [PubMed] [Google Scholar]
- 25. Parent N, Sane AT, Droin N, Bertrand R (2005) Procaspase-2S inhibits procaspase-3 processing and activation, preventing ROCK-1-mediated apoptotic blebbing and body formation in human B lymphoma Namalwa cells. Apoptosis 10: 313–322. [DOI] [PubMed] [Google Scholar]
- 26. De Matteis MA, Morrow JS (1998) The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol 10: 542–549. [DOI] [PubMed] [Google Scholar]
- 27. Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, et al. (1997) Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278: 294–298. [DOI] [PubMed] [Google Scholar]
- 28. Martin SJ, O'Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, et al. (1995) Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem 270: 6425–6428. [DOI] [PubMed] [Google Scholar]
- 29. Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, et al. (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319 (Pt 3): 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, et al. (1987) Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res 47: 414–418. [PubMed] [Google Scholar]
- 31. Andrews PA, Velury S, Mann SC, Howell SB (1988) cis-Diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res 48: 68–73. [PubMed] [Google Scholar]
- 32. Roberts D, Schick J, Conway S, Biade S, Laub PB, et al. (2005) Identification of genes associated with platinum drug sensitivity and resistance in human ovarian cancer cells. Br J Cancer 92: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DiSaia PJ, Sinkovics JG, Rutledge FN, Smith JP (1972) Cell-mediated immunity to human malignant cells. A brief review and further studies with two gynecologic tumors. Am J Obstet Gynecol 114: 979–989. [DOI] [PubMed] [Google Scholar]
- 34. Delmastro DA, Li J, Vaisman A, Solle M, Chaney SG (1997) DNA damage inducible-gene expression following platinum treatment in human ovarian carcinoma cell lines. Cancer Chemother Pharmacol 39: 245–253. [DOI] [PubMed] [Google Scholar]
- 35. Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, et al. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501. [DOI] [PubMed] [Google Scholar]
- 36. Kashima T, Vinters HV, Campagnoni AT (1995) Unexpected expression of intermediate filament protein genes in human oligodendroglioma cell lines. J Neuropathol Exp Neurol 54: 23–31. [DOI] [PubMed] [Google Scholar]
- 37. O'Reilly LA, Ekert P, Harvey N, Marsden V, Cullen L, et al. (2002) Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ 9: 832–841. [DOI] [PubMed] [Google Scholar]
- 38. Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM (2003) Overexpression of the anti-apoptotic caspase-2 short isoform in macrophage-derived foam cells of human atherosclerotic plaques. Am J Pathol 162: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Narine KA, Keuling AM, Gombos R, Tron VA, Andrew SE, et al. (2010) Defining the DNA mismatch repair-dependent apoptotic pathway in primary cells: evidence for p53-independence and involvement of centrosomal caspase 2. DNA Repair (Amst) 9: 161–168. [DOI] [PubMed] [Google Scholar]
- 40. Colussi PA, Harvey NL, Kumar S (1998) Prodomain-dependent nuclear localization of the caspase-2 (Nedd2) precursor. A novel function for a caspase prodomain. J Biol Chem 273: 24535–24542. [DOI] [PubMed] [Google Scholar]
- 41. McMahon LW, Walsh CE, Lambert MW (1999) Human alpha spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J Biol Chem 274: 32904–32908. [DOI] [PubMed] [Google Scholar]
- 42. Baliga BC, Read SH, Kumar S (2004) The biochemical mechanism of caspase-2 activation. Cell Death Differ 11: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 43. Paroni G, Henderson C, Schneider C, Brancolini C (2001) Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J Biol Chem 276: 21907–21915. [DOI] [PubMed] [Google Scholar]
- 44. Zhivotovsky B, Samali A, Gahm A, Orrenius S (1999) Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ 6: 644–651. [DOI] [PubMed] [Google Scholar]
- 45. Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, et al. (2000) Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol 149: 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shikama Y, U M, Miyashita T, Yamada M (2001) Comprehensive studies on subcellular localizations and cell death-inducing activities of eight GFP-tagged apoptosis-related caspases. Exp Cell Res 264: 315–325. [DOI] [PubMed] [Google Scholar]
- 47. Shirakura H, Hayashi N, Ogino S, Tsuruma K, Uehara T, et al. (2005) Caspase recruitment domain of procaspase-2 could be a target for SUMO-1 modification through Ubc9. Biochem Biophys Res Commun 331: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 48. Troy CM, Stefanis L, Greene LA, Shelanski ML (1997) Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells. J Neurosci 17: 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shimohama S, Tanino H, Fujimoto S (2001) Differential subcellular localization of caspase family proteins in the adult rat brain. Neurosci Lett 315: 125–128. [DOI] [PubMed] [Google Scholar]
- 50. Paroni G, Henderson C, Schneider C, Brancolini C (2002) Caspase-2 can trigger cytochrome C release and apoptosis from the nucleus. J Biol Chem 277: 15147–15161. [DOI] [PubMed] [Google Scholar]
- 51. Tinnikov AA, Samuels HH (2013) A novel cell lysis approach reveals that caspase-2 rapidly translocates from the nucleus to the cytoplasm in response to apoptotic stimuli. PLoS One 8: e61085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cotter TG, Lennon SV, Glynn JM, Green DR (1992) Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res 52: 997–1005. [PubMed] [Google Scholar]
- 53. Suarez-Huerta N, Mosselmans R, Dumont JE, Robaye B (2000) Actin depolymerization and polymerization are required during apoptosis in endothelial cells. J Cell Physiol 184: 239–245. [DOI] [PubMed] [Google Scholar]
- 54. Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, et al. (1998) Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem 273: 22490–22497. [DOI] [PubMed] [Google Scholar]
- 55. Hu RJ, Bennett V (1991) In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s). J Biol Chem 266: 18200–18205. [PubMed] [Google Scholar]
- 56. Janicke RU, Ng P, Sprengart ML, Porter AG (1998) Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem 273: 15540–15545. [DOI] [PubMed] [Google Scholar]
- 57. Brown TL, Patil S, Cianci CD, Morrow JS, Howe PH (1999) Transforming growth factor beta induces caspase 3-independent cleavage of alphaII-spectrin (alpha-fodrin) coincident with apoptosis. J Biol Chem 274: 23256–23262. [DOI] [PubMed] [Google Scholar]
- 58. Cryns VL, Bergeron L, Zhu H, Li H, Yuan J (1996) Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem 271: 31277–31282. [DOI] [PubMed] [Google Scholar]
- 59. Rotter B, Kroviarski Y, Nicolas G, Dhermy D, Lecomte MC (2004) AlphaII-spectrin is an in vitro target for caspase-2, and its cleavage is regulated by calmodulin binding. Biochem J 378: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rao JY, Jin YS, Zheng Q, Cheng J, Tai J, et al. (1999) Alterations of the actin polymerization status as an apoptotic morphological effector in HL-60 cells. J Cell Biochem 75: 686–697. [PubMed] [Google Scholar]
- 61. Suria H, Chau LA, Negrou E, Kelvin DJ, Madrenas J (1999) Cytoskeletal disruption induces T cell apoptosis by a caspase-3 mediated mechanism. Life Sci 65: 2697–2707. [DOI] [PubMed] [Google Scholar]
- 62. Yamazaki Y, Tsuruga M, Zhou D, Fujita Y, Shang X, et al. (2000) Cytoskeletal disruption accelerates caspase-3 activation and alters the intracellular membrane reorganization in DNA damage-induced apoptosis. Exp Cell Res 259: 64–78. [DOI] [PubMed] [Google Scholar]
- 63. Kumar S (2009) Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more? Nat Rev Cancer 9: 897–903. [DOI] [PubMed] [Google Scholar]
- 64. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, et al. (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3: 339–345. [DOI] [PubMed] [Google Scholar]