Abstract
Metastatic melanoma remains a difficult disease to treat, and long term survivors are rare. Over the past few years, however, breakthroughs in both immunotherapy as well as targeted agents have had a tremendous impact on patients diagnosed with this disease. This review summarizes recent advances in systemic therapies for melanoma, including immune modulators directed against cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and programmed death-1 (PD-1), as well as a number of targeted agents. These approaches hold great promise as the landscape of therapeutic options for advanced melanoma continues to evolve.
Keywords: metastatic melanoma, therapy, ipilimumab, PD-1, BRAF, MEK, KIT
Introduction
While a number of landmark advances have been made in recent years, metastatic melanoma remains a difficult disease to treat, and most patients ultimately succumb to their disease. Until recently, chemotherapy was the historical mainstay of treatment for the majority of patients, although no phase III trial demonstrated an overall survival benefit.1–3 Prior to 2011, high-dose interleukin-2 (HDIL-2) was the only other Food and Drug Administration (FDA) approved option, but response rates are low and most patients are not eligible due to the significant toxicity associated with the drug.4 Advances in the understanding of both immune regulation as well as oncogenic drivers in melanoma have led to the development of new therapies that have demonstrated an overall survival benefit in phase III trials. Ipilimumab, a cytotoxic-T-lymphocyte-associated antigen-4 (CTLA-4) antibody, was the first agent to demonstrate an overall survival benefit for metastatic melanoma in a phase III trial, ultimately leading to FDA approval in 2011.5 The discovery that mutated BRAF is a major contributor to oncogenesis in approximately half of all melanomas ultimately led to the development of selective BRAF inhibitors, which have also shown a survival benefit in phase III studies.6–8 This increased understanding has paved the way for newer approaches to therapy with novel immune modulators as well as the identification of other oncogenic targets.
Immunotherapy
The association between the immune system and melanoma is well-known, with spontaneous regression observed in primary lesions and rarely in cases of metastatic disease.9–14 The goal of immunotherapeutic approaches is to induce a T-cell response, though this has been limited in the past by insufficient knowledge regarding T-cell regulation. CTLA-4 is a regulatory molecule that serves as a checkpoint under normal conditions to limit T-cell activation and expansion, with an additional role in preventing autoimmunity.15 Other related pathways, including programmed death 1 (PD-1), function in the regulation of the immune response and are promising viable therapeutic targets in melanoma. A partial listing of key immunomodulator trials is shown in Table 1.
Table 1.
Selected immunomodulator trials in advanced melanoma.
| Study | Agent | Phase | Trial design | Pts | RR (%) | SD (%) | 2-yr OS (%) |
|---|---|---|---|---|---|---|---|
| Hodi et al5 | Ipilimumab | III | 3 arm randomized | 676 | |||
| Ipilimumab 3 mg/kg q 3 weeks with gp100 vaccine | 5.7 | 14.4 | 21.6 | ||||
| Ipilimumab 3 mg/kg q 3 weeks with gp100 vaccine placebo | 11 | 17.5 | 23.5 | ||||
| gp100 vaccine alone | 1.5 | 9.6 | 13.7 | ||||
| Robert et al18 | Ipilimumab | III | 2 arm randomized | 502 | |||
| Ipilimumab 10 mg/kg q 3 weeks + DTIC 850 mg/m2; ipilimumab 10 mg/kg maintenance q 12 weeks until PD | 15.2 | 18 | 28.5 | ||||
| Ipilimumab placebo + DTIC 850 mg/m2; ipilimumab placebo maintenance q 12 weeks until PD | 10.3 | 19.8 | 17.9 | ||||
| Topalian et al34 | Nivolumab | I | Single arm, dose escalation ranging from 0.1 to 10 mg/kg q 2 weeks | 94 | 28 | 6a | NA |
| Hamid et al35 | MK-3475 | I | Single arm 3 dose levels: 1 mg/kg, 3 mg/kg, 10 mg/kg |
85 | 51 | NR | NA |
Note:
Stable disease >24 weeks.
Abbreviations: NR, not reported; NA, not applicable as additional footnotes.
CTLA-4
Creating a targeted immune response against a specific antigen is a complex process requiring the coordination of several interconnected pathways. An initial signal occurs when a tumor-associated antigen is presented via the major histocompatibility complex on an antigen-presenting cell (APC) and binds to a T-cell receptor (TCR).16 A second, co-stimulatory signal is created upon the binding of B7 on the APC to CD28 on the T-cell. This in turn activates a number of pro-survival pathways and upregulation of transcription factors, ultimately leading to increased T-cell activation and expansion. In response to this TCR-activated complex, CTLA- 4, which is normally present in cytoplasmic vesicles, translocates to the membrane where it modulates the TCR/CD28 complex, likely through a number of mechanisms, including competitive binding of B7, interaction with TCR activity at the immune synapse, and direct inhibition of CD28.17
Ipilimumab is a fully human monoclonal CTLA-4 antibody that has demonstrated an overall survival (OS) benefit in two phase III studies in patients with metastatic melanoma.5,18 Initial phase I studies tested doses from 3–20 mg/kg, with some suggestion of a direct-dose response relationship, though potentially at the risk of increased toxicity.19 In an early trial in which patients with metastatic melanoma were given a single dose of ipilimumab at 3 mg/kg, a partial response was seen in 2 of 17 patients.20 A phase II study of pretreatment with ipilimumab at 10 mg/kg in 155 patients with advanced melanoma showed a 35% disease control rate, and a median overall survival (OS) of 10.2 months.21 Other studies have also shown promising response rates as well as the potential for durable responses. One dose-ranging study in which patients were treated at 0.3 mg/kg, 3 mg/kg, or 10 mg/kg demonstrated a direct relationship between ipilimumab dose- and response rates, with approximately 11% of patients in the highest dose cohort exhibiting a response.22 Toxicity also appeared to correlate with dose, with no grade 3 or 4 events observed at the lowest dose level; however, the toxicity profile was generally manageable for the other patient groups.22 A landmark phase III trial which demonstrated an OS benefit for patients receiving ipilimumab ultimately led to FDA approval of this agent in 2011.5 In this study, patients with previously treated unresectable or metastatic melanoma were randomized to one of three treatment arms: ipilimumab 3 mg/kg every 3 weeks for 4 doses with a gp100 vaccine, ipilimumab 3 mg/kg every 3 weeks for 4 doses with a vaccine placebo, or gp100 vaccine alone. A total of 676 patients were enrolled, and patients who received ipilimumab, with or without the vaccine, showed improved OS compared to the vaccine only group (10 months and 10.1 months, respectively, versus 6.4 months for vaccine alone, P < 0.001, P = 0.003, respectively).5 Importantly, there appeared to be evidence of long-term survivors in the two ipilimumab arms, with 21.6% of patients in the ipilimumab plus vaccine arm and 23.5% of patients who received ipilimumab alone still alive at 24 months, suggesting the potential for durable responses. There did not appear to be any significant differences in terms of survival between the two ipilimumab groups. Overall, ipilimumab appeared to be well-tolerated. The most common adverse events appeared to be immune-related, which occurred in 60% of patients who received ipilimumab and 32% of patients who received the vaccine as monotherapy, though only 10%–15% of patients experienced a grade 3 or 4 event. The most common immune-related adverse events (irAE) appeared to be dermatologic or gastrointestinal, with the development of a rash reported in 40% of patients and diarrhea in 30%. Other toxicities included hepatotoxicity and endocrinopathies. Immune-related events were generally responsive to treatment, including high-dose steroids, though occasionally tumor necrosis factor (TNF)-α blockade was required. Importantly, however, there were 12 treatment-related deaths in the ipilimumab group, 7 of which were thought to be immune-related. The benefit of ipilimumab in the first-line setting was subsequently confirmed in a phase III trial in which 502 patients were randomized to receive ipilimumab at 10 mg/kg with dacarbazine (DTIC) 850 mg/m2 followed by maintenance ipilimumab at 10 mg/kg every 12 weeks until progression, or ipilimumab placebo with DTIC followed by ipilimumab placebo every 12 weeks.18 OS was improved in patients who received ipilimumab and was 11.2 months versus 9.1 months for patients in the chemotherapy arm (HR 0.72, P < 0.01). Furthermore, durable responses were also observed, with 28.5% and 20.8% of patients in the ipilimumab arm surviving for 2 and 3 years, respectively. Adverse events, including irAEs, were similar to those previously reported for ipilimumab, including diarrhea and pruritis/rash. There was a higher incidence of aspartate aminotransferase (AST)/alanine aminotransferase (ALT) elevation, likely due to the combination of ipilimumab with DTIC. The consistent improvement in overall survival in two phase III studies, with a subset of patients experiencing durable responses, confirmed the benefit of ipilimumab in the setting of metastatic melanoma.
However, many questions remain in terms of dosing, schedule, and strategies for combination with other therapies. A randomized phase III trial comparing two different dose levels of ipilimumab, 3 mg/kg versus 10 mg/kg, (NCT01515189) recently completed accrual and will ultimately help define the optimal dose. Preclinical data have also suggested that selective inhibition of oncogenic BRAFV600 can lead to increased expression of melanocyte differentiation antigens (MDAs), potentially resulting in increased T-cell recognition.23 Furthermore, peripheral blood samples from patients with BRAFV600 mutated melanoma who were treated with the selective BRAF inhibitor GSK2118436 showed no impairment in immune function.24 Taken together, these findings suggest a possible synergy between targeted and immunotherapeutic agents. Currently, a clinical trial is underway in which patients with BRAFV600 mutated melanoma are being treated with vemurafenib for 6 weeks, followed by ipilimumab (NCT01673854) to further investigate this strategy.
Additionally, the assessment of response to therapy with ipilimumab presents unique challenges in that the response patterns to immune modulators appear to be distinct from those expected with traditional cytotoxic therapy. Thus, separate guidelines have been proposed in order to more accurately classify responses in patients receiving this class of agents.25 Four distinct patterns of response are observed: an initial response after the completion of therapy that would typically be expected with standard cytotoxic agents, disease that initially remains stable, but then exhibits a slow continued response after the cessation of therapy, a response after an initial increase in the size of tumor lesions, or a response after the development of new lesions. The latter two categories are designed to describe patients who clinically remain stable and exhibit radiologic progression only. Patients who become more symptomatic along with apparent progression on scans most likely represent true disease progression.
PD-1
PD-1 is another inhibitory receptor involved in regulating T cell activation and expansion. While PD-1 is also a member of the CD28 family, it is thought to have distinct signaling functions from CTLA-4 in mediating immunosuppression. PD-1 is primarily expressed on activated T cells. The two known ligands for PD-1, programmed death ligand 1 and 2 (PD-L1 and PD-L2), are expressed in a wide variety of tissues, including tumor cells, though PD-L1 appears to predominate.26,27 Binding of PD-1 with its ligand generates an inhibitory signal to limit continued T-cell activation.28 Furthermore, blockade of this interaction has been shown to augment T cell responses in vitro with resultant anti-tumor activity.29,30 Tumor expression of PD-L1 is one potential mechanism by which tumor cells evade immune surveillance, and tumor surface expression of PD-L1 has been shown to be a poor prognostic marker in retrospective case series.29,31,32 A number of antibodies directed against PD-1 are now in various phases of clinical development, including nivolumab (MDX-1106, BMS-936558), MK-3475, and AMP-224.
Nivolumab is a fully humanized IgG4 monoclonal antibody specific for PD-1 that has shown promising activity in early phase studies. In an initial report of a phase I study, which included 4 different dose cohorts (0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg), the half-life ranged from 12 days for the three lower doses compared to 20 days for the 10 mg/kg dose.33 The maximum concentration and area under the curve (AUC) appeared to correlate directly with dose. Pharmacodynamic data revealed that PD-1 receptor occupancy appeared to be dose-independent, with a mean peak occupancy of 85% (70%–97%) and a mean plateau occupancy of 72% (59%–81%) observed at 4–24 hours and ≥57 days, though discordance between serum levels and receptor occupancy was observed in some patients. In this study, which included 39 patients with previously treated solid tumors (10 with melanoma), no dose-limiting toxicities were observed after one dose, and a maximum tolerated dose (MTD) was not established. After one dose of nivolumab, no grade 3 irAEs were seen, although one patient developed grade 3 colitis after administration of 5 doses. The most common adverse events reported in this study were decreased CD4+ lymphocyte counts, lymphopenia, fatigue, and musculoskeletal events. One patient with metastatic colorectal carcinoma treated at 3 mg/kg had a complete response (CR), while 2 patients treated at the 10 mg/kg dose (including one with melanoma) had partial responses (PR). An updated analysis of this trial, which included data on 236 evaluable patients, confirmed the promising results observed in the initial phases of the study.34 In 94 patients with metastatic melanoma treated at any dose, 28% had an objective response that appeared to be durable for most of the patients. Overall, nivolumab appeared to be well-tolerated, though 5% of patients discontinued therapy secondary to adverse events. The most common treatment-related events reported included fatigue, rash, diarrhea, and pruritis. Notably, some of the immune adverse events associated with ipilimumab, including colitis, hypophysitis, and thyroiditis, were observed in less than 1% of patients. PD-L1 expression was also assessed in 42 available tumor specimens. In the 25 patients whose tumors expressed PD-L1, 36% had an objective response, while no responses were observed in patients whose tumors did not express PD-L1.
Recent data presented from an early phase trial of MK-3475, in which patients with advanced melanoma were randomized to receive one of 3 dosing regimens, has also shown promising results. A total of 132 patients have been enrolled, and in the 85 patients that were evaluable at the time of analysis, 51% showed an objective tumor response.35 In the 27 patients who had received prior therapy with ipilimumab, 11 patients had a PR. MK-3475 appeared to be well-tolerated, with a low incidence of grade 3/4 events. Overall, these results are very promising for melanoma as well as other tumor types, and a number of larger studies are planned or are currently underway.
Emerging Immunotherapies
While ipilimumab and PD-1 targeted therapies are the furthest along in clinical development, a number of advances have been made with other immunomodulatory targets, including CD40, OX40, CD137 (4-1BB), and PD-L1, among others (Table 2). These targets show great promise for building upon the foundation of CTLA-4- and PD-1-directed therapy, although early toxicity signals may limit the use of some of these agents. CD40 is an important mediator of the humoral immune response and has been shown to be expressed in different types of APCs as well as in various tumors.36–38 A number of monoclonal antibodies targeting CD40 are in various phases of clinical development, including CP-870,893, dacetuzumab, Chi Lob 7/4, and lucatumumab.38 A clinical trial testing the combination of CP-870,893 with tremelimumab in patients with metastatic melanoma is currently enrolling patients (NCT01103635). OX40 is a costimulatory molecule expressed on CD4+ and CD8+ T lymphocytes, as well as other cell types, including natural killer (NK) cells and neutrophils.39–41 A mouse anti-human agonistic monoclonal antibody has entered phase I testing, though no initial responses were seen in a trial of 30 patients.38 Given that elevated levels of neutralizing human anti-mouse antibodies were detected in patients, humanized anti-OX40 antibodies are currently being developed. CD137 (4-1BB) is a surface protein that regulates numerous immune activities.42,43 This protein was shown to be expressed in activated T cells, whose only known ligand (CD137-ligand) is expressed mainly on macrophages, activated B cells, and dendritic cells.44,45 Two monoclonal agonist antibodies have been developed and are currently under clinical phase testing, but only urelumab (BMS-663513) has completed accrual; other studies were stopped due to concerns regarding liver toxicity.46,47 Some clinical activity was observed in patients with melanoma, and new dose escalation trials with these agents are currently ongoing.
Table 2.
Selected immunomodulator targets and available drugs.
| Target | Agent class | Phase of development |
|---|---|---|
| CD40 | ||
| CP-870,893 | Receptor agonist | I |
| Dacetuzumab | Receptor agonist | I |
| Chi Lob 7/4 | Receptor agonist | I |
| Lucatumumab | Receptor antagonist | I |
| OX40 | ||
| Anti-OX40 | Receptor agonist | I; halted |
| CD137 (4-1BB) | ||
| Urelumab | Receptor agonist | I/II |
| GITR | ||
| TRX518 | Receptor agonist | I |
| CTLA-4 | ||
| Ipilimumab | Receptor antagonist | III; FDA approved |
| Tremelimumab | Receptor antagonist | III |
| PD-1 | ||
| Nivolumab | Receptor antagonist | III |
| MK-3475 | Receptor antagonist | III |
| TGF-β | ||
| Fresolimumab | Receptor antagonist | I |
Other agents, including those that target PD-L1, KIR, TGF-β, and the glucocorticoid-induced TNF receptor (GITR) are currently under development.38,47 These, along with the other drugs described, represent a new era in immunomodulatory therapy that have a significant potential to impact patient care.
Targeted agents
Along with the development of novel immunotherapeutic approaches, an increased understanding of the molecular drivers of melanomagenesis has ushered in a new era of targeted therapeutics for this disease. Notably, the discovery in 2002 that BRAF is mutated in a large proportion of melanomas opened the door for novel approaches targeting the mitogen-activated protein kinase (MAPK) pathway. Initially identified in a screen of cancer cell lines, mutated BRAF is now known to be an oncogenic driver in approximately half of all melanomas.6 Additionally, the role of targeted therapy continues to be defined for other genetic subsets of melanoma as well.48,49
BRAF
As part of the MAPK pathway, the three isoforms of RAF in humans, including ARAF, BRAF, and CRAF, all serve to transmit extracellular signals intracellularly to a variety of effectors, ultimately resulting in increased growth and proliferation.50 The RAS-RAF-MEK-ERK pathway remains one of the best described MAPK pathways, and dysregulation of this signaling cascade is implicated in a number of malignancies. Under normal circumstances, activation of BRAF is highly dependent on RAS; however, the substitution of a glutamic acid for a valine at amino acid position 600 (BRAFV600E) results in a constitutively active kinase, now known to be capable of driving melanoma growth and metastasis.6,51–53 Other mutations at position 600 can occur, including V600K, which accounts for approximately 15%–30% of all BRAF mutations.54 The hypothesis that BRAF could be sufficient for oncogenesis in melanoma was initially met with some skepticism, largely due to the fact both high levels of BRAF expression as well as mutations are seen in normal melanocytes.55,56 Functional analyses revealed that most BRAF mutants, including all of those at amino acid position 600, possessed upregulated basal kinase activity when compared to wild-type BRAF, resulting in RAS-independent phosphorylation of ERK.57 Thus, while the activation of BRAF through mutation appears to be an early event in melanoma tumorigenesis, it remains a critical pathway in advanced disease. Early attempts at targeting BRAF with sorafenib or other drugs were largely unsuccessful, likely because these agents had insufficient selectivity for the mutant form of BRAF.58–62 A unique structure-guided approach led to the development the highly selective BRAF inhibitor vemurafenib, which was shown to have an OS benefit in a phase III trial.7,63 Early trials with vemurafenib were very promising, and in the initial phase I trial of PLX4032 (now known as vemurafenib), 24 of 32 patients in the extension phase had a PR, while 2 patients had a CR.64 Median progression-free survival (PFS) was 7 months, and median OS was not reached. A phase II study in 132 previously untreated patients with metastatic melanoma demonstrated an overall response rate (ORR) of 53%, with a median duration of response of 6.7 months.65 A pivotal phase III trial in which 675 previously untreated patients were randomized to receive either vemurafenib 960 mg PO BID or DTIC 1000 mg/m2 demonstrated an overall survival benefit in favor of the vemurafenib arm, with a hazard ratio (HR) for death in the vemurafenib group of 0.37 (CI 0.26 v 0.55).7 Six month OS was also improved and was 84% (95% CI, 78–89) in the vemurafenib arm versus 64% (95% CI, 56–73) in the DTIC arm. An interim analysis led to early stopping of the trial, with crossover to the vemurafenib arm. Updated results were recently presented, and with longer follow-up the improvement in OS was still evident despite crossover, at 13.6 months in the vemurafenib arm versus 9.7 months in the DTIC arm.66 Dabrafenib, another highly selective BRAF inhibitor, has also shown potential in the treatment of patients with metastatic melanoma. Early-phase trials demonstrated high response rates, and data from a recently completed randomized phase III study confirmed the progression of a free survival benefit when compared to DTIC.8,67,68Table 3 summarizes the results of selected BRAF inhibitor trials.
Table 3.
Key BRAF inhibitor (BRAFi) trials in metastatic melanoma.
| Study | Trial design | Control arm | Patients in BRAFi arm | RR in BRAFi arm (%) | Median PFS (months) |
|---|---|---|---|---|---|
| Vemurafenib trials | |||||
| Flaherty et al64 | Single arm dose escalation/phase II extension cohort | NA | 32a | 81 | >7 |
| Sosman et al65 | Single arm phase II | NA | 132 | 53 | 6.8 |
| Chapman et al7 | Randomized phase III | DTIC 1000 mg/m2 IV every 3 weeks |
337 | 57 | 6.9 versus 1.6 (HR 0.38; 95% CI 0.32–0.46, P < 0.001) |
| Dabrafenib trials | |||||
| Kefford et al67 | Single arm dose escalation/phase II extension cohort | NA | 16b | 63 | NR |
| Trefzer et al68 | Single arm phase II | NA | 76V600E | 59 | 27 weeks |
| 16V600K | 13 | 20 weeks | |||
| Hauschild et al8 | Randomized phase III | DTIC 1000 mg/m2 IV every 3 weeks |
187 | 50 | 6.7 versus 2.9 (HR 0.35; 95% CI 0.20–0.69, P < 0.0001) |
Notes:
Patients in extension phase treated at the recommended phase II dose of 960 mg BID;
evaluable patients with BRAFV600 mutated melanoma, treated at recommended phase II dose or higher (at least 150 mg BID).
Toxicity
While BRAF inhibitor therapy is generally well-tolerated, there are some unique toxicities that require coordinated management of patients receiving these agents. The most common adverse events reported for vemurafenib include arthralgias, rash, fatigue, and nausea, although in most cases there were few grade 3 or 4 events.7 Notably, the development of cutaneous squamous cell carcinomas (cuSCCs) was observed in 12% of patients, and keratoacanthomas in 8%. It is therefore recommended that patients undergo periodic dermatologic surveillance, and those who develop a concerning lesion should seek prompt evaluation for definitive local therapy. Interestingly, many of these lesions may harbor mutations in RAS, resulting in reactivation of the MAPK pathway.69 Photosensitivity, which can potentially be severe, has also been reported, and patients should be advised to wear sunscreen and counseled in sun avoidance. Dabrafenib appears to have a similar toxicity profile, though lower rates of cuSCCs/keratoacanthomas have been reported.8 Additionally, pyrexia appears to be more common in patients receiving dabrafenib, although grade 3 events were rare.
Mechanisms of resistance to BRAF inhibitor therapy
The success of BRAF-targeted therapy represents a milestone in the treatment of metastatic melanoma. With high response rates that have the potential to result in a relatively quick clinical improvement, these agents are ideal for patients with rapidly progressive or symptomatic disease whose tumors harbor a BRAF mutation. However, there is a small subset of patients whose disease is initially refractory, and even in those who do respond, most will ultimately relapse within a year. This raises important questions regarding the mechanisms of both primary and acquired resistance. Initially thought to center solely around MAPK reactivation, it is now becoming apparent that a number of mechanisms likely contribute.
Though preclinical models have demonstrated that mutations in BRAF itself are capable of inducing resistance, the presence of such a mutation has yet to be identified in the patient samples analyzed to date.70 However, MAPK pathway dysregulation at points both upstream and downstream of BRAF likely play a critical role in acquired resistance. Activation of NRAS is one such mechanism, and it is thought that oncogenic mutations in NRAS result in sustained activation of CRAF, providing a bypass mechanism to BRAF inhibition. An analysis of PLX4032 resistant clones known to harbor the V600E mutation identified a de novo activating mutation in NRAS (Q61K) in one cell line.71 The same mutation was then found in two separate biopsies in a patient whose disease had relapsed on PLX4032. A more recent analysis of available patient samples for patients treated in a phase II trial with vemurafenib served as further confirmation of this mechanism.72 Of the thirteen patient samples available at the time of progression, 3 NRAS mutations were identified. It is well-known that MEK signaling is a critical driver in BRAF mutated melanoma, and it is likely that altered MEK signaling accounts for another potential mechanism of resistance. In an analysis of tumor samples from patients treated with the MEK inhibitor AZD6244, a MEK mutation was identified in a patient who had progressed on treatment.73 Interestingly, combined BRAF and MEK inhibition prevented the emergence of resistant clones. COT is another MAP kinase that has been shown to play a role in resistance to BRAF inhibition, as preclinical data have shown that it is capable of inducing ERK activation independently of RAF.74 Additional in vitro analyses suggest that high COT expression is associated with de novo resistance to BRAF inhibition, underscoring the fact that even within the same pathway multiple mechanisms are likely to contribute.
Outside of the MAPK pathway, the picture continues to be defined, as a number of pathways appear to play a role in the development of resistance to BRAF inhibition. One potential mediator includes the phosphatidylinositol-3-kinase (PI3K) pathway. The PI3K/AKT/mTOR pathway, a key mediator of cellular proliferation, apoptosis, and metabolism, is known to be dysregulated in a number of cancers, including melanoma.75 Preclinical studies have shown that BRAF and PI3K signaling work in concert to allow melanoma to escape apoptosis.76 Phosphatase and tensin homolog (PTEN), which normally serves to abrogate PI3K signaling, has been shown to be lost in approximately 10% of all melanomas, leading to sustained activation of prosurvival signals generated by AKT signaling.76 AKT itself may also be constitutively activated in melanoma irrespective of PTEN, and higher levels of expression are generally observed in more advanced stages of disease.77 Recent preclinical data shows that PTEN loss may lead to intrinsic resistance to BRAF inhibition through suppression of proapoptotic signals generated by BIM.78 BRAF mutated cell lines lacking functional PTEN were shown to induce significantly less apoptosis and increased levels of phosphorylated AKT in response to treatment with PLX4720 compared to analogous cell lines with intact PTEN. In examining downstream effectors of AKT that may mediate this effect, BIM expression appeared to be relatively suppressed in PTEN-negative cell lines in response to PLX4720 exposure. Furthermore, dual BRAF/PI3K inhibition increased BIM expression and appeared to enhance apoptosis in PTEN-negative cells, suggesting this strategy as a method for overcoming intrinsic resistance. Acquired BRAF resistance may also result from activation of the IGF-1R pathway, which has been shown to be involved in melanoma cell growth and survival.79 In an analysis of multiple receptor tyrosine kinases, IGF-1R expression appeared to be upregulated in resistant melanoma cell lines, although the exact mechanism of action remains to be defined. Treatment with two IGF-1R inhibitors resulted in increased apoptosis in cells that were resistant to BRAF inhibition.80 This appeared to be mediated through increased AKT signaling, with no appreciable effect observed on ERK phosphorylation. The same was observed in a sample from one patient who had progressive disease after treatment with PLX4032. Five paired sets of pre- and post-treatment samples were analyzed, and increased levels of both IGF-1R and pAKT were observed in the post treatment sample of one patient, lending further validation to the in vitro data. An additional potential mechanism for required resistance involving an alternative pathway is the role of PDGFRβ.71 Nazarian et al showed that in resistant cell lines harboring a V600E mutation, a number of receptor tyrosine kinases were differentially expressed compared to the parental line, including PDGFRβ. Subsequent analysis revealed that only PDGFRβ showed increased phosphorylation, consistent with its upregulated activity. Furthermore, in patient samples, over-expression of PDGFRβ was seen in tumor tissue relative to baseline. The underlying mechanisms of resistance to BRAF inhibitor therapy is a complex and heterogeneous process and combination strategies aimed at other targets in conjunction with BRAF inhibition is a promising strategy moving forward.
MEK
Trametinib, an allosteric inhibitor of MEK1 and MEK2, has recently been shown to improve OS when compared to cytotoxic chemotherapy in BRAF mutated melanoma, though response rates are lower than those observed with the single agent vemurafenib.81 Preclinical data suggested that MEK is a viable target in melanoma ,as MEK inhibition is capable of suppressing tumor growth in BRAFV600E xenografts.82 Similarly to BRAF inhibition, early attempts at targeting MEK in melanoma did not show promising results, potentially due to the narrow therapeutic window of many of the agents tested.83–86 In pharmacokinetic analyses, trametinib has a half-life of approximately 4 days and a low peak to trough ratio, properties which are thought to contribute to its improved toxicity profile when compared to other inhibitors in this class.87,88 In the initial phase I study which included patients with multiple tumor types, 2 mg daily was established as the recommended phase II dose. Overall, 10% of patients responded, and patients with BRAF mutated melanoma appeared to have the best responses. Given this early sign of promising activity, 97 patients with metastatic melanoma were ultimately included in the study. Of those, 36 were known to have a BRAFV600 mutation and six of these patients had received prior therapy with a BRAF inhibitor.89 In the 30 patient BRAF inhibitor-naïve cohort, the ORR was 33%, with 2 patients experiencing a complete response and a median PFS of 5.6 months (95% confidence interval [CI], 5.5–11.1 months). More modest levels of activity were observed for other cohorts, including those with BRAF wild-type disease. A phase II study also showed promising results in the BRAF inhibitor-naïve patient population.90 In this cohort of 57 patients with BRAFV600E/K melanoma, the response rate was 25%, with a median PFS of 4 months (95% CI, 3.6–5.6 months). No responses were seen in the 40 patients who had received prior therapy with a BRAF inhibitor, though a minority of patients experienced stable disease. A recent randomized phase III study comparing trametinib to cytotoxic chemotherapy in patients with BRAF mutated melanoma confirmed the promise seen in earlier studies.81 A total of 322 patients were randomized 2:1 to receive either trametinib or chemotherapy (dacarbazine or paclitaxel). The response rate for patients receiving trametinib was 22% (95% CI 17–28) versus 8% (95% CI 4–15, P = 0.01) for those who received chemotherapy. Median PFS was improved in the trametinib arm (4.8 months) compared to 1.5 months in the chemotherapy arm. At 6 months, OS in the trametinib arm was 81%, compared to 67% in the chemotherapy arm, even though crossover had been allowed. Trametinib appeared to be well-tolerated, with cutaneous toxicities being the most frequently reported side effect. Rash, including acneiform dermatitis, was reported in more than two-thirds of patients, with 8% reported as grade 3 or 4. Diarrhea, fatigue, and peripheral edema were also among the other commonly reported adverse events, though grade 3/4 events were rare and occurred in less than 10% of patients.
Role of MEK inhibition in combination therapy
The identification of MAPK reactivation as a major contributor to BRAF inhibitor resistance, as well as the single agent activity of trametinib, has driven a concerted effort aimed at dual BRAF + MEK inhibition. An initial phase I/II study testing the combination of dabrafenib and trametinib included multiple cohorts in order to assess drug-drug interactions, and then ultimately safety and efficacy in the BRAF mutated melanoma population.91 This study established that full monotherapy doses of each agent could be safely delivered (150 mg BID of dabrafenib, 2 mg daily of trametinib). An early analysis of the cohort which included 71 BRAF inhibitor-naïve patients showed that 5 had a complete response and 47% had a partial response.92 A recent report of 162 patients who were randomized into one of three arms (dabrafenib 150 mg twice daily alone; dabrafenib 150 mg twice daily + trametinib 1 mg daily; or dabrafenib 150 mg twice daily + trametinib 2 mg daily) showed that the full dose combination regimen resulted in an improved response rate when compared to dabrafenib monotherapy (76% vs. 54%). Median PFS was also improved with dual inhibition at 10.5 months [95% CI, 7.4–14.9 months] versus 5.6 months with single agent dabrafenib [95% CI, 4.5–7.4 months]. Overall, the combination appeared to be well-tolerated, and the most common side effects reported were pyrexia, rash, nausea, diarrhea, and fatigue. Interestingly, dual inhibition appears to reduce the incidence of hyperproliferative skin lesions, including the cuSCCs observed with selective BRAF inhibitor therapy. Two phase III studies comparing dual BRAF + MEK inhibition versus BRAF inhibitor monotherapy in patients with metastatic melanoma are currently accruing patients (NCT01584648 and NCT01597908) and additional trials are planned.
KIT
In addition to BRAF, mutations in KIT may also play an important role in melanomagenesis in some patients with melanoma, particularly those with mucosal and acral melanoma, as well as in those with melanomas arising from chronically sun-damaged skin.48,49,93 KIT is a transmembrane receptor tyrosine kinase that normally functions through the binding of stem cell factor to regulate numerous cellular processes including proliferation and inhibition of apoptosis. KIT has been shown to be dysregulated in other malignancies, including the vast majority of gastrointestinal stromal cell tumors (GIST), for which the efficacy of targeted inhibition has been well-established.94 The role of KIT as an oncogene in melanoma has begun to emerge in recent years. It is well-known to be essential for melanocyte development and survival, and now appears to be a critical oncogene in defined subsets of melanoma as well.95–97 A number of series have examined the incidence of aberrations in KIT in specific melanoma subtypes, and it appears that approximately 15%–20% of acral and 15%–35% mucosal melanomas, depending on anatomic subsite, harbor mutations in KIT. The most common mutations appear to be in exons 11 and 13, though mutations in exon 17 and others have also been observed. Initial trials of imatinib showed disappointing results with no significant clinical activity; however, these trials included a largely unselected patient population.98–100 Preclinical data suggesting sensitivity to KIT-targeted agents in melanoma cell lines along with isolated reports of dramatic clinical responses to imatinib in patients with specific mutations renewed interest in KIT as potential oncogenic target in melanoma.101–105 In a phase II study of imatinib in patients with advanced melanoma with KIT aberrations, 43 patients were treated with imatinib 400 mg BID.106 All patients had either KIT amplification or mutation, including exons 9,11,13,17, and 18. Eighteen patients experienced some tumor regression, and, notably, 9 out of the 10 PRs were in patients whose tumors had mutation in exons 11 or 13. Similar to GIST, it appeared that specific KIT mutations were predictive of imatinib sensitivity. An additional study conducted in the United States also included 25 evaluable patients whose tumors had KIT amplification or mutation.107 Two patients had a CR, 2 had a durable PR (53 weeks, 89 weeks, ongoing), and another 2 patients experienced a transient partial response. Interestingly, all patients who had a response possessed mutations in either exon 11 or 13. Additional studies are currently underway to determine whether KIT-targeted therapy, potentially with more selective agents, will lead to improved outcomes for this patient population.
Conclusions
Recent advances in systemic therapy have changed the landscape of treatment options for metastatic melanoma. With the established efficacy of both immune modulators and targeted agents, there is new promise for patients diagnosed with this devastating disease. Decades of translational and clinical work have laid the foundation for continued breakthroughs, though long term survivors are still rare. With continued development of novel compounds and combination strategies, it is possible that many more patients stand to benefit.
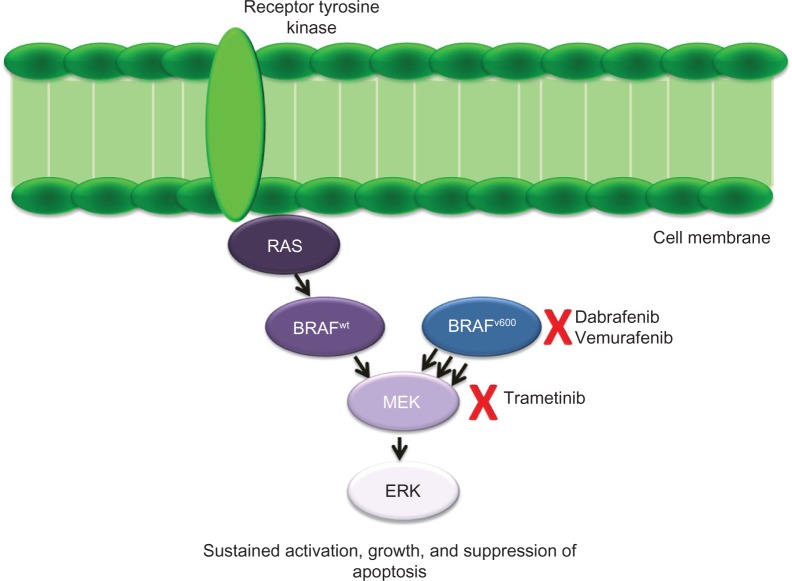
Figure 1.
Overview of the MAPK pathway and therapeutic targets.
Footnotes
Author Contributions
Analyzed the data: AKSS. Wrote the first draft of the manuscript: AKSS. Contributed to the writing of the manuscript: AKSS. Agree with manuscript results and conclusions: AKSS. Jointly developed the structure and arguments for the paper: AKSS. Made critical revisions and approved final version: AKSS. All authors reviewed and approved of the final manuscript.
Funding
Author discloses no funding sources.
Competing Interests
Dr. Salama has served as a consultant for Roche-Genentech and Bristol-Myers-Squibb (unpaid). Dr. Salama receives research funding from Bristol-Myers-Squibb.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
References
- 1.Atallah E, Flaherty L. Treatment of metastatic malignant melanoma. Curr Treat Options Oncol. 2005;6(3):185–93. doi: 10.1007/s11864-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 2.Mouawad R, Sebert M, Michels J, Bloch J, Spano J-P, Khayat D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2010;74(1):27–39. doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907–13. [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 9.Ronan SG, Eng AM, Briele HA, Shioura NN, Das Gupta TK. Thin malignant melanomas with regression and metastases. Arch Dermatol. 1987;123(10):1326–30. [PubMed] [Google Scholar]
- 10.Gromet MA, Epstein WL, Blois MS. The regressing thin malignant melanoma: a distinctive lesion with metastatic potential. Cancer. 1978;42(5):2282–92. doi: 10.1002/1097-0142(197811)42:5<2282::aid-cncr2820420528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97(2):197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 12.Cook MG. The significance of inflammation and regression in melanoma. Virchows Arch A Pathol Anat Histopathol. 1992;420(2):113–5. doi: 10.1007/BF02358800. [DOI] [PubMed] [Google Scholar]
- 13.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19(5):275–82. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- 14.Valenzano Menada M, Moioli M, Garaventa A, et al. Spontaneous regression of transplacental metastases from maternal melanoma in a newborn: case report and review of the literature. Melanoma Res. 2010;20(6):443–9. doi: 10.1097/CMR.0b013e32833faf6a. [DOI] [PubMed] [Google Scholar]
- 15.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17(14):4622–8. doi: 10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe AH, Abbas AK. T-cell costimulation—biology, therapeutic potential, and challenges. N Engl J Med. 2006;355(10):973–5. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 17.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 19.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26(32):5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 20.Tchekmedyian S, Glaspy J, Korman A, Keler T, Deo Y, Davis T. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma. Proc Am Soc Clin Oncol. 2002;21(Abstract 56):223. [Google Scholar]
- 21.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21(8):1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 23.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 24.Hong DS, Vence L, Falchook G, et al. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res. 2012;18(8):2326–35. doi: 10.1158/1078-0432.CCR-11-2515. [DOI] [PubMed] [Google Scholar]
- 25.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 27.Ishida M, Iwai Y, Tanaka Y, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84(1):57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 28.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 30.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 32.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamid O. Efficacy and Safety of MK-3475 in Patients with Advanced Melanoma. Paper presented at The Society for Melanoma Research 2012 Congress; Nov 8–11, 2012; Hollywood, CA. [Google Scholar]
- 36.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035–43. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliopoulos AG, Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4(4):360–7. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24(12):1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 40.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9(4):1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169(4):1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 43.May KF, Jr, Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62(12):3459–65. [PubMed] [Google Scholar]
- 44.Pollok KE, Kim YJ, Zhou Z, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150(3):771–81. [PubMed] [Google Scholar]
- 45.Lin W, Voskens CJ, Zhang X, et al. Fc-dependent expression of CD137 on human NK cells: insights into “agonistic” effects of anti-CD137 monoclonal antibodies. Blood. 2008;112(3):699–707. doi: 10.1182/blood-2007-11-122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sznol M, Hodi FS, Margolin K, McDermott DF, Ernstoff MS, Kirkwood J. Phase I study of BMS-663513, a fully human anti-CD137 agnonist monoclonal antibody, in patients (pts) with advanced cancer (CA) Journal of Clinical Oncology. 2008 May 20;26(Suppl) abstr 3007). [Google Scholar]
- 47.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19(5):1044–53. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 49.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 50.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63(17):5198–202. [PubMed] [Google Scholar]
- 52.Ikenoue T, Hikiba Y, Kanai F, et al. Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor κB pathway and cellular transformation. Cancer Res. 2004;64(10):3428–35. doi: 10.1158/0008-5472.CAN-03-3591. [DOI] [PubMed] [Google Scholar]
- 53.Ikenoue T, Kanai F, Hikiba Y, et al. Functional consequences of mutations in a putative Akt phosphorylation motif of B-raf in human cancers. Mol Carcinog. 2005;43(1):59–63. doi: 10.1002/mc.20102. [DOI] [PubMed] [Google Scholar]
- 54.Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012;18(12):3242–9. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- 55.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 56.Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121(5):1160–2. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- 57.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 58.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95(5):581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min CJ, Liebes LF, Escalon J, et al. Phase II trial of sorafenib (S[BAY 43-9006]) in metastatic melanoma (MM) including detection of BRAF with mutant specific-PCR (MS-PCR) and altered proliferation pathways-final outcome analysis. Journal of Clinical Oncology. 2008;26(Suppl) abstr 9072). [Google Scholar]
- 60.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27(17):2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 61.Amaravadi RK, Schuchter LM, McDermott DF, et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res. 2009;15(24):7711–8. doi: 10.1158/1078-0432.CCR-09-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ott PA, Hamilton A, Min C, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One. 2010;5(12):e15588. doi: 10.1371/journal.pone.0015588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman PB, Hauschild A, Robert C, et al. Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E-mutated melanoma. Journal of Clinical Oncology. 2012;30(Suppl) abstr 8502). [Google Scholar]
- 67.Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. Journal of Clinical Oncology. 2010;28(Suppl) 15s. abstr 8503). [Google Scholar]
- 68.Trefzer U, Minor D, Ribas A, et al. BREAK-2: a phase IIA trial of the selective BRAF kinase inhibitor GSK2118436 in patients with BRAF (V600E/K)-positive metastatic melanoma. Paper presented at: Presented at The Society for Melanoma Research 2011 Congress; Nov 9–13, 2011; Tampa, FL. [Google Scholar]
- 69.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whittaker S, Kirk R, Hayward R, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci Transl Med. 2010;2(35):35–41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 71.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sosman JA, Pavlick AC, Schuchter LM, et al. Analysis of molecular mechanisms of response and resistance to vemurafenib (vem) in BRAFV600E melanoma. Journal of Clinical Oncology. 2012;30(Suppl) abstr 8503). [Google Scholar]
- 73.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–6. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27(41):5511–26. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 76.Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25(35):4848–56. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 77.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64(19):7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 78.Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hilmi C, Larribere L, Giuliano S, et al. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol. 2008;128(6):1499–505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- 80.Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 82.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439(7074):358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18(2):555–67. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23(23):5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 85.LoRusso PM, Krishnamurthi SS, Rinehart JJ, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res. 2010;16(6):1924–37. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 86.Haura EB, Ricart AD, Larson TG, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16(8):2450–7. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 87.Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17(5):989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 88.Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 89.Falchoo GS, Lewis KD, Infante JR, et al. Lancet Oncol. 2012. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim KB, Lewis K, Pavlick AC, et al. A Phase II study of the MEK1/MEK2 inhibitor GSK1120212 in metastatic BRAF V600E or K mutant cutaneous melanoma patients previously treated with or without a BRAF inhibitor. Pigment Cell & Melanoma Research. 2011;24(5):1021. (Abst. LBA 1021–3). [Google Scholar]
- 91.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Infante JR, Falchook GS, Lawrence DP, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436) Journal of Clinical Oncology. 2011;29(Suppl) abstr CRA8503). [Google Scholar]
- 93.Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–8. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 94.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 95.Hou L, Panthier JJ, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development. 2000;127(24):5379–89. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 96.Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003;16(3):287–96. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 97.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126(5):1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 98.Wyman K, Atkins MB, Prieto V, et al. Multicenter Phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106(9):2005–11. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- 99.Ugurel S, Hildenbrand R, Zimpfer A, et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92(8):1398–405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim KB, Eton O, Davis DW, et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Brit J Cancer. 2008;99(5):734–40. doi: 10.1038/sj.bjc.6604482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26(12):2046–51. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 102.Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008;21(4):492–3. doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 103.Antonescu CR, Busam KJ, Francone TD, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121(2):257–64. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 104.Ashida A, Takata M, Murata H, Kido K, Saida T. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009;124(4):862–8. doi: 10.1002/ijc.24048. [DOI] [PubMed] [Google Scholar]
- 105.Jiang X, Zhou J, Yuen NK, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin Cancer Res. 2008;14(23):7726–32. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 106.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 107.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–34. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]