Abstract
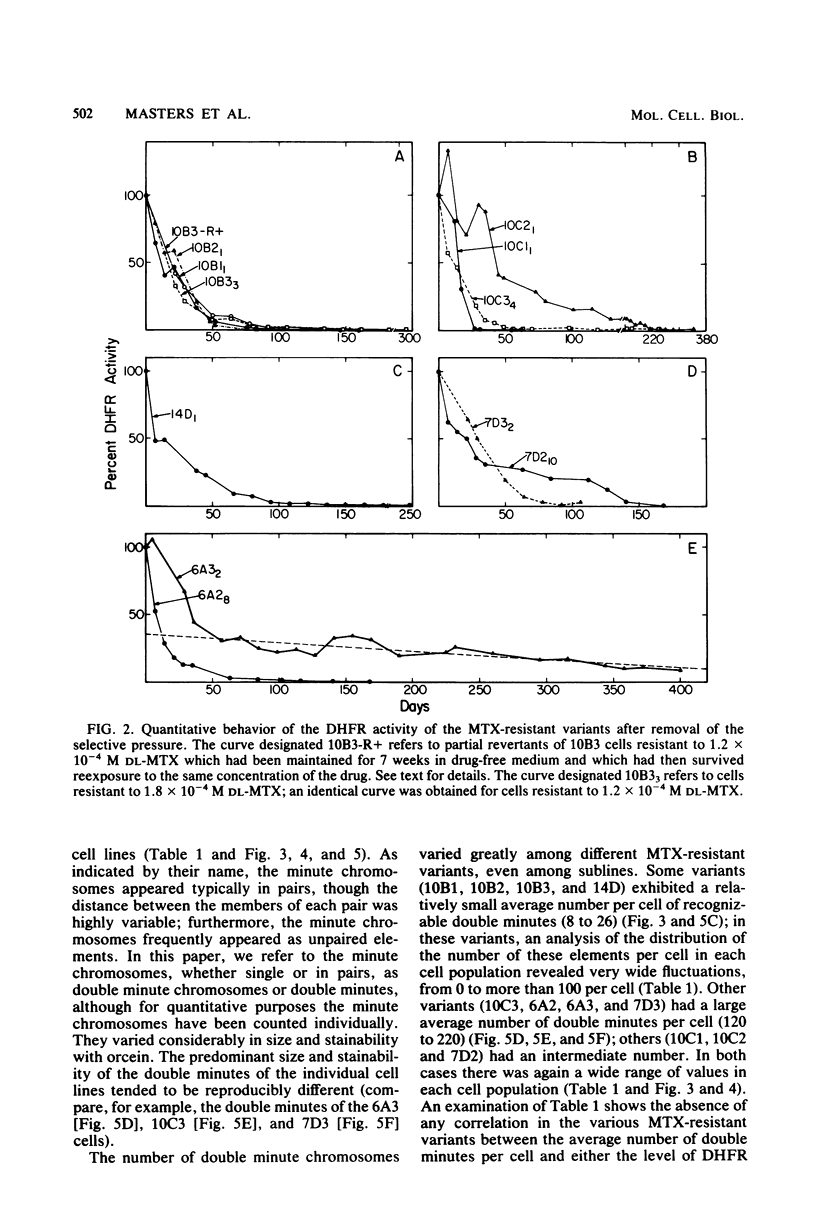
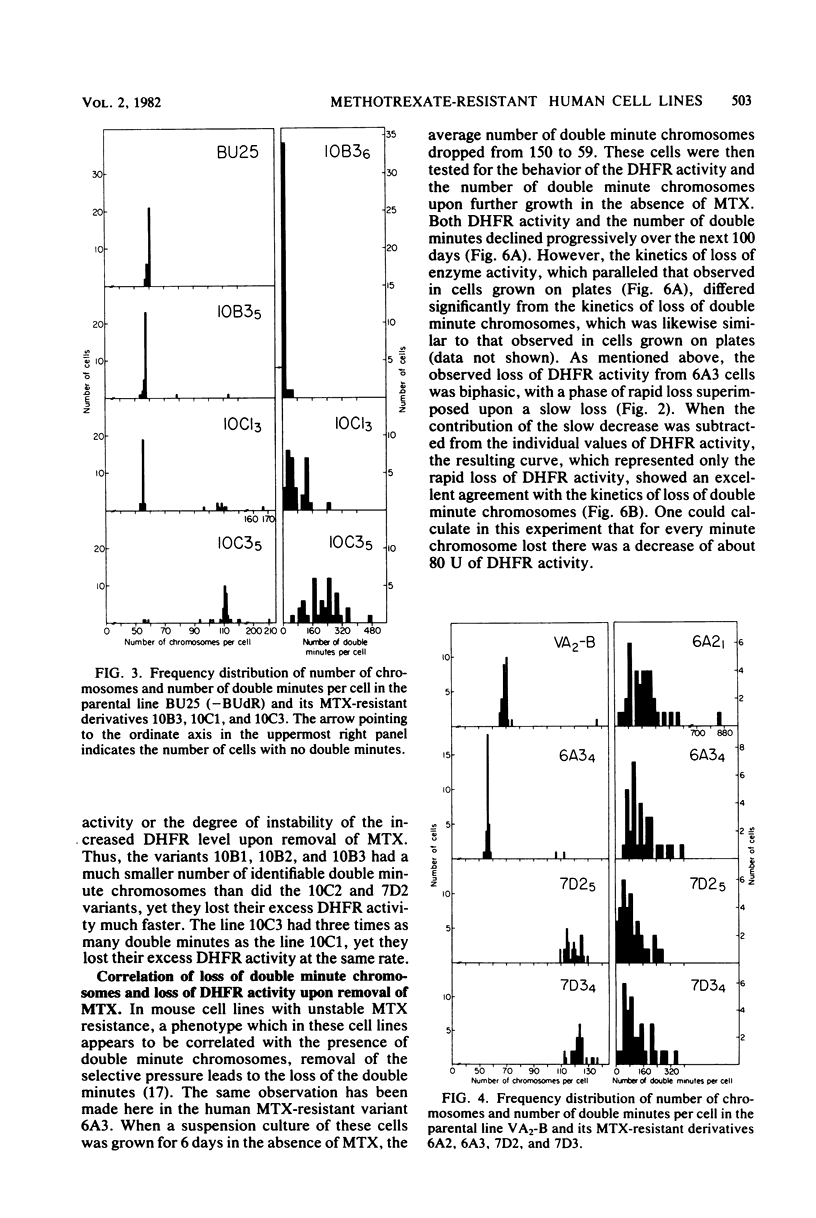
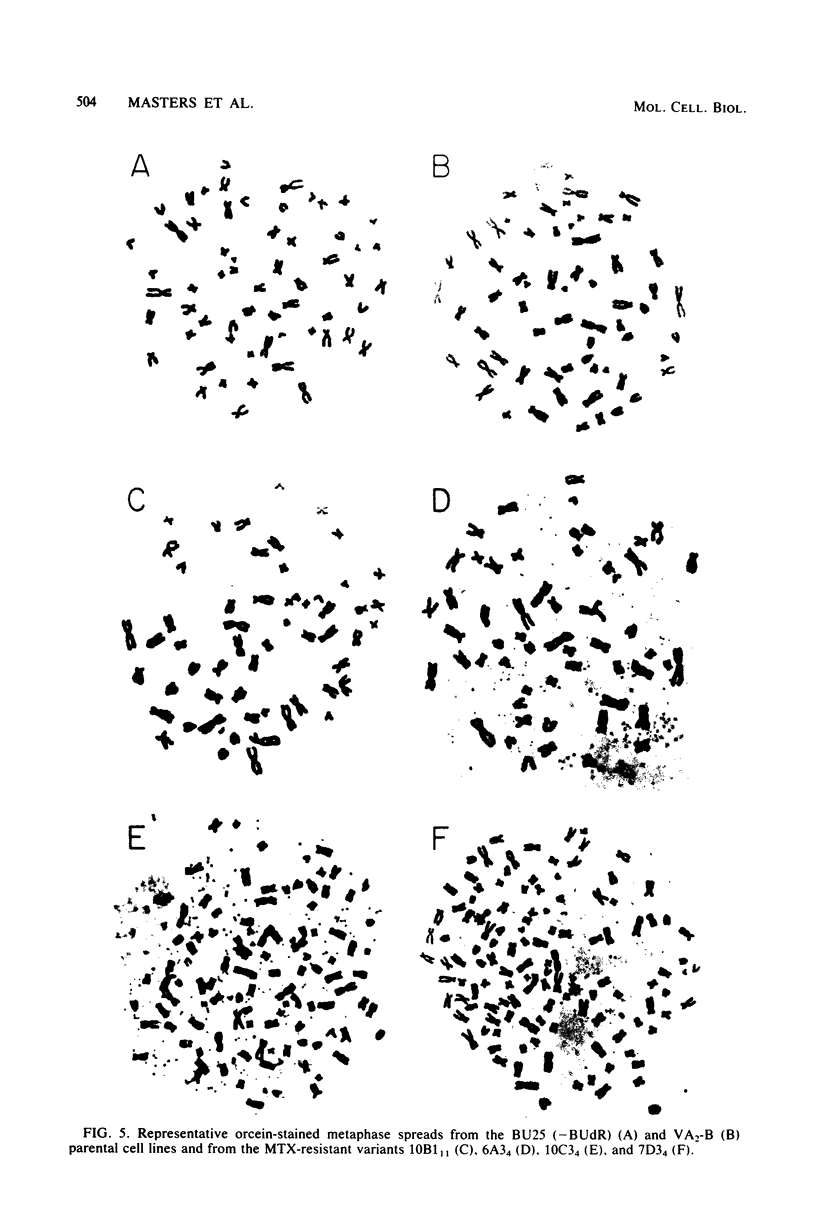
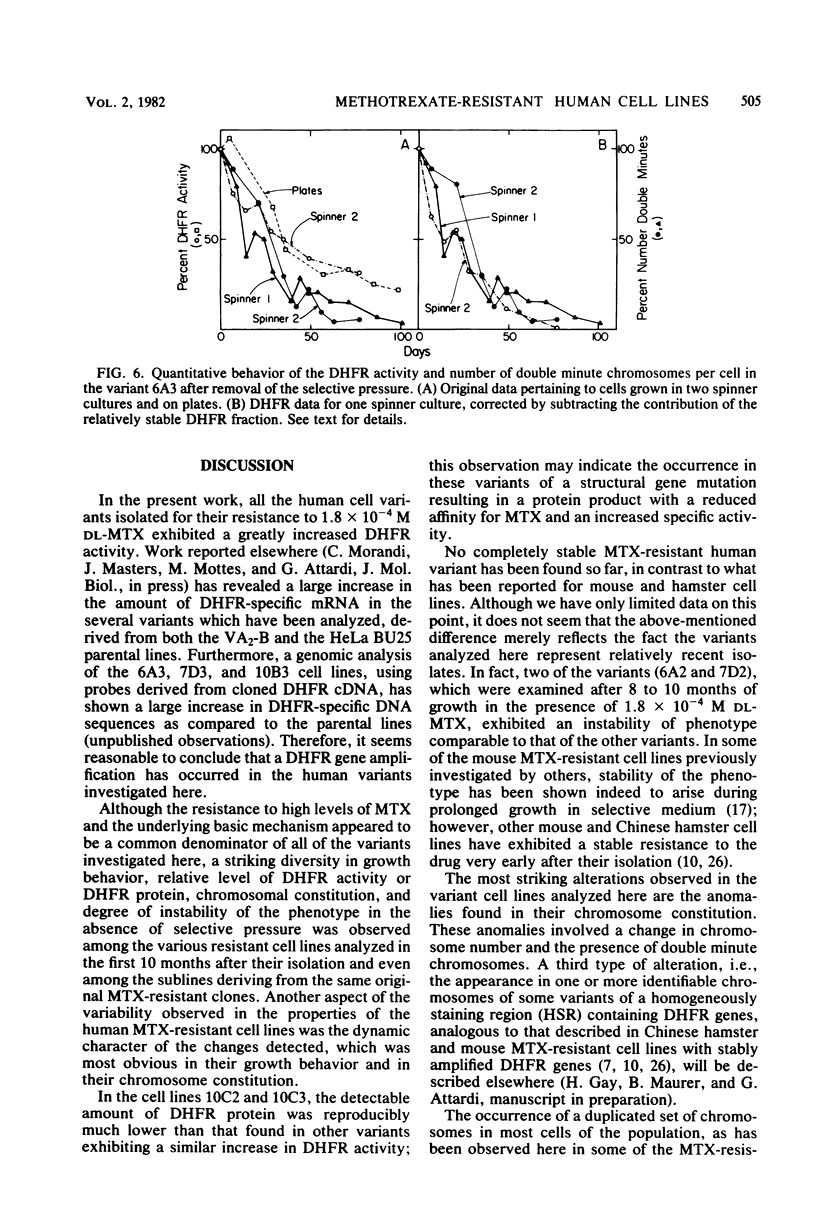
Several variants resistant to 1.8 x 10(-4) M DL-methotrexate (MTX) have been isolated from the human cell lines HeLa BU25 and VA2-B by exposing them to progressively increasing concentrations of the drug. A striking variability of phenotype and chromosome constitution was observed among the different variants. All resistant cell lines exhibited a greatly increased dihydrofolic acid reductase (DHFR) activity and DHFR content; however, the DHFR activity levels varied considerably among the variants, ranging between about 35 and 275 times the parental level. In the absence of selective pressure, the increased DHFR activity was unstable, and in all cell lines but one was completely lost over a period ranging in different variants between 25 and 200 days. The MTX-resistant cells lines showed anomalies in their chromosome constitution, which involved the occurrence of a duplicated set of chromosomes in most cells of some of the variants and the presence of double minute chromosomes in all cell lines. An analysis of the correlation of loss of double minute chromosomes and loss of DHFR activity in the absence of MTX has given results consistent with the idea that the double-minute chromosomes contain amplified DHFR genes. However, the most significant finding is that, in contrast to what has been reported in the mouse system, the recognizable double-minute chromosomes varied greatly in number in different variants without any relationship to either the level of DHFR activity or the degree of instability of MTX resistance in the absence of selective pressure. These and other observations point to the occurrence in the human MTX-resistant variants of another set of DHFR genes, representing a varied proportion of the total, which is associated with the regular chromosomes, and which may be unstable in the absence of selective pressure.
Full text
PDF

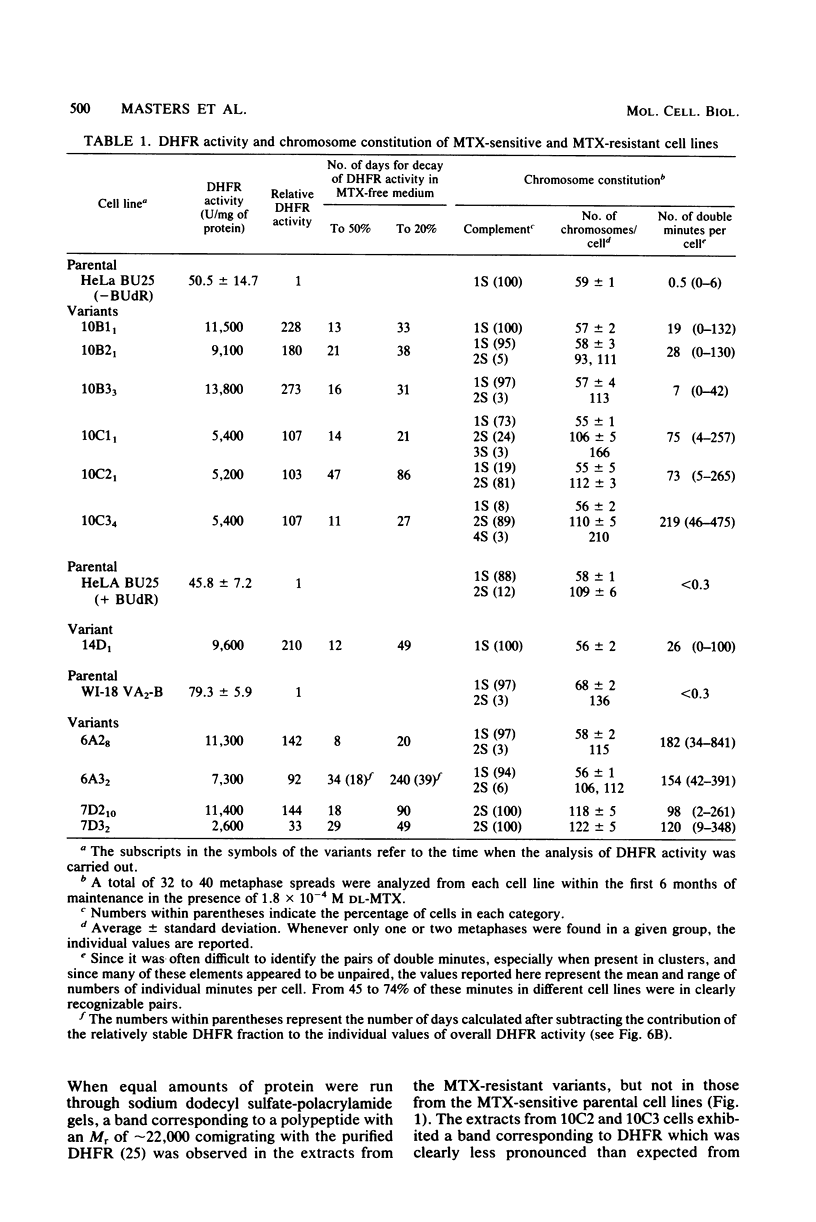
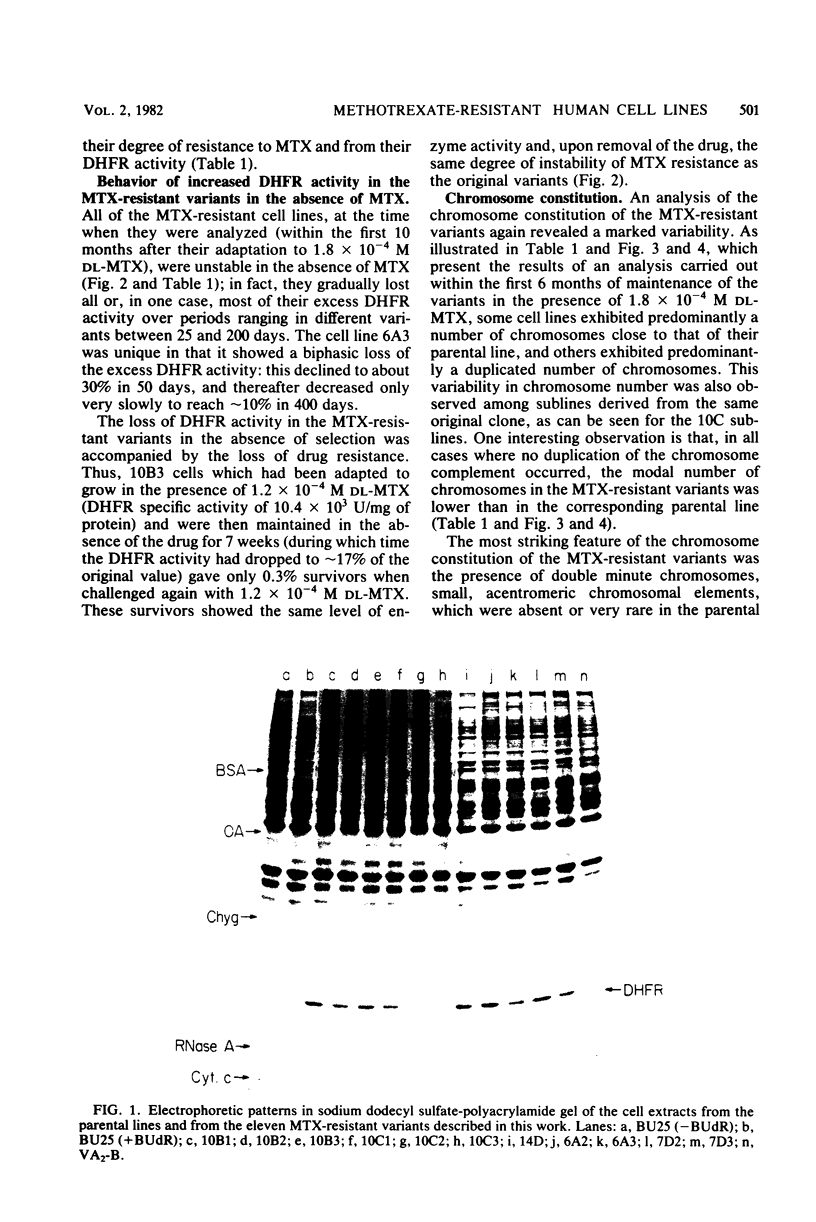


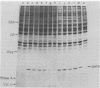
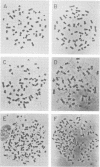
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Schimke R. T. Synthesis and degradation of folate reductase in sensitive and methotrexate-resistant lines of S-180 cells. J Biol Chem. 1976 May 25;251(10):3063–3074. [PubMed] [Google Scholar]
- Barker P. E., Hsu T. C. Double minutes in human carcinoma cell lines, with special reference to breast tumors. J Natl Cancer Inst. 1979 Feb;62(2):257–262. [PubMed] [Google Scholar]
- Baskin F., Rosenberg R. N., Dev V. Correlation of double-minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3654–3658. doi: 10.1073/pnas.78.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Albrecht A. M., Hutchison D. J., Spengler B. A. Drug response, dihydrofolate reductase, and cytogenetics of amethopterin-resistant Chinese hamster cells in vitro. Cancer Res. 1972 Jan;32(1):153–161. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Clark E. M., Harding N. G., Mounts P. M., Tyler-Smith C., van Heyningen V., Walker P. M. The development of resistance to methotrexate in a mouse melanoma cell line. I. Characterisation of the dihydrofolate reductases and chromosomes in sensitive and resistant cells. Chromosoma. 1979;74(2):153–177. doi: 10.1007/BF00292270. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Littlefield J. W. Elevated dihydrofolate reductase messenger RNA levels in methotrexate-resistant BHK cells. Cell. 1976 Mar;7(3):391–396. doi: 10.1016/0092-8674(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER G. A. Increased levels of folic acid reductase as a mechanism of resistance to amethopterin in leukemic cells. Biochem Pharmacol. 1961 Jul;7:75–77. doi: 10.1016/0006-2952(61)90128-9. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- George D. L., Powers V. E. Cloning of DNA from double minutes of Y1 mouse adrenocortical tumor cells: evidence for gene amplification. Cell. 1981 Apr;24(1):117–123. doi: 10.1016/0092-8674(81)90507-9. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., ZAKRZEWSKI S. F., NICHOL C. A. Relation of folic acid reductase to amethopterin resistance in cultured mammalian cells. J Biol Chem. 1961 Mar;236:952–958. [PubMed] [Google Scholar]
- Hänggi U. J., Littlefield J. W. Altered regulation of the rate of synthesis of dihydrofolate reductase in methotrexate-resistant hamster cells. J Biol Chem. 1976 May 25;251(10):3075–3080. [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. HeLa cells resistant to bromodeoxyuridine and deficient in thymidine kinase activity. Int J Cancer. 1966 Jan;1(1):19–30. doi: 10.1002/ijc.2910010105. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levan A., Levan G., Mitelman F. Chromosomes and cancer. Hereditas. 1977;86(1):15–30. doi: 10.1111/j.1601-5223.1977.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. Hybridization of hamster cells with high and low folate reductase activity. Proc Natl Acad Sci U S A. 1969 Jan;62(1):88–95. doi: 10.1073/pnas.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Lewis J. A., Biedler J. L., Hession C. Antifolate-resistant Chinese hamster cells. Evidence for dihydrofolate reductase gene amplification among independently derived sublines overproducing different dihydrofolate reductases. J Biol Chem. 1980 Jul 25;255(14):7024–7028. [PubMed] [Google Scholar]
- Mitchell C. H., Attardi G. Cytoplasmic transfer of chloramphenicol resistance in a human cell line. Somatic Cell Genet. 1978 Nov;4(6):737–744. doi: 10.1007/BF01543161. [DOI] [PubMed] [Google Scholar]
- Morandi C., Attardi G. Isolation and characterization of dihydrofolic acid reductase from methotrexate-sensitive and -resistant human cell lines. J Biol Chem. 1981 Oct 10;256(19):10169–10175. [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTEN J., JENSEN F., KOPROWSKI H. Morphological and virological investigation of human tissue cultures transformed with SV40. J Cell Comp Physiol. 1963 Apr;61:145–163. doi: 10.1002/jcp.1030610206. [DOI] [PubMed] [Google Scholar]
- Perkins J. P., Hillcoat B. L., Bertino J. R. Dihydrofolate reductase from a resistant subline of the L1210 lymphoma. Purification and properties. J Biol Chem. 1967 Oct 25;242(20):4771–4776. [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]