Abstract
Objective
To compare diagnostic accuracy of measures of breast cancer–related lymphedema (BCRL).
Design
Cross-sectional design comparing clinical measures with the criterion standard of previous diagnosis of BCRL.
Setting
University of California San Francisco Translational Science Clinical Research Center.
Participants
Women older than 18 years and more than 6 months posttreatment for breast cancer (n=141; 70 with BCRL, 71 without BCRL).
Interventions
Not applicable.
Main Outcome Measures
Sensitivity, specificity, receiver operator characteristic curve, and area under the curve (AUC) were used to evaluate accuracy.
Results
A total of 141 women were categorized as having (n=70) or not having (n=71) BCRL based on past diagnosis by a health care provider, which was used as the reference standard. Analyses of ROC curves for the continuous outcomes yielded AUC of .68 to .88 (P<.001); of the physical measures bioimpedance spectroscopy yielded the highest accuracy with an AUC of .88 (95% confidence interval, .80–.96) for women whose dominant arm was the affected arm. The lowest accuracy was found using the 2-cm diagnostic cutoff score to identify previously diagnosed BCRL (AUC, .54–.65).
Conclusions
Our findings support the use of bioimpedance spectroscopy in the assessment of existing BCRL. Refining diagnostic cutoff values may improve accuracy of diagnosis and warrant further investigation.
Keywords: Area under curve, Diagnosis, Lymphedema, Rehabilitation, Sensitivity and specificity, Upper extremity
Prevalence of breast cancer-related lymphedema is estimated at between 5% and 42%.1-5 Differences in diagnostic criteria and a lack of standardized assessment contribute to variability in prevalence statistics.6 After breast cancer treatment, women who develop lymphedema have greater limitations in shoulder function, have greater restrictions in activity, and report poorer quality of life than women without lymphedema.7-10 Accurate assessment may facilitate earlier diagnosis, limit sequelae, and improve ability to monitor treatment responses.
BCRL is the accumulation of protein-rich extracellular fluid caused by lymphatic disruption as a result of treatment for breast cancer.11 Physical assessment of BCRL is performed by comparing affected with unaffected limbs or by comparing postoperative with preoperative measurements. Physical measures of lymphedema include water displacement, perometry, and circumferential assessment with a tape measure, all of which evaluate total limb volume; and bioimpedance analysis, which is a measure of extracellular fluid.12-14 All have demonstrated excellent reliability12,15,16; however, because lymph is an extracellular fluid, bioimpedance may be a more accurate reflection of changes in lymphatic volume. Moreover, BIS has shown promise in assessment of segmental UE lymphedema.17
Of the measures that quantify whole-limb volume, water displacement during limb immersion has been considered the criterion standard.15,16 Interlimb differences of 10% or 200mL have been described as diagnostic criteria for BCRL.18 Volume measured through water displacement has been shown to be highly correlated to total cross-sectional area of the UE measured by computed tomography scan19; however, the water displacement method is unable to distinguish changes in muscle, adipose, or extracellular fluid volume, or to identify localized areas of swelling. Additionally, this technique is often viewed by clinicians as cumbersome and time-consuming, which may discourage its use.
Circumferential measurement may be the most common clinical assessment method.6 Circumference measures themselves may be used to monitor lymphedema, or volume can be calculated from circumference using the formula for the volume of a truncated cone.15 The most common criterion for lymphedema diagnosis is greater than or equal to 2cm interlimb difference at any single location, or greater than or equal to 200mL volume difference.6 Results may be confounded by changes in muscle and fat mass because circumference-based measurements can only assess whole-limb volume. Moreover, circumferential assessment of hand volumes is difficult because of the irregular shape of the hand.
Optoelectric volumetry (perometry) uses infrared light emitted from a frame that passes over the limb. Sensors measure limb diameters every few millimeters, and then volume is calculated.12 Perometry has demonstrated excellent reliability and has been validated against other assessment tools, including BIS,12 circumference,12,13 and water displacement.13 However, the cost of the optoelectric perometer precludes use in most clinical settings and therefore is not included in this study.
BIS measures impedance to an alternating current over a range of frequencies (4-1000kHz).20 High-frequency current passes through both intracellular and extracellular fluid compartments. However, low-frequency current conducts minimally through cells, passing selectively through ECF. Impedance at 0 frequency (mathematically extrapolated) is a reflection of ECF impedance and is inversely proportional to ECF volume, which includes lymph. Thus it is used as a marker for lymphedema. Impedance ratios of the unaffected to affected UE R0 are compared with baseline values or published norms.21
Bioimpedance analysis has been shown to be reliable and accurate in the assessment of BCRL.12,22,23 Cornish et al22 evaluated the ability of multiple frequency bioelectric impedance analysis to predict onset of BCRL in 102 patients after breast cancer treatment. BIS correctly identified BCRL up to 10 months earlier than did circumference assessment. Cornish22 estimated sensitivity and specificity of BIS at 1.0 and .98, respectively. Subsequently, Hayes et al23 used BIS as the criterion standard to calculate sensitivity and specificity of the SOAC and of self-report for assessment of BCRL. Three SDs above normative values for the BIS impedance ratio was considered diagnostic. The diagnostic criterion for SOAC of greater than 5cm interlimb difference resulted in sensitivity of .35 and specificity of .885. Sensitivity and specificity of SOAC of greater than 10% difference were .05 and 1.0, respectively. These low sensitivities indicate that SOAC missed many true-positive cases of BCRL. Regarding self-report, sensitivity was .65 and specificity .77. Czerniec et al12 compared circumferential assessment with tape measure, perometry, BIS, and self-report in 33 women with BCRL and 18 women without cancer or lymphedema. The authors found excellent reliability of and agreement between the physical measures. Self-report was found to correlate moderately with these physical measures; however, the reliability of self-report was poor.
Among self-report measures used to monitor BCRL is the NQ, originally developed and validated as a phone interview questionnaire to describe the signs and symptoms of BCRL.24 Sensitivity and specificity of the NQ were determined by comparison with circumferential measurements. Sensitivity was higher than specificity (.93–.96 vs .69–.75) to diagnose any stage of lymphedema.
The accuracy of a diagnostic test may be assessed by measures of validity. Several studies have investigated correlation and agreement between objective measures of BCRL.12-16,18,19,25,26 Others have evaluated sensitivity and specificity for circumference, volumetry, and bioimpedance.22,23,27,28 To our knowledge, none have evaluated ROC curves and AUC to describe and compare the accuracy of these assessment tools. The purpose of this study is to compare diagnostic accuracy of circumference, volume calculated from circumference, BIS, and the NQ in identifying previously diagnosed BCRL using sensitivity, specificity, ROC curves, and AUC. ROC curves are often used to evaluate the ability of a diagnostic test to discriminate between those with and those without the condition of interest.29 An advantage to ROC curves is the ability to display true positives and false positives at all cutoff levels for a diagnostic test, regardless of the decision threshold required for dichotomized sensitivity and specificity calculations. This can be useful in identifying optimal diagnostic cutoff points. Additionally, AUC can be calculated and used to compare overall performance of multiple tests.
METHODS
Participants
Women at least 18 years of age, at least 6 months posttreatment for unilateral breast cancer, with or without UE lymphedema were recruited. Women were excluded for bilateral breast cancer, current UE infection, lymphangitis, preexisting lymphedema, current breast cancer, or contraindications to BIS testing. Potential participants were recruited through the National Lymphedema Network website, San Francisco Bay–area hospitals, and breast cancer or lymphedema support groups and conferences.
Written informed consent was obtained prior to testing. The study was approved by the UCSF Committee on Human Research and the CTSI Clinical Research Center Advisory Committee.
Procedures
We used a cross-sectional study design. All participants attended a single evaluation session in the UCSF CTSI Clinical Research Center. One doctorally trained, experienced physical therapist (B.J.S.) performed all assessments.
A flexible, nonstretch tape measure was used to measure circumference of each UE, beginning at the ulnar styloid, designated as 0cm, and at 10-cm intervals proximally to 40cm. Volume was calculated using the formula for volume of a truncated cone,
where h is the length of each measured segment and C is the circumference at each end of that segment.25
The SFB7a, a single channel, tetra polar BIS device, was used to measure UE impedance. The women removed all jewelry; skin was prepped with alcohol wipe prior to surface electrode placement. Participants were positioned in supine for 10 minutes with no pillows, arms at sides, and lower extremities flat and slightly abducted. Electrodes were placed on the dorsum of the wrists adjacent to the ulnar styloid process, the dorsum of the hands just proximal to the third metacarpophalangeal joint, anterior to the ankle joints between the malleoli, and over the dorsum of the feet over the third metatarsal bone just proximal to the third metatarsophalangeal joint.20
Participants completed demographic, health, and symptom questionnaires. The women completed the NQ to report symptoms associated with lymphedema.24 Women were asked to rate difference in size for the hand, forearm, and upper arm. A value was assigned for each UE location at which size difference was reported: 1 (“very slight, you are the only person who would notice this”), 2 (“noticeable to people who know you well, but not to strangers”), and 3 (“very noticeable”). Scores were summed and categorized as no lymphedema (0), mild lymphedema (1–3), or at least moderate lymphedema (≥4).24
Data Analysis
Statistical analyses were performed using SPSS Version 18b and Stata/SE Release 11.1.c Means and SDs for interval data were obtained. Independent t tests were performed for normally distributed data. Chi-square was used for nominal and categoric variables.
To dichotomize BIS R0 ratios of the unaffected to affected limb, 3 SDs above the mean of published norms were used as the decision threshold (>1.139 if the affected arm was the dominant arm; >1.066 if the affected arm was the nondominant arm).17 For more complete analysis, additional decision thresholds were used: 1, 2, and 3 SDs above the mean ratio of our nonlymphedema group, regardless of hand dominance. The same was done for ratio of volume calculated from circumference, comparing affected with unaffected limbs.
To dichotomize interlimb volume difference, the cutoff point for diagnosis of lymphedema was greater than or equal to 200mL. For the difference in circumference, comparison was done for each measurement site, with greater than or equal to 2cm difference diagnostic of lymphedema.
The reference standard used for calculation of sensitivity and specificity was previous diagnosis of BCRL by a health care provider. Sensitivity represents the rate of true positives found by the index test, while specificity represents true negatives. Likelihood ratios were calculated:
An LR+ greater than 5 and an LR– less than 0.2 provide meaningful information about the likelihood of having the disorder.30
To provide insight into the performance of each assessment tool, we compared areas under the ROC curves. Sensitivity and 1 minus specificity data over a range of outcomes were used to construct the ROC curves, and AUC was calculated with 95% CIs for dichotomous and continuous variables. Higher AUC values represent greater accuracy.31,32 An AUC of 1.0 represents perfect sensitivity and specificity; an AUC of 0.5 represents an essentially worthless test.
RESULTS
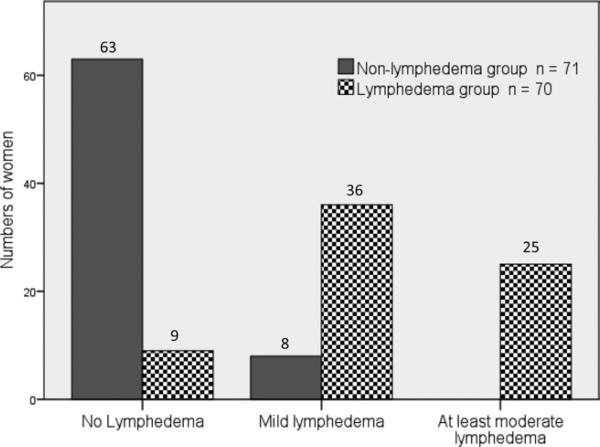
Data from 141 women were included, 70 of whom had been previously diagnosed with BCRL. This was used to categorize the participants into the lymphedema or nonlymphedema groups. Participant characteristics are presented in table 1. More women in the lymphedema group had axillary node dissection; women in the lymphedema group had greater numbers of nodes removed. Outcomes of physical measures used to assess lymphedema are presented in table 2. Large and significant interlimb volume differences were found between groups for each measure. Of the 70 women in the lymphedema group, 39% had interlimb volume differences greater than 200mL. None of the women in the nonlymphedema group had interlimb volume differences greater than 200mL. We also found significant differences between groups in distribution of the NQ scores (P<.05) (fig 1). In the lymphedema group, 9 women were categorized as no lymphedema (12.5%), 36 as mild (81.8%), and 25 as at least moderate (35.7%). In the nonlymphedema group, 63 women were categorized as not having lymphedema (87.5%), 8 as having mild lymphedema (18.2%), and none as having at least moderate. The 8 women who were categorized by the NQ as having mild lymphedema demonstrated interlimb volume differences less than 200mL (range, –26.23mL to 191.04mL), and mean bioimpedance ratio ± SD was .99±.02.
Table 1.
Patient Characteristics
| Characteristics | Nonlymphedema n=71 | Lymphedema n = 70 | Difference in Means (95% CI) | P |
|---|---|---|---|---|
| Age (y) | 55.2±8.8 | 57.7±10.1 | 2.5 (5.7 to –0.6) | .12† |
| Body mass index | 25.5±4.6 | 27.2±6.2 | 1.7 (3.5 to –0.1) | .07† |
| Affected side = dominant side | 38* | 35* | NA | .68‡ |
| Affected side = nondominant side | 33* | 35* | ||
| Years since cancer diagnosis | 5.0±4.1 | 7.6±6.2 | 2.6 (4.4 to 0.9) | .003† |
| Sentinel lymph node biopsy | 54* | 36* | NA | .002‡ |
| Axillary node dissection | 46* | 61* | NA | .002‡ |
| Number of nodes removed | 9±6 | 13± 7 | 4 (7 to 2) | <.001† |
| Received radiation therapy | 49* | 55* | NA | .20‡ |
| Received chemotherapy | 48* | 50* | NA | .62‡ |
NOTE. Values are numbers or mean ± SD.
Abbreviation: NA, not applicable.
Reported in numbers of women.
Independent t tests for differences in means.
χ2.
Table 2.
Differences Between Groups in Outcomes of Lymphedema Assessment
| Measure | Nonlymphedema Group n=71 Mean ± SD | Lymphedema Group n=70 Mean ± SD | Difference (95% CI) | P ‡ |
|---|---|---|---|---|
| BIS resistance ratio* | ||||
| All: | 0.99±0.04 | 1.11±0.15 | 0.12 (0.09–0.16) | <.001 |
| Dominant = affected | 1.00±0.04, n=33 | 1.11±0.13, n=35 | 0.11 (0.07–0.16) | |
| Dominant = unaffected | 0.98±0.04, n=38 | 1.11 ±0.17, n=35 | 0.13 (0.07–0.19) | |
| Volume difference (mL)† | –7.86±72.61 | 206.30±261.85 | 214.2 (150.4–278.0) | <.001 |
| Volume ratio* | 0.995±0.04 | 1.10±0.12 | 0.11 (0.08–0.14) | <.001 |
| Circumference difference, 0cm† | 0.05±0.30 | 0.58±1.25 | 0.53 (0.23–0.83) | .001 |
| Circumference difference, 10cm | –0.11 ±0.64 | 1.39±2.08 | 1.50 (0.99–2.01) | <.001 |
| Circumference difference, 20cm | –0.15±0.64 | 1.36±1.72 | 1.51 (1.07–1.96) | <.001 |
| Circumference difference, 30cm | 0.04±0.99 | 1.45±1.73 | 1.41 (0.94–1.88) | <.001 |
| Circumference difference, 40cm | –0.11±0.83 | 0.62±1.44 | 0.72 (0.33–1.12) | <.001 |
Ratio of unaffected–affected sides.
Differences represent affected – unaffected.
Independent t tests for differences in means.
Fig 1.
Classification of lymphedema based on the NQ.
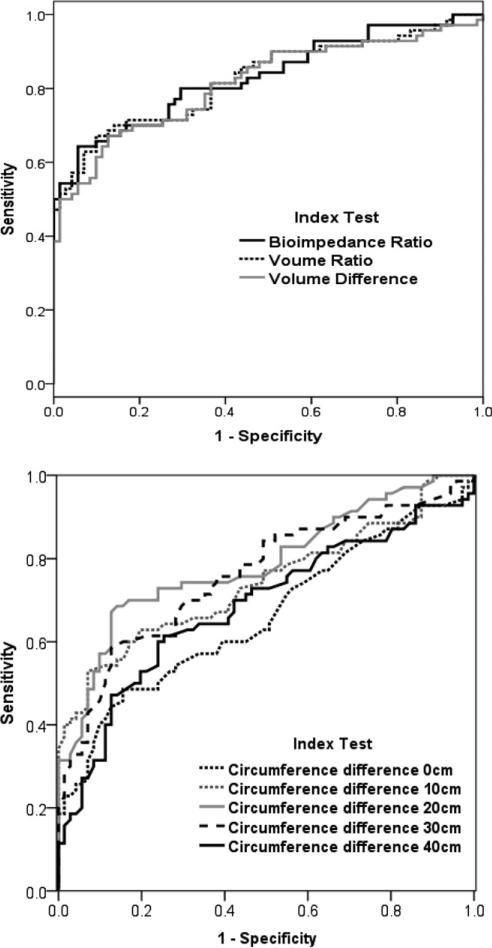
For continuous data, the ROC curves are displayed in figure 2, and AUC data are presented in table 3. Analyses of ROC curves for the continuous outcomes yielded AUCs of .68 to .88 (P≤.001). Accounting for limb dominance, the highest AUC was found for BIS R0 ratio when the dominant limb was the affected limb, at .88 (95% CI, .80–.96). However, the BIS R0 ratio when the dominant limb was the unaffected limb was .79 (95% CI, .68–.89). Without regard for limb dominance, the AUCs for BIS R0 ratio and for volume ratio were each .83 (95% CI, .76–.90). Volume difference (volume of affected limb – volume of unaffected limb) yielded an AUC of .82 (95% CI, .74–. 89). AUCs for circumference, using continuous data, produced lower values, ranging from .66 to .79.
Fig 2.
ROC curve for continuous variables.
Table 3.
Area Under the ROC Curve for Continuous Variables
| Index Test | AUC (95% CI) | P |
|---|---|---|
| BIS resistance ratio* regardless of limb dominance | .83 (.76–.90) | <.001 |
| BIS resistance ratio for dominant limb = affected limb | .88 (.80–.96) | <.001 |
| BIS resistance ratio for dominant limb = unaffected limb | .79(.68–.89) | <.001 |
| Volume ratio* | .83 (.76–.90) | <.001 |
| Volume difference† | .82 (.74–.89) | <.001 |
| Circumference difference at 0cm† | .66 (.57–.75) | .001 |
| Circumference difference at 10cm | .74 (.66–.83) | <.001 |
| Circumference difference at 20cm | .79 (.71–.86) | <.001 |
| Circumference difference at 30cm | .76 (.68–.84) | <.001 |
| Circumference difference at 40cm | .68 (.60–.77) | <.001 |
Ratio of unaffected to affected sides.
Differences represent affected to unaffected.
Table 4 presents sensitivity, specificity, LR+, LR–, and AUC for the dichotomized outcomes. These include the NQ, dichotomized as no lymphedema and any lymphedema; commonly used volume and circumference cutoff scores; and 1, 2, and 3 SDs above the nonlymphedema group mean for BIS and volume ratios. The highest AUC was found for the NQ (AUC=.88; 95% CI, .83–.93). The highest AUC for the dichotomized objective physical measures was for BIS, at a diagnostic cutoff of 2 SDs above our nonlymphedema group mean (AUC=.75; 95% CI, .69–.81). Next highest was volume ratio at 2 SDs above the nonlymphedema group mean (AUC=.74; 95% CI, .68–.81). The remaining values were less than or equal to .70, with the lowest for circumference using the 2-cm diagnostic cutoff.
Table 4.
Sensitivity, Specificity, Likelihood Ratios, and AUC for Dichotomous Outcomes Measures for Assessment of Lymphedema
| Dichotomous Test Variables | Sensitivity (95% CI) | Specificity (95% CI) | LR+* (95% CI) | LR- (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|
| NQ | 0.87 (0.77–0.94) | 0.89 (0.79–0.95) | 7.73 (4.0–14.9) | 0.15 (0.08–0.27) | 0.88 (0.83–0.93) |
| BIS, resistance ratio of unaffected limb/affected limb | |||||
| Cutoff at published values† | 0.37 (0.26–0.50) | 0.99 (0.92–1.00) | 26.4 (3.68–189) | 0.64 (0.53–0.77) | 0.68 (0.62–0.74) |
| Cutoff at 1 SD‡ | 0.87 (0.77–0.94) | 0.47 (0.35–0.56) | 1.63 (1.29–2.06) | 0.28 (0.14–0.54) | 0.67 (0.60–0.74) |
| Cutoff at 2 SD‡ | 0.51 (0.39–0.64) | 0.99 (0.92–1.00) | 36.5 (5.15–259) | 0.49 (0.39–0.63) | 0.75 (0.69–0.81) |
| Cutoff at 3 SD‡ | 0.31 (0.21–0.44) | 1.00 (0.95–1.00) | 310* | 0.69 (0.59–0.80) | 0.66 (0.60–0.71) |
| Volume ratio of affected/unaffected | |||||
| Cutoff at 1 SD‡ | 0.87 (0.77–0.94) | 0.54(0.41–0.66) | 1.87 (1.44–2.44) | 0.24 (0.13–0.46) | 0.70 (0.63–0.77) |
| Cutoff at 2 SD‡ | 0.51 (0.39–0.64) | 0.97 (0.90–1.00) | 18.3 (4.57–72.9) | 0.50 (0.39–0.64) | 0.74 (0.68–0.81) |
| Cutoff at 3 SD‡ | 0.34 (0.23–0.47) | 1.00 (0.95–1.00) | 340* | 0.66 (0.56–0.78) | 0.67 (0.62–0.73) |
| Volume calculated from circumference Difference = affected - unaffected | |||||
| –Volume difference ≥200 mL | 0.39 (0.27–0.51) | 1.00 (0.95–1.00) | 390* | 0.61 (0.51–0.74) | 0.69 (0.64–0.75) |
| Circumference difference = affected - unaffected | |||||
| At 0cm; ≥2cm cutoff | 0.07 (0.03–0.16) | 1.00 (0.95–1.00) | 70* | 0.93 (0.87–0.99) | 0.54 (0.51–0.57) |
| At 10cm; ≥2cm cutoff | 0.29 (0.18–0.41) | 1.00 (0.95–1.00) | 290* | 0.71 (0.62–0.83) | 0.64 (0.55–0.74) |
| At 20cm; ≥2cm cutoff | 0.29 (0.18–0.41) | 1.00 0.95–1.00) | 290* | 0.71 (0.62–0.83) | 0.64 (0.59–0.70) |
| At 30cm; ≥2cm cutoff | 0.36 (0.25–0.48) | 0.94 (0.86–0.98) | 6.34 (2.33–17.3) | 0.68 (0.57–0.82) | 0.65 (0.59–0.71) |
| At 40cm; ≥2cm cutoff | 0.14 (0.07–0.25) | 0.99 (0.92–1.00) | 10.1 (1.33–77.1) | 0.87 (0.79–0.96) | 0.56 (0.52–0.61) |
.999 was used for calculations of LR+ when specificity = 1.0.
1.139 for dominant = affected arm; 1.066 for dominant = unaffected arm.
Above mean of nonlymphedema group.
From each ROC curve for continuous outcomes, we determined the diagnostic cutoff with the highest AUCs. These values are reported in table 5, along with their sensitivities, specificities, and likelihood ratios. The highest AUC was found for volume ratio using a diagnostic cutoff of 1.04 (AUC=.78; 95% CI, .71–.85), and the highest AUC for BIS R0 was found using a 1.06 cutoff score (AUC=.76, 95% CI, .70–.83), without regard for limb dominance. The highest AUC for volume difference was found using a 75-mL cutoff value (AUC=.76; 95% CI, .69–.83). These were consistently higher than those in table 4, which included the more common diagnostic cutoff criteria. For example, for circumference, the highest AUC was found when .25cm interlimb difference at the 20-cm location of the forearm was used as the index test (AUC=.77; 95% CI, .70–.84). This is in contrast to that found for the 2-cm threshold, which yielded an AUC of .64.
Table 5.
Statistics for Cutoff Values for Index Tests Determined to Have the Highest AUC for Continuous Variables
| Index Test Cutoff Value | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR- (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|
| 0.25cm Interlimb difference (at 20cm forearm location) | 0.69 (0.56–0.79) | 0.85 (0.74–0.92) | 4.43 (2.51–7.79) | 0.37 (0.26–0.53) | 0.77 (0.70–0.84) |
| 75mL Interlimb volume difference | 0.67 (0.55–0.78) | 0.85 (0.74–0.92) | 4.33 (2.46–7.64) | 0.39 (0.27–0.55) | 0.76 (0.69–0.83) |
| 1.04 Limb volume ratio unaffected/affected | 0.67 (0.55–0.78) | 0.89 (0.79–0.95) | 5.96 (3.04–11.7) | 0.37 (0.26–0.52) | 0.78 (0.71–0.85) |
| 1.06 Bioimpedance ratio unaffected/affected | 0.54 (0.42–0.66) | 0.99 (0.92–1.00) | 38.5 (5.44–273) | 0.46 (0.36–0.60) | 0.76 (0.70–0.83) |
NOTE. Reference standard: previous diagnosis of lymphedema.
DISCUSSION
This study was retrospective in nature. We evaluated the accuracy of objective measures in identifying the presence of previously diagnosed lymphedema in a large cohort of women with and without lymphedema who had undergone breast cancer treatment. The women who had not been previously diagnosed with lymphedema served as our diagnosis-negative group. These women demonstrated BIS ratios that were similar to healthy controls used in previous studies. Ward et al33 reported healthy control group impedance ratio as 1.016±.046. The impedance ratio for our nonlymphedema group was .99±.041.
The NQ demonstrated high sensitivity, consistent with the findings of Norman et al,24 and the highest AUC. In the nonlymphedema group, 8 women were categorized by the NQ as having mild lymphedema. These 8 women reported mild symptoms but did not meet the objective criteria of having lymphedema based on published criteria. Interlimb volume differences were less than 150mL for 7 of the 8 women, with a mean ± SD of 42.03±84.39mL (range, –26.23mL to 191.04mL). Mean BIS ratio ± SD was .99±.02, and none of the 8 women had a BIS ratio greater than 1.02. Only 1 woman in the nonlymphedema group reported no symptoms on the NQ yet presented with objective findings of lymphedema (BIS ratio=1.08; affected side=nondominant side). Of the women with no symptoms of lymphedema, none had volume differences greater than 150mL. Because the NQ does not rely on significant physical changes to categorize BCRL, it may be a useful adjunct to early identification of symptoms associated with lymphedema and an important self-monitoring tool.
Additionally, 9 women who had been previously diagnosed with lymphedema were categorized by the NQ as having no lymphedema. All of these women had BIS ratios less than the published diagnostic cutoff values and interlimb volume differences less than 150mL. These cases may indeed have presented initially as mild lymphedema that subsequently resolved, which is consistent with the findings of Hayes et al.34 These results may also be reflective of response to treatment. Six of these 9 women had previously received lymphedema treatment.
Circumference measurement, water displacement, and perometry allow assessment of total limb volume but may not reflect changes in extracellular fluid volume and thus are not specific measures of lymphedema. Changes in bone, muscle, and fat will result in changes in total limb volume. In this study, circumference was the least accurate in identifying previously diagnosed lymphedema. BIS and volume calculated from circumference were the most accurate. However, BIS differentiates extracellular fluid from total limb volume and therefore may demonstrate better discrimination in assessment of lymphedema. Lymphedema has been shown to have a negative impact on UE function,7-10,34 activity, and quality of life. Assessment tools that can accurately detect lymphedema early in its course may improve outcomes and prevent long-term sequelae.
Circumference
Comparing accuracy using continuous scales is preferred because they are more precise. However, for clinical diagnosis of lymphedema, a cutoff score that defines a positive test is more useful. The 2-cm criterion resulted in a relatively low AUC of .54 to .65. Moreover, using greater than or equal to 2cm interlimb difference to define a positive test resulted in low sensitivity, consistent with the findings of Hayes.23 This implies that a negative test using this method alone may not provide enough information to rule out lymphedema. However, this method resulted in very high specificity and LR+, implying that after breast cancer treatment, a woman who presents with greater than 2cm difference between arms at any site very likely has lymphedema. Finding the ideal value at which to categorize a patient as having or not having lymphedema depends, in part, on the risk associated with inaccurate diagnosis. The trade-off between finding true positives and false positives must be balanced, and this balance changes at different cutoff scores. It appears that the 2-cm difference may be too large to capture milder cases of lymphedema. We found higher AUCs for lower cutoff values. The highest AUC was found for the cutoff value of an interlimb difference of .25cm. It is doubtful, however, that this difference in limb circumference exceeds the standard error of measurement for circumferences.
Volume Calculated From Circumference
Volume ratio and volume difference using continuous data demonstrated high accuracy. Again, however, diagnostic cutoff points are more useful clinically. The diagnostic criterion of 200mL, while having high specificity and LR+, had sensitivity of less than 0.5 and LR– of .61, meaning that in the face of a negative test, the patient is just as likely to have as not have the condition. Lower values for diagnostic criteria could potentially capture milder cases of BCRL. Our finding of a higher AUC at 75mL interlimb volume difference suggests that this value may be more accurate in monitoring lymphedema than other values. Lower lymphedema diagnostic cutoff scores for volume differences warrant further study (table 5).
The impact of hand dominance on volume and impedance has been documented in healthy controls.22,33 Ward33 reported 3.6±4.1% difference in limb size based on hand dominance. Cornish22 reported on average 3% lower impedance in the dominant arm in healthy controls. In spite of this, hand dominance is not routinely considered during whole-limb volume measurements. Stout Gergich et al35 compared women who developed BCRL after breast cancer surgery to a nonlymphedema group using infrared perometry and found significant differences in limb volume between groups. There were also significant differences between groups, however, with regard to height, hand dominance, and affected UE. In the control group, 60% of the women were left-handed, compared with 35% in the lymphedema group. To date, BIS is the only measure to account for the effect of hand dominance in assessment of BCRL.
The use of volume ratios in assessment of BCRL relies on comparison with baseline measures or with published results from healthy controls. Cornish et al36 evaluated volume ratios (calculated from circumference) of at-risk limb/healthy limb for women prior to breast cancer surgery. The presurgery volume ratio was 1.00±.03 for the 20 women who later went on to develop lymphedema. Mean volume ratio ± SD for these women after development of lymphedema was 1.14±.08. These findings are similar to ours in which the nonlymphedema group volume ratio was 0.995±.04 and the lymphedema group volume ratio was 1.10±.12. The nonlymphedema group was used as the comparison group in the present study. Presurgical baseline measures would be the optimal comparison; however, these may be unavailable to the practicing clinician. Therefore, comparison of volume ratio to published “controls” may have merit.
Bioimpedance Spectroscopy
Our finding of AUC of .83 to .88 (using continuous outcomes) supports the use of BIS in assessment of BCRL. Interestingly, affected side relative to dominant side mattered in terms of accuracy. Use of diagnostic cutoff values for BIS R0 ratios of 1.139 (dominant arm affected) and 1.066 (nondominant arm affected) have been reported in the literature.17 For the continuous outcomes, use of these ratios did improve accuracy, but only for the women whose dominant limb was the affected limb. Surprisingly, accuracy was lower when the dominant limb was the unaffected limb. When we used these cutoffs to dichotomize the index tests, AUC was lower and was similar to that obtained when we used the cutoff score of 3 SDs above our nonlymphedema mean ratio. We used as a reference standard the retrospective diagnosis of lymphedema. It is unknown how accurate these earlier diagnoses were, and it is doubtful that hand dominance was considered.
There was a statistically significant difference between bioimpedance resistance ratios of the nonlymphedema and lymphedema groups, consistent with the findings of others. Ridner et al14 compared 11 women with BCRL with 14 healthy controls. Impedance ratios for the healthy controls, determined by random assignment of limb as the affected side, were reported as .997, and for the lymphedema group, 1.17 (SD unreported). Resistance ratios for the nonlymphedema group in the present study were similar at .99, and for the lymphedema group, 1.11. This represents a 12% difference comparing the lymphedema group with the nonlymphedema group. The highest AUC (.76; 95% CI, .70–.83) for the dichotomized results was found for BIS ratio of 1.06 (table 5). This is 7% greater than the mean BIS ratio in the nonlymphedema group.
Study Limitations
The reference standard used for determination of sensitivity and specificity was previous diagnosis of lymphedema. The training and expertise of those performing and interpreting the reference tests were unknown; therefore, we were unable to determine the reliability of those findings. Measurement methods and diagnostic criteria were likely variable.
Blinding of the assessor to participant diagnosis was not possible. This could have contributed to test review bias. To minimize error and bias, testing protocols were standardized and techniques practiced prior to subject recruitment. Data entry and calculation were performed by other study personnel without regard to group membership.
The number of women with BCRL in our study is not reflective of the prevalence of the condition in the population. Our results may have been positively skewed because the likelihood of identifying true positives increases if prevalence is high.30 Additionally, most of the women in the lymphedema group had had lymphedema treatment, including education, compression, and complete decongestive therapy. Our data may, in part, reflect response to that treatment.
We did not factor hand dominance into measures of lymphedema that relied on circumference measures. Although there is a documented effect of hand dominance on size, volume, and impedance, dominance is not routinely incorporated into assessment in the clinic at present. Understanding the effect of hand dominance on volume change is important in accurate assessment and requires further consideration.
CONCLUSIONS
A challenge faced by researchers and clinicians is to determine and use diagnostic methods that accurately identify as many true-positive cases of BCRL as possible. Mild cases may potentially be missed when using current diagnostic decision thresholds that have low sensitivity and areas under the ROC curve. There continues to be a need to investigate appropriate diagnostic cutoff scores to identify lymphedema correctly.
Of the objective measures used in this study to evaluate lymphedema, circumference was the least accurate in identifying previously diagnosed lymphedema. BIS and volume calculated from circumference were the most accurate. BIS is easy to use, reliable, and accurate. Because differences in impedance represent differences in extracellular volume, BIS may be more a more appropriate measure of BCRL.
Acknowledgments
We thank the following individuals for their valuable contributions: Jane Armer, PhD (scientific and technical advice); Nancy Byl, PhD and Joanne Krasnoff, PhD (method and technical advice); and Bruce Cooper, PhD and Rob Slaughter, PhD (statistical consultation).
Supported by a grant from the National Institute of Nursing Research (NIH 1R21 NR0101282U) and by NIH/NCRR UCSF-CTSI grant no. UL1 RR024131.
List of Abbreviations
- AUC
area under the curve
- BIS
bioimpedance spectroscopy
- BCRL
breast cancer–related lymphedema
- CI
confidence interval
- CTSI
Clinical and Translational Science Institute
- ECF
extracellular fluid
- LR–
likelihood ratio–negative
- LR+
likelihood ratio–positive
- NQ
Norman Questionnaire
- R0
impedance ratio at 0 frequency
- ROC
receiver operator characteristic
- SOAC
sum of arm circumferences
- UCSF
University of California San Francisco
- UE
upper extremity
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
ImpediMed, Principal US Office, 5959 Cornerstone Ct W, Ste 100, San Diego, CA 92121.
SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606.
StataCorp LP, 4905 Lakeway Dr, College Station, TX 77845.
References
- 1.Deo SV, Ray S, Rath GK, et al. Prevalence and risk factors for development of lymphedema following breast cancer treatment. Indian J Cancer. 2004;41:8–12. [PubMed] [Google Scholar]
- 2.Nardone L, Palazzoni G, D'Angelo E, et al. Impact of dose and volume on lymphedema. Rays. 2005;30:149–55. [PubMed] [Google Scholar]
- 3.Johansson K, Holmstrom H, Nilsson I, Ingvar C, Albertsson M, Ekdahl C. Breast cancer patients’ experiences of lymphoedema. Scand J Caring Sci. 2003;17:35–42. doi: 10.1046/j.1471-6712.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–7. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23:76–83. [PubMed] [Google Scholar]
- 7.Smoot B, Wong J, Cooper B, et al. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010;4:167–78. doi: 10.1007/s11764-010-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–11. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 9.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242–8. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Dawes DJ, Meterissian S, Goldberg M, Mayo NE. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008;40:651–8. doi: 10.2340/16501977-0232. [DOI] [PubMed] [Google Scholar]
- 11.Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998;83:2798–802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Czerniec SA, Ward LC, Refshauge KM, et al. Assessment of breast cancer-related arm lymphedema--comparison of physical measurement methods and self-report. Cancer Invest. 2010;28:54–62. doi: 10.3109/07357900902918494. [DOI] [PubMed] [Google Scholar]
- 13.Deltombe T, Jamart J, Recloux S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40:26–34. [PubMed] [Google Scholar]
- 14.Ridner SH, Montgomery LD, Hepworth JT, Stewart BR, Armer JM. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007;40:35–46. [PubMed] [Google Scholar]
- 15.Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86:205–14. [PubMed] [Google Scholar]
- 16.Megens AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82:1639–44. doi: 10.1053/apmr.2001.26822. [DOI] [PubMed] [Google Scholar]
- 17.Czerniec SA, Ward LC, Lee MJ, Refshauge KM, Beith J, Kilbreath SL. Segmental measurement of breast cancer-related arm lymphoedema using perometry and bioimpedance spectroscopy. Support Care Cancer. 2010:1–8. doi: 10.1007/s00520-010-0896-8. (epub ahead of print May 15, 2010) [DOI] [PubMed] [Google Scholar]
- 18.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–17. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 19.Sagen A, Karesen R, Skaane P, Risberg MA. Validity for the simplified water displacement instrument to measure arm lymphedema as a result of breast cancer surgery. Arch Phys Med Rehabil. 2009;90:803–9. doi: 10.1016/j.apmr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Cornish BH, Chapman M, Thomas BJ, Ward LC, Bunce IH, Hirst C. Early diagnosis of lymphedema in postsurgery breast cancer patients. Ann N Y Acad Sci. 2000;904:571–5. doi: 10.1111/j.1749-6632.2000.tb06518.x. [DOI] [PubMed] [Google Scholar]
- 21.Warren AG, Janz BA, Slavin SA, Borud LJ. The use of bioimpedance analysis to evaluate lymphedema. Ann Plast Surg. 2007;58:541–3. doi: 10.1097/01.sap.0000244977.84130.cf. [DOI] [PubMed] [Google Scholar]
- 22.Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2–11. [PubMed] [Google Scholar]
- 23.Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005;89:221–6. doi: 10.1007/s10549-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 24.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81:1192–205. [PubMed] [Google Scholar]
- 25.Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82:1201–12. [PubMed] [Google Scholar]
- 26.Tewari N, Gill PG, Bochner MA, Kollias J. Comparison of volume displacement versus circumferential arm measurements for lymphoedema: implications for the SNAC trial. ANZ J Surg. 2008;78:889–93. doi: 10.1111/j.1445-2197.2008.04686.x. [DOI] [PubMed] [Google Scholar]
- 27.Godoy JM, Silva SH, Godoy MF. Sensitivity and specificity of combined perimetric and volumetric evaluations in the diagnosis of arm lymphedema. Prague Med Rep. 2007;108:243–7. [PubMed] [Google Scholar]
- 28.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res Treat. 2005;94:1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 29.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 30.Portney LG, Watkins MP. Foundations of clinical research, applications to practice. 3rd ed. Pearson Prentice Hall; Upper Saddle River, NJ: 2009. [Google Scholar]
- 31.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev. 2008;29(suppl 1):S83–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12:132–9. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 33.Ward LC, Czerniec S, Kilbreath SL. Quantitative bioimpedance spectroscopy for the assessment of lymphoedema. Breast Cancer Res Treat. 2009;117:541–7. doi: 10.1007/s10549-008-0258-0. [DOI] [PubMed] [Google Scholar]
- 34.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–42. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 35.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 36.Cornish BH, Thomas BJ, Ward LC, Hirst C, Bunce IH. A new technique for the quantification of peripheral edema with application in both unilateral and bilateral cases. Angiology. 2002;53:41–7. doi: 10.1177/000331970205300106. [DOI] [PubMed] [Google Scholar]