Abstract
Objective
To explore the feasibility and dosage of tramadol combined with fentanyl in awake endotracheal intubation.
Methods
Using Dixon’s up-and-down sequential design, the study enrolled patients from each of the 20-49, 50-60 and 70-and-above age groups scheduled for elective surgery under general anesthesia. The feasibility and dosage of tramadol combined with fentanyl in awake endotracheal intubation, guided by fiberoptic bronchoscopy, were verified.
Results
After intravenous injection with fentanyl 2.2 μg/kg and tramadol 2.0 mg/kg in the 20-49 age group, fentanyl 1.6 μg/kg and tramadol 1.9 mg/kg in the 50-69 age group and fentanyl 1 μg/kg and tramadol 1.8 mg/kg in those at the age of 70 or above, the patients achieved conscious sedation without obvious respiratory depression. Meanwhile, under these dosages, the patients could easily tolerate the thyrocricocentesis airway surface anesthesia and fiberoptic bronchoscope guided tracheal intubation. Postoperative follow-up showed that most patients had memory of the intubation process but without significant discomfort. No awake endotracheal intubation-related side effect was noted.
Conclusions
Fiberoptic bronchoscope guided nasotracheal intubation can be successfully completed with background administration of fentanyl and tramadol. However, the specific dosages need to be tailored in different age of patients.
KEY WORDS : Awake endotracheal intubation, fiberoptic bronchoscope, nasotracheal intubation, fentanyl, tramadol
Introduction
The management of a difficult airway has been challenging during the induction of anesthesia, and has been linked (1) with the safety and quality of anesthesia. Many serious anesthesia-related complications are resulted from improper management of the airway. Fiberoptic bronchoscopy-guided awake endotracheal intubation is a safe and effective approach to managing a difficult airway under non-urgent settings. However, patients have to experience unbearable pain receiving the intubation when they are fully awake. Ovassapian (2) believed that conscious sedation could partly improve their tolerability, though he stressed the importance to avoid respiratory depression whenever possible during endotracheal intubation, as it would be directly linked with the safety of anesthesia. As a strong opioid analgesic with proven analgesic effect, fentanyl has been widely used in the induction of general anesthesia to reduce patient response to endotracheal intubation. However, the intravenous administration is prone to cause respiratory depression, and application of this agent alone during endotracheal intubation may put the patients at risk of respiratory depression (3,4). Tramadol, another opioid with strong analgesic properties, can relieve general to severe pain by acting on μ-opioid receptors to affect the synaptic norepinephrine reuptake (5). At present, the perioperative administration of tramadol is mainly prescribed for postoperative pain control and elimination of chills, and this agent has not been used for endotracheal intubation in previous reports. Due to its strong analgesic effect yet satisfying safety and mild respiratory depression, tramadol is used in this study as an adjuvant during awake endotracheal intubation. The feasibility and respective dosage of combined application of tramadol and fentanyl in this procedure are analyzed as a potential option for managing difficult airways in clinical practice.
Materials and methods
The study is approved by the Ethics Committee of the Third Xiangya Hospital of Central South University.
First phase
Using Dixon’s up-and-down sequential design (6,7), the study enrolled 52 patients from each of the 20-49, 50-60 and 70-and-above age groups scheduled for elective surgery under general anesthesia at the first phase. The exclusion criteria (8,9) included: a significantly difficult airway confirmed before anesthesia (cervical spine injury, Mallampati class III-IV and limited mouth opening); severe cardiovascular disease; hypertension; preoperative difficulty breathing due to respiratory infections; long-term use of opioids; severe intestinal obstruction or full stomach; and an obesity index greater than 2.5. Atropine 0.5 mg plus 1.0 phenobarbital sodium was administered intramuscularly one hour before anesthesia. Continued inhalation of 2 L/min oxygen was delivered via a nasal cannula upon entry of the operating room, followed by establishment of venous access with monitoring of the blood pressure (BP), heart rate (HR), respiratory rate (RR) and digital pulse oximetry (SpO2). After intravenous replacement with about 5 mL/kg of Ringer’s lactate, the agents were applied for observation. Double blindness was guaranteed during the procedure, so that neither the patients nor the caregivers were informed of the dosage of medication. The initial dosage of intravenous fentanyl was based on routine practice and previous reports (10,11), and 2.0 μg/kg fentanyl was given to the 20-49 age group, 0.6 μg/kg to the 50-69 age group and 0.2 μg/kg to those at the age of 70 or above. The dose increments/decrements were fixed at 0.2 μg/kg regardless of age (diluted to 10 μg/mL with saline). The medication was delivered following the up-and-down sequential method, in which the doses for a certain subject would always be determined according to the reaction of his/her previous subject, except for the first patient. If no respiratory depression was present (SpO2 >92%) in the previous patient upon a certain dosage, an increment of 0.2 μg/kg would be applied to the current dose for the next subject. On the contrary, when severe respiratory depression (SpO2 ≤92%) (12-14) was observed, the dose would be reduced by 0.2 μg/kg, and so forth. To avoid severe hypoxia after the medication, mask-assisted ventilation would be delivered immediately in the case that SpO2 was found ≤92% until the indicator returned to normal.
After the end of the first phase, the mean value, standard deviation and 99% confidence interval of the minimal single dose (EC50) of intravenous fentanyl for respiratory depression, as well as the normal dosage ranges, in each age group were calculated.
Second phase
Dixon’s up-and-down sequential design method was also employed and 20 patients were selected from each of the 20-49, 50-60 and 70-and-above age groups scheduled for elective surgery under general anesthesia. The exclusion criteria were the same as those in the first phase. Medication, monitoring, infusion and other care before anesthesia were finished following the same procedures as in the previous phase. Continued inhalation of 2 L/min oxygen was delivered via a nasal cannula after medication until the completion of endotracheal intubation. At this phase, double blindness was also ensured, and the dosage of fentanyl was lower than the lowest range of minimal single doses that caused respiratory depression in the age groups at the first phase. With fentanyl as the background medication for the observation, different doses of tramadol were tested for fiberoptic bronchoscope guided awake endotracheal intubation. The initial intravenous dose of tramadol was determined at 1 mg/kg for all subjects based on clinical practice and existing reports (15,16), with increments and decrements fixed at 0.1 mg/kg. The medication was still administered following the up-and-down sequential method. Operation of awake endotracheal intubation: diluted fentanyl (10 μg/mL) and tramadol (10 mg/mL) were given intravenously. The turbinate was contracted with a swab soaked with 0.1 mg/mL adrenaline plus 1% lidocaine, and 5 mL of 2% lidocaine was injected through thyrocricocentesis. The patient was advised not to swallow or cough during the injection, and was asked to cough thereafter to strengthen the anesthetic effect on the airway surface. Nasal endotracheal intubation was then finished under the guidance of fiberoptic bronchoscopy.
Tolerability of awake endotracheal intubation was assessed according to the existing reports (17,18) and clinical experience. The procedure was considered intolerable in any of the following case: significant side effects after the medication, obvious pain during thyrocricocentesis or insertion of the endotracheal tube through the nose, significant choking and cough upon entry of the fiberoptic bronchoscope into the glottis during thyrocricocentesis or after completion of endotracheal intubation, or refused continuation of the procedure by the patient. On the contrary, it was considered tolerable if no significant side effect was present after the medication, no significant pain during thyrocricocentesis or insertion of the endotracheal tube through the nose, little to no choking and cough upon entry of the fiberoptic bronchoscope into the glottis during thyrocricocentesis or after completion of endotracheal intubation was observed, and the patient remained quiet and cooperative at the end of the procedure. The second phase was concluded by calculation of the mean value, standard deviation and 95% confidence interval of tramadol doses required for each age group to complete the fiberoptic bronchoscope guided nasotracheal intubation with background administration of fentanyl.
Third phase
Twenty patients from each of the 20-49, 50-60 and 70-and-above age groups scheduled for elective surgery under general anesthesia via awake nasal endotracheal intubation were selected for this phase. The exclusion criteria included uncontrolled concurrent severe cardiovascular disorders by assessment before anesthesia, high blood pressure, preoperative difficulty breathing due to respiratory infections, long-term use of opioids, and an obesity index of greater than 2.5. Anesthesia preparation and treatment was the same as those in the second phase, and double blindness was guaranteed throughout the procedure. Based on the drug dosages determined for each age group in the previous two phases, slow intravenous injection with fentanyl 2.2 μg/kg and tramadol 2.0 mg/kg was prescribed to the 20-49 age group, fentanyl 1.6 μg/kg and tramadol 1.9 mg/kg to the 50-69 age group and fentanyl 1 μg/kg and tramadol 1.8 mg/kg to those at the age of 70 or above. After nasal contraction with 0.1 mg/mL epinephrine plus 1% lidocaine, 5 mL of 2% lidocaine was injected through thyrocricocentesis.and nasal endotracheal intubation was finished under the guidance of fiberoptic bronchoscopy. Measurements of BP, HR, RR, SpO2, sedation score, nausea and vomiting score, airway status score, pain score, discomfort score, intubation score and post-intubation condition score (19-21) were recorded before medication and one, three, five minutes after medication, before thyrocricocentesis and one, three, five minutes after thyrocricocentesis, before fiberbronchoscope-guided endotracheal intubation, immediate during the intubation and one, three, five minutes after the intubation.
Upon completion of endotracheal intubation and confirmation of correct positioning, general anesthesia was immediately applied and surgery was performed. Relevant complications, awareness scores of awake endotracheal intubation and related adverse reactions were documented after surgery.
Statistical method
The collected parameters were assorted in Microsoft Excel and SPSS17.0. Measurement data were expressed as x̄±s. The mean value, standard deviation and 99% confidence interval of the minimal single dose (EC50) of intravenous fentanyl for respiratory depression, as well as the 99% normal dosage ranges, in each age group were calculated. The mean value, standard deviation and 95% confidence interval of tramadol doses required for each age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of fentanyl were calculated. Data of the third phase were subject to paired t-test with a P value of <0.05 indicating statistical difference.
Results
Dixon’s up-and-down sequential test, a simple and efficient method to determining the effective dose of medication, was applied in this study to approach the minimal single dose (EC50) of intravenous fentanyl to cause respiratory depression in each age group (Figure 1). The EC50 values in the 20-49, 50-60 and 70-and-above age groups were 94±0.28 2.43±0.30 and 1.62±0.22 μg/kg, 99% confidence interval (CI) 2.84-3.04 2.33-2.53 and 1.61-1.63 μg/kg, and 99% range 2.22-3.66 1.66-3.20 and 1.05-2.19 μg/kg, respectively.
Figure 1.
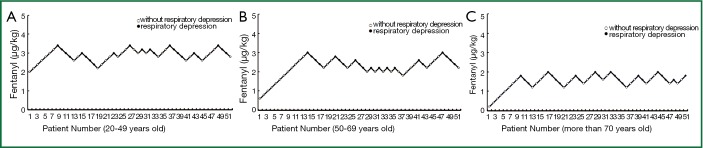
Up-and-down sequential minimal single doses of intravenous fentanyl for respiratory depression in each age group.
The EC50 of tramadol doses required for each age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of fentanyl was derived from the up-and-down sequential test (Figure 2). The EC50 of tramadol doses required for the 20-49 age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of 2.2 μg/kg fentanyl was 1.96±0.18 mg/kg. The EC50 of tramadol dose required for the 50-69 age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of 1.6 μg/kg fentanyl was 1.89±0.20 mg/kg. The EC70 of tramadol doses required for the 70-and-above age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of 1.0 μg/kg fentanyl was 1.78±0.17 mg/kg. The 95% CI values were 1.86-2.01 1.72-2.06 and 1.68-1.88 mg/kg, respectively.
Figure 2.
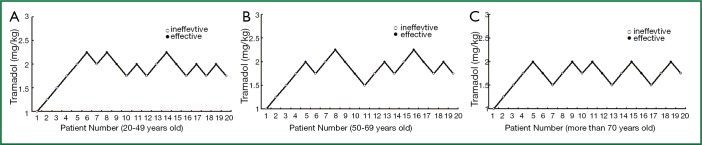
Up-and-down sequential EC50 of tramadol doses required for each age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of fentanyl.
Awake nasal fiberoptic bronchoscope guided tracheal intubation was successful for all subjects at the third phase. Significant difference was noticed in the changes of MAP, HR and SPO2 between the three groups (P<0.05), though they were within the normal physiological range (Table 1). Only one patient in the 50-69 age group had a SPO2 value lower than 92% due to breath holding during thyrocricocentesis, which was relieved after the patient was asked to take a deep breath.
Table 1. Changes of vital signs in three groups before and after tracheal intubation.
| MAP |
HR |
SpO2 |
|||||
|---|---|---|---|---|---|---|---|
| (mmHg) | P value | (beats.min-1) | P value | (%) | P value | ||
| Group I (20-49 years old patients) |
Baseline | 95.6 (11.7) | 78.5 (12.0) | 97.6 (1.5) | |||
| After fentanyl and tramadol administration | |||||||
| 1 min | 101.5 (13.2) | <0.001* | 81.3 (11.4) | 0.054 | 96.8 (2.2) | 0.217 | |
| 3 min | 97.6 (12.6) | 0.426 | 82.1 (12.4) | 0.001* | 96.2 (2.3) | 0.030* | |
| 5 min | 97.5 (11.5) | 0.423 | 80.7 (12.4) | 0.100 | 96.5 (2.3) | 0.068 | |
| After thyrocricoid puncture | |||||||
| 1 min | 100.3 (13.2) | 0.060 | 85.0 (14.2) | 0.013* | 97.2 (1.5) | 0.240 | |
| 3 min | 97.73 (11.2) | 0.254 | 82.1 (12.6) | 0.047* | 97.4 (1.8) | 0.585 | |
| 5 min | 99.0 (11.9) | 0.055 | 82.7 (12.2) | 0.106 | 97.8 (1.4) | 0.584 | |
| After fibreoptic nasal tracheal intubation | |||||||
| 1 min | 98.5 (13.0) | 0.308 | 81.5 (11.8) | 0.176 | 97.8 (1.4) | 0.697 | |
| 3 min | 97.6 (13.5) | 0.505 | 81.0 (11.1) | 0.271 | 98.2 (1.7) | 0.264 | |
| 5 min | 96.1 (11.9) | 0.875 | 81.1 (9.2) | 0.333 | 98.4 (1.6) | 0.094 | |
| Group II (50-69 years old patients) |
Baseline | 94.0 (13.9) | 74.8 (17.6) | 97.2 (0.9) | |||
| After fentanyl and tramadol administration | |||||||
| 1 min | 98.0 (11.9) | 0.062 | 75.7 (19.8) | 0.450 | 97.3 (1.2) | 0.166 | |
| 3 min | 99.0 (11.1) | 0.064 | 77.0 (17.2) | 0.177 | 95.6 (1.9) | 0.004* | |
| 5 min | 99.5 (13.7) | 0.002* | 77.7 (16.3) | 0.164 | 96.3 (1.6) | 0.085 | |
| After thyrocricoid puncture | |||||||
| 1 min | 100.5 (11.6) | 0.024* | 84.0 (19.6) | 0.040* | 95.2 (1.6) | <0.001* | |
| 3 min | 97.0 (10.5) | 0.172 | 78.6 (15.3) | 0.110 | 96.6 (0.8) | 0.007 | |
| 5 min | 97.2 (8.9) | 0.323 | 78.3 (16.5) | 0.130 | 97.3 (1.4) | 0.615 | |
| After fibreoptic nasal tracheal intubation | |||||||
| 1 min | 99.2 (12.1) | 0.031* | 80.5 (17.6) | 0.012* | 96.5 (0.5) | 0.039* | |
| 3 min | 97.0 (11.3) | 0.367 | 78.3 (17.1) | 0.087 | 97.5 (0.5) | 0.266 | |
| 5 min | 93.8 (7.8) | 0.970 | 77.0 (16.6) | 0.158 | 97.8 (0.9) | 0.220 | |
| Group III (more than 70 years old patients) |
Baseline | 113.3 (10.8) | 83.2 (8.7) | 96.3 (1.3) | |||
| After fentanyl and tramadol administration | |||||||
| 1 min | 114.5 (8.4) | 0.250 | 83.4 (10.4) | 0.785 | 96.3 (0.9) | 0.980 | |
| 3 min | 116.1 (2.9) | 0.260 | 81.6 (8.8) | 0.045* | 96.3 (1.3) | 1.000 | |
| 5 min | 112.2 (5.2) | 0.763 | 81.2 (12.3) | 0.173 | 96.4 (1.96) | 0.892 | |
| After thyrocricoid puncture | |||||||
| 1 min | 117.2 (4.2) | 0.114 | 82.8 (9.3) | 0.670 | 96.5 (1.7) | 0.772 | |
| 3 min | 114.5 (3.1) | 0.624 | 83.2 (9.6) | 0.889 | 97.2 (1.6) | 0.218 | |
| 5 min | 112.5 (5.2) | 0.770 | 82.0 (10.0) | 0.037* | 97.8 (1.3) | 0.014* | |
| After fibreoptic nasal tracheal intubation | |||||||
| 1 min | 116.8 (6.2) | 0.205 | 81.8 (9.3) | 0.268 | 97.3 (1.4) | 0.179 | |
| 3 min | 113.0 (7.2) | 0.823 | 81.2 (9.8) | 0.032* | 98.0 (1.3) | 0.030* | |
| 5 min | 111.0 (6.1) | 0.271 | 81.6 (9.6) | 0.045* | 98.5 (1.0) | 0.001* | |
MAP, mean arterial blood pressure; HR, heart rate;baseline, immediately before fentanyl and tramadol administration. *,compared with baseline, P<0.05.
The nausea scores of all three groups maintained within one point after administration of fentanyl and tramadol, and no noticeable nausea and vomiting was observed.
All subjects could make coughing and swallowing actions as per instruction during endotracheal intubation after medication. Few patients experienced dizziness but presented no significant drowsiness. The degree of sedation was not deep and patients remained cooperative after medication. Some patients complained of mild pain and discomfort during intubation but presented no apparent resistance. All patients were able to complete the process of fiberoptic bronchoscope guided tracheal intubation, suggesting that the procedure was well tolerated. State scores were satisfying after the intubation, and most patients stayed quiet and cooperative. A few subjects presented mild cough and choking when the endotracheal tube reached the glottis, which could be relieved by placation or intravenous injection of a small dose (20-30 mg) of propofol (sedation, airway condition, discomfort, intubation and pain during endotracheal intubation after medication and specific status scores after completion of the procedure are shown in Table 2).
Table 2. Relevant scores during endotracheal intubation.
| Consciousness score | Airway score | Discomfort score | Intubation score | Faces pain scale | Post intubation conditions |
|
|---|---|---|---|---|---|---|
| Group I (20-49 years old patients) (n=20) | 3.85±0.36 [3-4] | 2.90±0.31 [2-3] | 0.80±0.41 [0-1] | 0.40±0.75 [0-3] | 1.70±0.97 [0-4] | 1.10±0.30 [1-2] |
| Group II (50-69 years old patients)(n=20) | 3.60±0.50 [3-4] | 2.85±0.36 [2-3] | 0.70±0.47 [0-1] | 0.80±0.95 [0-3] | 1.90±1.02 [0-4] | 1.05±0.02 [1-2] |
| Group III (more than 70 years old patients) (n=20) | 3.85±0.36 [3-4] | 2.70±0.31 [2-3] | 0.80±0.41 [0-1] | 0.35±0.74 [0-3] | 1.78±0.97 [0-4] | 1.05±0.22 [1-2] |
On the first day after surgery, all three groups presented a high level of memory of the events before anesthesia, the local anesthesia, the bronchoscopic operation and intubation (100%, 100%, 80% and 30% for the 20-49 year-old age group; 100%, 90%, 70% and 10% for the 50-69 age group, and 90%, 80%, 60% and 5% for the 70-and-above group, respectively). Nonetheless, all subjects were satisfied with the anesthetic effect and there was no chief compliant of significant discomfort.
Discussion
Fiberoptic bronchoscopy guided awake endotracheal intubation has been considered as an effective means to manage difficult airways. Woodall (22) reported remarkable elevated blood pressure levels in 23% patients undergoing this procedure when lidocaine was administered alone for airway surface anesthesia, and half the patients experienced heart rate increase by 20% or more, resulting in a relatively high intubation failure rate (at about 10%). Endotracheal intubation may often cause airway mucosal injury and is associated with a high incidence of local anesthesia-related adverse reactions due to the need for a large dose of lidocaine (about 9 mg/kg) to deepen the surface anesthetic effect. In view of these, the authors believe that the risk of awake endotracheal intubation, in which surface anesthesia is applied alone without combining sedative analgesics, will outweigh the benefit.
Conscious sedation has been regarded as a conducive factor for the completion of endotracheal intubation by many clinical observation studies (23,24). However, it is a technically demanding component of anesthesia during awake endotracheal intubation because the patients have to be so moderately sedated that they can be quietly cooperative with reduced throat and cough reflex during the airway surface anesthesia and endotracheal tube placement, and they also maintain satisfying spontaneous breathing, awareness and ability to vomit and expectorate on their own for safety concerns. Any inappropriate treatment may easily lead to excessively deep sedation, difficulty in waking, uncooperative restlessness, lack of communication or even aggravated respiratory depression, where a non-acute airway can evolve into an acute one that greatly increases the risk of induction. Sutherlan et al. (25) reported successful cases of awake nasal endotracheal intubation with the combination of fentanyl (1.4±0.6 μg/kg) and midazolam (1.9±1.8 mg/kg). Puchner et al. (26) reported that most patients could tolerate awake nasotracheal intubation under surface anesthesia by intravenous administration with 1.5 μg/kg fentanyl in addition to 5-10 μg as a supplement dose based on their reactions. Although the impact of age on sedative analgesics during awake endotracheal intubation has not been thoroughly analyzed in most of the existing studies, it is related to the administration of fentanyl both pharmacokinetically and pharmacodynamically. Respiratory depression may occur in the elderly group at a significantly different dose from that in the younger group. This study has also revealed an age-dependent minimal single dose for respiratory depression across different age groups, which gradually decreases as age increases. To minimize the risk of fentanyl-induced respiratory depression during awake endotracheal intubation, the 99% normal range of the minimal single fentanyl dose by intravenous administration to cause respiratory depression in each group was calculated through statistical analysis in this study. Doses lower than the lowest limit of this range were used for background intravenous application. As a result, no case of significant respiratory depression was reported during awake endotracheal intubation.
Tramadol has strong analgesic and certain antitussive effects; it does neither inhibit breathing nor affect the respiratory rate and tidal volume at therapeutic doses (27). To compensate for the lower dose of fentanyl compared to that in single-agent application, this study introduced tramadol as a supplemental sedative analgesic agent for awake endotracheal intubation. Excitingly, the tramadol doses required for each age group to complete fiberoptic bronchoscope guided nasotracheal intubation with background administration of fentanyl were derived from the up-and-down sequential test. At the third phase, fiberoptic bronchoscope guided awake nasal endotracheal intubation was performed under conscious sedation with the combination of tramadol and fentanyl in each age group. The results showed stable vital signs across those groups after treatment. A few patients experienced dizziness but presented no significant drowsiness. It has been reported (28) that intravenous injection of tramadol is likely to cause nausea and vomiting. In this study, however, no case of significant nausea and vomiting was observed, which was presumably due to a consistent quiet status without obvious predisposing factors for all subjects.
The Dixon’s sequential test requires at least five up-and-down cycles to generate the target dose (29). In this study, the first phase had a larger number of subjects (52 vs. 20) and more up-and-down cycles (9 vs. 5) compared with the second phase, which was mainly due to the larger individual differences and higher risk of respiratory depression associated with fentanyl than tramadol. For safety concerns, more subjects were included for determining the minimal dose of respiratory depression to obtain more accurate information.
Adrenaline has a strong mucosal contraction effect and is widely used in nasal surgery. Hence, we combine adrenaline with lidocaine for nasal mucosal contraction in this cohort to minimize resistance during nasal intubation and mucosal injury. As a result, all subjects presented stable signs without obvious pain during nasal insertion except for only two patients with mild nasal mucosal injury and bleeding. Meanwhile, it is shown that a small dose of adrenaline had limited impact on the heart rate and blood pressure. Nonetheless, caution should be given when administering to hypertensive patients and use in combination with drugs to control the blood pressure and heart rate is recommended.
At present, direct injection of topical anesthetic agents via fiberoptic bronchoscopy is adopted in most studies (30,31) that advise against thyrocricocentesis because it is an invasive approach and may cause airway bleeding and other complications. However, topical anesthesia requires a long period before action, while an increased dose may put the patients at risk of local anesthetic toxicity. Furthermore, repeated introduction of fiberoptic bronchoscope for drug injection or its stay in the glottis or airway for the anesthetics to take action may place a higher risk of mucosal injury. After the administration of fentayl and tramadol in this study, nasal mucosal contraction was performed simultaneously with airway surface anesthesia via thyrocricocentesis. To avoid mucosal injury by the puncture needle, a 0.5×20 RW LB needle was used for thyrocricocentesis and injection. Fractional injection of local anesthetics was used for patients with swallowing or choking reaction to avoid mucosal injury. Following the combined application of tramadol and fentanyl, none of the subjects presented cough or choking during airway topical anesthesia except for a few suffering swallowing or slight cough reactions. Besides, no airway bleeding or significant sore throat was observed after surgery. Hence, the small puncture needle would not have significant impact on the airway if the patient has no severe choking action.
Studies (32,33) have attached importance to the quiet, cooperative status of patients and avoidance of severe cough after the completion of fiberoptic bronchoscopy guided endotracheal intubation, because such action may compromise the tube positioning under bronchoscopy. Meanwhile, for patients with cervical instability, preventing severe cough is helpful to reduce the risk of further cervical injury. Increased dosage of general anesthetics can undoubtedly improve tolerance of endotracheal intubation, but it also greatly increases the risk of excessive sedation and respiratory depression. In addition, despite the significance of conscious sedation for tolerability of intubation, either the doses used in this study or those recommended in some studies are still insufficient to generate the depth of anesthesia for eliminating airway responsiveness after intubation. Therefore, a major approach to avoiding cough reaction during awake endotracheal intubation should be adequate airway topical anesthesia. The primary purpose of conscious sedation is to eliminate or reduce the discomfort during airway surface anesthesia and minimize discomfort during intubation. This study has shown satisfying tolerability of patients to airway surface anesthesia after application of tramadol combined with fentanyl. Following thyrocricocentesis, patients may cough spontaneously for diffusion of the local anesthetics in the airway and around the glottis. With conscious sedation and complete surface anesthesia, the patients in this study manifested stable vital signs without obvious pain or cough during endotracheal intubation, and presented favorable tolerance to the tube after intubation. We also found a simple and accurate way to confirm full diffusion of local anesthetics in the throat by identifying bitter feeling in the mouth after spontaneous cough.
This study also shows that most patients had memory of the intubation process, which may be explained by the absence of obvious amnesia properties of pre-anesthetic medication, fentanyl and tramadol. It has been reported (34,35) that the addition of midazolam or propofol before awake endotracheal intubation may result in better amnesia effect. These agents are not used in this study because we wanted to ensure safety and that patients were fully awake so that we identify the effect of tramadol in the induction of anesthesia during awake endotracheal intubation. Further research will be needed to provide more evidence in this regard. Although most patients were aware of the intubation, this study has shown that they were generally satisfied with the induction process, and there was no other adverse reaction. This study is mainly limited as it does not provide data of pathologically obese patients and those with extreme physical weakness. Therefore, the doses of fentanyl and tramadol should be properly reduced for these patients according to the specific circumstances to avoid overdose. In addition, further studies based on a larger sample will be needed to determine the medical normal values and verify their reliability, as the dose ranges given in this study can serve as clinical reference only.
Based on the findings of this study, fiberoptic bronchoscope guided nasotracheal intubation can be successfully completed with background administration of fentanyl and tramadol for airway surface anesthesia. Since age has been taken into account as a confounding factor in the first place, safety was ensured and none of the subjects in this cohort presented obvious lethargy or difficulty in waking. In sum, this study provides an option for managing difficult airways, which should be verified in further clinical practice.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Braz LG, Braz DG, Cruz DS, et al. Mortality in anesthesia: a systematic review. Clinics (Sao Paulo) 2009;64:999-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovassapian A.The flexible bronchoscope. A tool for anesthesiologists. Clin Chest Med 2001;22:281-99 [DOI] [PubMed] [Google Scholar]
- 3.Kanowitz A, Dunn TM, Kanowitz EM, et al. Safety and effectiveness of fentanyl administration for prehospital pain management. Prehosp Emerg Care 2006;10:1-7 [DOI] [PubMed] [Google Scholar]
- 4.Skulska A, Kała M, Parczewski A.Fentanyl and its analogues in clinical and forensic toxicology. Przegl Lek 2005;62:581-4 [PubMed] [Google Scholar]
- 5.Keskinbora K, Aydinli I.An atypical opioid analgesic: tramadol. Agri 2006;18:5-19 [PubMed] [Google Scholar]
- 6.Lipnick RL, Cotruvo JA, Hill RN, et al. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol 1995;33:223-31 [DOI] [PubMed] [Google Scholar]
- 7.Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47-50 [DOI] [PubMed] [Google Scholar]
- 8.Kim MK, Lee JW, Jang DJ, et al. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia 2009;64:136-40 [DOI] [PubMed] [Google Scholar]
- 9.Boutonnet M, Faitot V, Keïta H.Airway management in obstetrics. Ann Fr Anesth Reanim 2011;30:651-64 [DOI] [PubMed] [Google Scholar]
- 10.Della Puppa A, Andreula C, Frass M.Assisted sedation: a safe and easy method for pain-free percutaneous vertebroplasty. Minerva Anestesiol 2008;74:57-62 [PubMed] [Google Scholar]
- 11.Cathelin M, Vignes R, Malki A, et al. Comparison between the side-effects of fentanyl and morphine in conscious man. Anesth Analg (Paris) 1980;37:265-73 [PubMed] [Google Scholar]
- 12.Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology 2000;92:1229-36 [DOI] [PubMed] [Google Scholar]
- 13.Miner JR, Gray RO, Bahr J, et al. Randomized clinical trial of propofol versus ketamine for procedural sedation in the emergency department. Acad Emerg Med 2010;17:604-11 [DOI] [PubMed] [Google Scholar]
- 14.Dargin J, Medzon R.Emergency department management of the airway in obese adults. Ann Emerg Med 2010;56:95-104 [DOI] [PubMed] [Google Scholar]
- 15.Hoda MQ, Khan MU, Abbas MQ, et al. Haemodynamic response of intravenous tramadol and intravenous morphine during laryngoscopy and endotracheal intubation. J Pak Med Assoc 2008;58:30-3 [PubMed] [Google Scholar]
- 16.Nikolaus T, Zeyfang A.Pharmacological treatments for persistent non-malignant pain in older persons. Drugs Aging 2004;21:19-41 [DOI] [PubMed] [Google Scholar]
- 17.Machata AM, Gonano C, Holzer A, et al. Awake nasotracheal fiberoptic intubation: patient comfort, intubating conditions, and hemodynamic stability during conscious sedation with remifentanil. Anesth Analg 2003;97:904-8 [DOI] [PubMed] [Google Scholar]
- 18.Lallo A, Billard V, Bourgain JL. A comparison of propofol and remifentanil target-controlled infusions to facilitate fiberoptic nasotracheal intubation. Anesth Analg 2009;108:852-7 [DOI] [PubMed] [Google Scholar]
- 19.Rai MR, Parry TM, Dombrovskis A, et al. Remifentanil target-controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: a double-blinded randomized controlled trial. Br J Anaesth 2008;100:125-30 [DOI] [PubMed] [Google Scholar]
- 20.Jennings PA, Cameron P, Bernard S. Measuring acute pain in the prehospital setting. Emerg Med J 2009;26:552-5 [DOI] [PubMed] [Google Scholar]
- 21.Fujii Y, Tanaka H, Toyooka H.Reduction of postoperative nausea and vomiting with granisetron. Can J Anaesth 1994;41:291-4 [DOI] [PubMed] [Google Scholar]
- 22.Woodall NM, Harwood RJ, Barker GL. Complications of awake fibreoptic intubation without sedation in 200 healthy anaesthetists attending a training course. Br J Anaesth 2008;100:850-5 [DOI] [PubMed] [Google Scholar]
- 23.Ahmed-Nusrath A, Mushambi M.Airway management in patients. Br J Hosp Med (Lond) 2009;70:365. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Liu JX, Jiang H, et al. Cardiovascular responses and airway complications following awake nasal intubation with blind intubation device and fibreoptic bronchoscope: a randomized controlled study. Eur J Anaesthesiol 2010;27:461-7 [DOI] [PubMed] [Google Scholar]
- 25.Sutherland AD, Williams RT. Cardiovascular responses and lidocaine absorption in fiberoptic-assisted awake intubation. Anesth Analg 1986;65:389-91 [PubMed] [Google Scholar]
- 26.Puchner W, Egger P, Pühringer F, et al. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand 2002;46:350-4 [DOI] [PubMed] [Google Scholar]
- 27.Bertsche T, Mikus G.Adverse drug reactions and drug interactions in analgesic therapy. Ther Umsch 2011;68:19-26 [DOI] [PubMed] [Google Scholar]
- 28.le Roux PJ, Coetzee JF. Tramadol today. Curr Opin Anaesthesiol 2000;13:457-61 [DOI] [PubMed] [Google Scholar]
- 29.Xue FS, He N, Liao X, et al. Clinical assessment of awake endotracheal intubation using the lightwand technique alone in patients with difficult airways. Chin Med J (Engl) 2009;122:408-15 [PubMed] [Google Scholar]
- 30.Cafiero T, Esposito F, Fraioli G, et al. Remifentanil-TCI and propofol-TCI for conscious sedation during fibreoptic intubation in the acromegalic patient. Eur J Anaesthesiol 2008;25:670-4 [DOI] [PubMed] [Google Scholar]
- 31.Donaldson AB, Meyer-Witting M, Roux A. Awake fibreoptic intubation under remifentanil and propofol target-controlled infusion. Anaesth Intensive Care 2002;30:93-5 [DOI] [PubMed] [Google Scholar]
- 32.Durga P, Sahu BP, Mantha S, et al. Development and validation of predictors of respiratory insufficiency and mortality scores: simple bedside additive scores for prediction of ventilation and in-hospital mortality in acute cervical spine injury. Anesth Analg 2010;110:134-40 [DOI] [PubMed] [Google Scholar]
- 33.Langford RA, Leslie K. Awake fibreoptic intubation in neurosurgery. J Clin Neurosci 2009;16:366-72 [DOI] [PubMed] [Google Scholar]
- 34.Piepho T, Thierbach AR, Göbler SM, et al. Comparison of two different techniques of fibreoptic intubation. Eur J Anaesthesiol 2009;26:328-32 [DOI] [PubMed] [Google Scholar]
- 35.Kim MK, Lee JW, Jang DJ, et al. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia 2009;64:136-40 [DOI] [PubMed] [Google Scholar]


