Abstract
Objective
To evaluate the application of immediate breast reconstruction (IBR) with silicon prosthetic implantation following bilateral nipple-preserving subcutaneous mammary gland excision in the treatment of young patients with early breast cancer.
Methods
We retrospectively analyzed the clinical data of 21 patients with breast cancer who were performed on IBR following bilateral nipple-preserving subcutaneous mammary gland excision in our hospital from January 2006 to March 2011.
Results
The operations were successful in all the 21 patients. Also, the treatment provided a good cosmetic effect. No local recurrence or distant metastasis was found in these 21 patients during the 6-66-month follow-up.
Conclusions
For the young patients with early breast cancer, mammary gland excision on the affected side along with prophylactic excision of the contralateral side, namely IBR following bilateral nipple-preserving subcutaneous mammary gland excision, provides good clinical effectiveness and cosmetological effects.
KEY WORDS : Breast cancer, prophylactic mastectomy, immediate breast reconstruction
Introduction
According to clinical studies, breast cancer patients are associated with poorer outcomes at a younger age, who have a higher risk of high grade tumors, relapse and metastasis when compared with patients older than 35 years (1,2). Meanwhile, age is an independent prognostic factor for local recurrence following breast conserving surgery, where younger patients (younger or at the age of 35 years) are more prone to local recurrence (3,4). In addition, younger women often have more physical demands. Therefore, we have adopted a more radical surgical treatment for the special population of young breast cancer patients – with informed consent of the patients, mastectomy is performed along with preventative removal of the contralateral breast (i.e. bilateral mastectomy sparing the skin and nipple areola complex), followed by immediate breast reconstruction with prosthesis implants. As a result, satisfactory clinical outcomes have been achieved, and the related data are summarized as follows.
Subjects and methods
General information
Twenty-one young women were included in the study, with a mean age of 28 years (22-34 years). Bilateral breast ultrasonography, mammography and breast MRI were conducted before surgery. The primary tumors ranged from 1-3 cm. The lesions were located in the left breasts in seven cases, right breasts in nine cases, and involving both breasts in five cases. There 15 patients with invasive breast cancer, 2 with mucinous breast cancer, and 4 with ductal carcinoma, including 3 with extensive ductal carcinoma in situ. One patient had received neoadjuvant chemotherapy. Based on TNM classification, there were 4 cases of Stage 0, 12 cases of Stage I, and 5 cases of Stage II.
Surgical methods
Axillary sentinel lymph node biopsy and mastectomy sparing the skin and nipple areola complex
Before surgery, skin folds under both breasts and bilateral axillary lines were marked for each patient in the sitting position. After general anesthesia was completed with the patient in the supine position, the range of mastectomy was marked, and curved incisions were designed along the external margin of the breast (Figure 1) which were bilaterally symmetrical so that they extended smoothly to the armpit for axillary sentinel lymph node biopsy (SLNB) or axillary dissection (Figure 2). About 8-10 minutes after subcutaneous injection of 0.5-1.5 mL methylene blue at the ipsilateral areola, a lateral incision was made along the affected breast to identify blue-stained lymphatic vessels and lymph nodes, which were removed for pathological testing, including enlarged yet not blue-stained lymph nodes. A positive frozen pathological result during the sentinel lymph node surgery would warrant axillary dissection. Otherwise, mastectomy sparing the skin and nipple areola complex was performed without axillary dissection (Figure 3).
Figure 1.
Design of surgical incisions.
Figure 2.

The incisions can be extended smoothly to the armpit for axillary sentinel lymph node biopsy (SLNB).
Figure 3.

The mammary gland tissue was removed.
Immediate breast reconstruction with prosthesis implants under bilateral pectoralis major
The base width, breast width and protrusion were measured before reconstruction as the basis for selecting the appropriate size of prostheses/dilators. Following mastectomy sparing the skin and nipple areola complex, immediate breast reconstruction was implemented with prosthesis/dilator implants. The space between the pectoralis major and the pectoralis minor was bluntly divided with fingers through the lateral border of the pectoralis major, and some of the muscle fibers could be removed and cut off at the lower starting point of pectoralis major. When dividing at the lower part of the lateral pectoralis major, the fascia and fibers of serratus anterior were lifted for implantation of the prosthesis, which was then adjusted according to the skin folds and anterior axillary line marked before surgery. The outer edge of pectoralis major and the serratus anterior muscle were then closed with interrupted sutures so that the surface of the prosthesis/dilator was covered by the muscles to prevent slipping and shift (Figure 4). Suction drainage was placed under the flap and pectoralis major. Compression bandages were applied with moderate pressure on the chest after the skin was sutured.
Figure 4.
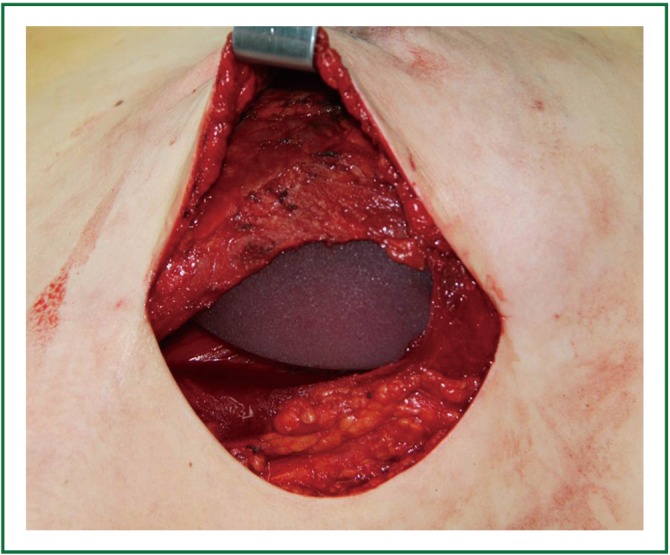
The expander underneath the pectoralis major.
Postoperative comprehensive treatment
Appropriate protocols were selected for postoperative chemotherapy and endocrine therapy based on with immunohistochemical results in accordance with the NCCN Guidelines. Since no axillary lymph node was metastasis found in any patient in the present cohort, postoperative radiotherapy was not delivered.
Results
All subjects were followed up until March 2011, with a median follow-up period of 30 months. No death, local recurrence or distant metastasis was observed. No significant complication was found in any patient, including skin incision infection or necrosis, prosthesis upward shift, prosthetic capsular contracture and other serious complications. Breast tightness was reported by six patients in three months after surgery. The nipple and areola complexes were satisfyingly retained in 17 patients, and partial nipple necrosis was observed in four patients, though it did not compromise the reconstructed appearance after decrustation. The aesthetic effects were evaluated at 6 and 12 months after immediate breast construction with prosthetic implants following mastectomy sparing the skin and nipple areola complex. The bilateral breast symmetry, natural appearance, surgical scar and other parameters were assessed by both the clinicians and patients. As a result, the excellent rate was 90% (Figure 5).
Figure 5.

Left breast cancer before (A) and after (B) IBR followingbilateral nipple preserving subcutaneous mammary gland excision.
Discussion
Modified radical mastectomy is the mostly used approach for patients with breast cancer in China. However, the large scar and absence of a breast will seriously compromise the physical integrity and quality of life of every patient undergoing this operation, especially for younger patients who will have to deal with greater physical and mental suffering. An ideal surgical treatment should take into account both the social and psychological needs of a single patient.
Theoretically, breast-conserving surgery can maintain the basic appearance of the breasts, reduce chest wall deformity, and reduce psychological trauma. This technique has been employed as a mainstream option for treating early breast cancer in Europe, US and other developed countries. However, the justification of breast-conserving surgery in the younger patients (at the age of 35 years or younger) remains controversial. There is evidence that age is an independent factor of local recurrence after breast-conserving surgery, and younger patients are associated with a relatively high risk of relapse and recurrence of breast cancer (5,6). In addition to complete removal of the tumor (negative margins), a successful breast-conserving surgery needs be able to yield satisfying cosmetic outcomes. Most Asian women, however, are unlikely to meet “demanding conditions” for breast-conserving surgery.
Bilateral symmetry is the most essential aesthetic factor of breast reconstruction. No matter how well a unilateral breast reconstruction is done, symmetrical appearance to the contralateral side is always extremely arduous. In the present cohort of 21 patients, bilateral mastectomy with immediate reconstruction was performed, and the position of skin folds under breasts, breast fullness and sagging of both sides were basically identical, resulting in the highest possible symmetry and best aesthetic appearance. The patients were satisfied with the outcomes, and their self-confidence, as well as the quality of life, was improved.
In terms of the therapeutic efficacy, clinical studies have shown that prophylactic mastectomy reduces the incidence of breast cancer (7,8). Patients with unilateral breast cancer have a higher risk of breast cancer at the contralateral side, with an annual increase of risk by 0.5-0.75%, which is independent of time (9-11). Age is a risk factor of recurrence of the contralateral breast cancer, where younger patients have a significantly higher risk of contralateral breast tumors. For fear of the occurrence of this condition, some patients with unilateral breast cancer choose to receive contralateral prophylactic mastectomy (CPM). At present, whether CPM improves the long-term survival has not been demonstrated by clinical studies. Considering that the risk of distant metastases may exceed the risk of contralateral breast cancer, however, most patients may not benefit from CPM. Studies have shown that CPM can reduce the incidence of breast cancer by 90% in women carrying the BRCA1 or BRCA2 mutation (12).
According to the report by Tuttle TM (13), the number of CPM implemented for breast cancer at all stages has tripled in the United States from 1998 to 2003, while the application of breast-conserving surgery (BCS) was gradually growing, which suggested that an increasing number of patients preferred smaller (BCS) or more radical (CPM) options, rather than unilateral mastectomy. Moreover, CPM was more often conducted in younger patients with relatively smaller lesions, negative lymph nodes and lower tumor grade. We believe that it can be explained by the longer expected survival, and higher risk of contralateral breast cancer in these patients, which makes them more likley to benefit from the procedure. A study in 2010 showed that CPM could slightly improve the 5-year tumor-related survival in ER-negative young patients at the early stage (14).
All of the 21 subjects in this study were young women with early breast cancer, aged 22-34 years, with primary tumors 1-3 cm in size and no lymph node metastasis. We adopted a radical option by performing bilateral mastectomy sparing the skin and nipple areola complex, followed by breast reconstruction with prosthesis implantation. The treatment provided a good cosmetic effect, and benefited those patients at least in terms of the physical appearance, and the quality of life. Due to the relatively small number of this cohort, and limited follow-up period, however, whether this treatment improves their long-term survival still remains uncertain.
Acknowledgements
Disclosure: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Livi L, Meattini I, Saieva C, et al. The impact of young age on breast cancer outcome. Eur J Surg Oncol 2010;36:639-45 [PubMed] [Google Scholar]
- 2.Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol 2011;21:26-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han W, Kang SY, Korean Breast Cancer Society Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat 2010;119:193-200 [DOI] [PubMed] [Google Scholar]
- 5.Bollet MA, Sigal-Zafrani B, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol 2007;82:272-80 [DOI] [PubMed] [Google Scholar]
- 6.de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer 2006;42:351-6 [DOI] [PubMed] [Google Scholar]
- 7.Paradiso A, Formenti S.Hereditary breast cancer: clinical features and risk reduction strategies. Ann Oncol 2011;22Suppl 1:i31-6 [DOI] [PubMed] [Google Scholar]
- 8.Stuckey A, Dizon D, Scalia Wilbur J, et al. Clinical characteristics and choices regarding risk-reducing surgery in BRCA mutation carriers. Gynecol Obstet Invest 2010;69:270-3 [DOI] [PubMed] [Google Scholar]
- 9.Rubino C, Arriagada R, Delaloge S, et al. Relation of risk of contralateral breast cancer to the interval since the first primary tumour. Br J Cancer 2010;102:213-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollias J, Ellis IO, Elston CW, et al. Clinical and histological predictors of contralateral breast cancer. Eur J Surg Oncol 1999;25:584-9 [DOI] [PubMed] [Google Scholar]
- 11.Yi M, Meric-Bernstam F, Middleton LP, et al. Predictors of contralateral breast cancer in patients with unilateral breast cancer undergoing contralateral prophylactic mastectomy. Cancer 2009;115:962-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaas R, Verhoef S, Wesseling J, et al. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Ann Surg 2010;251:488-92 [DOI] [PubMed] [Google Scholar]
- 13.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9 [DOI] [PubMed] [Google Scholar]
- 14.Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst 2010;102:401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]



