Background
Cryotherapy has been employed as a modality in the airways for many years. It’s use in the airway has been limited to the cryoprobe which requires direct contact with the tissue (1,2). Endoscopic spray cryotherapy (SCT) using liquid nitrogen as the cryogen was initially developed for endoscopic use in mucosal ablation in 1999 with subsequent indications for the treatment of esophageal malignancies (3,4). This novel spray cryotherapy technology allows for delivery of liquid nitrogen droplets to the endoluminal surface through a flexible 7 French disposable catheter producing a distinct visible frosting as it instantly flash freezes the tissue. As the liquid nitrogen droplets transform to gas in the endoluminal space, this gas needs to be evacuated from the space to prevent barotrauma. In the esophagus and stomach, an additional suction tube is placed to evacuate the gas. This is not practical in bronchoscopic procedures as there is less room for additional tubes and the active suctioning may remove needed oxygen from the patient’s lungs. Therefore, evacuation of the nitrogen gas must be accomplished through passive venting of the gas through the rigid bronchoscope or around and through the endotracheal tube with the cuff deflated and disconnected from the ventilator circuit. It is critical that an unobstructed route is available for the gas to exit and as long as an unobstructed pathway is maintained, pressure differentials would favor gas egress through the mouth. This concept of passive venting SCT is fundamentally different from the active venting SCT in the GI practice and is a driving force in the development of safe and effective techniques for use in the central airways.
The current device received a general use approval for the destruction of unwanted tissue from the FDA in 2012 (TruFreeze system, CSA Medical Inc., USA). This system provides liquid nitrogen at low pressures through a 7 French flexible catheter at adjustable flow rates delivering a liquid nitrogen spray which flash freezes a 2-3 cm oval target area depending on the flow setting and distance to the target (Figure 1 and Video 1). The previous generation device delivered liquid nitrogen only at 25 watts of energy (normal flow) while the current device also allows for delivery at 12.5 watts of energy (low flow). Depth of freeze is determined by the duration of the freeze (up to 5 mm). This new adjustable lower flow setting allows for a wider margin of safety in the airways by delivering the liquid nitrogen at a slower rate, allowing more time to recognize the build up of trapped nitrogen gas and make adjustments to the spray and/or the gas ventilation route. We report the first experience with this adjustable flow SCT in 4 of our initial patients with a diverse spectrum of central airway disease.
Figure 1.
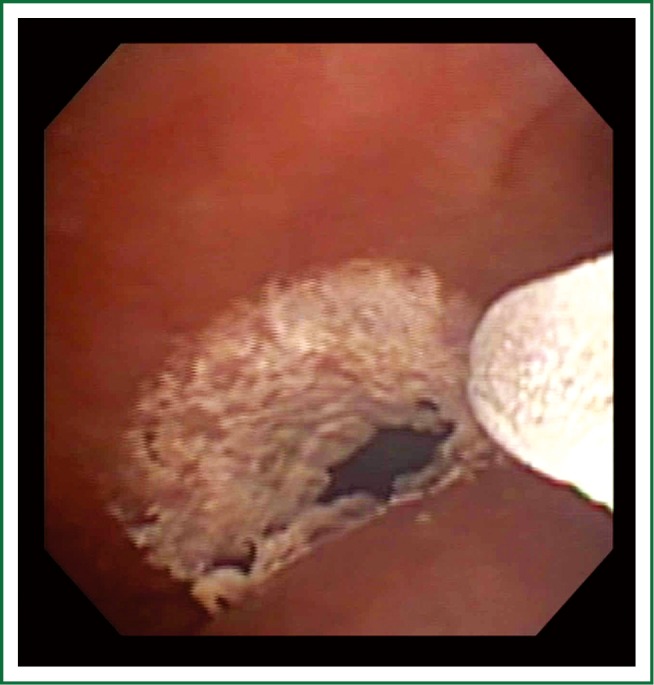
SCT applied to infiltrating tumor to the right main bronchus tumor.
Video 1.

SCT
Case 1 is a 70-year-old Caucasian woman and former smoker with known stage IV pulmonary adenocarcinoma that was no longer responding to chemotherapy. A hybrid nitinol/silicone stent had been placed in the right main bronchus to relieve obstruction from the tumor originating in the right upper lobe. This was successful in treating her dyspnea until the tumor progressed and partially obstructed the proximal end of the stent. Attempts at mechanical debulking resulted in significant bleeding. Normal flow SCT was applied to the bleeding mucosa in 3 separate sprays each allowing for 5 seconds of visible freezing of the mucosa achieving hemostasis. Serial applications of low flow SCT were used on a 4-week schedule to further debulk and prevent proximal obstruction of the stent. Over a 3-month period, with concurrent chemotherapy and radiation, the tumor in the airway regressed. At month 4, there was an increase in abnormal tissue at the proximal end of the stent. Biopsies confirmed this tissue to be benign granulation tissue. Low flow SCT in 5-10 second freezes were applied to the granulation tissue in serial bronchoscopies every 4 to 6 weeks maintaining patency of the stent and preventing further granulation tissue formation. The SCT did not result in any injury or alteration to the stent.
Case 2 is a 58-year-old Caucasian never-smoker woman with stage IV pulmonary adenocarcinoma. She presented with hypoxia and dyspnea from a left upper lobe mass that was causing almost complete obstruction of the left lower lobe secondary to extrinsic compression at the left hilum. An uncovered nitinol stent was placed in the left lower lobe basilar segment extending to the left main bronchus to restore patency to the airway without iatrogenic obstruction of the left lower lobe superior segment. This resulted in significant improvement in her dyspnea. The low flow setting of SCT was serially applied in cycles of 5-10 second freezes through the stent walls to the non-obstructing but infiltrating mucosal tumor to prevent tumor ingrowth through the stent. No degradation to the stent was noted and stent obstruction from tumor ingrowth was prevented with serial treatments at 4-6 week intervals.
Case 3 is a 59-year-old Caucasian gentleman current smoker with stage IV pulmonary adenocarcinoma, complete obstruction of the right upper lobe and extensive infiltrating tumor throughout the trachea and bilateral main bronchi. The patient complained of intractable cough refractory to medication, including significant narcotics, which disrupted his sleep and every aspect of his daily life. SCT was applied on the normal flow setting to the infiltrating tumor in the trachea and proximal bilateral main bronchi. Low flow SCT was used to treat the right bronchus intermedius and the distal left main bronchus. At one week follow up, the patient reported significant improvement in his cough with restoration of sleep and significant decrease in cough suppressant medication use. Two serial applications of SCT at 4-week interval resulted in resolution of his cough despite persistent right upper lobe obstruction.
Case 4 is a 32-year-old Caucasian female who had been treated for asthma for most of her life without significant improvement in her symptoms. Bronchoscopic evaluation revealed an area of tracheal stenosis with 60% obstruction extending approximately 5 mm in width. This was thought to be the result of prolonged intubation as an infant after a severe meconium aspiration. SCT was used to successfully treat the area of stenosis prior to and post balloon dilation. The result was improvement in the stenosis to less than 10% obstruction and significant symptomatic and spirometric improvement in her breathing. No contraction or scarring was noted on subsequent follow up bronchoscopy.
Discussion
The current generation device for Spray cryotherapy has recently been approved for general use. We report the first use of this novel device in the airway and its adjustable flow settings. Unlike probe cryotherapy and other therapeutic tools we have available in interventional pulmonology, spray cryotherapy does not make use of the Joule-Thompson effect for treatment, but instead uniquely provides a uniform and planar distribution of the liquid nitrogen droplets to the target tissue effectively flash freezing (–196 degrees Celsius) a relatively large area of the central airways despite the irregular surfaces often encountered in endobronchial disease. Understanding the mechanism and potential risks for this new therapy is essential for its safe application to patients. When the liquid nitrogen is delivered to the airway, it undergoes phase transformation and becomes nitrogen gas as its energy is delivered to the tissue. This results in the immediate formation of nitrogen gas in the airways that has the potential to displace oxygen and expand the lungs to a volume that might exceed their capacity at which point, pneumothorax or barotrauma may occur. Initial reports of this technology in patients with endobronchial disease noted a significant rate of complications that may have been the result of insufficient venting of nitrogen gas produced during the phase change of nitrogen from liquid to gas phase. These complications included hypotension, bradycardia, pneumothorax, and operative and post-operative death (5). Both the additional gas volume and the displacement of oxygen from the liquid nitrogen phase change in the lungs are critical factors to recognize and manage for safe and effective use of this technology.
Unlike the GI practice which uses active suction venting during SCT, airway procedures rely on passive venting of the nitrogen gas. Success of passive venting is heavily dependent upon the relationship between the outer diameter of the bronchoscope, the inner diameter of the endotracheal tube or rigid scope, and an unobstructed exist route to the atmosphere from the site of SCT application and are critical elements for safe use of this technology. At our institution, using the previous generation of SCT, we preferred to use rigid bronchoscopy for the procedure to ensure an uncompromised passive venting route. In the new adjustable system we have been regularly using an endotracheal tube (minimum 8.0 mm inner diameter, larger if possible) with a therapeutic flexible bronchoscope (6 mm diameter). The ETT was always disconnected from the ventilator and cuff deflated prior to each spray. During the spray, active monitoring for frosting mist venting from the mouth and ETT was performed (Figure 2 and Video 2) and the chest wall observed for abnormal rise. If at anytime there was concern that passive ventilation was compromised, the spray was immediately aborted and the scope immediately removed from the ETT. Even with the new lower flow rate on the SCT, appropriate passive venting can only be assured by restricting targets to the central airways only and mitigating any endobronchial obstruction that might interfere with passive venting on the route from the distal tip of the catheter to the atmosphere. On the lower flow setting, the liquid nitrogen is delivered at half the rate of the normal flow and thus the rate of volume expansion in the lungs is drastically reduced allowing more time to safely allow passive ventilation of the nitrogen gas. Unfortunately, nothing is without a cost, this extra margin of safety for passive ventilation comes with the cost of a longer time to deliver the same amount of liquid nitrogen to the tissues compared with the normal flow. Patients with very low respiratory reserve may be unable to tolerate the longer time off of positive pressure ventilation that is required to deliver the cryogen on the low flow setting. In these cases, the normal flow is necessary to minimize the time off ventilation.
Figure 2.
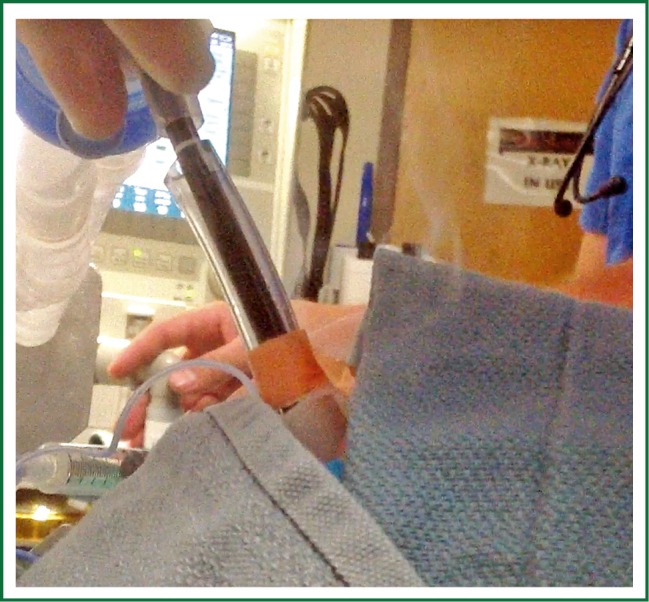
Passive venting during SCT application with endotracheal tube cuff deflated and disconnected from the ventilator.
Video 2.

Passive Venting
Another important factor for safely using this technology is to allow time for nitrogen to wash out of the lungs between sprays and be replaced by oxygen, very similar to principles of preoxygenation of the lungs prior to elective intubation. While oxygen saturation levels are usually indicative of the respiratory status of the patient, after the SCT, the ratio of oxygen to nitrogen levels may be markedly reduced even in the setting of high O2 saturations. If this is not recognized, each subsequent spray may result in quicker and more severe desaturations. In the severely compromised patient population that we are faced with, failure to mitigate these procedurally related effects could be catastrophic. The ability to adjust the flow rate allows for a slower rate of nitrogen gas production buildup in the airways in the low flow setting allowing more time for passive venting of the gas and a higher margin of safety in cases where venting may be more difficult.
Our mitigation strategy and clinical practice was to first allow the patient 3-5 minutes on 100% oxygen, then to check baseline oxygen saturation and expired FIO2 as indicated on the ventilator prior to the spray. The lower the baseline oxygen saturation and expired FIO2, the shorter the duration of spray considered. Typically, 5 to 10 second cycles (normal and low flow settings respectively) of freezing were well tolerated. After the spray, the scope was completely removed to improve gas mixture as well as allow for maximum ventilation through the ETT. The next spray was not initiated until the expired FIO2 measured on the ventilator had returned to the pre spray baseline thus (usually about 1 to 3 minutes), ensuring optimal oxygenation prior to the introduction of nitrogen gas inherent to SCT. If rigid bronchoscopy and jet ventilation were used, a period of 2 to 3 minutes was allowed after the oxygen saturations returned to pre spray baseline prior to performing the next spray.
The introduction of spray cryotherapy appears to provide us with an effective tool that can be utilized in all stages of endobronchial disease without some of the substantive limitations of the common heat therapies. SCT has been shown to preserve cartilage and other structural tissues, result in less scar tissue, and have greater normal tissue regrowth in treated areas than “hot” therapies like laser, argon plasma coagulation and electrocautery (6-8). Early disease may be treated with SCT and it appears to produce less damage to structural components of the airways with less risk of residual scarring and stenosis than some of the other modalities. The cases above were selected to demonstrate some novel applications of this new therapy including treatment of endobronchial obstruction and tumor around all types of stents, controlling superficial bleeding, debulking tumor, palliative treatment of debilitating malignant cough, and even benign diseases like tracheal stenosis. SCT can safely be used in conjunction with both covered metal and silicone airway stents without the risk of ignition. Unlike the probe cryotherapy devices which require direct contact and can easily freeze to and accidentally dislodge a stent when the probe is moved, SCT is a non contact method that can be used at a distance of several centimeters from the target and allow for safe treatment in and around stents. In uncovered stents, SCT provides a safe tool to treat endobronchial disease that would otherwise grow through the stent wall over time. In selected cases, SCT may make it possible to use uncovered stents in malignant obstructing airway tumors, minimizing the obstruction of any patent branching airways as well as reducing biofilm, infection risks, and obliterated mucociliary action from the covered or solid stents. SCT is safe for use in with pacemakers, defibrillators, or any implanted electrical devices in contrast to electrosurgical devices.
Cryotherapy has been a useful tool in bronchoscopy for several decades, but its use has always been limited by the inefficiencies and impracticality of the devices available to deliver the cryogen in the airways. With the introduction of this new device, adjustable flow SCT provides a safe and novel tool for the airways that offers unique treatment options to treat a broad spectrum of malignant and benign endobronchial diseases.
Acknowledgements
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Except Dr Sarkar has received financial compensation as a consultant for CSA.
Disclosure: Dr. Sarkar has received financial compensation as a consultant for CSA. The other authors have no conflict of interest to declare.
References
- 1.Mathur PN. Application of Laser, Electrocautery, Argon Plasma Coagulation, and Cryotherapy in Flexible Bronchoscopy. In: Wang KP, Mehta A, Turner JF. eds. Flexible Bronchoscopy, 3rd edition. Blackwell Science©, 2012:206-9. [Google Scholar]
- 2.Lee SH, Choi WJ, Sung SW, et al. Endoscopic cryotherapy of lung and bronchial tumors: a systematic review. Korean J Intern Med 2011;26:137-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston CM, Schoenfeld LP, Mysore JV, et al. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc 1999;50:86-92 [DOI] [PubMed] [Google Scholar]
- 4.Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett’s esophagus: a pilot study. Gastrointest Endosc 2005;62:842-8 [DOI] [PubMed] [Google Scholar]
- 5.Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4 [DOI] [PubMed] [Google Scholar]
- 6.Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012;15:580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krimsky WS, Sarkar S, Harely D, et al. A signal center experience with spray cryotherapy in the aerodigestive tract and chest. Chest 2009;136:140S-a-140S [Google Scholar]
- 8.Fernando HC, Dekeratry D, Downie G, et al. Feasibility of spray cryotherapy and balloon dilation [DOI] [PubMed]


