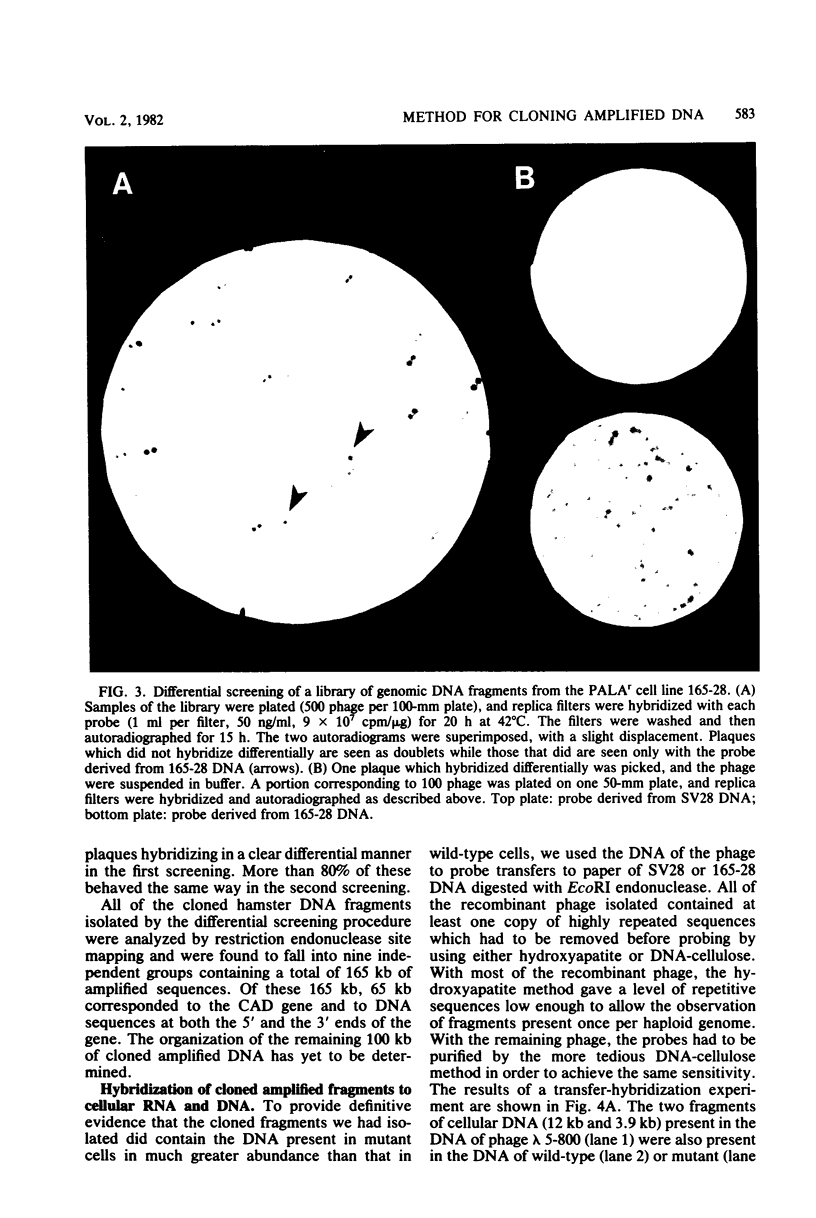
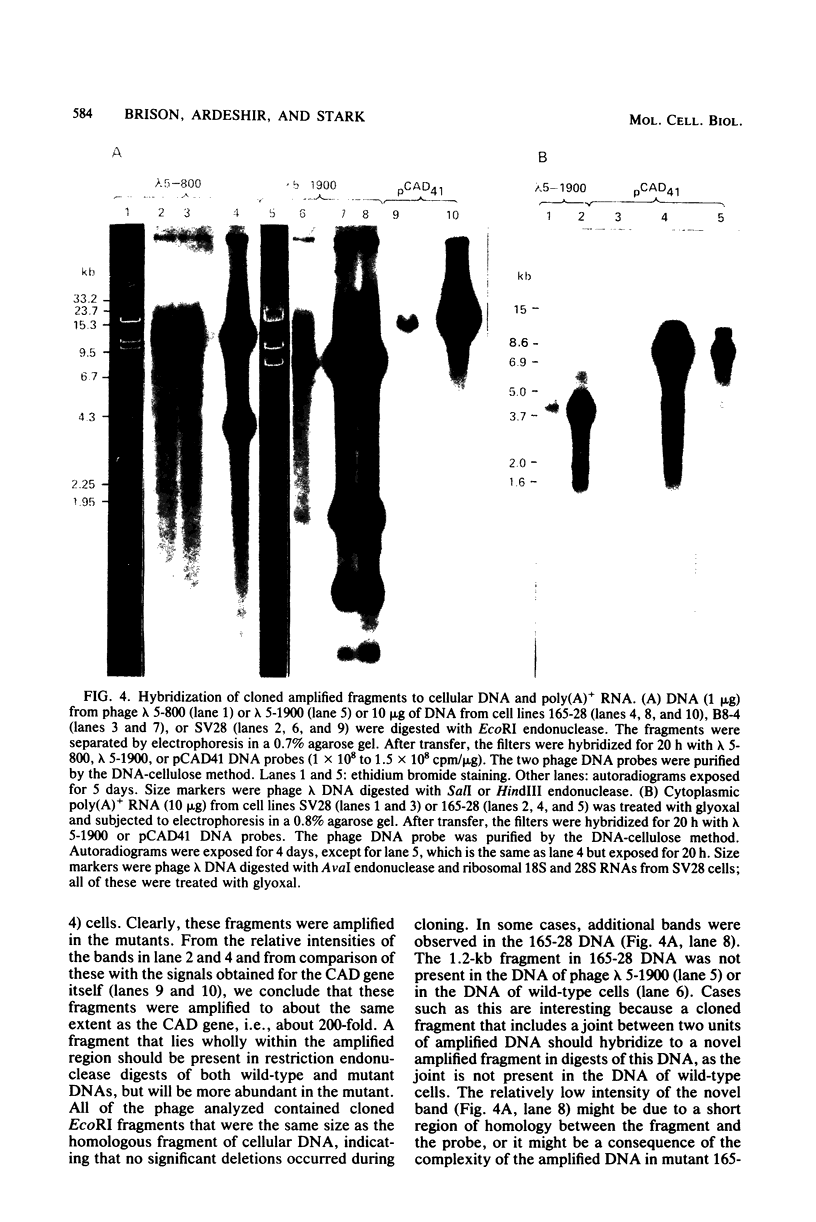
Abstract
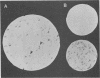
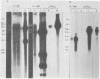
Mutant Syrian hamster cell lines resistant to N-(phosphonacetyl)-L-aspartate, a potent and specific inhibitor of aspartate transcarbamylase, have amplified the gene coding for the multifunctional protein (CAD) that includes this activity. The average amount of DNA amplified is approximately 500 kilobases per gene copy, about 20 times the length of the CAD gene itself. A differential screening method which uses genomic DNAs as probes was developed to isolate recombinant phage containing fragments of amplified DNA. One probe was prepared by reassociating fragments of total genomic DNA from 165-28, a mutant cell line with 190 times the wild-type complement of CAD genes, until all of the sequences repeated about 200 times were annealed and then isolating the double-stranded DNA with hydroxyapatite.This DNA was highly enriched in sequences from the entire amplified region, whereas the same sequences were very rare in DNA prepared similarly from wild-type cells. After both DNAs were labeled by nick translation, highly repeated sequences were removed by hybridization to immobilized total genomic DNA from wild-type cells. A library of cloned DNA fragments from mutant 165-28 was screened with both probes, and nine independent fragments containing about 165 kilobases of amplified DNA, including the CAD gene, have been isolated so far. These cloned DNAs can be used to study the structure of the amplified region, to evaluate the nature of the amplification event, and to investigate gene expression from the amplified DNA. For example, one amplified fragment included a gene coding for a 3.8-kilobase, cytoplasmic, polyadenylated RNA which was overproduced greatly in cells resistant to N-(phosphonacetyl)-L-aspartate. The method for cloning amplified DNA is general and can be used to evaluate the possible involvement of gene amplification in phenomena such as drug resistance, transformation, or differentiation. DNA fragments corresponding to any region amplified about 10-fold or more can be cloned, even if no function for the region is known. The method for removing highly repetitive sequences from genomic DNA probes should also be of general use.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker P. E., Stubblefield E. Ultrastructure of double minutes from a human tumor cell line. J Cell Biol. 1979 Dec;83(3):663–666. doi: 10.1083/jcb.83.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin F., Rosenberg R. N., Dev V. Correlation of double-minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3654–3658. doi: 10.1073/pnas.78.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Galau G. A., Angerer R. C., Britten R. J. Comparative aspects of DNA organization in Metazoa. Chromosoma. 1975 Jul 21;51(3):253–259. doi: 10.1007/BF00284818. [DOI] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Evans D., Birnstiel M. L. Localization of amplified ribosomal DNA in the oocyte of Xenopus laevis. Biochim Biophys Acta. 1968 Aug 23;166(1):274–276. doi: 10.1016/0005-2787(68)90517-0. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Cloning of DNA from double minutes of Y1 mouse adrenocortical tumor cells: evidence for gene amplification. Cell. 1981 Apr;24(1):117–123. doi: 10.1016/0092-8674(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Carrano A. V., Burkhart-Schultz K., Dozy A. M., Yu L. C., Kan Y. W. Assignment of human beta-, gamma-, and delta-globin genes to the short arm of chromosome 11 by chromosome sorting and DNA restriction enzyme analysis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5804–5808. doi: 10.1073/pnas.76.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Properties of dispersed, highly repeated DNA within and near the hamster CAD gene. Mol Cell Biol. 1982 Mar;2(3):302–307. doi: 10.1128/mcb.2.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Structure of the gene for CAD, the multifunctional protein that initiates UMP synthesis in Syrian hamster cells. Mol Cell Biol. 1982 Mar;2(3):293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Gene-amplification model of carcinogenesis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2465–2468. doi: 10.1073/pnas.78.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Swyryd E. A., Seaver S. S., Stark G. R. N-(phosphonacetyl)-L-aspartate, a potent transition state analog inhibitor of aspartate transcarbamylase, blocks proliferation of mammalian cells in culture. J Biol Chem. 1974 Nov 10;249(21):6945–6950. [PubMed] [Google Scholar]
- Tashima M., Calabretta B., Torelli G., Scofield M., Maizel A., Saunders G. F. Presence of a highly repetitive and widely dispersed DNA sequence in the human genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1508–1512. doi: 10.1073/pnas.78.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. A., Lewis W. H., Parfett C. L. Somatic cell genetics: a review of drug resistance, lectin resistance and gene transfer in mammalian cells in culture. Can J Genet Cytol. 1980;22(4):443–496. doi: 10.1139/g80-056. [DOI] [PubMed] [Google Scholar]