Abstract
Proton magnetic resonance spectroscopy (1H MRS) has been applied to numerous clinical studies, especially for neurological disorders. This technique can non-invasively evaluate brain metabolites and neurochemicals in selected brain regions and is particularly useful for assessing neuroinflammatory disorders. Neurometabolites assessed with MRS include the neuronal markers N-acetylasparate (NAA) and glutamate (Glu), as well as the glial marker myoinositol (MI). Therefore, the concentrations of these metabolites typically correspond to disease severity and often correlate well with clinical variables in the various brain disorders. Neuroinflammation with activated astrocytes and microglia in brain disorders are often associated with elevated MI, and to a lesser extent elevated total creatine (tCr) and choline containing compounds (Cho), which are found in higher concentrations in glia than neurons, while neuronal injury is indicated by lower than normal levels of NAA and Glu. This review summarizes the neurometabolite abnormalities found in MRS studies performed in patients with neuroinflammatory disorders or neuropathic pain, which also may be associated with neuroinflammation. These brain disorders include multiple sclerosis, neuroviral infections (including Human Immunodeficiency virus and Hepatitis C), degenerative brain disorders (including Alzheimer’s disease and Parkinson’s disease), stimulant abuse (including methamphetamine and cocaine) as well as several chronic pain syndromes.
Keywords: Magnetic Resonance Spectroscopy, Neuroinflammation, Neuropathic Pain
Introduction
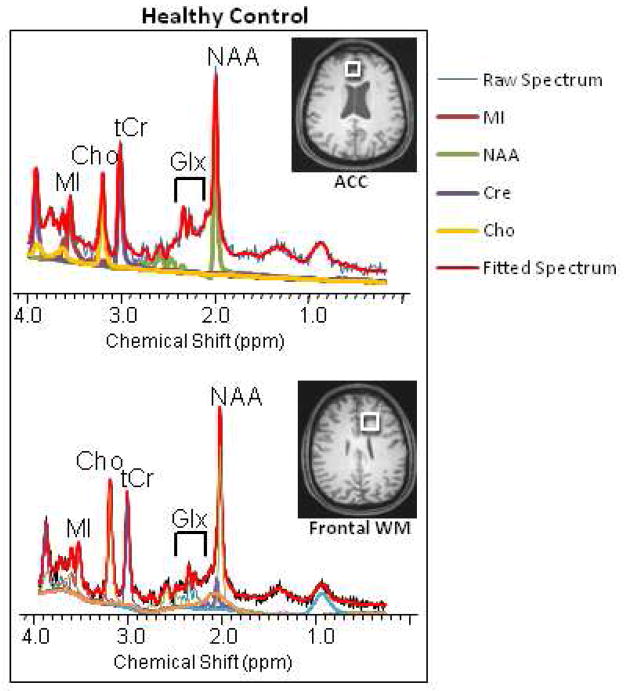
Magnetic resonance spectroscopy (MRS) is a non-invasive analytical technique that can measure metabolite concentrations in tissues and in living persons without using ionizing radiation. Both phosphorus (31P) and proton (1H) MRS have been used to study changes in brain metabolite levels associated with neuroinflammation and neurodegenerative diseases, but 1H MRS is used in the clinical setting and in the overwhelming majority of clinical studies. While 31P MRS provides measurements of energy metabolites, 1H MRS accurately measures the levels of brain metabolites that reflect neuronal and glial density. This review will focus only on studies that used 1H MRS. Figure 1 shows typical proton spectra obtained from the anterior cingulate cortex (ACC) and from the frontal white matter, in a healthy adult. Of note, proton spectra show a prominent resonance of water (at 4.7ppm) that is 100,000 times higher than the resonance signals of the brain metabolites; the water resonance has to be suppressed to enable evaluation of metabolites of interest. The spectral peak at 2.02 ppm associated with N-acetyl-aspartate (NAA) and NAA-glutamyl (NAAG) is widely used as a marker of neuronal density since NAA is found within mature neurons, while the resonance peak for myo-inositol (MI at 3.56 ppm) is a putative glial marker since it is primarily present in glial cells. The peak for choline compounds (Cho at 3.2 ppm) includes soluble choline-containing compounds and is a marker for cell membrane metabolism and cellular turnover. Cho is typically higher in the white matter than in gray matter, such as that shown in the ACC (see Figure 1). The total creatine (tCr at 3.0 ppm) resonance peak includes proton resonances from creatine and phosphocreatine, which reflects the levels of cellular energy metabolites, and is thought to be relatively stable and hence is often used as a reference marker in MRS studies. However, both Cho and tCr are two to three fold higher concentrations in glial cells than in neurons (Brand et al, 1993), and may be elevated in conditions of neuroinflammation. Other neurochemicals, such as glutamate (Glu), glutamine (Gln), Glu+Gln (Glx), gama-aminobutyric acid (GABA) and glutathione (GSH) can also be assessed, but typically require specialized editing techniques due to the overlapping resonances of Glu and Gln, as well as the overlap of the cysteinyl moiety of the GSH molecule with the higher-concentration creatine resonance at 2.95 ppm. These special techniques will be discussed below in the application of 1H MRS to evaluate changes in brain glutamate levels in neuroinflammatory conditions.
Figure 1.
Representative MR spectra from the frontal gray matter (anterior cingulate cortex or ACC) and the frontal white matter (bottom) from a healthy volunteer. Note the relatively higher Choline peak in the frontal white matter compared to that in the ACC.
MRS is one of the few techniques that can assess the chemical composition of the brain in living individuals and can be used as a biomarker to evaluate neuroinflammation or to monitor treatment effects. Metabolite concentrations measured by MRS are often reported as metabolite ratios using tCr or Cho as reference chemicals. However, both tCr and Cho can change in disease states (Chang et al, 2002) and with age (Chang et al, 1996) which may lead to misinterpretation of spectral data that rely on metabolite ratios (Jansen et al, 2006). Therefore, absolute quantification of metabolites by MRS is necessary. In addition, correction of the partial volume of cerebral spinal fluid (CSF), which does not contain major brain metabolites, should be performed when the volumes of interest (or voxels) include sulci in the brain tissue, which may be variable across subjects. Furthermore, since the metabolite concentrations are different between gray and white matter, correction for the variable proportions of gray and white matter within each voxel is also needed.
Proton MRS can non-invasively evaluate neuronal integrity and glial content. Neuronal injury or loss is reflected by decreased NAA concentration, while inflammation in the brain leads to glial activation and subsequent elevated MI level on MRS. Myo-inositol functions as an osmolyte that maintains glial cell volumes; hence, activated glia with enlarged cell volumes tends to have elevated MI. Inflammatory mediators released by activated glia may be neurotoxic; therefore, in addition to elevated MI, the neuronal marker NAA may be reduced, reflecting neuronal injury, dysfunction or neuronal loss. We will review MRS findings in several categories of brain disorders that have associated neuroinflammation, such as the well documented neuroinflammatory diseases (e.g., multiple sclerosis, infection with human immunodeficiency virus (HIV) or other viral infections of the brain), neurodegenerative disorders (e.g., Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease), stimulant abuse and neuroinflammation associated with neuropathic pain.
MRS Studies in Patients with Multiple Sclerosis (MS)
MS affects more than 350,000 people in the United States and 2.5 million worldwide. MS is the prototypical autoimmune disorder of the central nervous system, in which neuroinflammation plays a major role in the pathophysiological processes that leads to demyelination and subsequent neuronal and axonal damage. Therefore, it is not surprising that the glial markers (MI, Cho and tCr) are often elevated in patients with MS (Table 1). Since MI is primarily localized in glia, activated glia show elevated MI, which has been observed in almost all studies of MS patients. Elevated MI (or MI/tCr, MI/NAA, MI/Cho) were found in the corpus calosum and in normal appearing white matter (NAWM) even in patients with relapsing remitting (RR) MS without recent relapses (Kirov et al, 2009), and also in patients with primary progressive and secondary progressive MS (Bagory et al, 2011). One large study of 96 patients with clinically isolated syndrome also found elevated MI in the posterior parietal white matter within 6 months of the CIS onset, and especially if they had abnormal brain MRI at baseline (Fernando et al, 2004). These CIS patients showed elevated MI regardless whether they met McDonald criteria at their 3 months follow-up (Fernando et al, 2004). One study of MS patients with contrast-enhancing (active) lesions also found elevated MI (Srinivasan et al, 2005); however, MI was decreased in an acute tumefactive MS lesion, and became elevated only during recovery (Ernst et al, 1998) (Figure 2). Overall, the elevated MI reflects gliosis and ongoing neuroinflammation and is most prominent in the secondary progressive MS patients (Geurts et al, 2006). Elevated MI also correlated with total brain T2 lesion load in the thalamus and hippocampus of MS patients (Geurts et al, 2006).
Table 1.
Metabolite Abnormalities in Selected Brain Regions Evaluated in Multiple Sclerosis
| Myo-inositol or MI/Cr | NAA or NAA/Cr | Total Creatine or PCr | Choline or Cho/Cr or Cho/NAA | Glutamate, Glu/tCr, Glx or Glx/tCr | |
|---|---|---|---|---|---|
|
| |||||
| Clinically isolated syndrome | ⇑ MI in parietal NAWM | NAA not different than controls | ⇑ tCr in parietal NAWM | normal Cho | Glx not different than control |
| Primary progressive MS | ⇑ MI and MI/tCr in NAWM and lesions | normal NAA in cortex or NAWM but ⇓ tNAA in thalamus; ⇓ tNAA/tCr in NAWM and lesions | ⇑ tCr or normal in NAWM and lesions | ⇑ or nornal Cho and Cho/NAA in NAWM and lesions | Slightly lower Glu or normal |
| Secondary progressive MS | ⇑ MI, MI/tCr, MI/NAA, MI/Cho in NAWM, lesions, thalamus and hippocampus | normal or ⇓ tNAA but ⇓ tNAA/tCr in NAWM and lesions | ⇑ tCr in NAWM | ⇑ Cho/NAA or ⇓ tCho in NAWM and lesions | Slightly lower Glu or normal |
| Relapsing-remitting MS | ⇑ MI or MI/Cr in NAWM and Spinal cord | ⇓ NAA or NAA/tCr in NAWM or whole brain NAA ⇓ NAAor tNAA/Cho and NAA/Cr in the spinal cord |
⇑ tCr in NAWM | ⇑ or normal Cho in NAWM ⇓ Cho/tCr in the spinal cord |
Slightly lower Glu or normal |
| Acute demyelinating lesions | ⇑ acute WM lesions | ⇓ NAA in Brain lesions | ⇓ tCr in WM lesions | ⇑ Cho WM lesions | ⇑ Glu in acute lesions and WM |
| Chronic lesions | ⇓ MI in WM legions | ⇓ NAA in WM lesions | not different | not different | Slightly lower Glu or normal |
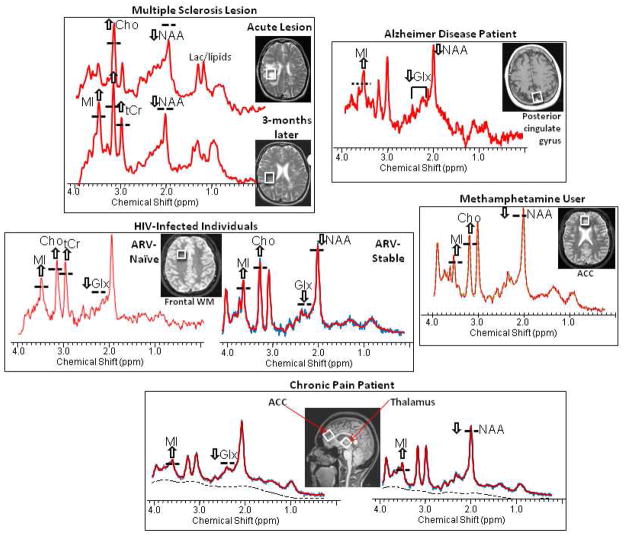
Figure 2.
Representative MR spectra and the MRI showing the voxel location of the spectra from the brains of patients with various neuroinflammatory disorders, including multiple sclerosis, Alzheimer’s disease, HIV-infection, methamphetamine dependence, and a chronic pain syndrome (painful neuropathy). All of these disorders demonstrate elevated glial markers, especially myo-inositol (MI) and less often choline-containing compounds (Cho) and total creatine (tCr). In addition, the neuronal markers, N-acetylaspartate (NAA) and glutamate (Glu), may be decreased also which may be related directly to the disorder, but also may be exacerbated by neuroinflammation.
Although Cho is also higher in glial cells, it is found in cell membranes of all brain cells, and may only be slightly elevated in patients with MS (Vrenken et al, 2005). Except for patients with CIS and in gray matter regions of MS patients, all other subtypes of MS show elevated Cho or Cho/tCr in the NAWM and in the lesions, especially in acute demyelinating lesions. In patients with RR MS, both Cho and tCr may be elevated diffusely in NAWM (Inglese et al, 2003). However, in MS patients with normal appearing MRI scans (i.e., no active disease), Cho was found to be normal (Vrenken et al, 2005) or even decreased in the normal appearing white matter (NAWM) (Gustafsson et al, 2007). Therefore, Cho elevation may also be a useful predictive marker of active disease in the brain of MS patients.
Similar to the brain, the spinal cord of patients with relapsing remitting MS also showed decreased NAA/tCr and increased MI/tCr (Marliani et al, 2010), suggesting neuronal loss and glial activation, but the interpretation of metabolite ratios is difficult since these abnormal findings might be related to elevated tCr also. However, these absolute concentrations of glial markers may be useful objective biomarkers for functional disabilities since levels of MI (Ciccarelli et al, 2007; Gustafsson et al, 2007), Cho (Ciccarelli et al, 2007) and tCr (Ciccarelli et al, 2007), correlated with scores on the Expanded Disability Status Scale (EDSS). These correlations support the notion that neuroinflammation, particularly glial activation, contributes to brain dysfunction as identified through the EDSS.
Depending on the extent of the neuronal damage, NAA levels may be normal in MS patients with primarily demyelination, despite changes in the other metabolites (Bagory et al, 2011). However, some of the neurochemical changes may occur regionally and may not be detected easily with localized MRS if the affected brain region was not assessed. Using whole brain measurements, NAA levels were shown to decrease significantly in patients with remitting relapsing MS (Rigotti et al, 2012). Decreased white matter NAA levels represent neuronal (or axonal) dysfunction, which may lessen with treatment (Kirov et al, 2013).
Another neuronal metabolite that might be altered in MS is glutamate since the majority of the neurons are glutamatergic. Studies using a special MRS sequence (i.e., echo-time-averaged Point Resolved Spectroscopy or TE-averaged PRESS) found elevated levels of glutamate only in acute lesions and normal-appearing white matter, but not in chronic lesions; conversely, NAA was lower in chronic lesions than in acute lesions and normal-appearing white matter (Srinivasan et al, 2005). Therefore, altered glutamatergic metabolism is present in patients with MS and may precede reduction in NAA. Furthermore, recent advances in diffusion tensor spectroscopy (DTS) conducted on higher magnetic field MRS (7 Tesla) showed lower axial diffusion of NAA, which suggests axonal injury (Wood et al, 2012). Together with proton MRS, DTS may provide new approaches to distinguish axonopathy from other processes such as inflammation, edema, demyelination and gliosis.
MRS Studies of Neuroviral Infections
MRS has been applied to study various neuroinfections, but only the two more common viral infections (HIV and HCV) and several opportunistic viral infections (JC virus and CMV) will be discussed below. Table 2 summarizes the neurometabolite abnormalities associated with these neuroviral infections.
Table 2.
Metabolite Abnormalities in Selected Brain Regions Evaluated in Viral Brain Infections
| Viral Infection | Myo-inositol or MI/Cr | NAA or NAA/Cr | Total Creatine | Choline or Cho/Cr or Cho/NAA | Glutamate, Glx, Glu/Cr or Glx/tCr |
|---|---|---|---|---|---|
|
| |||||
| HIV | |||||
| Acute or early HIV infection | ⇑ MI or MI/tCr in Basal Ganglia | ⇓ NAA frontal cortex | not measured | ⇓ Cho, Cho/tCr, Cho/NAA in ACC & frontal WM but normalize or increased after two months; ⇑ Cho/tCr occipital GM | ⇓ Glx in Frontal GM; ⇑ Glx in basal ganglia after 6 months |
| Chronic HIV | ⇑ MI or MI/tCr Frontal GM, WM and Basal Ganglia | ⇓ NAA in white matter and NAA/tCr correlate inversely with CD16+ and CD8+ T cells | normal or mildly elevated frontal WM but may ⇓ in HAD | ⇑ Cho or Cho/tCr Frontal GM, frontal or parietal WM and basal ganglia | ⇓ Glx or Glx/tCr in ACC, frontal WM and parietal GM |
| HAART naïve | ⇑ MI in frontal WM, correlated with higher viral load and lower CD4 | ⇓ Frontal WM NAA only in HAD | ⇑ tCr in frontal WM | ⇑ Cho frontal WM & GM, correlated with CSF viral load | ⇓ Glx in Frontal GM |
| > 6 month HAART | MI or MI/tCr may normalize or remain elevated | ⇓ NAA/tCr Frontal cortex | tCr may normalize | Cho and Cho/tCr may normalize or remain elevated | Glx may normalize in basal ganglia |
| HAND (HAD) | ⇑ MI or MI/tCr frontal GM, WM and Basal Ganglia | ⇓ NAA or NAA/tCr frontal WM & basal ganglia | normal or elevated in WM | normal of elevated in WM | ⇓ Glu in Frontal & parietal GM |
| HAND (MND) | ⇑ MI or MI/tCr cortical GM | mild ⇓ NAA or NAA/tCr White matter & basal ganglia | normal or mildly elevated frontal WM | normal of mildly elevated in WM | ⇓ Glu in Frontal & parietal GM; ⇓ Glx/tCr Frontal WM |
| Neuro-asymptomatic | ⇑ MI or MI/tCr in frontal WM | normal in frontal cortex, WM and basal ganglia | normal in frontal cortex, WM and basal ganglia | normal in frontal cortex, WM and basal ganglia | ⇑ Glu Basal ganglia; ⇓ Glx/tCr Frontal WM |
| Hepatitis C | ⇑ MI/tCr Basal ganglia | ⇑ NAA Basal ganglia but ⇓ NAA/tCr CSO | ⇑ tCr Basal ganglia | ⇑ Cho in basal ganglia and parietal WM | ⇑ Glx in parietal WM |
| HCV without liver disease | normal MI/tCr | ⇓ NAA/tCr in PCG | not assessed | normal Cho/tCr | not assessed |
| HIV/HCV Co-infection | ⇑ MI/tCr Basal ganglia | ⇓ NAA or ⇓ NAA/tCr in frontal WM and parietal WM | not assessed | not different from control | not assessed |
| HCV post treatment | ⇓ MI/tCr (normalize) in Basal ganglia | no change | not assessed | ⇓ Cho/tCr (normalize) in Basal ganglia; Persistent ⇑ Cho/tCr in nonresponders | not assessed |
| Opportunistic viral infection | |||||
| PML (JC Virus) | ⇑ MI or MI/tCr in WM lesions only in those who recovered | ⇓ NAA, NAA/tCr or NAA/Cho in WM lesions | ⇓ tCr in lesions | Cho or Cho/tCr ⇑ WM lesions | ⇓ Glx in lesions and NAWM |
| Cytomegalovirus | Slightly ⇑ MI in WM lesions | ⇓ NAA in WM lesions | not different from control | ⇓ Cho in WM lesions | not assessed |
ACC = anterior cingulate cortex
PCG = posterior cingulate cortex
CSO = centrum semiovale
Human Immunodeficiency Virus (HIV)
Since the AIDS epidemic began in 1981, nearly 30 million people have died of AIDS-related causes worldwide. Approximately 34 million people are currently living with HIV, with an estimated 1.2 million infected in the U.S. Similar to MS, neuroinflammation is a neuropathological hallmark of HIV-infection. The virus enters the brain early during the infection and develops viral reservoirs in the brain, while reactive glial activation along with ongoing viral activity propagates the neuroinflammatory processes. MRS has been applied to evaluate brain inflammation and neuronal injury in HIV patients since the 1980’s; see Review (Chang, 2012). Similar to MS patients with acute demyelination or active disease, acutely infected HIV patients (averaged 15 days post exposure) already showed elevated ratio of Cho/tCr in the occipital gray matter along with elevated CSF neopterin, a neuroinflammatory protein indicative of macrophage activation (Valcour et al, 2012). Elevated Cho also may reflect cell membrane injury. In longitudinal studies of acutely infected HIV patients, Cho and Cho/ tCr or Cho/NAA ratios were found to be lower than those in seronegative controls initially, but may increase or normalize with treatment, and the Cho levels may remain elevated or become higher than normal after 3–6 months of ARV treatment (Chang et al, 2004a; Lentz et al, 2011). Similarly, the glial marker MI (or MI/tCr), along with Cho (or Cho/tCr), were typically elevated in chronically HIV-infected individuals, and were found in frontal gray or white matter, and basal ganglia regions (Chang et al, 2004b; Harezlak et al, 2011; Letendre et al, 2011). In antiretroviral naïve HIV-infected individuals, plasma and CSF viral loads typically correlated with these glial markers (MI, Cho) (Chang et al, 2002); however, these correlations are seldom found in those well maintained on stable combination antiretroviral therapy since the majority of these HIV infected individuals would have undetectable levels of virus. Furthermore, in HIV patients effectively viral suppressed with highly active antiretroviral treatment (HAART), the initially elevated Cho and MI (Figure 2) typically normalize approximately 6 months after treatment, suggesting lesser neuroinflammation (Chang et al, 1999c; Tarasow et al, 2004).
Neuronal injury associated with HIV brain infection may lead to lower levels of NAA or NAA/tCr. Lower NAA was found primarily in the frontal white matter and basal ganglia of chronically infected HIV patients, and only in those with more severe HIV-associated dementia (Chang et al, 1999b; Chang et al, 2004b). However, more recent studies of antiretroviral stable HIV patients who had been infected for longer durations found reduced NAA in the white matter (Schweinsburg et al, 2005; Chang et al, 2006) or in the basal ganglia (Mohamed et al, 2010), even in those with milder forms of HIV-associated neurocognitive disorders (HAND). Lower NAA/tCr was also found in those chronically infected with only mild cognitive problems, with AIDS dementia complex stage 0.5 (Harezlak et al, 2011), see also Figure 2. One study showed improvements in the NAA/tCr ratios after neuroprotective treatment with memantine (Schifitto et al, 2007); the increased ratio of NAA/tCr after treatment may reflect reversible neuronal injury or it may in fact be due to normalization of the initially elevated tCr associated with the lesser neuroinflammation or glial activation.
Also similar to MS, brain glutamate levels may be altered in HIV-infected individuals with early stages of neuronal injury. HIV-infected individuals, especially those with milder forms of HAND, had normal NAA but lower levels of parietal and frontal gray matter glutamate (Ernst et al, 2010) or higher glutamate+glutamine (Glx) in the frontal white mater (Mohamed et al 2010), and these individuals typically also had higher levels of the glial marker MI. Similarly, a multicenter study also found decreased Glx/tCr with normal NAA/tCr in the frontal white matter of neuroasymptomatic HIV patients (Harezlak et al, 2011); these findings likely reflect lower Glx and higher tCr. Lower levels of Glu or Glx, along with elevated MI and tCr, indicate that neuroinflammation likely contributed to the excitotoxic glutamatergic injury, since activated glia may be unable to reuptake the released glutamate from the extracellular space (Ernst et al, 2010; Mohamed et al, 2010). The lower brain glutamate levels were also associated with greater number of nucleoside reverse transcriptase inhibitors (NRTIs) used (Ernst et al, 2010) and poorer cognitive performance in the HIV patients in both of these studies (Ernst et al, 2010; Mohamed et al, 2010). These findings suggest that use of NRTIs may lead to greater neuroinflammation, which in turn may lead to cognitive deficits.
To further evaluate the relationships amongst cellular and soluble markers of inflammation and pathophysiological processes in the brains of HIV patients, several studies measured cytokine levels in relation to the MRS markers. In antiretroviral-naïve HIV patients, 3- months of highly active antiretroviral therapy (HAART) did not lead to significant changes in metabolites measured with MRS, despite marked improvement on systemic variables (CD4, viral load, macrophage chemotactic protein or MCP-1) and CSF variables (viral load, MCP-1) (Chang et al, 2003). However, CSF MCP-1, but not serum MCP-1, correlated inversely with the neuronal component before HAART and positively with the glial component after HAART (Chang et al, 2004a). These findings suggest that HAART led to persistent activation of glia (with persistent elevation of MI in the brain) that might have been beneficial to the patients after 3 months. These findings are consistent with another study that found higher MCP-1in HIV patients with lower NAA/Cr in the FWM and parietal cortex, while interferon gamma-induced protein 10 (IP-10) correlated with lower neuronal pattern scores and higher basal ganglia and inflammatory pattern scores (Letendre et al, 2011). Another study of HIV patients during the first year of their infection found lower NAA and higher Cho levels that correlated with increased CD16+ monocytes (Lentz et al, 2011), which belong to the monocyte subset that traverses the blood brain barrier to disseminate HIV in the brain (Williams et al, 2012). Similarly, lower NAA in the frontal cortex correlated with higher CD8+ T cells, specifically effector T cells (Lentz et al, 2009), which are neurotoxic during HIV-infection and are thought to contribute to HIV-related neurodegeneration (Liu et al, 2009). These studies demonstrate that MRS markers are useful for monitoring treatment effects and can be used to non-invasively assess how both central and peripheral inflammatory mediators might impact the brain.
Hepatitis C Virus (HCV) Infection
HCV is more prevalent than HIV, with an estimate of 3% (170 million) of the world’s population and approximately 2.7 million Americans being chronically infected with the virus. HCV often co-infects those with HIV infection since the route of transmission is similar in the high risk population (i.e., high risk sexual behavior and illegal drug use). Up to 50% of HCV infected patients have symptoms of fatigue, depression and variable degrees of cognitive deficits, affecting attention, learning and memory, that are independent of the viral load or clearance of the virus after antiretroviral treatment (Pattullo et al, 2011). Post-mortem studies of patients with HCV indicate that the brain might act as a separate compartment for viral replication, and microglia may be the locus for infection and subsequent neuroinflammatory activity (Forton et al, 2004). Individuals infected with HCV indeed showed evidence of neuroinflammation on MRS, such as elevated Cho/tCr in the basal ganglia and white matter compared to healthy controls and to those with hepatitis B, and these cerebral metabolite abnormalities were independent of hepatic encephalopathy or a history of intravenous drug abuse (Forton et al, 2001). Microglia activation was also demonstrated on PET imaging with increased update of (11) C-(R)-PK11195 tracer in the caudate and thalamus, especially in those with HCV genotype-1 infection (Grover et al, 2012). The same individuals who had the elevated microglial marker radiotracer uptake also showed elevated MRS glial markers MI/tCr and Cho/tCr in the basal ganglia (Grover et al, 2012), which further confirmed that these makers are related to neuroinflammation with microglial activation. Similar to studies in patients with HIV, HCV patients who had higher levels of the glial marker MI/tCr also showed more cognitive deficits, such as slower working memory reaction times (Forton et al, 2008). HCV infected patients also showed elevated Cho/tCr in the BG (Forton et al, 2002; Grover et al, 2012) and in the white matter (Forton et al, 2002), indicating presence of glial activation in these brain regions. In a one-year follow-up study, those who responded to antiretroviral treatments and had viral clearance showed further elevation of Cho/tCr that did not correlate with improvement in cognitive function (Pattullo et al, 2011). However, the increased Cho/tCr may be due to either further elevation of Cho from glial activation and/or acute cell membrane injury, or to reduced tCr which would reflect decreased inflammation. Again, changes in metabolite ratios are often difficult to interpret.
Several studies found lower NAA/tCr in the basal ganglia (Grover et al, 2012) and lower NAA in the centrum semiovale (McAndrews et al, 2005) of patients with HCV infection, which suggested neuronal injury or loss in these brain regions. The lower NAA/tCr was also found in the occipito-parietal cortical region of HCV patients with no signs of liver disease, but had neuropsychiatric symptoms, such as fatigue, depression, anxiety and cognitive deficits (Weissenborn et al, 2004). Therefore, HCV may lead to brain injury or neuroinflammation independent of the liver. In contrast, higher than normal NAA concentrations, along with elevated Cho and tCr, were observed in the basal ganglia of patients with HCV, which indicate ongoing neuroinflammation such as glial activation and macrophage infiltration (Bokemeyer et al, 2011). The investigators also found an inverse correlation between fatigue and the brain metabolites, suggesting that the neuroinflammation may be a compensatory response. Two studies evaluated individuals with HIV and HCV co-infection; one study found only a trend for further elevation of MI/tCr in the basal ganglia (Garvey et al, 2012) and the other study found lower NAA/tCr in the ACC of those who were co-infected (Bladowska et al, 2013). The variable findings across studies may be due to differences in the subject characteristics (e.g., age, sex, disease severity) and the relatively small sample size in some of the studies.
After treatment with pegylated interferon/ribavirin, the MRS glial marker ratios (MI/tCr and Cho/tCr) in the basal ganglia decreased more in the virological responders compared to the non-responders or relapsers, indicative of reduced viral infection and neuroinflammation after effective response to the treatment (Byrnes et al, 2012). Furthermore, the responders showed significant improvements in verbal learning, memory, and visuo-spatial memory, highlighting the beneficial effects of eradicating HCV in the brain to prevent ongoing neuroinflammation and cognitive impairment (Byrnes et al, 2012).
MRS in Opportunistic Brain Lesions (Table 2)
Specific neurochemical profiles of the metabolites measured on MRS may be used to differentiate brain lesions due to toxoplasmic abscesses, progressive multifocal leukoencephalopathy (PML), CNS lymphoma and cryptococommas (Chang et al, 1995). PML is often a fatal demyelinating brain disease caused by the JC virus and remains to be a significant opportunistic infection in patients infected with HIV, as well as those who receive immunosuppressive therapy such as the humanized monoclonal antibody therapy for autoimmune inflammatory diseases (e.g., MS and Crohn’s disease). Several MRS studies of PML patients found characteristic metabolite profiles, with lower NAA levels, higher glial metabolites (MI, Cho, tCr) and lipids levels, as well as presence of lactate, in acute demyelinating white matter lesions relative to contralateral normal appearing white matter and to healthy controls (Chang et al, 1997a; Iranzo et al, 1999; Yoon et al, 2007). These neurometabolite abnormalities are consistent with neuropathology that found neuronal loss, cell membrane and myelin breakdown, and increased glial activity in PML lesions. Some of these neurochemical profiles may change during disease progression or recovery. For instance, longitudinal follow up of PML patients showed progressive changes in the glial marker MI, which was highest in those with the longest survival (Chang et al, 1997a). Other opportunistic viral infections of the brain, such as cytomegalovirus (CMV), may show similar cerebral metabolite abnormalities. One study found lower NAA and Cho levels in the parietal white matter, with slightly elevated glial marker MI, in the brains of children infected congenitally with CMV (van der Voorn et al, 2009).
Neurodegenerative Diseases
The different primary neurodegenerative disorders each have characteristic clinical syndromes and pathological hallmarks; however, many of these disorders are associated with significant neuroinflammation. The typical neuroinflammatory processes in these disorders include microglial and astroglial activation, which indicate the brain’s response to neurotoxic processes or to the neuronal degeneration associated with each of these diseases. However, the strong glial responses may become aberrant and further contribute to brain injury. MRS can assess both the glial response and the neuronal injury in these conditions, and may be useful for monitoring resolution of neuroinflammation during treatments. Table 3 shows the neurometabolite abnormalities found in patients with Alzheimer’s disease, frontotemporal dementia and Parkinson’s disease; some of the studies will be discussed below.
Table 3.
Metabolite Abnormalities in Selected Brain Regions Evaluated in Degenerative Brain Disorders
| Disorder | Myo-inositol, MI/tCr or syllo-inositol | NAA or NAA/tCr | Creatine or PCr | Choline or Cho/tCr or Cho/NAA | Glutamate, Glu/Cr, Glx or Glx/tCr | GABA |
|---|---|---|---|---|---|---|
|
| ||||||
| Alzheimer’s disease | ⇑ MI or MI/tCr in Parietal (GM > WM) and temporoparieta l region | ⇓ NAA and NAA/tCr in Parietal GM > WM; ⇓ NAA/tCr in hippocampus and anterior temporal lobe (correlated with MMSE, CDR, and clock drawing test) | ⇓ tCr in PCG and in whole brain GM | no change in PCG or whole brain GM; ⇓ Cho/tCr in Hippocamp us | ⇓ Glu/tCr in PCG and hippocampus | not assessed |
| MCI (including amnestic) | ⇑ syllo-inositol/tCr and MI/tCr in PCG | NA/tCr not different from controls | normal in PCG | no change | ⇓ Glu in PCG, but normal Glu in hippocampus | not assessed |
| Mild AD | ⇑ Temporo-parietal; ⇑ syllo-inositol/tCr and MI/tCr in PCG | ⇓ or NAA or NAA/tCr in PCG | ⇓ tCr in PCG | slightly lower Cho in temporal pole | ⇓ Glu in PCG | not assessed |
| AD with behavioral symptoms | ⇑ MI/tCr in ACC orrelated with worse behavior; PCG MI/tCr correlated with poorer cognition; | ⇓ NAA/tCr in ACC correlated with worse behavior, PCG NAA/tCr correlated with poorer cognition | probably ⇓ tCr in PCG | not different | not assessed | not assessed |
|
| ||||||
| Frontotempor al dementia | ⇑ MI and MI/tCr ACC and temporoparietal | ⇓ NAA and NAA/tCr in ACC | not different from controls | not different from controls | ⇓ Glx in ACC | not measured |
| Parkinson’s disease (PD) | not assessed | Normal or ⇓ NAA in Sustantia nigra; normal or ⇓ NAA/tCr in PCG and basal ganglia; ⇓ NAA/tCr in occipital cortex | ⇓ tCr Sustantia nigra | ⇑ Cho Sustantia nigra | ⇓ Glu Sustantia nigra | ⇑ GABA/rC r in putamen and pons |
| PD (normal cognition) | not assessed | normal NAA/tCr in PCG; ⇓ NAA/tCr Occipital lobe | not assessed | normal Cho/tCr | ⇓ Glu/tCr PCG | not assessed |
GM = gray matter; WM = white matter
MCI = mild cognitive impairment
PCG = posterior cingulate gyrus
Alzheimer’s disease (AD)
AD is the most common neurodegenerative disorder in the aging population, affecting 5.4 million Americans and another 10 million in the coming decade due to the aging “baby boomers”. Although the etiology of AD is still unknown, numerous postmortem studies and structural brain imaging studies consistently documented specific neurodegenerative changes and patterns of brain atrophy in patients with AD. The neurodegenerative changes are partly due to neurotoxic misfolded proteins that aggregate in the amyloid plaques in the brain parenchyma (Hardy and Allsop, 1991). On neuropathology, in addition to the hallmark findings of beta-amyloid plaques and neurofibrillary tangles from the abnormally phosphorylated tau proteins, neuronal loss and reactive gliosis including microglial activation are well documented. In postmortem studies, NAA levels measured with ex vivo NMR spectroscopy correlated negatively with the number of senile plaques and neurofibrillary tangles in the adjacent brain tissue of Alzheimer patients (Klunk et al, 1992). Therefore, in vivo MRS in AD patients may assess the severity of the neuropathology.
Brain regions particularly vulnerable or affected include the posterior cingulate gyrus (PCG), along with the basal medial temporal limbic areas, inferior parietal lobule and the inferior temporal gyri (Brun and Englund, 1981). Hence, early studies of proton MRS of Alzheimer patients performed localized measurements in the parietal, typically PCG, or temporoparietal regions (Miller et al, 1993; Moats et al, 1994; Ernst et al, 1997). Most MRS studies consistently demonstrated neuroinflammatory changes with elevated glial marker MI or MI/tCr and lower levels of neuronal marker NAA or NAA/tCr in patients with Alzheimer’s disease, especially in the parietal regions (Moats et al, 1994; Ernst et al, 1997; Griffith et al, 2008a), see Figure 2, but also in the temporoparietal region (Ernst et al, 1997; Rami et al, 2007) and in the hippocampus (Jessen et al, 2009).
Patients with AD and those with mild cognitive impairment (MCI) both showed signs for neuronal dysfunction or loss with lower levels of NAA or NAA/tCr, as well as lower levels of tCr and Glu, in the PCG or in the temporoparietal cortex (Rami et al, 2007). The lower NAA or NAA/tCr in AD patients typically correlated with more advanced stages of dementia on the clinical dementia rating (CDR) (Ernst et al, 1997), and poorer cognitive performance on the Mini-Mental State examinations (MMSE) (Frederick et al, 2004), on the clock drawing test (Griffith et al 2007) and story recall test (Shinno et al, 2007).
Signs for neuroinflammation with elevated MI or MI/tCr were consistently found in subjects with MCI, a precursor to AD (Griffith et al, 2007), as well as in those with the clinical diagnosis of AD (Miller et al, 1993; Ernst et al, 1997; Rami et al, 2007). Furthermore, elevated scyllo-inositol (sI)/tCr was reported in the PCG of AD patients, both in those with mild AD and in those with amnestic MCI (Griffith et al, 2007). Similar to MI, sI is an inositol stereoisomer capable of binding Aβ42 and attenuating Aβ42 neurotoxicity (McLaurin et al, 2000). Therefore, elevated MI or MI/tCr in AD patients may reflect more than glial or microglial activation, possibly a response to Aβ42 aggregates. Similar to the correlation between neuronal injury and decreased NAA or NAA/tCr, higher levels of MI, MI/tCr or sI/tCr correlated with more advance disease, as assessed by the CDR (Ernst et al, 1997) and by the clock drawing test (Griffith et al, 2007). AD patients with higher MI/tCr in the ACC also had more behavioral symptoms, including delusional thoughts and activity disturbances (Shinno et al, 2007).
Furthermore, studies that used absolute quantification methods and carefully chosen voxel locations showed MRS to be useful for differential diagnoses between the different dementia syndromes. For example, patients with frontotemporal dementia showed significantly reduced NAA and Glx levels and markedly elevated glial marker MI in the frontal cortex, while patients with early AD showed relatively normal metabolites in the frontal region and only mildly elevated MI in the temporoparietal region (Ernst et al, 1997). Furthermore, AD patients showed lower than normal levels of hippocampal Glu, Glu/tCr, Glu/mI and Glu/NAA, and lower NAA/tCr, as well as lower Glu/mI in AD compared to those with MCI (Rupsingh et al, 2011). Therefore, similar to the lower brain glutamate levels in other neuroinflammatory disorders discussed above, decreased intracellular glutamate may result partly from neuroinflammation, and may be a sensitive early marker for brain injury.
Changes in NAA/tCr were used as a primary outcome measure to evaluate the efficacy of memantine in mildly demented AD patients (Ashford et al, 2011). Another recent study evaluated brain metabolite changes after 4 months of treatment with galantamine, a cholinesterase inhibitor and allosteric potentiating ligand modulating presynaptic nicotinic acetylcholine receptors. The levels of Glu, Glu/Cr, and Glu/NAA all increased in the hippocampal region after the treatment with galantamine, and the increases correlated with improvements on the MMSE and on the ADAS-cog scores, although no significant changes in MMSE or ADAS-cog scores were detected in this small sample of AD patients (Penner et al, 2010). Therefore, MRS may provide useful surrogate biomarkers to monitor treatment effects in AD.
Parkinson’s disease (PD)
PD is estimated to affect seven to 10 million people worldwide, and is 1.5 times more prevalent in men than women. The etiology of sporadic PD also remains unknown; however, both the clinical syndrome and neuropathological studies demonstrated that the dopaminergic system is particularly vulnerable with loss of 50–80% of nigra neurons in the symptomatic patients. Degenerating dopaminergic neurons show the pathognomonic Lewy bodies, which are intracytoplasmic filamentous inclusions containing abnormally ubiquinated alpha-synuclein proteins (Tofaris et al, 2003). These aberrantly aggregated proteins in turn activate microglia to release inflammatory cytokines and other products that further propagate the neuroinflammation (Mogi et al, 1994), as well as the proinflammatory innate response (Zhang et al, 2005). Therefore, MRS may be useful in detecting neurometabolite abnormalities that would reflect neuronal loss and neuroinflammation in the brains of PD patients. Table 3 shows the brain metabolite abnormalities reported in patients with PD.
Although the neuropathology of PD involves dopamingergic neurons, which have their cell bodies in the substantia nigra, not all the MRS studies evaluated this brain region. Two earlier small studies that evaluated the substantia nigra found lower tCr (O’Neill et al, 2002) and trends for lower Glu, NAA, and GSH, and higher Cho (Oz et al, 2006) in PD patients compared to controls. Other small studies that evaluated the posterior cingulate gyrus found either normal NAA/tCr (Griffith et al, 2008c) or lower NAA/tCr (Camicioli et al, 2004; Oz et al, 2006), which correlated with dementia severity as assessed by the MMSE (Griffith et al, 2008b). A more recent large study of PD patients with normal cognition (n=70) or mild cognitive impairment (n=66) found normal NAA/tCr levels in the substantia nigra and basal ganglia, but lower NAA/tCr in the occipital lobe (Nie et al, 2013). Another study of PD patients with normal cognitive function also found normal NAA/tCr but lower ratio of Glu/tCr in the posterior cingulate gyrus (Griffith et al, 2008c). Using TE-averaged PRESS, which can provide more accurate assessment of Glx (mainly glutamate with minimal contributions of glutamine, NAA and GABA), the ratio of Glx/water was found to be comparable in the lentiform nuclei of PD patients and healthy controls (Kickler et al, 2007). This discrepancy between the two studies that evaluated Glu/tCr and Glx/water was likely due to the different brain regions assessed (PCG vs. lentiform nucleus) and the different sensitivities of the methods used for the measurements (PRESS vs. TE-averaged PRESS). The lower Glx/tCr in the first study may be due to either lower Glu or higher tCr, or both, reflecting greater than normal age-related changes, since Glu decreases and tCr increases with age (Chang et al, 2009). However, studies that evaluated tCr levels in PD patients actually found lower tCr in the substantia nigra (O’Neill et al, 2002) and a trend for lower tCr in the posterior cingulate gyrus (Kickler et al, 2007). These findings again highlight the difficulty of interpreting metabolite ratios, and that tCr may vary in disease conditions and should not be used as a reference metabolite. Using ultra-high field MRS (at 7 Tesla), PD patients with mild to moderate dementia showed elevated GABA levels, more so in the pons (+64%) than in the putamen (+32%), but no other neurometabolites abnormalities (Emir et al, 2012). These findings are consistent with prior postmortem studies (Kish et al, 1986) and an animal model of PD (Chassain et al, 2010) that found elevated GABA in the striatum, suggesting the role of GABAergic regulation of non-dopaminergic neurons of the pons in the pathophysiology of PD.
Taken together, the majority of MRS studies in PD patients found signs for neuronal loss, primarily in brain regions other than the substantia nigra, especially in PD patients with more cognitive symptoms. These MRS studies also found minimal signs for neuroinflammation, since only lower tCr and trends for elevated Cho were observed. Despite the pathological findings of microglial activation in selected brain regions, none of the studies found abnormalities in the glial marker myo-inositol.
MRS in Stimulant Abusers
Stimulants, such as methamphetamine (Meth) or cocaine, can lead to oxidative stress, dysregulation of neurotrophic factors, excitotoxicity, and neuroinflammation in preclinical studies (Yamamoto et al, 2010). Table 4 shows neurometabolites abnormalities reported in individuals who abused or were dependent on the various stimulants, including Meth, cocaine, “Ecstasy”, and those with co-morbid conditions of HIV-infection and Meth abuse. Several MRS studies of Meth users indeed showed signs of neuroinflammation, with elevated glial markers of myo-inositol and Cho in frontal cortex or white matter (Ernst et al, 2000; Sung et al, 2007) (Figure 2). The neuronal marker NAA or NAA/tCr was often found to be reduced in various brain regions, including the anterior cingulate cortex (ACC), frontal white matter, basal ganglia and even the occipital cortex of Meth users (Ernst et al, 2000; Nordahl et al, 2002; Chang et al, 2005a). Furthermore, the levels of NAA or NAA/tCr were typically lower in those who used greater cumulative amounts of Meth or shorter duration of abstinence (Ernst et al, 2000; Sung et al, 2007). However, individuals with sustained abstinence may show normalization of NAA/tCr in the ACC (Salo et al, 2011) but higher levels of Cho in the ACC and higher MI in the basal ganglia (Taylor et al, 2007). Another neuronal metabolite, glutamate plus glutamine (Glx), was also found to be decreased in Meth subjects during early abstinence, but Glx may normalize with sustained abstinence (Ernst and Chang, 2008). Taken together, these findings indicate significant neuroinflammation associated with chronic Meth use, especially during active usage of the drug, and that the neuronal injury or dysfunction associated with chronic Meth abuse may normalize but the neuroinflammatory changes may persist for some time with cessation of drug abuse.
Table 4.
Metabolite Abnormalities in Selected Brain Regions Evaluated in Stimulant Users
| Disorder | Myo-inositol or MI/Cr | NAA, NAA/tCr or NAA/Cho | Total Creatine | Choline or Cho/Cr or Cho/NAA | Glx, Glx/Cr or Glu | GABA |
|---|---|---|---|---|---|---|
|
| ||||||
| Methamphet amine (Meth) | ⇑ MI in ACC and frontal WM, and in basal ganglia of longer usage | ⇓ NAA or NAA/tCr in ACC, basal ganglia and frontal WM | ⇓ tCr and tCr/Cho in Basal ganglia | ⇑ Cho and Cho/NAA in ACC | not measuerd or not different in ACC, BG & FWM | not measured |
| Meth (early abstinence < 6 months) | ⇑ MI in ACC and frontal WM | ⇓ NAA/tCr in ACC | not measured | ⇑ Cho/NAA in ACC | ⇓ Glx in ACC | not measured |
| Meth (sustained abstinence) | not assessed | NAA/tCr normal in ACC | not different in ACC and visual cortex | Normal Cho/N AA in ACC | Glx normal in ACC | not measured |
| Meth+HIV | ⇑⇑ MI frontal WM | ⇓⇓ NAA in frontal WM, ACC and basal ganglia | ⇓⇓ tCr Basal ganglia | ⇑⇑ Cho in FWM | not measured | not measured |
| Cocaine | ⇑ MI in FWM & ACC | ⇓ NAA in ACC; ⇓ NAA & NAA/tCr in DLPFC and thalamus | ⇑ tCr in FWM of AA men | ⇓ Cho/tCr in thalamus | ⇓ Glu/tCr in prefrontal cortex | lower GABA in prefrontal cortex |
| Cocaine users (drug administratio n studies) | not measured | 0.4 mg/kg cocaine led to sustained increases in NAA in Basal ganglia | not measured | 0.4 mg/kg cocaine led to sustained increases in Cho in Basal ganglia | ⇑ Glu in Left dorsal ACC, decreased after 2400 mg of NAC | not measured |
| MDMA | ⇑ MI in Parietal WM and MI correlated with usage | ⇓ NAA/tCr and NAA/Cho in ACC | no difference in parietal WM, Occipital GM & ACC | no difference in parietal WM, Occipital GM & ACC | no difference in parietal WM, Occipital GM & ACC | not measured |
AA = African American
MDMA = methylene-dioxy-methamphetamine
NAC = N-acetylcysteine
Similarly, chronic cocaine abuse may lead to significant neuroinflammation in the brain. Chronic cocaine users showed elevated glial markers, MI and tCr, in the frontal white matter and those who used the drug longer or were female had even higher glial marker MI (Chang et al, 1997b; Chang et al, 1999d). Although preclinical studies indicate that acute administration of cocaine is not neurotoxic, chronic cocaine users showed lower NAA in the frontal brain regions (Chang et al, 1999d; Meyerhoff et al, 1999) and thalamus (Li et al, 1999), suggesting neuronal injury or dysfunction which may be related to the ongoing neuroinflammation. On TE-averaged PRESS, cocaine users also showed lower than normal Glu/tCr in the prefrontal cortex that correlated with years of cocaine use (Yang et al, 2009). The lower Glu/tCr may reflect decreased reuptake of glutamate by activated glia, as well as elevated tCr concentrations that were observed in frontal lobes of cocaine users (Chang et al, 1997b; Chang et al, 1999d).
Another commonly abused psychostimulant is 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”). Chronic users of MDMA also showed elevated glial metabolites MI and MI/tCr levels in the frontal and parietal regions without evidence for neuronal injury (Chang et al, 1999a). However, other investigators found lower ratios of NAA/tCr and NAA/Cho in the ACC that correlated with greater lifetime MDMA use (Reneman et al, 2002) and poorer performance on verbal memory (Reneman et al, 2001), and suggested associated neuronal dysfunction. However, these lower ratios also may reflect neuroinflammation with elevated tCr and Cho instead.
Psychostimulants are commonly abused amongst HIV-infected individuals, and both stimulants and HIV may be associated with neuronal injury and neuroinflammatory changes. MRS of HIV-infected individuals who abused Meth indeed showed additive effects on the decreased neuronal maker NAA in ACC, frontal white matter and basal ganglia regions, decreased tCr in basal ganglia and elevated glial marker MI in the frontal white matter (Chang et al, 2005b). However, another MRS study did not find such additive effects between Meth and HIV (Taylor et al, 2007), which might be due to differences in subject characteristics (e.g., duration of Meth abstinence, viral burden or antiretroviral treatment status) between the two studies. No data are available on the additive or interactive effects of cocaine or MDMA with HIV on brain metabolites.
MRS in Chronic Pain Syndromes
The perception of pain in the brain is mediated through the spinothalamic pathway and may be modulated by higher cortical brain regions, such as the ACC. Recent studies also indicate that chronic pain may be mediated or perpetuated by neuroinflammation, such as microglial activation and autoantibodies in those with chronic regional pain syndrome (Blaes et al, 2004; Alexander et al, 2007). Such neuroinflammation has been shown on PET, with increased uptake of the carbon-11(R)-PK11195 tracer, a marker of activated microglia, in the thalamus of chronic pain patients with limb denervation or spinal cord injury, including those who had the injuries many years earlier (Banati, 2003). However, this PET tracer is not readily available in the routine clinical setting. Therefore, objective biomarkers such as neurometabolites measured with MRS in the thalamic or the ACC may be more accessible for evaluating neuroinflammation associated with pain syndromes (Figure 2). Only a small number of studies have used MRS to evaluate chronic pain syndromes. Table 5 shows that patients with chronic pain from various etiologies (low back pain, complex regional pain syndrome or CRPS, painful diabetic neuropathy, migraines, trigeminal neuralgia, spinal cord injury, fibromyalgia and temporomandibular disorder) commonly have elevated glial marker MI and/or lower levels of the neuronal metabolites, NAA or NAA/tCr and tCr, glutamate or Glx/MI. These metabolite alterations have been found in the thalamus, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC) and the insula.
Table 5.
Metabolite Abnormalities in Selected Brain Regions Evaluated in Chronic Pain Syndromes
| Disorder | Myo-inositol or MI/tCr | NAA or NAA/tCr | Total Creatine | Choline or Cho/tCr or Cho/NAA | Glutamate, Glu/tCr, Glx or Glx/tCr | GABA |
|---|---|---|---|---|---|---|
|
| ||||||
| Low back pain | not different | ⇓ NAA/tCr in DLPFC | not measured | not different from controls | not different | not measured |
| Chronic Regional Pain Syndrome | ⇑ MI/tCr in L orbitofrontal | ⇓ NAA/tCr in contralateral Thalamus and bilateral DLPFC | not measured | not different from controls | not measured | not measured |
| Diabetic neuropathy with pain | not measured | ⇓ NAA in thalamus; normal NAA in ACC | ⇓ tCr DLPFC | not different from controls | not measured | not measured |
| Migraine with aura | not measured | ⇓ NAA/Cho and NAA/tCr in Thalamus | not measured | not different from controls | not measured | not measured |
| Trigeminal Neuraglia | not measured | ⇓ NAA/Cho and NAA/tCr in Thalamus | not measured | not different from controls | not measured | not measured |
| Spinal cord injury (with pain or high psychosocial impact) | ⇑ MI in thalamus and ACC | ⇓ NAA in thalamus; normal NAA but ⇓ NAA/Ins in ACC | ⇑ tCr in ACC | ⇑ Cho in ACC | ⇓ Glx/Ins ACC | not measured |
| Fibromyalgia | no correlation with pain | no correlation with pain | not measured | no correlation with pain | ⇑ Glx in insula; change in Glu/tCr and Glx/tCr in isula correlated inversely with pain | ⇓ in insula |
| Temporo-mandibular disorder | not measured | ⇑ NAA in posterior insula | not measured | ⇑ Cho in posterior insula | ⇑ Gln in Right insula; ⇓ Gln in left insula after pain testing | not measured |
Earlier studies of chronic pain syndromes focused on evaluating neurometabolite abnormalities in the thalamus, and found that lower neuronal marker NAA and higher glial marker MI correlated with pain intensity in patients with spinal cord injury (Pattany et al, 2002). These findings suggest that neuronal dysfunction of inhibitory neurons (decreased NAA), possibly as a result of deafferentation, and glial activation (elevated MI) in the thalamus may mediate neuropathic pain. Likewise, lower than normal levels of NAA were reported in the thalamus of patients with CRPS (Fukui et al, 2006) and diabetic patients with chronic neuropathic pain (Sorensen et al, 2008). Lower than normal ratios of NAA/Cho and NAA/tCr in the thalamus were also found in patients with migraine with aura and in those with trigeminal neuralgia (Gu et al, 2008). Therefore, reductions in NAA, and possibly elevated glial metabolites Cho and tCr, in the thalamus may play important roles in neuropathic pain.
More recent studies focused on the role of other cortical brain regions on the perception of pain; therefore, brain regions such as the ACC and the insula were evaluated. Elevated glial metabolites (MI, tCr and Cho) and lower neuronal metabolites (NAA and Glx) were found in the ACC of chronic pain patients with spinal cord injury, especially those with high psychosocial impact, suggesting neuroinflammation along with decreased glutamatergic metabolism (Widerstrom-Noga et al, 2013). However, others found elevated Glx/tCr in various brain regions of chronic pain patients with fibromyalgia (Harris et al, 2008; Harris and Clauw, 2012). Interestingly, glutamate levels also may vary depending on the pain severity or with treatment. For example, Glu and Gln levels in the ACC were elevated proportionately to the perceived pain after an acute painful stimulus in healthy individuals (Mullins et al, 2005) while decreased Glx/tCr levels in the insula also correlated with decreased pain response in patients with fibromyalgia who received acupuncture (Harris et al, 2008) and after treatment with pregabalin (Harris and Clauw, 2012). Another small study of patients with temporomandibular disorder also found no difference in Glu but elevated Gln in the right insula in all patients; however, those with greater pain response to pressure-pain testing had lower levels of Gln (Gerstner et al, 2012). These altered Glu or Gln levels are consistent with preclinical models that implicate the role of glutamate in mediating chronic pain through central sensitization of both the inotropic and metabotropic glutamatergic receptors (Latremoliere and Woolf, 2009). Although Glu levels measured using MRS represent primarily the intracellular or neuronal compartment, it remains unclear whether the findings reflect the metabolic pool or the vesicular pool of Glu (for synaptic transmission), making the interpretation of the altered glutamate in relation to pain physiology difficult. In contrast, GABA levels, which may reflect neuronal activity more closely than glutamate, were lower in the insula of patients with fibromyalgia compared to pain-free individuals (Foerster et al, 2012). Therefore, levels of Glu, Gln and GABA assessed with MRS may be particularly useful for assessing pain and monitoring treatments for pain.
Technical Issues and Future Directions
To apply MRS as a routine clinical tool to assess neuroinflammation and possibly neuropathic pain, several technical issues need to be considered. First, it is apparent from the studies discussed above that none of the major brain metabolites can be assumed to be normal in these neuroinflammatory disorders. Consequently, interpretation of metabolite ratios (e.g., using tCr or Cho as a reference) will be difficult, since an altered metabolite ratio may be due to an altered concentration of the numerator or denominator metabolite, or both. Employing optimized methods to measure metabolite concentrations is necessary to determine the actual neurometabolite alterations in a given patient. This can be achieved using a variety of techniques, for instance, by relating metabolite signals to that of the water (the tissue water concentration appears to be more stable than metabolite concentrations in major brain diseases).
Second, using MRS as a routine clinical tool would require that the metabolite measurements are stable over time, and can be compared across sites and scanners. Stability of metabolite measures over extended time periods (months to years) can be achieved on most modern MR scanners, at least during periods without major scanner or software upgrades. Likewise, metabolite results are fairly reproducible (at the 10% level) across studies and subjects on a given scanner. However, for routine clinical use of spectroscopy, metabolite concentrations would need to be comparable across scanner platforms, i.e., independent of the manufacturer or field strength, similar to other clinical laboratory tests that are comparable across laboratories. Achieving this for MR spectroscopy would require a level of standardization and sophistication that currently is not available in the clinical setting. In fact, even carefully designed multi-center MRS research studies commonly find inter-site biases in metabolite levels that need to be corrected statistically to perform valid group comparisons.
Finally, normative metabolite concentrations are dependent on important clinical variables, such age and possibly gender, which vary from one patient to another. For instance, normal brain aging is associated with increased neuroinflammation, which in turn may lead to higher levels of glial metabolites (especially myo-inositol and tCr) in some regions. Therefore, metabolite concentrations in a given patient have to be compared to normative values from controls at the same age, same sex, and from the same brain region. For instance, metabolite level for a specific brain region for an individual may be expressed as a “z-score”, i.e. number of standard deviations above or below a norm that is corrected for age and sex. However, such normative curves are not generally available. Other inter-individual differences that might affect the metabolite measurements include differences in partial volume effects from the proportions of gray and white matter, or the percentage of CSF in the volume of tissue measured. Corrections and standardization for these additional inter-subject differences between the metabolite measurements will require additional time and more optimized software that is not routinely available in the current clinical scanners.
Conclusion
MRS can provide useful non-invasive biomarkers for assessing neuroinflammation and neuropathic pain. Some technical issues still need to be optimized or standardized across various clinical scanners before MRS can be used routinely to assess neuroinflammatory disorders and neuropathic pain. However, recent studies of patients with various neuroinflammatory disorders suggest that elevated glial metabolites, such as myo-inositol and to a lesser degree tCr and Cho, can be detected in brain regions that are expected to show activation of microglia and astrocytes, while decreased neuronal metabolites, especially glutamate and later NAA, also may reflect additional neuronal and glial dysfunction. Since decreased intracellular glutamate may result from activated glial cells, which have decreased transporter function for reuptake of the released extracellular glutamate, decreased glutamate on MRS may be a sensitive early marker for brain injury and may reflect neuroinflammation.
Because MRS is non-invasive, it can be performed repeatedly to monitor treatment effects. The many MRS studies performed over the years in the various neuroinflammatory brain disorders have provided new insights into the pathophysiology of these disorders. A common pattern is that neuroinflammation typically occurs early in the course of these brain disorders. With progression and chronicity of the neuroinflammation and glial activation, neuronal injury starting with lower glutamate followed later by lower NAA, which may contribute to cognitive and behavioral symptoms in these patients.
Acknowledgments
Funding:
This work was supported by the National Institute on Drug Abuse (K24-DA016170), the National Institute on Neurological Disorders and Strokes (U54-NS056883) and the National Institutes on Minority Health and Health Disparities (G12 MD007601).
We thank Dr. Steven Buchthal who generated the MR spectra shown in the figures in this manuscript.
Footnotes
Disclosure statements:
The authors have no actual or potential conflicts of interest.
References
- Alexander GM, Perreault MJ, Reichenberger ER, Schwartzman RJ. Changes in immune and glial markers in the CSF of patients with Complex Regional Pain Syndrome. Brain Behav Immun. 2007;21:668–76. doi: 10.1016/j.bbi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Ashford JW, Adamson M, Beale T, La D, Hernandez B, Noda A, Rosen A, O’Hara R, Fairchild JK, Spielman D, Yesavage JA. MR spectroscopy for assessment of memantine treatment in mild to moderate Alzheimer dementia. J Alzheimers Dis. 2011;26(Suppl 3):331–6. doi: 10.3233/JAD-2011-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagory M, Durand-Dubief F, Ibarrola D, Comte JC, Cotton F, Confavreux C, Sappey-Marinier D. Implementation of an absolute brain (1)H-MRS quantification method to assess different tissue alterations in multiple sclerosis. IEEE Trans Biomed Eng. 2011 doi: 10.1109/TBME.2011.2161609. [DOI] [PubMed] [Google Scholar]
- Banati RB. Neuropathological imaging: in vivo detection of glial activation as a measure of disease and adaptive change in the brain. Br Med Bull. 2003;65:121–31. doi: 10.1093/bmb/65.1.121. [DOI] [PubMed] [Google Scholar]
- Bladowska J, Zimny A, Koltowska A, Szewczyk P, Knysz B, Gasiorowski J, Furdal M, Sasiadek MJ. Evaluation of metabolic changes within the normal appearing gray and white matters in neurologically asymptomatic HIV-1-positive and HCV-positive patients: Magnetic resonance spectroscopy and immunologic correlation. Eur J Radiol. 2013;82:686–92. doi: 10.1016/j.ejrad.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Blaes F, Schmitz K, Tschernatsch M, Kaps M, Krasenbrink I, Hempelmann G, Brau ME. Autoimmune etiology of complex regional pain syndrome (M. Sudeck) Neurology. 2004;63:1734–6. doi: 10.1212/01.wnl.0000143066.58498.ba. [DOI] [PubMed] [Google Scholar]
- Bokemeyer M, Ding XQ, Goldbecker A, Raab P, Heeren M, Arvanitis D, Tillmann HL, Lanfermann H, Weissenborn K. Evidence for neuroinflammation and neuroprotection in HCV infection-associated encephalopathy. Gut. 2011;60:370–7. doi: 10.1136/gut.2010.217976. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology. 1981;5:549–64. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Byrnes V, Miller A, Lowry D, Hill E, Weinstein C, Alsop D, Lenkinski R, Afdhal NH. Effects of antiviral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56:549–56. doi: 10.1016/j.jhep.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Camicioli RM, Korzan JR, Foster SL, Fisher NJ, Emery DJ, Bastos AC, Hanstock CC. Posterior cingulate metabolic changes occur in Parkinson’s disease patients without dementia. Neurosci Lett. 2004;354:177–80. doi: 10.1016/j.neulet.2003.09.076. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005a;57:967–74. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3, 4- methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J Magn Reson Imaging. 1999a;10:521–6. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999b;52:100–8. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999c;53:782–9. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–56. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005b;162:361–9. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004a;9:431–40. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999d;156:716–22. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, Singer E, Cornford M. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997a;48:836–45. doi: 10.1212/wnl.48.4.836. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–48. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- Chang L, Feger U, Ernst T. Neuroimaging. In: Gendelman HE, Ikezu T, editors. Neuroimmune pharmacology. Springer; New York, NY: 2012. p. 1.p. 827. [Google Scholar]
- Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–5. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004b;23:1336–47. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Mehringer CM, Ernst T, Melchor R, Myers H, Forney D, Satz P. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997b;42:1105–14. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Miller BL, McBride D, Cornford M, Oropilla G, Buchthal S, Chiang F, Aronow H, Beck CK, Ernst T. Brain lesions in patients with AIDS: H-1 MR spectroscopy. Radiology. 1995;197:525–31. doi: 10.1148/radiology.197.2.7480706. [DOI] [PubMed] [Google Scholar]
- Chassain C, Bielicki G, Keller C, Renou JP, Durif F. Metabolic changes detected in vivo by 1H MRS in the MPTP-intoxicated mouse. NMR Biomed. 2010;23:547–53. doi: 10.1002/nbm.1504. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Wheeler-Kingshott CA, McLean MA, Cercignani M, Wimpey K, Miller DH, Thompson AJ. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220–31. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- Emir UE, Tuite PJ, Oz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS One. 2012;7:e30918. doi: 10.1371/journal.pone.0030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–72. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203:829–36. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Walot I, Huff K. Physiologic MRI of a tumefactive multiple sclerosis lesion. Neurology. 1998;51:1486–8. doi: 10.1212/wnl.51.5.1486. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–53. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MS, O’Brien JT, Perry RH, English P, Forster G, McMeekin W, Slade JY, Golkhar A, Matthews FE, Barber R, Kalaria RN, Ince PG. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol. 2004;30:385–95. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–83. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–9. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- Forton DM, Hamilton G, Allsop JM, Grover VP, Wesnes K, O’Sullivan C, Thomas HC, Taylor-Robinson SD. Cerebral immune activation in chronic hepatitis C infection: a magnetic resonance spectroscopy study. J Hepatol. 2008;49:316–22. doi: 10.1016/j.jhep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, Wesnes KA, Taylor-Robinson SD. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–9. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- Forton DM, Thomas HC, Taylor-Robinson SD. Central nervous system involvement in hepatitis C virus infection. Metab Brain Dis. 2004;19:383–91. doi: 10.1023/b:mebr.0000043983.42843.ac. [DOI] [PubMed] [Google Scholar]
- Frederick BD, Lyoo IK, Satlin A, Ahn KH, Kim MJ, Yurgelun-Todd DA, Cohen BM, Renshaw PF. In vivo proton magnetic resonance spectroscopy of the temporal lobe in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1313–22. doi: 10.1016/j.pnpbp.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24:75–9. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Ramlackhansingh A, Thomson E, Allsop JM, Politis M, Kulasegaram R, Main J, Brooks DJ, Taylor-Robinson SD, Winston A. Acute HCV/HIV coinfection is associated with cognitive dysfunction and cerebral metabolite disturbance, but not increased microglial cell activation. PLoS One. 2012;7:e38980. doi: 10.1371/journal.pone.0038980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner GE, Gracely RH, Deebajah A, Ichesco E, Quintero A, Clauw DJ, Sundgren PC. Posterior insular molecular changes in myofascial pain. J Dent Res. 2012;91:485–90. doi: 10.1177/0022034512443366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts JJ, Reuling IE, Vrenken H, Uitdehaag BM, Polman CH, Castelijns JA, Barkhof F, Pouwels PJ. MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn Reson Med. 2006;55:478–83. doi: 10.1002/mrm.20792. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Okonkwo OC, O’Brien T, Watts RL, Marson DC. Brain metabolism differs in Alzheimer’s disease and Parkinson’s disease dementia. Alzheimers Dement. 2008a;4:421–7. doi: 10.1016/j.jalz.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Okonkwo OC, O’Brien T, Watts RL, Marson DC. Brain Nacetylaspartate is reduced in Parkinson disease with dementia. Alzheimer Dis Assoc Disord. 2008b;22:54–60. doi: 10.1097/WAD.0b013e3181611011. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Stewart CC, Evanochko WT, Buchthal SD, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Elevated brain scyllo-inositol concentrations in patients with Alzheimer’s disease. NMR Biomed. 2007;20:709–16. doi: 10.1002/nbm.1132. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Okonkwo OC, O’Brien T, Hollander JA. Reduced brain glutamate in patients with Parkinson’s disease. NMR Biomed. 2008c;21:381–7. doi: 10.1002/nbm.1203. [DOI] [PubMed] [Google Scholar]
- Grover VP, Pavese N, Koh SB, Wylezinska M, Saxby BK, Gerhard A, Forton DM, Brooks DJ, Thomas HC, Taylor-Robinson SD. Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J Viral Hepat. 2012;19:e89–96. doi: 10.1111/j.1365-2893.2011.01510.x. [DOI] [PubMed] [Google Scholar]
- Gu T, Ma XX, Xu YH, Xiu JJ, Li CF. Metabolite concentration ratios in thalami of patients with migraine and trigeminal neuralgia measured with 1H-MRS. Neurol Res. 2008;30:229–33. doi: 10.1179/016164107X235473. [DOI] [PubMed] [Google Scholar]
- Gustafsson MC, Dahlqvist O, Jaworski J, Lundberg P, Landtblom AM. Low choline concentrations in normal-appearing white matter of patients with multiple sclerosis and normal MR imaging brain scans. AJNR Am J Neuroradiol. 2007;28:1306–12. doi: 10.3174/ajnr.A0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–33. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci Lett. 2012;520:192–6. doi: 10.1016/j.neulet.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–7. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190–5. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Moreno A, Pujol J, Marti-Fabregas J, Domingo P, Molet J, Ris J, Cadafalch J. Proton magnetic resonance spectroscopy pattern of progressive multifocal leukoencephalopathy in AIDS. J Neurol Neurosurg Psychiatry. 1999;66:520–3. doi: 10.1136/jnnp.66.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–32. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Jessen F, Gur O, Block W, Ende G, Frolich L, Hammen T, Wiltfang J, Kucinski T, Jahn H, Heun R, Maier W, Kolsch H, Kornhuber J, Traber F. A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI. Neurology. 2009;72:1735–40. doi: 10.1212/WNL.0b013e3181a60a20. [DOI] [PubMed] [Google Scholar]
- Kickler N, Krack P, Fraix V, Lebas JF, Lamalle L, Durif F, Krainik A, Remy C, Segebarth C, Pollak P. Glutamate measurement in Parkinson’s disease using MRS at 3 T field strength. NMR Biomed. 2007;20:757–62. doi: 10.1002/nbm.1141. [DOI] [PubMed] [Google Scholar]
- Kirov, Patil V, Babb JS, Rusinek H, Herbert J, Gonen O. MR spectroscopy indicates diffuse multiple sclerosis activity during remission. J Neurol Neurosurg Psychiatry. 2009;80:1330–6. doi: 10.1136/jnnp.2009.176263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov, Tal A, Babb JS, Herbert J, Gonen O. Serial proton MR spectroscopy of gray and white matter in relapsing-remitting MS. Neurology. 2013;80:39–46. doi: 10.1212/WNL.0b013e31827b1a8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Rajput A, Gilbert J, Rozdilsky B, Chang LJ, Shannak K, Hornykiewicz O. Elevated gamma-aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson’s disease: correlation with striatal dopamine loss. Ann Neurol. 1986;20:26–31. doi: 10.1002/ana.410200106. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Panchalingam K, Moossy J, McClure RJ, Pettegrew JW. N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 1992;42:1578–85. doi: 10.1212/wnl.42.8.1578. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, Halpern EF, Rosenberg ES, Williams K, Gonzalez RG. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011;17:220–9. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, Williams K, Rosenberg ES, Gonzalez RG. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–72. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, Marquie-Beck J, Navia B. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–9. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Wang Y, Pankiewicz J, Stein EA. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry. 1999;45:1481–7. doi: 10.1016/s0006-3223(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182:3855–65. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliani AF, Clementi V, Albini Riccioli L, Agati R, Carpenzano M, Salvi F, Leonardi M. Quantitative cervical spinal cord 3T proton MR spectroscopy in multiple sclerosis. AJNR Am J Neuroradiol. 2010;31:180–4. doi: 10.3174/ajnr.A1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–8. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta -induced toxicity. J Biol Chem. 2000;275:18495–502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Schuff N, Ezekiel F, Norman D, Clark W, Weiner MW, Fein G. Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addict Biol. 1999;4:405–19. doi: 10.1080/13556219971399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–7. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Moats RA, Ernst T, Shonk TK, Ross BD. Abnormal cerebral metabolite concentrations in patients with probable Alzheimer disease. Magn Reson Med. 1994;32:110–5. doi: 10.1002/mrm.1910320115. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, Sacktor NC. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28:1251–7. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, Rowland LM, Jung RE, Sibbitt WL., Jr A novel technique to study the brain’s response to pain: proton magnetic resonance spectroscopy. Neuroimage. 2005;26:642–6. doi: 10.1016/j.neuroimage.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Nie K, Zhang Y, Huang B, Wang L, Zhao J, Huang Z, Gan R. Marked N-acetylaspartate and choline metabolite changes in Parkinson’s disease patients with mild cognitive impairment. Parkinsonism Relat Disord. 2013;19:329–34. doi: 10.1016/j.parkreldis.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Schuff N, Marks WJ, Jr, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002;17:917–27. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- Oz G, Terpstra M, Tkac I, Aia P, Lowary J, Tuite PJ, Gruetter R. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006;55:296–301. doi: 10.1002/mrm.20761. [DOI] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–5. [PMC free article] [PubMed] [Google Scholar]
- Pattullo V, McAndrews MP, Damyanovich A, Heathcote EJ. Influence of hepatitis C virus on neurocognitive function in patients free from other risk factors: validation from therapeutic outcomes. Liver Int. 2011;31:1028–38. doi: 10.1111/j.1478-3231.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- Penner J, Rupsingh R, Smith M, Wells JL, Borrie MJ, Bartha R. Increased glutamate in the hippocampus after galantamine treatment for Alzheimer disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:104–10. doi: 10.1016/j.pnpbp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Rami L, Gomez-Anson B, Bosch B, Sanchez-Valle R, Monte GC, Villar A, Molinuevo JL. Cortical brain metabolism as measured by proton spectroscopy is related to memory performance in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:274–9. doi: 10.1159/000107487. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Flick H, den Heeten GJ. Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. AJNR Am J Neuroradiol. 2002;23:231–7. [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Schmand B, van den Brink W, den Heeten GJ. Prefrontal Nacetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry. 2001;50:550–4. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- Rigotti DJ, Inglese M, Kirov, Gorynski E, Perry NN, Babb JS, Herbert J, Grossman RI, Gonen O. Two-year serial whole-brain N-acetyl-L-aspartate in patients with relapsing-remitting multiple sclerosis. Neurology. 2012;78:1383–9. doi: 10.1212/WNL.0b013e318253d609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32:802–10. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Galloway GP, Nordahl TE. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend. 2011;113:133–8. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ, Kolson DL, Simpson D, Nath A, Berger J, Shriver SL, Millar LL, Colquhoun D, Lenkinski R, Gonzalez RG, Lipton SA. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–86. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL, Grant I. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–64. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Shinno H, Inagaki T, Miyaoka T, Okazaki S, Kawamukai T, Utani E, Inami Y, Horiguchi J. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer’s disease. J Neurol Sci. 2007;260:132–8. doi: 10.1016/j.jns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–1. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–25. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tarasow E, Wiercinska-Drapalo A, Jaroszewicz J, Orzechowska-Bobkiewicz A, Dzienis W, Prokopowicz D, Walecki J. Antiretroviral therapy and its influence on the stage of brain damage in patients with HIV - 1H MRS evaluation. Med Sci Monit. 2004;10(Suppl 3):101–6. [PubMed] [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13:150–9. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–11. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J. CNS viral invasion and inflammation during acute HIV. J Infect Dis. 2012 doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voorn JP, Pouwels PJ, Vermeulen RJ, Barkhof F, van der Knaap MS. Quantitative MR imaging and spectroscopy in congenital cytomegalovirus infection and periventricular leukomalacia suggests a comparable neuropathological substrate of the cerebral white matter lesions. Neuropediatrics. 2009;40:168–73. doi: 10.1055/s-0029-1243228. [DOI] [PubMed] [Google Scholar]
- Vrenken H, Barkhof F, Uitdehaag BM, Castelijns JA, Polman CH, Pouwels PJ. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Med. 2005;53:256–66. doi: 10.1002/mrm.20366. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, Ahl B, Manns MP, Boker KW. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41:845–51. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154:204–12. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401–15. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ET, Ronen I, Techawiboonwong A, Jones CK, Barker PB, Calabresi P, Harrison D, Reich DS. Investigating axonal damage in multiple sclerosis by diffusion tensor spectroscopy. J Neurosci. 2012;32:6665–9. doi: 10.1523/JNEUROSCI.0044-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–21. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174:171–6. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Bang OY, Kim HS. Progressive Multifocal Leukoencephalopathy in AIDS: Proton MR Spectroscopy Patterns of Asynchronous Lesions Confirmed by Serial Diffusion-Weighted Imaging and Apparent Diffusion Coefficient Mapping. J Clin Neurol. 2007;3:200–3. doi: 10.3988/jcn.2007.3.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]