Abstract
Invasion ecology has much advanced since its early beginnings. Nevertheless, explanation, prediction, and management of biological invasions remain difficult. We argue that progress in invasion research can be accelerated by, first, pointing out difficulties this field is currently facing and, second, looking for measures to overcome them. We see basic and applied research in invasion ecology confronted with difficulties arising from (A) societal issues, e.g., disparate perceptions of invasive species; (B) the peculiarity of the invasion process, e.g., its complexity and context dependency; and (C) the scientific methodology, e.g., imprecise hypotheses. To overcome these difficulties, we propose three key measures: (1) a checklist for definitions to encourage explicit definitions; (2) implementation of a hierarchy of hypotheses (HoH), where general hypotheses branch into specific and precisely testable hypotheses; and (3) platforms for improved communication. These measures may significantly increase conceptual clarity and enhance communication, thus advancing invasion ecology.
Keywords: Communication platforms, Definitions and terminology, Hierarchy of hypotheses, Invasive alien species, Synthesis, Transdisciplinarity
Introduction
Studying biological invasions can yield insights into numerous basic ecological, evolutionary, and biogeographical topics (Sax et al. 2005). As some invasive species threaten biodiversity, are vectors of human diseases, and cause socio-economic costs, their investigation also has an applied focus. From its beginning, invasion ecology has combined these basic and applied aspects. The first written accounts of invasive species date back to the eighteenth century (Chew 2006), but the publication of Elton’s (1958) book “The ecology of invasions by animals and plants”, which conveys an explicit conservation point of view, is generally considered to be the starting point of focused research on biological invasions (Richardson and Pyšek 2008). In the 1980s, invasion ecology emerged as a specific research field (Richardson and Pyšek 2007). This was in part due to the international program of the Scientific Committee on Problems of the Environment (SCOPE) on biological invasions (Drake et al. 1989). The program focused on three questions, again addressing basic as well as applied aspects: (i) What factors determine whether a species will become an invader or not? (ii) What are the properties that determine whether an ecological community is vulnerable or resistant to invasions? (iii) How should effective management strategies be developed?
Some answers to these questions are now available, and have been summarized in various journal articles and books (Lockwood et al. 2007; Blackburn et al. 2009; Davis 2009; Richardson 2011a). Based on Elton’s work and the SCOPE program, invasion ecologists have produced plenty of hypotheses and data. New methods such as modelling approaches, multi-scale comparisons and molecular methods are being applied, and new topics such as propagule pressure (the pattern in which propagules arrive; Simberloff 2009) and post-introduction evolution have been raised (Richardson and Pyšek 2008). It should be expected, thus, that knowledge has increased considerably since the beginning of invasion research. Nevertheless, progress towards satisfactory explanation and prediction of invasions as well as management of invasive species is rather slow (Puth and Post 2005; Lockwood et al. 2007; Blackburn et al. 2009; Davis 2009; Richardson 2011b; Moles et al. 2012).
Several authors have already called for an improvement of the implementation of existing knowledge into policies and management (Hulme 2006; Lodge et al. 2006). In this paper, we focus on invasion science itself: We think there is much potential for improving the effectiveness of basic and applied research on invasions. We argue that progress in invasion ecology can be accelerated by, first, explicating difficulties that basic and applied research on invasions are facing today and, second, developing measures to overcome them. By difficulties, we here mean circumstances that hinder or complicate basic or applied research. Difficulties for invasion ecology arise from: (A) society’s impact and perception; (B) the peculiarity of the invasion process; and (C) the scientific methodology. Overlaps between these three domains exist, but this classification is helpful to structure our considerations. In Tables 1, 2, and 3, we summarize difficulties of all three domains as well as measures to overcome them. Some of these difficulties and measures have been pointed out before and are covered by the references provided. Here, our focus is on new possibilities to improve the effectiveness of basic and applied research on biological invasions, especially regarding domain C.
Table 1.
Invasion ecology is confronted with three domains of difficulties. Domain A: Difficulties arising from society and its relationship to biological invasions, measures that can be taken to overcome them and consequences for the scientific approach of invasion ecology; letters and numbers in parentheses refer to Table 3
| Difficulty | Measures | Consequences for the scientific approach | |
|---|---|---|---|
| A1 | Deliberate introductions, influenced by commercial interests and changing fashions | Risk assessment protocols Black, white, and gray listsa International cooperation to prevent trade with risky speciesb Raising public awarenessc |
Commercial interests and changing fashions should be considered for explanation and prediction (C8) |
| A2 | Accidental introductions, promoted by globalization | Quarantine measuresd International cooperation to prevent accidental introductionsb Raising public awarenessc |
Changes in transportation pathways should be considered for explanation and prediction (C8) |
| A3 | Inconsistent evaluation of invasive species | Development of management strategies based on knowledge about public attitudese | Public attitudes should be investigated and considered (C8) |
| A4 | Little motivation for management measures due to little prospect of successf | Improve information about feasibility of management strategiesg | Need for clear management guidelines (C6) |
Table 2.
Invasion ecology is confronted with three domains of difficulties. Domain B: Difficulties caused by the peculiarity of the invasion process, and consequences for the scientific approach of invasion ecology; letters and numbers in parentheses refer to Table 3
| Difficulty | Consequences for the scientific approach | |
|---|---|---|
| B1 | Complexity: many different factors interact in determining invasion success | Synthesis needed to integrate the interacting influence of multiple factors (C2) |
| B2 | Context dependence: invader success varies in time and space | Historic data are relevant (C4) Case studies needed, but also synthesis (C2) |
| B3 | Cultural influences at each stage of the process | Socio-cultural sciences have to be integrated for explanation and prediction (C8) |
Table 3.
Invasion ecology is confronted with three domains of difficulties. Domain C: Conceptual and methodological difficulties, and measures to meet them. C1–C3 relate to the conceptual basis and theory of invasion ecology, C4 and C5 to empirical research, and C6–C8 to the need of integration with other scientific disciplines and societal groups. Letters and numbers in parentheses refer to difficulties given in Tables 1 and 2
| Difficulty | Measures | |
|---|---|---|
| C1 | Terminology: unclear concepts and definitions | Explicit definitions (see checklist in Box 1) |
| C2 | Insufficient synthesis; sub-division of invasion ecology (e.g., taxonomic groups) | Hierarchy of hypotheses (HoH) with precise, testable hypotheses at lowest level |
| C3 | Imprecise hypotheses (a) different versions of hypotheses (b) lack of testability |
HoH |
| C4 | Lack of data to test hypotheses (a) lack of data on unsuccessful introductions (b) lack of large-scale experimental data (c) lack of long-term data |
Funding of large-scale and long-term research ‘Indirect’ methods (e.g., retrospective analyses and model simulations instead of long-term experiments) Online databases Citizen science and monitoring programs by the general public |
| C5 | Bias in data collection (a) invasion events (most research on successful species in areas with high density of researchers) (b) methods of data collection |
Frequent reviews with connection to HoH; aim: identification of gaps and biases |
| C6 | Necessity of communication of research results to concerned stakeholders (A4) | Focus on output valuable for applications Up-to-date networks and platforms Joint conferences and discussions |
| C7 | Complexity (B1) creates the need to integrate other biological subdisciplines | Integration of HoH into other disciplines Joint conferences and discussions |
| C8 | Influence of socio-economic and cultural processes on invasions (A1, A2, B3) creates the need for transdisciplinary research | Communication and collaboration with researchers of humanities and social sciences Joint conferences and discussions |
Domain A: Difficulties Arising from Society and Its Relationship to Biological Invasions
Society causes biological invasions, and biological invasions influence society. This feedback not only complicates effective prevention and management (A1–A4 in Table 1) but also has consequences for the scientific approach (right column in Table 1). An example is the perception of invasive species by the general public. The general public has only limited knowledge of the phenomenon of biological invasions (Gellis Communications 2008), and perception as well as evaluation of invasions are not at all homogeneous across societal groups (Fischer and van der Wal 2007; Gherardi 2011; Lambert and Rotherham 2011) (A3 in Table 1). Especially in case of deliberate introductions related to agriculture, forestry, fisheries, and biological control, species can cause benefits as well as costs (Gozlan 2008). Thus, species ranked as highly problematic by conservation scientists sometimes are regarded as not harmful or even desirable by the public. For example, conservation scientists perceive the tree of heaven (Ailanthus altissima) as a harmful invader with the potential to threaten native species; on the other hand, many people on the Mediterranean islands appreciate its ability to grow on dry soils and to provide shade (Bardsley and Edwards-Jones 2007).
Such disparate perceptions have consequences for applied research on invasions: research on managing invasions and strategies tailored to address actual societal needs cannot be efficient unless these needs are uncovered. An increasing amount of work already aims to include social and economic demands into invasion research (Fischer and van der Wal 2007; Berghöfer et al. 2010; Perrings et al. 2010a). Such efforts are in high demand, and more inter- and transdisciplinary collaborations should be established to foster them (Richardson 2011b; see below).
Domain B: Difficulties Arising from the Peculiarity of the Invasion Process
In addition to problems related to society, a major obstacle for research is that invasion processes are notedly difficult to analyze, explain, and predict. Invasion processes are complex (Lodge 1993; Hayes and Barry 2008; B1 in Table 2) and context-dependent (Zedler and Kercher 2004; Gurevitch et al. 2008; Blackburn et al. 2009) (B2 in Table 2). This creates the need for methods that are able to explain and predict multiple interacting influences (Heger and Trepl 2003), and to take into account the history of current invasions for their explanation (Cassey et al. 2005) (see right column in Table 2).
Global transportation networks and other socio-cultural activities (such as horticulture or fishery) not only cause difficulties for the prevention and management of invasive species, but also create the need to integrate socio-cultural sciences into research (Kowarik 2003; Niggemann et al. 2009; Tatem 2009) (B3 in Table 2). One example is the spread of New Zealand bittercress (Cardamine corymbosa) to Europe and the U.S., which is largely due to a combination of ecological traits (e.g., active short-distance seed dispersal) and socio-economic activities that include international plant auctions and exchanges of container-grown plants among nurseries, garden centers, and private gardens (Hoste et al. 2008). An increasing number of studies already integrate socio-cultural analyses into approaches to study invasions (Dehnen-Schmutz and Williamson 2006; Skou et al., in press), and invasion ecologists increasingly collaborate with socio-cultural scientists. An example is the workshop organized by C. Kueffer, in Bielefeld, Germany, August 2012 (http://www.uni-bielefeld.de/(en)/ZIF/AG/2012/08-27-Kueffer.html), where half of the participants where socio-cultural scientists and the other half natural scientists. To improve effectiveness of explanation, prediction and management, similar efforts should be strengthened (see key measure 3 below).
Domain C: Conceptual and Methodological Difficulties
Invasion ecology has to cope with several conceptual and methodological difficulties, many of which are related to or produced by society and the peculiarity of the invasion process (see right columns in Tables 1 and 2). The scientific methodology in invasion research is facing difficulties concerning the conceptual basis and theory of invasion ecology (C1–C3 in Table 3), empirical research (C4 and C5 in Table 3), and the need for integration with other scientific disciplines and societal groups (C6–C8 in Table 3). We will focus on some particularly important difficulties and propose three key measures to overcome them.
Terminology: Unclear Terms and Concepts
As many other research fields, invasion ecology is still plagued by the ambiguous use of terms and unclear concepts (Richardson et al. 2011; McGeoch et al. 2012) (C1 in Table 3). Inconsistent terminology can cause difficulties when it comes to the communication of research rationales and results, both within science, and between science and the broader public; therefore explicit definitions are needed. However, they are not equally necessary for all publications. General treatments of biological invasions (such as this publication) can cover different definitions of invasive species, whereas comparisons of sets of native and invasive species need explicit definitions and consistent applications of underlying concepts (van Kleunen et al. 2010).
Creating a single set of definitions that suits all purposes seems impossible (Hodges 2008), as different research goals create different ideas of what is peculiar about invasions (Küffer and Hirsch Hadorn 2008). We therefore suggest to accept that different stakeholders use different definitions (cf. Heger et al., in press). However, it is important to clarify how alien or invasive species is defined by a given person or text. We propose to use the following checklist to achieve such clarity.
Key Measure 1: Checklist for Explicit Definitions
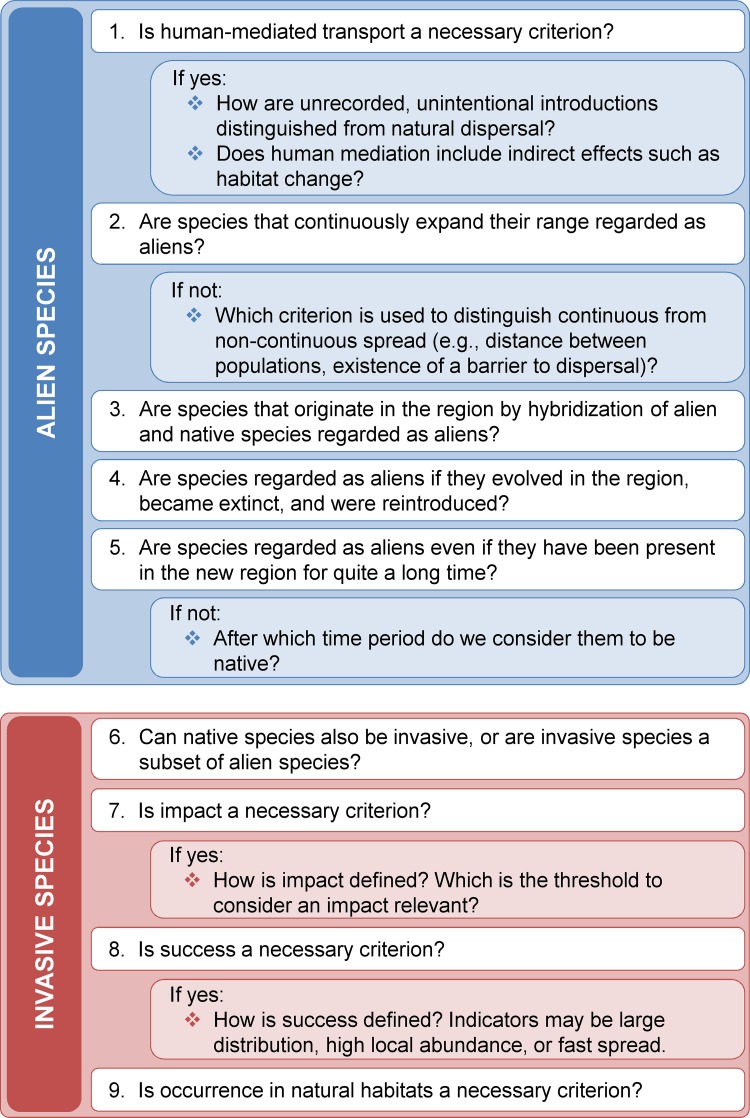
The checklist we suggest consists of five questions that are important to define alien species, and four additional questions for defining invasive species (Box 1). Depending on the research context (e.g., basic or applied focus), different answers are possible for each question. The references included below can help deciding which answers are most reasonable for a given context.
Box 1.
Checklist for definitions: questions that should be answered when defining alien or invasive species
Question 1:How did the species arrive in areas beyond their native range? Is human-mediated transport regarded a condition to call a species alien? If the answer is ‘yes’, it should be stated how unintentional species introductions are distinguished from natural dispersal events. In cases where information on the pathway is lacking, proxies can be used (e.g., geographical distribution, see Webb 1985). Additionally, it is helpful to state what is meant by human-mediated transport: are indirect effects of human action, e.g., habitat change, included or excluded? An excellent example clarifying this and similar aspects can be found in Pyšek et al. (2004).
Question 2:Are continuously spreading species (‘leading edge dispersal’, Wilson et al. 2009) regarded as alien? Climate change alters species distributions; hence spontaneous colonization events from neighboring geographic regions may become more frequent in the near future (Walther et al. 2009). If continuously spreading species are not viewed as alien, the definition will need to include a criterion to distinguish continuous from non-continuous spread. For example, Richardson et al. (2000) suggested that a new occurrence of plant species should be regarded as alien if it is more than about 100 km away from the closest native population. Another option is to consider species as alien as soon as they overcome a species-specific barrier to dispersal (Heger and Trepl 2003).
Question 3: Are species that originate in the region by hybridization of alien and native species regarded as aliens? In the strict sense of many definitions of alien species, these hybrids have to be regarded as natives, because they evolved in the region. If authors do not agree with this view, it should be stated clearly (see e.g., Pyšek et al. 2004).
Question 4: Are species regarded as alien if they evolved in the region, became extinct, and were re-introduced? When answering this question, the time scale has to be explained. Some authors argue species that were native in an area but became extinct during the last glaciation should be viewed as alien (Webb 1985; Pyšek et al. 2004).
Question 5:Is residence time within an area regarded as an important criterion? In this case, it is useful to specify after which residence time a species is considered to be native (see Carthey and Banks 2012).
The previous questions all relate to the term alien species; questions 6–9 can be used to clarify definitions of invasive species.
Question 6:Can native species also be called invasive? The term invasive species is sometimes used for species expanding their range, no matter whether they are alien or native (Myster 1993; Valéry et al. 2008; Catford et al. 2009; Carey et al. 2012). Davis (2009), as an example, proposes to focus on similarities between processes of species redistributions instead of trying to separate aliens from range-expanding native species (SPRED-ecology, pp. 191–192). It is useful to state whether this view is shared, or invasive species are regarded as a subset of alien species (see e.g., ISSG 2000; Richardson et al. 2011 for respective definitions).
Question 7:Do invasive species necessarily have a negative impact in their new environment? Some definitions apply the term invasive to those alien species that spread, regardless of their effects in the new environment (e.g., Heger and Trepl 2003). If impact is used as a condition (as e.g., in ISSG 2000), it should be specified what kind of impact is meant, e.g., economic, social, and/or ecological impact, and which is the threshold to consider the impact relevant.
Question 8:Do invasive species have to be successful? Some authors propose that success is an important criterion to define invasive species (Valéry et al. 2008). As success can be indicated by a large distribution, high local abundance, dominance, fast spread, or a combination of these, it should be explained which measure of success the definition uses.
Question 9:Do invasive species have to occur in semi-natural communities? As some alien species at first only occur in heavily modified habitats (Richardson et al. 2000), the colonization of semi-natural or natural habitats is sometimes viewed as a useful criterion to define invasive species (Reichard and Hamilton 1997). According to such definitions, alien species quickly spreading in agricultural habitats are excluded from the invasive species category.
Explicitly answering these nine questions can help solving the problem of unclear terminology. The implementation of this checklist could, for instance, be accomplished in a working group or regular symposia. Increased consciousness of a growing number of authors, editors, and reviewers will help to minimize misunderstandings and misinterpretations.
Invasion Theory: Lack of Synthesis and Imprecise Hypotheses
Each of the many existing hypotheses in invasion ecology covers specific aspects of the general mechanisms behind biological invasions. Some recent studies offer ideas for a synthesis of invasion theory (Colautti and MacIsaac 2004; Blumenthal 2006; Catford et al. 2009; Davis 2009; Gurevitch et al. 2011). These approaches each put together different pieces of available knowledge in a specific and valuable way, but each approach is limited in what it covers. Additionally, invasion ecology still struggles to overcome a taxonomic bias, especially a division into plant-oriented studies on the one hand and animal-oriented studies on the other hand (Pyšek et al. 2008; Jeschke et al. 2012a). As a result, our overall knowledge about the mechanisms driving invasions is still patchy. Although a few treatments of both invasive plants and animals are available (Blackburn et al. 2011), a general synthesis of invasion ecology is still missing (C2 in Table 3).
As an additional difficulty, studies testing widely used hypotheses often report contradictory results (Jeschke et al. 2012b; Moles et al. 2012). This is oftentimes due to the context dependency of invasions (see above). Contradictory results become a problem as soon as the respective hypothesis is at stake: it is not clear if hypotheses with ambiguous evidence are worth keeping, or if they should be discarded (cf. Jeschke et al. 2012b). For example, the biotic resistance hypothesis (also known as ‘diversity-invasibility hypothesis’) states that ecosystems with a high biodiversity are more resistant to invaders than ecosystems with a low biodiversity (Elton 1958; Levine and D’Antonio 1999; Mack et al. 2000; Fridley et al. 2007; Davis 2009). Several small-scale experiments have supported this hypothesis, whereas large-scale studies hardly ever do so (Fridley et al. 2007). The latter sometimes even show the opposite pattern of what is predicted (Levine 2000; Stohlgren et al. 2003, 2006). Second, the enemy release hypothesis (Keane and Crawley 2002), which states that the absence of enemies is one cause of invasion success, is supported by several studies (Wolfe 2002; Mitchell and Power 2003), but questioned by others (Frenzel and Brandl 2003; te Beest et al. 2009).
One reason for these contradictory results is that considerable variation exists with respect to the wording of many current hypotheses (C3a in Table 3), and studies addressing them are not always explicit about which version they focus on. If two studies claim to test a certain hypothesis but are in fact testing different variants of this hypothesis, they may have opposite conclusions even if their empirical results are similar. The biotic resistance hypothesis, for example, is sometimes formulated as above, stating that ecosystems with a high biodiversity are more resistant to invaders than ecosystems with a low biodiversity. According to another, very general formulation of this hypothesis, ecosystems with a high biodiversity and a low level of disturbance should be more resistant to invaders than ecosystems with a low biodiversity and a high level of disturbance (Jeschke and Genovesi 2011). Yet another formulation focuses on disturbance and leaves out diversity (Mack et al. 2000), and other factors have also been tested to see if they influence an ecosystem’s resistance to invaders, e.g., the presence of keystone predators (Carlsson et al. 2010).
A related difficulty is that many existing versions of hypotheses are too imprecise to be actually testable (C3b in Table 3). In fact, the number of variants of some hypotheses probably keeps rising exactly because existing versions are not testable. The biotic resistance hypothesis in the version stating that ecosystems with a high biodiversity are more resistant to invaders than ecosystems with a low biodiversity can be tested only if ‘biodiversity’ and ‘resistance’ are specified. Existing studies have quantified biodiversity in different ways, for example by measuring richness of native species (Arndt 2006; Capers et al. 2007) or evenness (Wilsey and Polley 2002; Mattingly et al. 2007). Resistance has also been quantified in different ways, for example, by counting the number of invasive species (assuming that fewer invasive species will be found in resistant ecosystems as compared to other ecosystems; e.g., Arndt 2006; Capers et al. 2007), or by calculating the fraction of introduced species that have become established (Blackburn and Duncan 2001; Jeschke and Genovesi 2011). Existing studies have thus focused on different forms of biodiversity and resistance (see also Jeschke et al. 2012b), and have consequently tested different formulations of the resistance hypothesis, in most cases without stating which exact version of the hypothesis has been addressed.
Another example is the enemy release hypothesis. Its general version contains several different possible mechanisms and processes, hence no single study can be designed to test it in its full extent. Studies addressing enemy release can only focus on some of its aspects, and often do so without explicitly discussing this limitation. For example, some studies compare populations of invasive species in the new range to populations of the same species in the indigenous range and quantify infestation, i.e., abundance or diversity of predators or parasites that can be found on the species (Mitchell and Power 2003; Vignon et al. 2009). Other studies use the same comparison but quantify damage typically caused by predators, e.g., leaf damage (Lewis et al. 2006; Ebeling et al. 2008). Another approach is to compare invasive to similar or related native species, and again, in some cases infestation is quantified (Frenzel and Brandl 2003; Blakeslee and Byers 2008), in others damage (Carpenter and Cappuccino 2005; Sugiura 2010). The case is even more complicated by the fact that some comparisons analyze the importance of generalist predators (Jogesh et al. 2008), others that of specialist predators (Memmott et al. 2000; Liu et al. 2007). It is often stated that the data confirm or reject the enemy release hypothesis without stating that only some aspects have been tested (see also Davis 2011).
Key Measure 2: A Hierarchy of Hypotheses (HoH)
The difficulty of imprecise hypotheses and lacking synthesis can be overcome by what we call a hierarchy of hypotheses (HoH). We suggest arranging hypotheses in an inverted tree-like structure, in which general hypotheses (i.e., hypotheses including too many aspects to be tested in single case studies) at the top branch into more and more specific hypotheses at the bottom. The most specific hypotheses (at the bottom) are very precise, and each can be approached with case studies. An accumulation of evidence for or against individual hypotheses can then help evaluate the more general predictions represented by this branch (cf. Jeschke et al. 2012b).
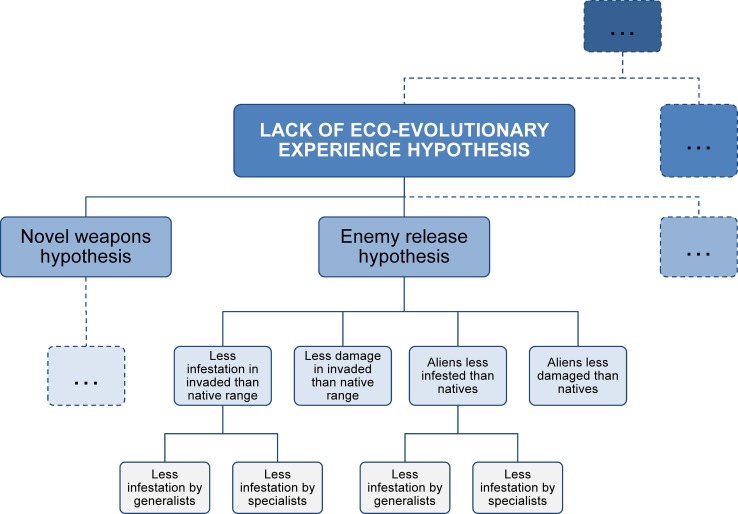
A HoH is able to structure the various aspects contained within many existing hypotheses. Let us use the enemy release hypothesis as an example. Its general formulation can be situated at the top of a branch (Fig. 1). A hypothesis addressing the rate of infestation in the new compared to the native range could be situated below, and further branch into hypotheses focused on generalist or specialist predators only (Fig. 1). Other lower-level hypotheses and aspects of the enemy release hypothesis could be fanned out in the same way; where necessary, hypotheses could also be specified with respect to certain taxa or habitats.
Fig. 1.
Sketch of a possible hierarchy of hypotheses (HoH) for invasion ecology. Overarching ideas branch into more precise, better testable hypotheses at lower levels. ‘Infestation’ means abundance or diversity of predators or parasites that can be found on the species. Empty boxes indicate that the hierarchy may be extended
Such an explicit formulation of testable lower-level hypotheses could be used to structure research on biological invasions. Every study could explicitly state which lower-level hypothesis is tested, whether it is confirmed or rejected, and what that means for higher-level hypotheses. To construct a HoH for invasion ecology will not be easy, and it has to be worked out how exactly the lower-level hypotheses contribute to the rejection or confirmation of the higher-level hypotheses. We think of a HoH as an evolving structure, at all times able to integrate new insights. As soon as it is constructed, it will be much easier than it is today to see whether lower-level hypotheses for a given higher-level hypothesis reach similar levels of empirical support, or whether certain lower-level hypotheses are better supported than others. Furthermore, it would be possible to see which hypotheses apply under which environmental conditions, for which scales, for which taxonomic groups and habitats. In other words, important information would be available to decide which hypotheses are valuable as a basis for prediction and management for given conditions.
Within a HoH, higher-level hypotheses are also connected to each other. For example, the enemy release hypothesis is connected to the novel weapons hypothesis. The latter hypothesis suggests that invasive species can have a competitive advantage over native species because they possess a trait that the native species are not evolutionarily adapted to and therefore affects them negatively (Callaway and Ridenour 2004). A shared idea is that missing eco-evolutionary ‘experience’ of the resident species with the invader can be advantageous for alien species. We suggest calling this the ‘lack of eco-evolutionary experience hypothesis’ (Fig. 1).
In a HoH for invasion ecology, every existing hypothesis would find its place within an interlinked system of other hypotheses. Every hypothesis could be classified as a basic building block at a lower level (i.e., testable but with small cover and extent) or be located at a higher level. Different formulations of similar ideas (e.g., formulations of the biotic resistance hypothesis described above) could be neighbors on one level and be integrated into an overarching idea at a higher level. In this way, a novel possibility for synthesis becomes visible. Research could aim at precisely determining which hypotheses hold in which situations, finding more and more interconnections among hypotheses and ideas, and search for more higher-level theories synthesizing those at lower levels. Future research should focus on building and maintaining such a HoH. It could be implemented as an online tool and updated regularly to integrate new data and hypotheses.
Empirical Evidence: Lack of Data and Biases in Data Collection
In addition to conceptual issues, a lack of data to test hypotheses (McGeoch et al. 2010) is a difficulty in invasion ecology (C4 in Table 3). For example, information on failed invasions following accidental introductions is often not available, especially for plants and invertebrates, sometimes not even for vertebrates. This problem affects many hypotheses in invasion ecology (Jeschke 2009; Lockwood et al. 2009; Rodriguez-Cabal et al. 2009). Invasion ecology also lacks homogeneous data at large spatial scales, and long-term data are rare as well (but see Meiners et al. 2004). While short-term effects of invasive species are often known, their long-term effects are rarely investigated and hard to predict (Strayer et al. 2006). The history of invasion processes sometimes can be recovered through the study of herbarium specimens in combination with molecular research and literature reviews. Model simulations can additionally help fill this gap to some degree (Strayer et al. 2006). The study of ongoing changes in the effects of invasive species is necessary for predicting future effects. Unfortunately, the collection of long-term data is often hampered by difficulties to acquire funding for more than a few years. Citizen science has proven useful to gather large amounts of data, with a spatial and temporal coverage that would be hard to achieve for individual research teams (Dickinson et al. 2012). More citizen science programs to engage the general public into invasion research should be started. Online databases such as DAISIE (Delivering Alien Invasive Species Inventories for Europe; http://www.europe-aliens.org), GISD (Global Invasive Species Database; http://www.issg.org/database), or NOBANIS (European Network on Invasive Alien Species; http://www.nobanis.org) have proven very useful, but they can only summarize data that are actually available.
Another difficulty for data analysis is that data collection is often biased, e.g., taxonomically, geographically, or methodologically (C5 in Table 3). Research on successful invaders is concentrated in those areas where most funding is available (Wilson et al. 2007; Pyšek et al. 2008). Similarly, researchers preferentially use those research methods that are easier to put into practice. Finally, initial introduction seems to be much less studied than other phases of the invasion process (Puth and Post 2005). These difficulties could be overcome, at least partly, if review studies that summarize existing data and identify research gaps and biases, such as the one by Pyšek et al. (2008), would be undertaken more frequently. A coherent framework, like the hierarchy of hypotheses suggested above, could help structure such summaries.
Lack of Communication with the Public, and with Other Scientific Disciplines
In addition to the discussed possibilities for improvement of the scientific methodology of invasion ecology concerning theory and data, there is a considerable potential for improvement concerning communication. Enhanced communication of applied research results to relevant stakeholders could help advance implementation of existing knowledge into policy and management (see Driscoll et al. 2011; Jones-Walters and Çil 2011) (C6 in Table 3), and invasion ecology could profit considerably from an improved communication among scientists of different disciplines (e.g., community ecology, macroecology, biological control, weed science, conservation biology, global change biology, biogeography, and evolutionary biology; Davis et al. 2001; C7 in Table 3). A hierarchy of hypotheses could help implement knowledge exchange: a similar hierarchy could be developed for other disciplines, and these HoHs could be interconnected on a higher level.
As pointed out above, invasion processes are influenced by socio-economic and cultural activities in many different ways, which also creates the need for transdisciplinary research (C8 in Table 3). An increasing number of studies already advance in that direction, e.g., by analyzing historic catalogues (Dehnen-Schmutz et al. 2007; Blackburn et al. 2010), by explaining patterns in alien species richness based on indicators of current and historic socio-economic conditions (Hulme 2009; Essl et al. 2010), or by considering factors like economic value of species and invasions (Born et al. 2005; Gozlan et al. 2010). Another way to bridge the gap between ecology and social sciences is to combine vector science (Carlton and Ruiz 2005) with the study of continually shifting global decentralized networks (Barabási 2002).
Key Measure 3: Platforms for Improved Communication
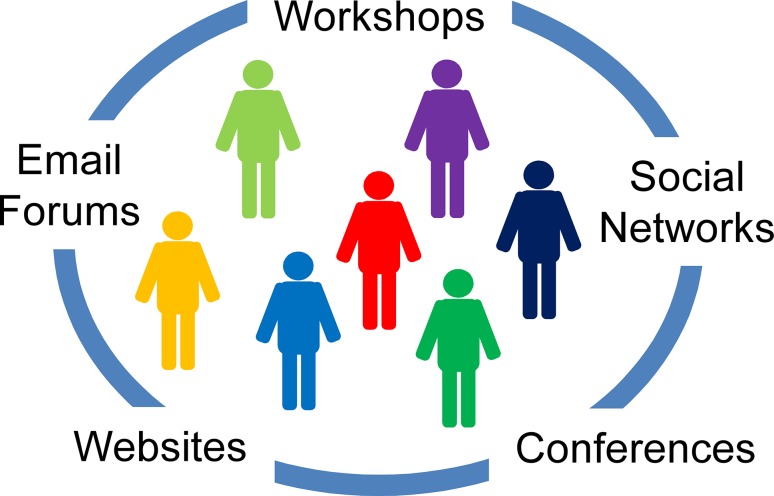
We suggest to establish platforms for improved communication among scientists of different disciplines and with other societal groups (Fig. 2). Conference series explicitly addressing biological invasions already exist (e.g., ‘Neobiota’ or ‘Biolief’). By inviting contributions from non-ecological disciplines, especially social sciences, these conferences could be used as forums for integrative, transdisciplinary research. Such transdisciplinary conferences would also benefit from frequent opportunities for open discussions. Moderated discussions in small groups can strongly promote the exchange of ideas and views, and are able to yield valuable insights. Smaller workshops addressing specific inter- or transdisciplinary questions would foster exchange of views and the development of novel approaches to invasion research. To permanently establish a culture of inter- and transdisciplinary communication at invasion conferences, it might be necessary to have one or more institutions guiding the process (cf. Aronson et al. 2010 concerning integrative communication in ecological economics). Therefore, existing organizations such as Neobiota (http://www.oekosys.tu-berlin.de/menue/neobiota/) should be used as a starting ground for such inter- and transdisciplinary efforts. Establishing an international transdisciplinary society for invasion science could be the next step.
Fig. 2.
Possibilities to improve communication among scientists of different disciplines, managers, politicians, and other stakeholders (represented by different colors)
The internet is providing possibilities for communication that should be better utilized for invasion research. In particular, social networks could be used for increasing communication among invasion scientists (cf. Nisbet et al. 2010 for similar recommendations to enhance communication regarding climate change research). Websites can also be set-up for citizen science approaches where volunteers can post the observations of alien species on a website (Dickinson et al. 2012; http://www.waarnemingen.be). Websites and apps of networks that connect science and policy can be very helpful as well, e.g., the Network-Forum for Biodiversity Research Germany (NeFo, http://www.biodiversity.de). It has been shown that stakeholders prefer free and easily accessible information on biological invasions (Bayliss et al. 2012). Two recently established websites (http://www.lifewatch.eu and http://www.congressgenetics.eu/) offer a combination of easily accessible information and communication platforms for researchers and stakeholders involved in biodiversity management. These initiatives could serve as a guide for launching a similar website for biological invasions. The HoH as described above could become the basis for such a website. It could become an evolving online platform, integrating knowledge from different subdisciplines and providing easy access to existing knowledge for other societal groups. Email forums, integrated in existing or newly founded organizations and invigorated at workshops and symposia, could further enhance communication within science as well as among scientists and other stakeholders.
Conclusion
This contribution is meant to increase awareness about existing difficulties in basic and applied invasion research, and to motivate efforts to more efficiently push to the limits of explanation, prediction, and management. Much can be done to increase clarity in communication, within science as well as between science, management, and the public. The proposed checklist for definitions can be useful to find a common language, and the proposed networking activities will provide opportunities to meet and exchange knowledge and ideas. Finally, the implementation of a hierarchy of hypotheses in invasion ecology can sharpen and synthesize existing hypotheses and can make scientific knowledge better available and thus more useful for understanding and managing invasions.
Acknowledgments
This paper summarizes the results of many fruitful discussions during the workshop “Tackling the emerging crisis of invasion biology: How can ecological theory, experiments, and field studies be combined to achieve major progress?” (March 2010 in Benediktbeuern, Germany; workshop of the specialist group “Theory in Ecology” of the Ecological Society of Germany, Austria and Switzerland, GfÖ), organized by TH, SH, ATP, and JMJ. We thank Laura Aquiloni, Silvia Bertocchi, Sara Brusconi, Alberto Inghilesi, Christiane Koch, Giuseppe Mazza, Roberto Merciai, Gabriele Orioli, and Elena Tricarico for their valuable contributions during this workshop. Katharina Dehnen-Schmutz as well as anonymous reviewers provided many critical comments that helped to improve the manuscript. The manuscript was reviewed through Peerage of Science (http://www.peerageofscience.org). Stella Copeland provided language corrections. JMJ acknowledges financial support from the DFG (JE 288/4-1).
Biographies
Tina Heger
is a postdoctoral researcher at Technische Universität München, Germany and University of California, Davis, USA. She is interested in conceptual ecology and biological invasions. Her current research focusses on the influence of evolutionary legacy on invasion success.
Anna T. Pahl
is a doctoral candidate in Restoration Ecology at the Technische Universität München. Her research in invasion ecology is mainly focused on the relevance of changes in the community context for the invasion success of alien plants.
Zoltan Botta-Dukát
is a senior researcher at the MTA Centre for Ecological Research. His main research topics are community assembly and biological invasion.
Francesca Gherardi
was a professor of zoology and conservation biology at the Department of Biology, University of Florence, Italy. Her interests were focused on invasion biology, biodiversity conservation, and eco-ethology. She passed away, and we miss her positive, motivating attitude as well as her valuable scientific contributions.
Christina Hoppe
is a doctoral candidate at the Institute of Ecology, Evolution and Diversity at the Goethe University of Frankfurt. Her main research interests include seed dispersal strategies of plant species, invasion ecology and conservation biology.
Ivan Hoste
studied history at Ghent University and has a job at the National Botanic Garden of Belgium since 1997. His research interests include the alien flora of Western Europe and the nineteenth- and twentieth-century history of botany in Belgium.
Kurt Jax
is a senior scientist at the Helmholtz-Centre for Environmental Research - UFZ, Germany, and professor of ecology at the Technische Universität München. His research interests are the theory and philosophy of ecology, with a special emphasis on linking the natural sciences and the humanities with respect to these fields.
Leena Lindström
is a University lecturer in Ecology and Evolutionary Biology at the University of Jyväskylä and her team is part of the Finnish Academy funded Centre of Excellence in Biological Interactions Research. Her research interests include evolutionary ecology of species invasions.
Pieter Boets
is a PhD student at the department of Applied Ecology, Laboratory of Environmental Toxicology and Aquatic Ecology at Ghent University, Belgium. His research interest is related to the impact and spread of alien macroinvertebrates in inland waters in Flanders (Belgium). Based on a combination of modelling techniques and laboratory experiments, he tries to gain insight into the complex ecology of invasive species.
Sylvia Haider
is a postdoctoral researcher at the Martin Luther University Halle Wittenberg, Germany. Her research focus lies on plant invasions in mountains with a special emphasis on distribution patterns and adaptation processes. She is part of the Consortium of the Mountain Invasion Research Network (MIREN, http://www.miren.ethz.ch).
Johannes Kollmann
is a professor in restoration ecology at the Technische Universität München. His scientific interest is focused on various aspects of ecological restoration, including plant provenances used for restoration and invasive alien plants.
Meike J. Wittmann
is a PhD student in biology at the University of Munich (LMU) where she develops stochastic models for the dynamics of introduced populations. More generally, she is interested in mathematical modelling in ecology and population genetics.
Jonathan M. Jeschke
is Principal Investigator at Technische Universität München, Germany, and Visiting Scientist at the Cary Institute of Ecosystem Studies, Millbrook, New York, USA. He held research positions at Ludwig-Maximilians-Universität München, Germany; Cary Institute of Ecosystem Studies, Millbrook, New York, USA; and University of Helsinki, Finland. His research interests focus on biological invasions, other novel organisms, biodiversity, predator–prey interactions, and other basic and applied ecological topics.
Contributor Information
Tina Heger, Phone: ++49-8161-714142, FAX: ++49-8161-714143, Email: t.heger@wzw.tum.de.
Anna T. Pahl, Email: a.pahl@wzw.tum.de
Zoltan Botta-Dukát, Email: botta-dukat.zoltan@okologia.mta.hu.
Christina Hoppe, Email: hoppe@bio.uni-frankfurt.de.
Ivan Hoste, Email: ivan.hoste@br.fgov.be.
Kurt Jax, Email: kurt.jax@ufz.de.
Leena Lindström, Email: leena.m.lindstrom@jyu.fi.
Pieter Boets, Email: pieter.boets@ugent.be.
Sylvia Haider, Email: Sylvia.haider@botanik.uni-halle.de.
Johannes Kollmann, Email: jkollmann@wzw.tum.de.
Meike J. Wittmann, Email: wittmann@bio.lmu.de
Jonathan M. Jeschke, Email: jonathan.jeschke@gmx.net
References
- Andreu J, Vilà M, Hulme PE. An assessment of stakeholder perception and management of noxious alien plants in Spain. Environmental Management. 2009;43:1244–1255. doi: 10.1007/s00267-009-9280-1. [DOI] [PubMed] [Google Scholar]
- Arndt E. Niche occupation by invasive ground-dwelling predator species in Canarian laurel forests. Biological Invasions. 2006;8:893–902. doi: 10.1007/s10530-005-0423-x. [DOI] [Google Scholar]
- Aronson, J., J.N. Blignaut, R.S. de Groot, A. Clewell, P.P. Lowry II, P. Woodworth, R.M. Cowling, D. Renison, et al. 2010. The road to sustainability must bridge three great divides. In Ecological economics reviews, ed. K. Limburg, and R. Costanza, 225–236. Boston: Blackwell. [DOI] [PubMed]
- Barabási A-L. Linked. The new science of networks. Cambridge: Perseus Publishing; 2002. [Google Scholar]
- Bardsley DK, Edwards-Jones G. Invasive species policy and climate change: Social perceptions of environmental change in the Mediterranean. Environmental Science & Policy. 2007;10:230–242. doi: 10.1016/j.envsci.2006.12.002. [DOI] [Google Scholar]
- Bayliss HR, Wilcox A, Stewart GB, Randall NP. Does research information meet the needs of stakeholders? Exploring evidence selection in the global management of invasive species. Evidence & Policy. 2012;8:37–56. doi: 10.1332/174426412X620128. [DOI] [Google Scholar]
- Berghöfer, U., R. Rozzi, and K. Jax. 2010. Many eyes on nature: Diverse perspectives in the Cap Horn Biosphere Reserve and their relevance for conservation. Ecology and Society 15: Art. 18. http://www.ecologyandsociety.org/vol15/iss1/art18/.
- Blackburn TM, Duncan RP. Determinants of establishment success in introduced birds. Nature. 2001;414:195–197. doi: 10.1038/35102557. [DOI] [PubMed] [Google Scholar]
- Blackburn TM, Lockwood JL, Cassey P. Avian invasions. Oxford: Oxford University Press; 2009. [Google Scholar]
- Blackburn TM, Gaston KJ, Parnell M. Changes in non-randomness in the expanding introduced avifauna of the world. Ecography. 2010;33:168–174. doi: 10.1111/j.1600-0587.2009.05882.x. [DOI] [Google Scholar]
- Blackburn TM, Pysek P, Bacher S, Carlton JT, Duncan RP, Jarosík V, Wilson JRU, Richardson DM. A proposed unified framework for biological invasions. Trends in Ecology & Evolution. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Blakeslee AMH, Byers JE. Using parasites to inform ecological history: Comparisons among three congeneric marine snails. Ecology. 2008;89:1068–1078. doi: 10.1890/07-0832.1. [DOI] [PubMed] [Google Scholar]
- Blumenthal DM. Interactions between resource availability and enemy release in plant invasion. Ecology Letters. 2006;9:887–895. doi: 10.1111/j.1461-0248.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- Bodey TW, Bearhop S, Roy SS, Newton J, McDonald RA. Behavioural responses of invasive American mink Neovison vison to an eradication campaign, revealed by stable isotope analysis. Journal of Applied Ecology. 2010;47:114–120. doi: 10.1111/j.1365-2664.2009.01739.x. [DOI] [Google Scholar]
- Born W, Rauschmayer F, Bräuer I. Economic evaluation of biological invasions—A survey. Ecological Economics. 2005;55:321–336. doi: 10.1016/j.ecolecon.2005.08.014. [DOI] [Google Scholar]
- Bremner A, Park K. Public attitude to the management of invasive non-native species in Scotland. Biological Conservation. 2007;139:306–314. doi: 10.1016/j.biocon.2007.07.005. [DOI] [Google Scholar]
- Burt J, Muir A, Piovia-Scott J, Veblen K, Chang A, Grossman J, Weiskel H. Preventing horticultural introductions of invasive plants: Potential efficacy of voluntary initiatives. Biological Invasions. 2007;9:909–923. doi: 10.1007/s10530-007-9090-4. [DOI] [Google Scholar]
- Byron J. Safe alternatives to replace invasives in California gardens. California Agriculture. 2008;62:88–89. [Google Scholar]
- Callaway RM, Ridenour WM. Novel weapons: Invasive success and the evolution of increased competitive ability. Frontiers in Ecology and the Environment. 2004;2:436–443. doi: 10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2. [DOI] [Google Scholar]
- Capers RS, Selsky R, Bugbee GJ, White JC. Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology. 2007;88:3135–3143. doi: 10.1890/06-1911.1. [DOI] [PubMed] [Google Scholar]
- Carey MP, Sanderson BL, Barnas KA, Olden JD. Native invaders—Challenges for science, management, policy, and society. Frontiers in Ecology and the Environment. 2012;10:373–381. doi: 10.1890/110060. [DOI] [Google Scholar]
- Carlsson NOL, Jeschke JM, Holmqvist N, Kindberg J. Long-term data on invaders: When the fox is away, the mink will play. Biological Invasions. 2010;12:633–641. doi: 10.1007/s10530-009-9470-z. [DOI] [Google Scholar]
- Carlton, J.T., and G.M. Ruiz. 2005. Vector science and integrated vector management in bioinvasion ecology: Conceptual frameworks. In Invasive alien species. A new synthesis, ed. H.A. Mooney, R.N. Mack, J.A. McNeely, L.E. Neville, P.J. Schei, and J.K. Waage, 36–58. Washington, DC: Island Press.
- Carpenter D, Cappuccino N. Herbivory, time since introduction and the invasiveness of exotic plants. Journal of Ecology. 2005;93:315–321. doi: 10.1111/j.1365-2745.2005.00973.x. [DOI] [Google Scholar]
- Carthey AJR, Banks PB. When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE. 2012;7:e31804. doi: 10.1371/journal.pone.0031804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassey P, Blackburn TM, Duncan RP, Lockwood JL. Lessons from the establishment of exotic species: A meta-analytical case study using birds. Journal of Animal Ecology. 2005;74:250–258. doi: 10.1111/j.1365-2656.2005.00918.x. [DOI] [Google Scholar]
- Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions. 2009;15:22–40. doi: 10.1111/j.1472-4642.2008.00521.x. [DOI] [Google Scholar]
- Chew M. Ending with Elton: Preludes to invasion biology. Tempe: Arizona State University; 2006. [Google Scholar]
- Colautti RI, MacIsaac HJ. A neutral terminology to define ‘invasive’ species. Diversity and Distributions. 2004;10:135–141. doi: 10.1111/j.1366-9516.2004.00061.x. [DOI] [Google Scholar]
- Davis MA. Invasion biology. Oxford: Oxford University Press; 2009. [Google Scholar]
- Davis, M.A. 2011. Researching invasive species 50 years after Elton: A cautionary tale. In Fifty years of invasion ecology: The legacy of Charles Elton, ed. D.M. Richardson, 269–276. Oxford: Blackwell Publishing.
- Davis MA, Thompson K, Grime JP. Charles S. Elton and the dissociation of invasion ecology from the rest of ecology. Diversity and Distributions. 2001;7:97–102. doi: 10.1046/j.1472-4642.2001.00099.x. [DOI] [Google Scholar]
- Dehnen-Schmutz K, Williamson M. Rhododendron ponticum in Britain and Ireland: social, economic and ecological factors in its successful invasion. Environment and History. 2006;12:325–350. doi: 10.3197/096734006778226355. [DOI] [Google Scholar]
- Dehnen-Schmutz K, Touza J, Perrings C, Williamson M. The horticultural trade and ornamental plant invasions in Britain. Conservation Biology. 2007;21:224–231. doi: 10.1111/j.1523-1739.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- Dickinson JL, Shirk J, Bonter D, Bonney R, Crain RL, Martin J, Phillips T, Purcell K. The current state of citizen science as a tool for ecological research and public engagement. Frontiers in Ecology and the Environment. 2012;10:291–297. doi: 10.1890/110236. [DOI] [Google Scholar]
- Drake JA, Mooney HA, Di Castri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M, editors. Biological invasions: A global perspective. Chichester: Wiley; 1989. [Google Scholar]
- Driscoll CT, Kathy Fallon L, Weathers KC. Integrating science and policy: A case study of the Hubbard Brook Research Foundation Science Links Program. BioScience. 2011;61:791–801. doi: 10.1525/bio.2011.61.10.9. [DOI] [Google Scholar]
- Ebeling SK, Hensen I, Auge H. The invasive shrub Buddleja davidii performs better in its introduced range. Diversity and Distributions. 2008;14:225–233. doi: 10.1111/j.1472-4642.2007.00422.x. [DOI] [Google Scholar]
- Elton CS. The ecology of invasions by animals and plants. London: Methuen & Co. Ltd.; 1958. [Google Scholar]
- Essl F, Dullinger S, Rabitsch W, Hulme PE, Hülber K, Jarošík V, Kleinbauer I, Krausmann F, et al. Socioeconomic legacy yields an invasion debt. Proceedings of the National Academy of Sciences of the United States of America. 2010;108:203–207. doi: 10.1073/pnas.1011728108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, van der Wal R. Invasive plant suppresses charismatic seabird—The construction of attitudes towards biodiversity management options. Biological Conservation. 2007;135:256–267. doi: 10.1016/j.biocon.2006.10.026. [DOI] [Google Scholar]
- Frenzel M, Brandl R. Diversity and abundance patterns of phytophagous insect communities on alien and native host plants in the Brassicaceae. Ecography. 2003;26:723–730. doi: 10.1111/j.0906-7590.2003.03649.x. [DOI] [Google Scholar]
- Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, et al. The invasion paradox: Reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gellis Communications. 2008. Scoping study for an EU wide communications campaign on biodiversity and nature. Final report to the Commission/DG ENV.
- Gherardi, F. 2011. Public perception of invasive alien species in Mediterranean Europe. In Invasive and introduced plants and animals: Human perceptions, attitudes and approaches to management, ed. R.A. Lambert, and I.D. Rotherham, 185–200. London: Earthscan.
- Gozlan RE. Introduction of non native freshwater fish: Is it all bad? Fish and Fisheries. 2008;9:106–115. doi: 10.1111/j.1467-2979.2007.00267.x. [DOI] [Google Scholar]
- Gozlan RE, Britton JR, Cowx I, Copp GH. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology. 2010;76:751–786. doi: 10.1111/j.1095-8649.2010.02566.x. [DOI] [Google Scholar]
- Gurevitch J, Howard IW, Ashton EA, Leger EA, Howe E, Woo E, Lerdau M. Effects of experimental manipulation of light and nutrients on establishment of seedlings of native and invasive woody species in Long Island, NY forests. Biological Invasions. 2008;10:821–831. doi: 10.1007/s10530-008-9241-2. [DOI] [Google Scholar]
- Gurevitch J, Fox GA, Wardle GM, Inderjit, Taub D. Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecology Letters. 2011;14:407–418. doi: 10.1111/j.1461-0248.2011.01594.x. [DOI] [PubMed] [Google Scholar]
- Hayes KR, Barry SC. Are there any consistent predictors of invasion success? Biological Invasions. 2008;10:483–506. doi: 10.1007/s10530-007-9146-5. [DOI] [Google Scholar]
- Heger, T., W.-C. Saul, and L. Trepl. in press. What biological invasions ‘are’ is a matter of perspective. Journal for Nature Conservation. doi:10.1016/j.jnc.2012.11.002.
- Heger T, Trepl L. Predicting biological invasions. Biological Invasions. 2003;5:313–321. doi: 10.1023/B:BINV.0000005568.44154.12. [DOI] [Google Scholar]
- Hodges KE. Defining the problem: Terminology and progress in ecology. Frontiers in Ecology and the Environment. 2008;6:35–42. doi: 10.1890/060108. [DOI] [Google Scholar]
- Hoste I, van Moorsel R, Barendse R. Een nieuwkomer in sierteeltbedrijven en tuinen: Cardamine corymbosa in Nederland en België. Dumortiera. 2008;93:15–24. [Google Scholar]
- Hulme PE. Beyond control: Wider implications for the management of biological invasions. Journal of Applied Ecology. 2006;43:835–847. doi: 10.1111/j.1365-2664.2006.01227.x. [DOI] [Google Scholar]
- Hulme PE. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. Journal of Applied Ecology. 2009;46:10–18. doi: 10.1111/j.1365-2664.2008.01600.x. [DOI] [Google Scholar]
- ISSG. 2000. IUCN Guidelines for the prevention of biodiversity loss caused by alien invasive species. http://intranet.iucn.org/webfiles/doc/SSC/SSCwebsite/Policy_statements/IUCN_Guidelines_for_the_Prevention_of_Biodiversity_Loss_caused_by_Alien_Invasive_Species.pdf.
- Jeschke JM. Across islands and continents, mammals are more successful invaders than birds (Reply to Rodriguez-Cabal et al.) Diversity and Distributions. 2009;15:913–914. doi: 10.1111/j.1472-4642.2009.00584.x. [DOI] [Google Scholar]
- Jeschke JM, Genovesi P. Do biodiversity and human impact influence the introduction or establishment of alien mammals? Oikos. 2011;120:57–64. doi: 10.1111/j.1600-0706.2010.18621.x. [DOI] [Google Scholar]
- Jeschke JM, Gómez Aparicio L, Haider S, Heger T, Lortie CJ, Pyšek P, Strayer DL. Taxonomic bias and lack of cross-taxonomic studies in invasion biology. Frontiers in Ecology and the Environment. 2012;10:349–350. doi: 10.1890/12.WB.016. [DOI] [Google Scholar]
- Jeschke JM, Gómez Aparicio L, Haider S, Heger T, Lortie CJ, Pyšek P, Strayer DL. Support for major hypotheses in invasion biology is uneven and declining. NeoBiota. 2012;14:1–20. doi: 10.3897/neobiota.14.3435. [DOI] [Google Scholar]
- Jogesh T, Carpenter D, Cappuccino N. Herbivory on invasive exotic plants and their non-invasive relatives. Biological Invasions. 2008;10:797–804. doi: 10.1007/s10530-008-9236-z. [DOI] [Google Scholar]
- Jones-Walters L, Çil A. Biodiversity and stakeholder participation. Journal for Nature Conservation. 2011;19:327–329. doi: 10.1016/j.jnc.2011.09.001. [DOI] [Google Scholar]
- Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. doi: 10.1016/S0169-5347(02)02499-0. [DOI] [Google Scholar]
- Kowarik I. Human agency in biological invasions: Secondary releases foster naturalisation and population expansion of alien plant species. Biological Invasions. 2003;5:293–312. [Google Scholar]
- Küffer, C., and G. Hirsch Hadorn. 2008. How to achieve effectiveness in problem-oriented landscape research: The example of research on biotic invasions. Living Reviews in Landscape Research 2: 2. http://landscaperesearch.livingreviews.org/Articles/lrlr-2008-2/.
- Lambert RA, Rotherham ID, editors. Invasive and introduced plants and animals: Human perceptions, attitudes and approaches to management. London: Earthscan; 2011. [Google Scholar]
- Levine JM. Species diversity and biological invasions: Relating local process to community pattern. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- Levine JM, D’Antonio CM. Elton revisited: A review of evidence linking diversity and invasibility. Oikos. 1999;87:15–26. doi: 10.2307/3546992. [DOI] [Google Scholar]
- Lewis KC, Bazzaz FA, Liao Q, Orians CM. Geographic patterns of herbivory and resource allocation to defense, growth, and reproduction in an invasive biennial, Alliaria petiolata. Oecologia. 2006;148:384–395. doi: 10.1007/s00442-006-0380-9. [DOI] [PubMed] [Google Scholar]
- Liu H, Stiling P, Pemberton RW. Does enemy release matter for invasive plants? Evidence from a comparison of insect herbivore damage among invasive, non-invasive and native congeners. Biological Invasions. 2007;9:773–781. doi: 10.1007/s10530-006-9074-9. [DOI] [Google Scholar]
- Lockwood JL, Hoopes MF, Marchetti MP. Invasion ecology. Malden: Blackwell Publishing; 2007. [Google Scholar]
- Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Diversity and Distributions. 2009;15:904–910. doi: 10.1111/j.1472-4642.2009.00594.x. [DOI] [Google Scholar]
- Lodge DM. Biological invasions: Lessons for ecology. Trends in Ecology & Evolution. 1993;8:133–137. doi: 10.1016/0169-5347(93)90025-K. [DOI] [PubMed] [Google Scholar]
- Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard SE, Mack RN, Moyle PB, et al. Biological invasions: Recommendations for U.S. policy and management. Ecological Applications. 2006;16:2035–2054. doi: 10.1890/1051-0761(2006)016[2035:BIRFUP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2. [DOI] [Google Scholar]
- Mattingly BW, Hewlate R, Reynolds HL. Species evenness and invasion resistance of experimental grassland communities. Oikos. 2007;116:1164–1170. doi: 10.1111/j.0030-1299.2007.15406.x. [DOI] [Google Scholar]
- McGeoch MA, Butchardt SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M. Global indicators of biological invasions: Species numbers, biodiversity impact and policy responses. Diversity and Distributions. 2010;16:95–108. doi: 10.1111/j.1472-4642.2009.00633.x. [DOI] [Google Scholar]
- McGeoch MA, Spear D, Kleynhans EJ, Marais E. Uncertainty in invasive alien species listing. Ecological Applications. 2012;22:959–971. doi: 10.1890/11-1252.1. [DOI] [PubMed] [Google Scholar]
- Meiners SJ, Cadenasso ML, Pickett STA. Beyond biodiversity: Individualistic controls of invasion in a self-assembled community. Ecology Letters. 2004;7:121–126. doi: 10.1111/j.1461-0248.2003.00563.x. [DOI] [Google Scholar]
- Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P. The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecologica. 2000;21:213–222. doi: 10.1016/S1146-609X(00)00124-7. [DOI] [Google Scholar]
- Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- Moles AT, Flores-Moreno H, Bonser SP, Warton DI, Helm A, Warman L, Eldridge DJ, Jurado E, et al. Invasions: The trail behind, the path ahead, and a test of a disturbing idea. Journal of Ecology. 2012;100:116–127. doi: 10.1111/j.1365-2745.2011.01915.x. [DOI] [Google Scholar]
- Moore JL, Rout TM, Hauser CE, Moro D, Jones M, Wilcox C, Possingham HP. Protecting islands from pest invasion: Optimal allocation of biosecurity resources between quarantine and surveillance. Biological Conservation. 2010;143:1068–1078. doi: 10.1016/j.biocon.2010.01.019. [DOI] [Google Scholar]
- Myster RM. Tree invasion and establishment in old fields at Hutcheson Memorial Forest. Botanical Reviews. 1993;59:251–272. doi: 10.1007/BF02857418. [DOI] [Google Scholar]
- Niggemann M, Jetzkowitz J, Brunzel S, Wichmann MC, Bialozyt R. Distribution patterns of plants explained by human movement behaviour. Ecological Modelling. 2009;220:1339–1346. doi: 10.1016/j.ecolmodel.2009.02.018. [DOI] [Google Scholar]
- Nisbet MC, Hixon MA, Moore KD, Nelson M. Four cultures: New synergies for engaging society on climate change. Frontiers in Ecology and the Environment. 2010;8:329–331. doi: 10.1890/1540-9295-8.6.329. [DOI] [Google Scholar]
- Perrings C, Mooney HA, Williamson M, editors. Bioinvasions and globalization. Ecology, economics, management, and policy. Oxford: Oxford University Press; 2010. [Google Scholar]
- Perrings, C., S. Burgiel, M. Lonsdale, H.A. Mooney, and M. Williamson. 2010b. Globalization and bioinvasions: The international policy problem. In Bioinvasions and globalization. Ecology, economics, management, and policy, ed. C. Perrings, H.A. Mooney, and M. Williamson, 235–250. Oxford: Oxford University Press.
- Puth LM, Post DM. Studying invasion: Have we missed the boat? Ecology Letters. 2005;8:715–721. doi: 10.1111/j.1461-0248.2005.00774.x. [DOI] [Google Scholar]
- Pyšek P, Richardson DM, Rejmánek M, Webster GL, Williamson M, Kirscher J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon. 2004;53:131–143. doi: 10.2307/4135498. [DOI] [Google Scholar]
- Pyšek P, Richardson DM, Pergl J, Jarosik V, Sixtova Z, Weber E. Geographical and taxonomic biases in invasion ecology. Trends in Ecology & Evolution. 2008;23:237–244. doi: 10.1016/j.tree.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Reichard SH, Hamilton CW. Predicting invasions of woody plants introduced into North America. Conservation Biology. 1997;11:193–203. doi: 10.1046/j.1523-1739.1997.95473.x. [DOI] [Google Scholar]
- Richardson DM, editor. Fifty years of invasion ecology: The legacy of Charles Elton. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- Richardson, D.M. 2011b. Invasion science: The roads travelled and the roads ahead. In Fifty years of invasion ecology: The legacy of Charles Elton, ed. D.M. Richardson, 397–407. Chichester: Wiley-Blackwell.
- Richardson DM, Pyšek P. Elton, C.S. 1958: The ecology of invasions by animals and plants. London: Methuen. Progress in Physical Geography. 2007;31:659–666. doi: 10.1177/0309133307087089. [DOI] [Google Scholar]
- Richardson DM, Pyšek P. Fifty years of invasion ecology: The legacy of Charles Elton. Diversity and Distributions. 2008;14:161–168. doi: 10.1111/j.1472-4642.2007.00464.x. [DOI] [Google Scholar]
- Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ. Naturalization and invasion of alien plants: Concepts and definitions. Diversity and Distributions. 2000;6:93–107. doi: 10.1046/j.1472-4642.2000.00083.x. [DOI] [Google Scholar]
- Richardson DM, Pyšek P, Carlton JT. A compendium of essential concepts and terminology in invasion ecology. In: Richardson DM, editor. Fifty years of invasion ecology: The legacy of Charles Elton. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- Rodriguez-Cabal M, Barrios-Garcia N, Simberloff D. Across island and continents, mammals are more successful invaders than birds (Reply) Diversity and Distributions. 2009;15:911–912. doi: 10.1111/j.1472-4642.2009.00583.x. [DOI] [Google Scholar]
- Sax, D.F., J.H. Brown, E.P. White, and S.D. Gaines. 2005. The dynamics of species invasions. Insights into the mechanisms that limit species diversity. In Species invasions: Insights into ecology, evolution, and biogeography, ed. D.F. Sax, J.J. Stachowicz, and S.D. Gaines, 447–465. Sunderland: Sinauer.
- Simberloff D. The role of propagule pressure in biological invasions. Annual Review of Ecology Evolution and Systematics. 2009;40:81–102. doi: 10.1146/annurev.ecolsys.110308.120304. [DOI] [Google Scholar]
- Skou, A.M.T., S. Pauleit, and J. Kollmann. in press. Tracing the introduction history of a potentially invasive ornamental shrub—Variation in frost hardiness and climate change. Nordic Journal of Botany. doi:10.1111/j.1756-1051.2012.01399.x.
- Stohlgren TJ, Barnett DT, Kartesz J. The rich get richer: Patterns of plant invasions in the United States. Frontiers in Ecology and the Environment. 2003;1:11–14. doi: 10.1890/1540-9295(2003)001[0011:TRGRPO]2.0.CO;2. [DOI] [Google Scholar]
- Stohlgren TJ, Jarnevich C, Chong GW, Evangelista PH. Scale and plant invasions: A theory of biotic acceptance. Preslia. 2006;78:405–426. [Google Scholar]
- Strayer DL, Eviner VT, Jeschke JM, Pace ML. Understanding the long-term effects of species invasions. Trends in Ecology & Evolution. 2006;21:645–651. doi: 10.1016/j.tree.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Sugiura S. Associations of leaf miners and leaf gallers with island plants of different residency histories. Journal of Biogeography. 2010;37:237–244. doi: 10.1111/j.1365-2699.2009.02199.x. [DOI] [Google Scholar]
- Tatem AJ. The worldwide airline network and the dispersal of exotic species: 2007–2010. Ecography. 2009;32:94–102. doi: 10.1111/j.1600-0587.2008.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Beest M, Stevens N, Olff H, van der Putten WH. Plant–soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. Journal of Ecology. 2009;97:1281–1290. doi: 10.1111/j.1365-2745.2009.01574.x. [DOI] [Google Scholar]
- Valéry L, Hervé F, Levfeuvre J-C, Simberloff D. In search of a real definition of the biological invasion phenomenon itself. Biological Invasions. 2008;10:1345–1351. doi: 10.1007/s10530-007-9209-7. [DOI] [Google Scholar]
- van Kleunen M, Dawson W, Schlaepfer D, Jeschke JM, Fischer M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecology Letters. 2010;13:947–958. doi: 10.1111/j.1461-0248.2010.01503.x. [DOI] [PubMed] [Google Scholar]
- Verbrugge LNH, Leuven RSEW, Velde Gvd. Evaluation of international risk assessment protocols for exotic species. Series of Reports on Environmental Science: Radboud University Nijmegen; 2010. [Google Scholar]
- Vignon M, Sasal P, Galzin R. Host introduction and parasites: A case study on the parasite community of the peacock grouper Cephalopholis argus (Serranidae) in the Hawaiian Islands. Parasitology Research. 2009;104:775–782. doi: 10.1007/s00436-008-1254-3. [DOI] [PubMed] [Google Scholar]
- Walther G-R, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, et al. Alien species in a warmer world: Risks and opportunities. Trends in Ecology & Evolution. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Webb DA. What are the criteria for presuming native status? Watsonia. 1985;15:231–236. [Google Scholar]
- Wilsey BJ, Polley HW. Reductions in grassland species evenness increase dicot seedling invasion and spittle bug infestation. Ecology Letters. 2002;5:676–684. doi: 10.1046/j.1461-0248.2002.00372.x. [DOI] [Google Scholar]
- Wilson JRU, Proches S, Braschler B, Dixon ES, Richardson DM. The (bio)diversity of science reflects the interests of society. Frontiers in Ecology and the Environment. 2007;5:409–414. [Google Scholar]
- Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM. Something in the way you move: Dispersal pathways affect invasion success. Trends in Ecology & Evolution. 2009;24:136–144. doi: 10.1016/j.tree.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Wolfe LM. Why alien invaders succeed: Support for the escape-from-enemy hypothesis. The American Naturalist. 2002;160:705–711. doi: 10.1086/343872. [DOI] [PubMed] [Google Scholar]
- Zedler JB, Kercher S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences. 2004;23:431–452. doi: 10.1080/07352680490514673. [DOI] [Google Scholar]