Abstract
Purpose
Normal progression of osteoporosis or the rigid reinforcement of the fractured vertebral body with polymethyl methacrylate (PMMA) cement is being discussed as a cause for adjacent-level fractures after vertebroplasty. The purpose of this study was to investigate whether augmentation with low stiffness cement can decrease the risk of adjacent-level fractures in low-quality bone.
Methods
Eighteen female osteoporotic lumbar specimens (L1–L5) were harvested and divided into three groups according to bone mineral density: (I) native; (II) PMMA; (III) modified PMMA (lower stiffness). For the PMMA and modified PMMA groups, a compression fracture was first mechanically induced in L3, and then the fracture received vertebroplasty treatment. The cement stiffness reduction of the modified PMMA group was achieved via an addition of 8 mL of serum to the typical PMMA base. All specimens were exposed to cyclic loading (4 Hz) and a stepwise increasing applied peak force. Cement stiffness was tested according to ISO 5833.
Results
A 51 % decrease in cement stiffness was achieved in the modified PMMA group (954 ± 141 vs. 1,937 ± 478 MPa, p < 0.001). Fatigue fracture force (the force level during cyclic loading at which the deformation experienced a sudden increase; FFF) was significantly affected by bone quality (r2 = 0.39, p = 0.006) and by the initial fracture force (the force necessary to create the initial fracture in L3 prior to augmentation; r2 = 0.82, p < 0.001). Using initial fracture force as a covariate, the FFF of the modified PMMA group (1,764 ± 49 N) was significantly higher than in the PMMA group (1,544 ± 55 N; p = 0.03).
Conclusions
A possible method to reduce adjacent-level fractures after vertebroplasty in patients with reduced bone quality could be the use of a lower modulus cement. Therefore, mixing cement with biocompatible fluids could prove useful to tailor cement properties in the operating theater.
Keywords: Vertebroplasty, Osteoporosis, Adjacent-level fracture, Bone cement, Cement stiffness
Introduction
Vertebral fractures are recognized as an indicator of osteoporosis and represent a significant burden to the individual and public health system worldwide [1–3]. A conservative therapy leads to loss of fitness and therefore to objectionable side effects [4]. A successful and established way of treating vertebral compression fracture is percutaneous vertebral augmentation [5–9].
Even with the success of vertebroplasty, there is one looming negative side effect that has been shown: vertebral body fractures adjacent to augmented vertebrae appear sooner than non-adjacent fractures [10–12]. There are two prevalent theories: they could either be due to normal progression of osteoporosis [10] or they could be provoked by the rigid reinforcement of the fractured vertebral body with PMMA [13, 14]. PMMA is known to have a seven to ten times higher elastic modulus than cancellous bone [13]. This might be a reason for a change of the biomechanical properties in the augmented spinal segment, which then leads to an increasing risk of adjacent fractures, especially in osteoporotic patients [15].
If the stiffness increase caused by PMMA was provoking the onset of adjacent-level fracture, then one strategy to reduce the incidence of adjacent segment fractures could be to modify the cement used in vertebroplasty.
Several groups have undertaken different approaches in an attempt to decrease the stiffness of bone cement. Ahn et al. [16] mixed PMMA with human blood and were able to show a stepwise decrease of the Young`s modulus from 915.5 to 545.6 MPa. Boger et al. [13] created a low-modulus cement (470 ± 30 MPa) by mixing commercially available PMMA with an aqueous fraction of 35 % sodium hyaluronate. They then tested the modified cement in non-fractured, single functional spine units (FSU) and compared the failure strength to both a group treated with normal PMMA and a native group. They did not show any significant difference in failure strength of the prophylactically treated single FSUs. This study leads to a belief that a reduction in the cement stiffness to this degree (74 %) will not significantly reduce the stability achieved by a vertebroplasty [13].
The aim of this study was to investigate whether augmentation with low-modulus cement in a multi-segmental osteoporotic spine using a reproducible fracture model can decrease the risk of adjacent-level fractures without early re-failure of the fractured vertebra by the evaluation of fatigue fracture force (FFF). For the first time, commercially available PMMA was mixed with fetal bovine serum as a biocompatible, pore-forming fluid to decrease the Young's modulus near to that of cancellous bone (50–800 MPa) [17].
Materials and methods
Specimens and preparation
Eighteen osteoporotic (t score −3.3 ± 0.67) lumbar (L1−L5) specimens were harvested from female donors after informed consent. All donors were over 60 years of age (78.3 ± 9.96 years). Directly after harvesting, specimens were sealed in plastic bags and stored at −22 °C until testing. Specimens were assessed via X-ray and manual inspection prior to testing, and those with pathologic changes (i.e., prior fractures or metastasis) were excluded. Spines were allocated to three groups on the basis of achieving a similar average BMD in each group: (I) native; (II) PMMA; (III) modified PMMA (lower stiffness).
The geometry of the specimens and their BMD were recorded by pQCT (XCT-2000; Stratec Medizintechnik, Pforzheim, Germany). The pQCT machine was calibrated using a standard phantom and a cone phantom provided by the manufacturer. A 2-mm-thick single tomographic slice (pixel size 0.59 × 0.59 mm) of L3 was captured in the transverse plane which passed through the midpoint of the vertebral body. Image processing and BMD calculation (T score and total bone mineral density) were completed using the manufacturer’s software package CXCT550 (version 5.50D).
Prior to testing, all specimens were defrosted overnight. All preparations were done on the testing day. The soft tissues including the vertebral discs on T12/L1 and L5/S1 were dissected, preserving all ligaments. L1 and L5 were embedded using a potting frame to ensure the parallel alignment of the cranial L3 endplate with the embedding plates. To preserve soft tissue constitution, the specimen was sprayed with Ringer solution throughout preparation and testing.
Creation of compression fracture
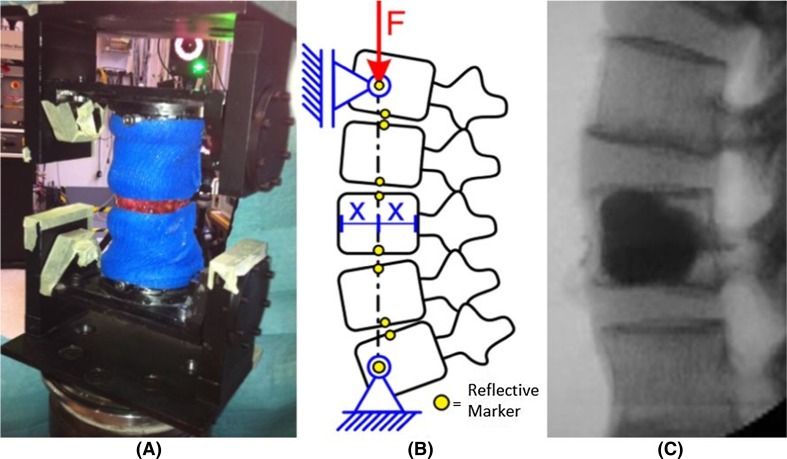
For the PMMA and the modified PMMA groups, a method introduced by Huber et al. [18] was used to create a defined compression fracture in L3 within a multi-segmental spinal specimen (L1–L5). Three holes (Ø 2.7 mm) were drilled into the anterior shell of the L3 vertebra. The functional spinal units L1/L2 and L4/L5 were supported by plaster casts to ensure fracture only at L3 (Fig. 1a). The entire construct was then exposed to a ramp loading using a servo-hydraulic testing machine (858 Bionix®, MTS, Eden Prairie, MN, USA) which induced unrestricted, coupled flexion–compression forces on the specimen via two rotational axes located on the cranial and caudal ends of the specimen until failure (Fig. 1b). The effective compressive load vector passed through the center of the anterior column of L3. After fracture, the specimen was exposed to 1,000 cycles with 100 N axial load at 4 Hz to create a wedge-shaped fracture. The plaster casts were removed after procedure completion.
Fig. 1.
a Specimen wrapped in a plaster cast with L3 exposed before undergoing compressive loading to produce the initial fracture. b Schematic of mechanical test setup for fracture initiation and fatigue loading. c Representative radiograph of a specimen after vertebroplasty augmentation of the induced fracture of L3
Cement augmentation
Cement used for the PMMA group was a commercially available cement (Vertecem V+, Synthes GmbH, Oberdorf, Switzerland; Young’s modulus approximately 1,800 MPa; 26 g powder and 10 mL liquid). For the modified PMMA group, 8 mL fetal bovine serum (Invitrogen GmbH, Karlsruhe, Germany) was added when mixing the powder and the liquid to achieve the desired stiffness reduction which would be closer to that of the natural bone. The stiffness of both the standard and the modified cements were determined using ISO 5833.
The fractured vertebral body (L3) was augmented via vertebroplasty for the PMMA and modified PMMA groups (Fig. 1c). Cements were mixed according to the manufacturer’s instructions. Cement was injected into the vertebral body until a sufficient filling from endplate to endplate could be observed to achieve the biomechanically adequate volume of 6.0 ± 2.3 mL for the lumbar spine [19]. Therefore, the amount of cement injected depended on the vertebral volume. The augmentation was done under fluoroscopy guidance (Fig. 1c) by an experienced spine surgeon.
Biomechanical testing
Specimens were mounted in the same test rig used for fracture creation. Fatigue experiments were started 120 min after cement application. All specimens were exposed to force-controlled, cyclic loading (4 Hz) which induced coupled flexion–compression forces. The increasing load forced the specimens to a greater height loss (creep) as well as an increase in kyphosis of the specimen. The applied peak force was increased stepwise using a Locati [20] test design by 100 N every 1,000 cycles starting from 100 N. The Locati method was chosen because it induces fatigue failure sooner than the typical fatigue test which uses a constant peak load. This was desirable to stimulate specimen failure prior to tissue degradation. Testing was stopped at a displacement of 30 mm. Specimens were tested at room temperature, soaked in Ringer solution and wrapped in plastic wrap to keep tissue moisture during testing.
Data analysis
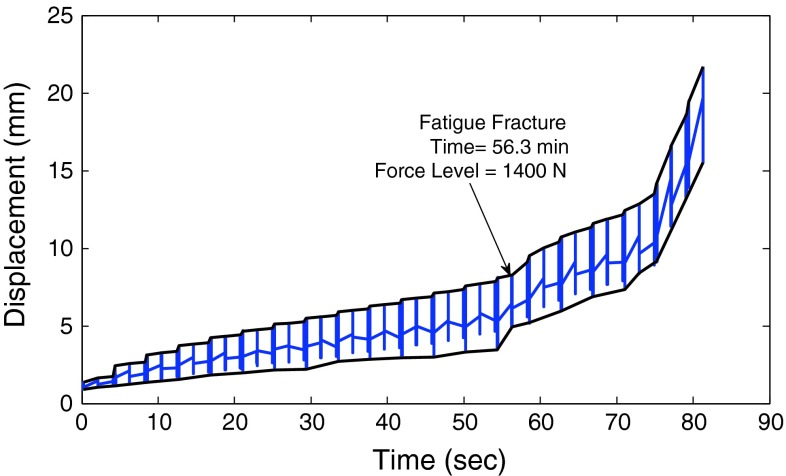
For the two vertebroplasty groups (PMMA and modified PMMA) which underwent the initial fracture of L3, both the initial fracture force (IFF) and the initial specimen stiffness were determined from data gathered during fracture creation. The IFF was defined as the peak force, which was followed by a drop in force of greater than 5 % during fracture creation. The initial specimen stiffness was determined from the slope of the force–displacement curve within the linear region (omitted first 20 % and last 15 % of data points before the IFF). For all specimens, the FFF was defined as the force level during cyclic loading at which the deformation experienced a sudden increase in the seating of the specimen (exhibited as a distinct increase in the displacement versus time graph, Fig. 2). This sudden increase in displacement has been shown previously to indicate bony fracture [21, 22]. After fatigue loading was completed, the fracture levels were determined by physical examination of the specimens.
Fig. 2.
A representative displacement versus time curve highlighting the point of fatigue fracture of the specimen. The fatigue fracture force was defined as the force level during cyclic loading at which the deformation experienced a sudden increase in the seating of the specimen
Statistical analysis was performed using a one-way ANOVA with testing group being the between samples factor with three levels (native, PMMA, modified PMMA). All r2 values presented are adjusted r2 determined via a linear regression analysis. A type I error probability of 5 % was used for all analysis (PASW Statistics 18, Chicago, IL, USA). A one-way analysis of covariance (ANCOVA) was used to determine the effects of testing group on FFF while adjusting for the presence of the covariate IFF. The independent variable, testing group, included only two levels, PMMA and modified PMMA, because the native group did not undergo an initial fracture. All assumptions were met, particularly homogeneity of the regression slopes was present for the covariate (IFF) and the covariate was linearly related to FFF.
Results
For each group, the specimen characteristics are given in Table 1. Two spines had to be excluded—due to a previously existing fracture in L3 and to a posterior ligament rupture during fracture creation. The specimens were equally divided into three groups (native, PMMA, modified PMMA) according to BMD (p = 0.99).
Table 1.
Specimen characteristics classified by experimental group with averages and standard deviations
| Group | N | Age (years) | T-score | BMD (mg/cm2) | BMD × endplate area (mg) |
|---|---|---|---|---|---|
| Native | 5 | 82.8 ± 7.7 | −3.34 ± 0.87 | 185.8 ± 51.2 | 2,651 ± 759 |
| PMMA | 5 | 72.4 ± 10.2 | −3.28 ± 0.73 | 189.7 ± 41.9 | 2,762 ± 484 |
| Modified PMMA | 6 | 79.5 ± 10.5 | −3.35 ± 0.70 | 185.5 ± 40.9 | 2,537 ± 623 |
A defined initial fracture in L3 was achieved in all specimens in both fracture groups [PMMA (n = 5) and modified PMMA (n = 6)]. The IFF (the force necessary to create the initial fracture in L3 prior to augmentation) showed a biasing tendency toward a 31 % higher fracture force for the PMMA group (2,854 ± 648 N) compared to the modified PMMA group (1,980 ± 786 N, p = 0.08, Table 2). IFF significantly depended on both specimen stiffness (r2 = 0.34, p = 0.04) and age (r2 = 0.40, p = 0.02, Table 2), but not on the BMD × endplate area (r2 = 0.20, p = 0.09). Specimen characteristics of age and BMD × endplate area were not significantly different between groups (p > 0.66).
Table 2.
Tabular overview of the averages and standard deviations of the results of mechanical testing without adjustment for covariates after both the initial fracture creation (PMMA and Modified PMMA groups only) and after fatigue testing broken down by experimental group
| Group | N | Initial fracture force (N) | Initial specimen stiffness (N/mm) | Fatigue fracture force (N) | Cement stiffness (MPa) |
|---|---|---|---|---|---|
| Native | 5 | NA | NA | 1,440 ± 590 | NA |
| PMMA | 5 | 2,854 ± 648 | 502 ± 129 | 1,760 ± 251 | 1,937 ± 478 |
| Modified PMMA | 6 | 1,980 ± 786 | 336 ± 125 | 1,583 ± 407 | 955 ± 141 |
The cement modulus for the commercial PMMA was 1,937 ± 78 MPa and the modified PMMA cement was 955 ± 141 MPa (p < 0.001, Table 2). Therefore, a reduction of 51 % in cement modulus was obtained for the modified PMMA in comparison to the unmodified cement. No differences were seen in the incidence of cement leakage with the addition of the serum. After hardening, a macroscopic, closed porous structure with the serum completely enclosed in the PMMA was seen in the modified PMMA group.
The total mean volume of the cement used was 7.1 ± 1.5 mL. There was no significant difference in the amount of cement that was used when comparing the PMMA (6.7 ± 1.7 mL) and the modified PMMA groups (7.6 ± 1.2 mL, p = 0.50).
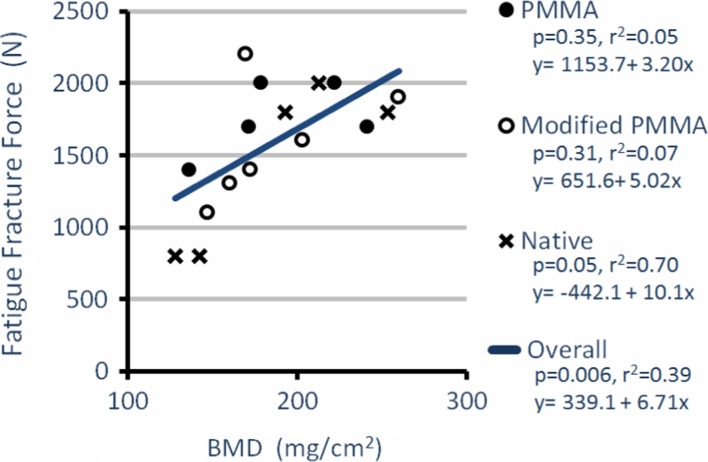
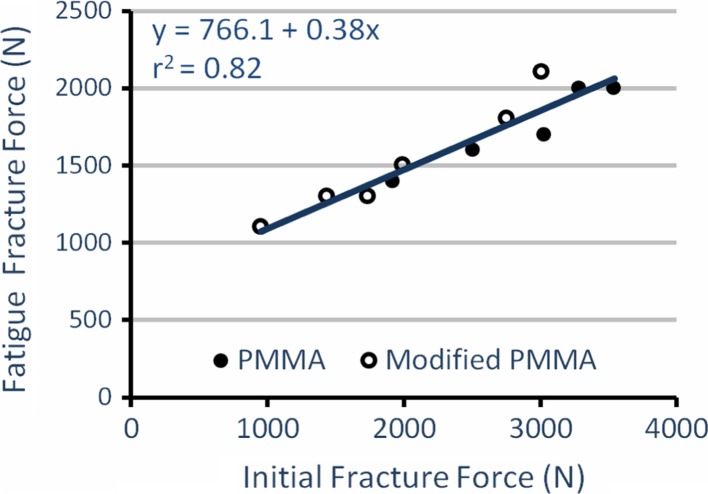
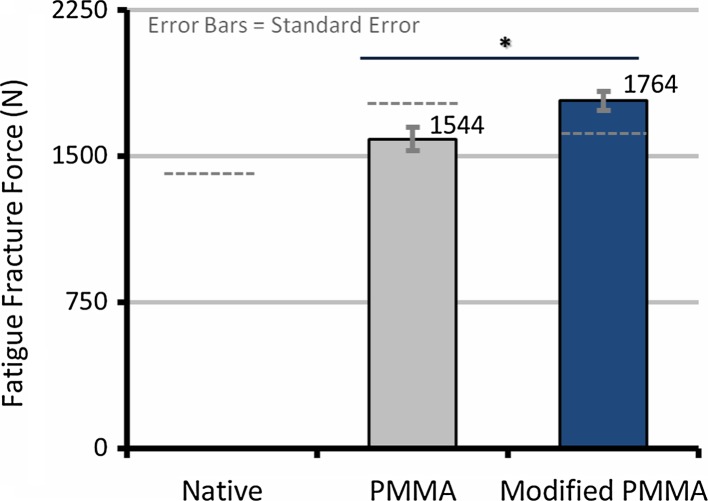
After treating the fracture in L3 with vertebroplasty and performing cyclic testing, the PMMA group showed the highest average FFF (1,760 ± 251 N, n = 5) compared to the native (1,440 ± 590 N, n = 5) and the modified PMMA (1,583 ± 407 N, n = 6) groups (Table 2). However, there was no overall significant difference between the groups (p > 0.50). The FFF was significantly affected by the bone quality (BMD, r2 = 0.39, p = 0.006, Fig. 3), BMD × endplate area (r2 = 0.27, p = 0.02) and most by IFF (r2 = 0.82, p < 0.001, Fig. 4). Using the IFF as a covariate in an ANCOVA, the difference in adjusted FFF between groups was significant with modified PMMA (1,764 ± 49 N, n = 6) now having a larger FFF than PMMA (1,544 ± 55 N, n = 5; F(1,8) = 7.57, η2 = 0.49, p = 0.03, Fig. 5).
Fig. 3.
Fatigue fracture force versus bone mineral density (BMD); fatigue fracture force depends significantly on the bone quality of the specimens (r2 = 0.39, p = 0.006, PMMA n = 5, modified PMMA n = 6, native n = 5)
Fig. 4.
Fatigue fracture force versus initial fracture force; fatigue fracture force depends significantly on the initial fracture force of the specimens (r2 = 0.82, p < 0.001, PMMA n = 5, modified PMMA n = 6)
Fig. 5.
Average fatigue fracture force adjusted for initial fracture force; the difference in fatigue fracture force between groups is significant (p = 0.03) with modified PMMA (1,764 ± 49 N) having a larger fatigue fracture force than PMMA (1,544 ± 55 N). Gray dashed lines represent unadjusted average fatigue fracture force
The fatigue failure pattern of the vertebrae is given in Table 3. After completion of fatigue testing, a total of six re-fractures in L3 occurred, as well as ten adjacent fractures in L2, and only two in L4. L4 exhibited only one sole fracture, whereas L2 exhibited seven. A combination of fractures occurred in L2 + L3 two times and in L3 + L4 once. Eighty percent of the tested specimens in the PMMA group had an adjacent-level fracture, while the modified PMMA group had adjacent-level fractures in 67 %.
Table 3.
Fracture pattern exhibited by the specimens after fatigue loading of the spine. After testing there was a total of seven re-fractures in L3 as well as ten adjacent fractures in L2. A combination of fractures occurred in L2 + L3 two times and in L3 + L4 once. Fractures of L4 were rare with only two fractures occurring
| Group | L2 | L2 + 3 | L3 | L3 + 4 | L4 |
|---|---|---|---|---|---|
| Native | 2 | 2 | 1 | 0 | 0 |
| PMMA | 3 | 0 | 1 | 0 | 1 |
| Modified PMMA | 3 | 0 | 2 | 1 | 0 |
Discussion
The aim of this study was to test the common theory that increased stiffness from augmentation leads to adjacent-level fractures. This raises the question of whether the properties of the cements that are used in vertebroplasty are optimum for their purpose. Due to the aging population and, therefore, the growing number of people suffering from osteoporosis, therapy particularly in osteoporotic related fractures needs to be adapted.
Luo et al. [23] showed that adjacent fractures were provoked due to low BMD causing greater stiffness changes and an increase in load sharing. They postulate that cement type has little influence in patients of normal bone quality and only plays a role in low BMD. The osteoporotic spines used in this present study also showed the influence of bone quality on the FFF after augmentation.
One possible explanation for the mechanical origin of adjacent-level fractures is given by Kayanja et al. [24]. They showed that augmentation of experimental osteoporotic compression fractures led to a reduced stiffness of the fractured spinal segment, while the adjacent vertebra exhibited an increase in superior and inferior cortical strain. They concluded that there was an increased central deformation of the adjacent-level endplates after augmentation, and that this deformation leads to fracture. Here, cement is postulated to be a load transfer device [24]. Reducing the stiffness of the cement, therefore, might lead to a decreased load transfer and reduce the central deformation in the adjacent vertebra as well as providing stability and some elasticity to the endplate area.
The hypothesis that a reduction in cement stiffness could reduce re-fracture rate of adjacent vertebra is not new. Boger et al. [13] tested a low-modulus PMMA, in which PMMA was mixed with a aqueous fraction of 35 % to achieve reduced stiffness. They did not see any benefit in using modified cement. Here, the modified cement was only tested in a single FSU without a fracture model. Nouda et al. [25] tested a bioresorbable calcium phosphate cement against PMMA in a similar setting to the current method. They showed that the incidence of vertebral body fractures following vertebroplasty was lower with calcium phosphate than with PMMA. On the other hand, they do see a big disadvantage that vertebrae augmented with calcium phosphate are subject to collapse. This was not the case in this study; the results showed that even after an initial fracture both cement types were able to stabilize the initial fracture back to levels at least as high as the intact spine. The comparison of FFF between the three test groups showed that the PMMA and modified PMMA groups had a higher average FFF than the native group (Table 2), but this difference was not significant (p > 0.50).
Our hypothesis was supported by the fact that the mean of the modified PMMA fatigue fracture force adjusted for IFF was significantly higher than that of the unmodified PMMA (p = 0.03, Fig. 5).1 However, this result was not seen in the unadjusted mean (p > 0.50, Table 2). In hindsight, dividing experimental groups by their IFF instead of BMD would have been ideal due to the fact that this covariate masked the effects of the low stiffness cement.
Mixing of 8 mL of serum with PMMA easily and reproducibly achieved the desired reduction in cement stiffness (51 %) close to that of cancellous bone (50–800 MPa) [17]. Similar to the results of Boger et al. [13], the reduction of cement stiffness still provided stability. In support of the current study, they did not see any differences in failure strength after reducing the cement stiffness by about 74 % [13]. Therefore, it is hypothesized that the biomechanical stability in the FSU is changed as little as possible.
In the framework of this in vitro study, fetal bovine serum as a liquid, which is easily accessible in laboratories, was used to reduce cement stiffness. For clinical studies, the use of bovine serum is clearly not an option due to unevaluated potential risks. However, after suitable investigations into their safety, any biocompatible, incompressible fluid could be investigated for their use in tailoring cement stiffness. The appropriate method to tailor cement stiffness will have to be provided by the cement producers.
The IFF (r2 = 0.82) was a significantly better predictor of FFF than overall BMD (p = 0.01, Figs. 3, 4). The overall bone quality (BMD, r2 = 0.39) could only partially predict the FFF. Introducing the stabilizing PMMA reduced the magnitude of the effect that BMD has on the FFF. This is shown by the augmented samples of the PMMA and modified PMMA groups having higher intercepts and lower slopes than the unaugmented samples (native group) in the FFF versus BMD regression equations (Fig. 3). When only considering the native group, the variance explained (r2 = 0.70) is comparable with the previously reported data on the correlations of fracture force and BMD [26].
The limitations of the study include the use of a small sample size due to the limitation of specimen availability and a large natural variability between specimens. This limited sample size may have ultimately reduced the power of the tests.
In conclusion, a possible method of reducing adjacent-level fractures after vertebroplasty in patients with reduced bone quality could be the use of a lower modulus cement. The idea of mixing cement with readily available non-toxic, biocompatible fluids could prove useful in the tailoring of cement properties.
Acknowledgments
Funding from the state of Hamburg and the Marie Curie ITN project SpineFX is kindly acknowledged. The authors would also like to thank Synthes GmbH for providing the cement.
Conflict of interest
Prof. Dr. Andreas Boger was formerly a Senior Project Scientist at Synthes GmbH until September 2011. No other authors have any declarations.
Footnotes
FFF for the modified PMMA group was also significantly higher than the PMMA group when the FFF was normalized with the initial fracture force (FFF/IFF) rather than using the IFF as a covariate (p = 0.03). To account for differences of individual specimen geometry, an ANCOVA analysis comparing the change in apparent fatigue strength (FFF/endplate area) adjusted for apparent initial strength (IFF/endplate area) was performed. In accordance with the FFF analysis, the modified PMMA fatigue strength (1.30 ± 0.041 MPa, n = 6) was also significantly higher than in the PMMA group (1.07 ± 0.045 MPa, n = 5, F(1,8) = 11.99, η2 = 0.60, p = 0.009, Fig. 5).
Contributor Information
Jan Philipp Kolb, Phone: +49-175-1623314, FAX: +49-40-741053847, Email: j.kolb@uke.de.
Rebecca A. Kueny, Phone: +49-40-428783712, FAX: +49 40-428782996, Email: rebecca.kueny@tuhh.de
Klaus Püschel, Phone: +49-40-74105213, FAX: +49-40-741059383, Email: pueschel@uke.de.
Andreas Boger, Phone: +49-981-4877399, FAX: +49-981-4877416, Email: andreas.boger@hs-ansbach.de.
Johannes M. Rueger, Phone: +49-40-741053459, FAX: +49-40-741054569, Email: rueger@uke.de
Michael M. Morlock, Phone: +49-40-428783053, FAX: +49-40-428782996, Email: morlock@tuhh.de
Gerd Huber, Phone: +49-40-428783153, FAX: +49-40-428782996, Email: g.huber@tu-harburg.de.
Wolfgang Lehmann, Phone: +49-40-741056691, FAX: +49-40-741053847, Email: wlehmann@uke.de.
References
- 1.Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17(5):505–511. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States. 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Bartl R, Gradinger R. Current diagnosis and therapy of osteoporosis on the basis of “European guidance 2008”. Orthopade. 2009;38(4):365–379. doi: 10.1007/s00132-008-1404-4. [DOI] [PubMed] [Google Scholar]
- 4.Arabmotlagh M, Rauschmann M. Filler materials for augmentation of osteoporotic vertebral fractures. Orthopade. 2010;39(7):687–692. doi: 10.1007/s00132-010-1619-z. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137(9):1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 6.Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18(2):311–320. doi: 10.1148/radiographics.18.2.9536480. [DOI] [PubMed] [Google Scholar]
- 7.Heini PF, Berlemann U, Kaufmann M, et al. Augmentation of mechanical properties in osteoporotic vertebral bones—a biomechanical investigation of vertebroplasty efficacy with different bone cements. Euro Spine J. 2001;10(2):164–171. doi: 10.1007/s005860000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22(2):373–381. [PMC free article] [PubMed] [Google Scholar]
- 9.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 10.Klazen CAH, Venmans A, de Fies J. Percutaneous vertebroplasty is not a risk factor for new osteoporotic compression fractures: results from Vertos II. AJNR Am J Neuroradiol. 2010;31:1447–1450. doi: 10.3174/ajnr.A2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27(1):217–223. [PMC free article] [PubMed] [Google Scholar]
- 12.Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226(1):119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 13.Boger A, Heini P, Windolf M, Schneider E. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Euro Spine J. 2007;16(12):2118–2125. doi: 10.1007/s00586-007-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkoff SM, Mathis JM, Erbe EM, Fenton DC. Biomechanical evaluation of a new bone cement for use in vertebroplasty. Spine. 2000;25(9):1061–1064. doi: 10.1097/00007632-200005010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hadley C, Awan OA, Zoarski GH. Biomechanics of vertebral bone augmentation. Neuroimaging Clin N Am. 2010;20(2):159–167. doi: 10.1016/j.nic.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Ahn DK, Lee S, Choi DJ, et al. Mechanical properties of blood-mixed polymethyl metacrylate in percutaneous vertebroplasty. Asian Spine J. 2009;3(2):45–52. doi: 10.4184/asj.2009.3.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002;17(9):1621–1628. doi: 10.1359/jbmr.2002.17.9.1621. [DOI] [PubMed] [Google Scholar]
- 18.Huber G, Müller-Bergen L, Sellenschloh K, et al. (2008) The influence of cement augmentation method on the fracture strength of augmented and adjacent vertebral bodies. In: 53rd Annual Meeting of the Orthopaedic Research Society, San Francisco
- 19.Broszyk Volume matters: a review of procedural details of two randomized controlled vertebroplasty trials in 2009. Eur Spine J. 2010;19:1837–1840. doi: 10.1007/s00586-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locati L. Le prove di fatica come ausilio alla progettazione ed alla produzione. Matallurgia It. 1955;47(9):301–308. [Google Scholar]
- 21.Brinkmann P, Biggermann M, Hilweg D. Fatigue fracture of human lumbar vertebrae. Clin Biomech. 1988;3:1–23. doi: 10.1016/S0268-0033(88)80001-9. [DOI] [PubMed] [Google Scholar]
- 22.Huber G, Skrzypiec DM, Klein A, Püschel K, Morlock MM. High cycle fatigue behaviour of functional spinal units. Ind Health. 2012;48(5):550–556. doi: 10.2486/indhealth.MSWBVI-11. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Skrzypiec DM, Pollintine P, et al. Mechanical efficacy of vertebroplasty: influence of cement type, BMD, fracture severity, and disc degeneration. Bone. 2006;40(4):1110–1119. doi: 10.1016/j.bone.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Kayanja MM, Togawa D, Lieberman IH. Biomechanical changes after the augmentation of experimental osteoporotic vertebral compression fractures in the cadaveric thoracic spine. Spine J. 2005;5(1):55–63. doi: 10.1016/j.spinee.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Nouda S, Tomita S, Kin A, et al. Adjacent vertebral body fracture following vertebroplasty with polymethyl methacrylate or calcium phosphate cement. Spine. 2009;34(24):2613–2618. doi: 10.1097/BRS.0b013e3181abc150. [DOI] [PubMed] [Google Scholar]
- 26.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(3):13–18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]