Abstract
Purpose
The majority of prognostic studies on postpartum lumbopelvic pain have investigated factors during pregnancy. Since the majority of women recover within the first few months of delivery, it is unknown if the same predictors are valid for long-term consequences. It is also important to investigate predictors within subgroups of patients with pregnancy-related lumbopelvic pain due to their different clinical courses. The aim of this study was to identify predictors for disability 15 months postpartum in women with persistent postpartum pelvic girdle pain (PGP).
Methods
Data were obtained by clinical tests and questionnaires 3 months postpartum. The outcome 15 months postpartum was disability measured with the Oswestry Disability Index.
Results
A multiple linear regression analysis identified two significant two-way interaction effects that were predictive of disability 15 months postpartum: (a) age + trunk flexor endurance, and (b) disability + hip extensor strength.
Conclusions
Age, muscle function and disability seem to influence the long-term outcome on disability in women with persistent postpartum PGP. It may be important to consider the possibility of different variables impact on each other when predicting long-term disability. In addition, further studies are needed to investigate the impact of interaction effects on long-term consequences in women with persistent postpartum PGP.
Keywords: Muscle function, Pregnancy-related low back pain, Prognostic factors, Risk factors
Introduction
Lumbopelvic pain affects almost half of all pregnant women [1]. Although there is a decline in the prevalence of lumbopelvic pain during the first few months after delivery in the majority of the women [2], a significant number of women have persistent pain 3 months postpartum [1]. In a previous study, women with severe lumbopelvic pain during pregnancy had an increased risk for long-term pain [3]. In addition, retrospective studies have shown that up to 20 % of women with recurrent lumbopelvic pain relate their first episode of pain to pregnancy [4, 5]. Thus, pregnancy seems to represent a risk for long-term lumbopelvic pain.
Several predictors of the development of and recovery from pregnancy-related lumbopelvic pain have been identified, although no clear picture has been presented [6]. In a review, Wu et al. [1] identified 12 potential risk factors for postpartum lumbopelvic pain. Strong evidence was found that predictors included strenuous work, previous lumbopelvic pain, and previous pelvic girdle pain (PGP) [1]. They also concluded that maternal age and maternal ethnicity could not be established as risk factors due to conflicting results. Other factors that have been found to be predictive of postpartum lumbopelvic pain are body mass index (BMI), hypermobility, pain onset, and pain level [7].
Recent studies of predictors have defined subgroups of patients with pregnancy-related lumbopelvic pain and have focused on PGP [2, 8–10]. Factors such as older age, work dissatisfaction, and pain location have been proposed as important predictors for postpartum PGP [2]. An association between muscle dysfunction and pregnancy-related PGP has also been proposed [11, 12]. To our knowledge, only one study has investigated muscle function as a predictor for postpartum PGP [2]. Gutke et al. [2] found that poor endurance of the trunk flexors early in pregnancy is predictive of persistent PGP 3 months postpartum. Other clinical signs that have been found to be predictive of disability and pain in women with postpartum PGP are the active straight leg raise (ASLR) test [10] and the sum of pelvic pain provocation tests [8].
However, since the majority of women recover within the first few months after delivery, there is a need to investigate whether the same predictors are valid for long-term consequences. To our knowledge, all but one previous study [10] investigated predictors identified during pregnancy. Due to the different clinical course of pregnancy-related lumbopelvic pain [2], there is also a need to further investigate predictors for different subgroups of patients with pregnancy-related lumbopelvic pain. The aim of this study was to identify predictors of disability 15 months postpartum in women with persistent postpartum PGP.
Materials and methods
Study participants
Women with persistent postpartum PGP and PGP in combination with lumbar pain (combined pain) were recruited approximately 3 months after delivery. They were included in a randomized controlled trial evaluating home-based specific stabilizing exercises (SSE) [13]. Women were excluded if they had a systemic locomotor disease; a verified diagnosis of spinal problems in the previous 2 months; a history of fracture, neoplasm, or previous surgery of the spine, pelvis, or femur; insufficient Swedish language skills; or ongoing pregnancy. All participants received oral and written information about the study before they provided oral consent. The study was approved by the regional ethics committee.
Clinical examination and assessment
All participants completed questionnaires, underwent clinical examinations and physical evaluations at the baseline (approximately 3 months postpartum) and again 12 months later (approximately 15 months postpartum). The questionnaire included background data, activity level [14], urinary leakage (yes/no), health-related quality of life measured with the EuroQol [15], general health measured with a visual analogue scale (VAS; 0–100 mm, low value indicating high well-being), well-being (very well, well, fairly well, fairly bad, bad, very bad), pain intensity (VAS; 0–100 mm, low value indicating no pain), pain location (pain drawing), and disability measured with the Oswerstry Disability Index (ODI) version 2.0 [16]. We also collected answers to questions regarding patients’ symptom satisfaction (delighted to mostly satisfied, or mixed to terrible feelings) [17] and expectations of treatment (completely restored to quite improved, or no expectations of being restored but hoping to get some relief, to no expectations of being restored or getting some relief).
The classification of PGP and combined pain was based on a clinical examination, including pelvic pain provocation tests and the Mechanical Diagnosis and Therapy (MDT) protocol, described in detail by Gutke et al. [18]. Criteria for PGP and combined pain are presented in Table 1. In addition, the ASLR test (4-point scale, summed score range from 0 to 6) [19] was performed.
Table 1.
Criteria for pelvic girdle pain group and combined pain
| Pelvic girdle pain |
| Pain experienced between the posterior iliac crest and the gluteal fold. With or without radiation in the posterior thigh and calf, and with or without pain in the symphysis |
| Pain reproducible by at least two out of the five pelvic pain provocation tests (two tests performed bilaterally) |
| No centralization or peripheralization phenomena and no change of pain or range of motion from repeated movements or different positions of the lumbar spine according to the MDT classification |
| Onset of pain during pregnancy or within 3 weeks from delivery |
| Combined pain |
| Pain experienced between the posterior iliac crest and the gluteal fold. With or without radiation in the posterior thigh and calf, and with or without pain in the symphysis |
| Pain reproducible by at least two out of the five pelvic pain provocation tests (2 test bilaterally) |
| Pain and/or change in range of motion from repeated movements or different positions of the lumbar spine, or experienced centralization and/or peripheralization according to the MDT classification |
| Onset of pain during pregnancy or within 3 weeks from delivery |
Pelvic girdle pain in combination with lumbar pain
MDT mechanical diagnosis and therapy
Physical functioning
For the gait test (modified from Ljungqvist et al.), women were asked to walk barefoot for a distance of 20 m “at a comfortable speed” on an indoor floor [11]. The number of seconds it took to walk the distance was recorded.
Maximal voluntary isometric hip extension strength was measured by a dynamometer (Chatillon CSD 500 strength dynamometer; Ametek, Largo, FL, USA) with a fixed sensor [11]. A sling was placed on the women’s thigh at the distal end of the femur and pulled in extension. The women were then instructed to pull as hard as they could. Two training repetitions were performed. The mean of the next three repetitions was used for analyses. Each repetition consisted of 5 s of work and 5–10 s of rest. The procedure was performed on both legs: all the women started with the right leg, but only the strongest leg was included in the analyses.
Isometric endurance of the trunk flexors was tested with women in the supine position with arms crossed over their chest, hips bent, and knees and feet apart (modified from McQuade et al.). They were asked to nod and to continue to lift their head and shoulders until the inferior angle of the scapula was lifted from the examination bench, and to hold the position for as long as possible [11]. The time that the position was maintained was recorded in seconds, and the test was interrupted after a maximum of 120 s.
Statistical analyses
Multiple linear regression analysis was performed on disability measured with the ODI at 15 months postpartum, to determine predictors from measures collected 3 months postpartum. The ODI was treated as a continuous scale. The initial choice of possible predictors was based on the hypothesis of an association between muscle dysfunction and PGP, as well as on previous findings in the literature [1, 2, 7, 10, 11, 20–22].
Pearson’s r was used to analyze correlations between factors and outcome, as well as between factors (Table 2). To control for possible multicollinearity, a selection between highly intercorrelated (r > 0.7) factors was made. The selection between the highly intercorrelated factors was based on which of the two factors had the highest correlation with the outcome. Average pain intensity during the previous week, general health, and mean hip extensor strength was excluded from the analysis due to high intercorrelation with other factors and lower correlation with the outcome. The EQ-5D score and EQ-VAS were both kept in the analysis, even though the correlation was 0.51. This decision was based on the fact that the two scores are different measurements of the same health-related quality of life instrument (EuroQol).
Table 2.
Bivariate correlation matrix between Oswestry Disability Index 15 months postpartum and potential predictive factors
| ODI 15 mm pp | Age | Wellbeing | Pain intensity | Pain drawing | ODI | EQ-5D score | EQ VAS | Gait speed | Hip extensor strength | Trunk flexor endurance | ASLR test | Lumbar pain | Treatment | SUI | Activity level | Pain frequency | Symptom satisfaction | Expectations on treatment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ODI 15 mm ppd | – | 0.05 | 0.23 | 0.14 | 0.17 | 0.37 | −0.41 | −0.21 | 0.06 | 0.28 | 0.23 | 0.18 | −0.80 | 0.09 | −0.14 | 0.11 | 0.11 | 0.05 | 0.12 |
| – | 0.71 | 0.11 | 0.34 | 0.25 | 0.01 | <0.01 | 0.14 | 0.68 | 0.85 | 0.10 | 0.20 | 0.58 | 0.56 | 0.34 | 0.46 | 0.45 | 0.76 | 0.42 | |
| Age | 0.54 | – | 0.10 | 0.05 | 0.30 | 0.07 | 0.06 | −0.01 | 0.17 | 0.07 | 0.14 | −0.27 | −0.12 | 0.17 | 0.14 | 0.08 | 0.08 | −0.03 | −0.01 |
| 0.71 | – | 0.51 | 0.74 | 0.04 | 0.65 | 0.69 | 0.92 | 0.25 | 0.63 | 0.33 | 0.06 | 0.40 | 0.24 | 0.35 | 0.60 | 0.58 | 0.86 | 0.97 | |
| Well-beinga | 0.23 | 0.10 | – | 0.22 | −0.08 | 0.48 | −0.39 | −0.56 | 0.15 | −0.29 | −0.08 | 0.07 | 0.19 | 0.13 | −0.10 | −0.05 | 0.34 | 0.36 | −0.21 |
| 0.11 | 0.51 | – | 0.12 | 0.58 | <0.01 | 0.01 | <0.01 | 0.30 | 0.04 | 0.60 | 0.62 | 0.19 | 0.39 | 0.50 | 0.74 | 0.02 | 0.01 | 0.14 | |
| Pain intensityb | 0.14 | 0.05 | 0.22 | – | 0.39 | 0.45 | −0.32 | −0.45 | 0.19 | −0.23 | 0.03 | 0.15 | −0.02 | −0.09 | −0.26 | −0.03 | 0.21 | 0.30 | −0.05 |
| 0.34 | 0.74 | 0.12 | – | 0.01 | <0.01 | 0.02 | <0.01 | 0.19 | 0.11 | 0.82 | 0.30 | 0.91 | 0.55 | 0.07 | 0.86 | 0.14 | 0.03 | 0.74 | |
| Pain drawingc | 0.17 | 0.30 | −0.8 | 0.39 | – | 0.43 | −0.25 | −0.17 | 0.30 | −0.02 | 0.15 | 0.09 | −0.06 | −0.04 | 0.06 | 0.02 | 0.23 | 0.11 | 0.08 |
| 0.25 | 0.04 | 0.58 | 0.01 | – | <0.01 | 0.08 | 0.23 | 0.04 | 0.91 | 0.28 | 0.53 | 0.69 | 0.77 | 0.66 | 0.88 | 0.10 | 0.46 | 0.57 | |
| ODId | 0.37 | 0.07 | 0.48 | 0.45 | 0.43 | – | −0.47 | −0.40 | 0.36 | −0.19 | 0.03 | 0.11 | 0.14 | 0.23 | 0.04 | −0.10 | 0.40 | 0.47 | <0.01 |
| 0.01 | 0.65 | <0.01 | <0.01 | <0.01 | – | <0.01 | <0.01 | 0.01 | 0.19 | 0.85 | 0.43 | 0.32 | 0.11 | 0.79 | 0.49 | <0.01 | <0.01 | 0.98 | |
| EQ-5D scoree | −0.41 | −0.06 | −0.39 | −0.32 | −0.25 | −0.47 | – | 0.51 | −0.06 | <0.01 | −0.13 | −0.06 | 0.02 | −0.05 | 0.03 | 0.13 | −0.30 | −0.38 | <0.01 |
| <0.01 | 0.65 | 0.01 | 0.02 | 0.08 | <0.01 | – | <0.01 | 0.66 | 1.00 | 0.36 | 0.69 | 0.91 | 0.76 | 0.83 | 0.35 | 0.03 | <0.01 | 0.98 | |
| EQ VASf | −0.21 | −0.14 | −0.56 | −0.45 | 0.17 | 0.40 | 0.51 | – | −0.22 | 0.34 | <0.01 | −0.14 | −0.12 | 0.02 | 0.21 | 0.04 | −0.07 | −0.39 | 0.09 |
| 0.14 | 0.92 | <0.01 | <0.01 | 0.23 | <0.01 | <0.01 | – | 0.13 | 0.01 | 0.98 | 0.34 | 0.43 | 0.90 | 0.15 | 0.76 | 0.62 | <0.01 | 0.53 | |
| Gait speedg | 0.06 | 0.17 | 0.15 | 0.19 | 0.30 | 0.38 | −0.06 | −0.22 | – | −0.26 | −0.17 | 0.11 | 0.01 | 0.23 | 0.15 | −0.01 | 0.05 | 0.09 | 0.22 |
| 0.68 | 0.25 | 0.30 | 0.19 | 0.04 | 0.01 | 0.66 | 0.13 | – | 0.07 | 0.23 | 0.43 | 0.95 | 0.11 | 0.31 | 0.92 | 0.74 | 0.54 | 0.13 | |
| Hip extensor strengthh | 0.28 | 0.07 | −0.29 | −0.23 | −0.02 | −0.19 | <0.01 | 0.34 | −0.26 | – | 0.46 | 0.03 | −0.24 | −0.15 | 0.09 | 0.32 | 0.06 | 0.37 | 0.24 |
| 0.05 | 0.63 | 0.04 | 0.11 | 0.91 | 0.19 | 1.00 | 0.01 | 0.07 | – | <0.01 | 0.83 | 0.09 | 0.30 | 0.54 | 0.02 | 0.66 | <0.01 | 0.09 | |
| Trunk flexor endurancei | 0.24 | 0.14 | −0.08 | 0.03 | 0.16 | 0.03 | −0.13 | <0.01 | 0.17 | 0.46 | – | −0.18 | −0.25 | −0.02 | −0.26 | 0.47 | −0.21 | −0.09 | 0.09 |
| 0.10 | 0.33 | 0.60 | 0.82 | 0.28 | 0.35 | 0.36 | 0.98 | 0.23 | <0.01 | – | 0.22 | 0.09 | 0.89 | 0.07 | <0.01 | 0.15 | 0.52 | 0.55 | |
| ASLR testj | 0.18 | −0.27 | 0.07 | 0.15 | 0.09 | 0.11 | −0.06 | −0.14 | 0.11 | 0.03 | −0.18 | – | −0.16 | 0.22 | 0.09 | 0.11 | −0.16 | 0.01 | 0.06 |
| 0.20 | 0.06 | 0.62 | 0.30 | 0.53 | 0.43 | 0.69 | 0.34 | 0.43 | 0.83 | 0.22 | – | 0.27 | 0.13 | 0.54 | 0.47 | 0.26 | 0.94 | 0.69 | |
| Lumbar paink | −0.80 | −0.12 | 0.19 | −0.16 | −0.06 | 0.14 | 0.02 | −0.12 | 0.01 | −0.24 | −0.25 | −0.16 | – | −0.07 | −0.07 | −0.32 | 0.12 | 0.14 | −0.11 |
| 0.58 | 0.40 | 0.19 | 0.91 | 0.69 | 0.32 | 0.91 | 0.43 | 0.95 | 0.09 | 0.09 | 0.27 | – | 0.66 | 0.65 | 0.03 | 0.41 | 0.34 | 0.46 | |
| Treatmentl | 0.09 | 0.17 | 0.13 | −0.09 | −0.04 | 0.23 | −0.05 | 0.02 | 0.23 | −0.15 | −0.20 | 0.22 | −0.07 | – | −0.04 | <0.01 | −0.17 | 0.01 | 0.06 |
| 0.56 | 0.24 | 0.39 | 0.55 | 0.77 | 0.11 | 0.76 | 0.90 | 0.11 | 0.30 | 0.89 | 0.13 | 0.66 | – | 0.98 | 0.98 | 0.26 | 0.94 | 0.69 | |
| SUIm | −0.14 | 0.14 | −0.10 | −0.26 | 0.06 | 0.04 | 0.03 | 0.21 | 0.15 | 0.88 | −0.26 | 0.09 | −0.07 | <0.01 | – | 0.10 | −0.07 | −0.02 | 0.01 |
| 0.34 | 0.35 | 0.50 | 0.07 | 0.66 | 0.79 | 0.83 | 0.15 | 0.31 | 0.54 | 0.07 | 0.54 | 0.65 | 0.98 | – | 0.49 | 0.65 | 0.88 | 0.97 | |
| Activity leveln | 0.11 | 0.08 | −0.05 | −0.03 | 0.02 | −0.10 | 0.13 | 0.04 | −0.01 | 0.32 | 0.47 | 0.11 | −0.32 | <0.01 | 0.10 | – | −0.04 | −0.03 | 0.34 |
| 0.46 | 0.60 | 0.74 | 0.86 | 0.90 | 0.49 | 0.35 | 0.76 | 0.92 | 0.02 | <0.01 | 0.47 | 0.03 | 0.98 | 0.49 | – | 0.78 | 0.86 | 0.02 | |
| Pain frequencyo | 0.11 | 0.08 | 0.34 | 0.21 | 0.23 | 0.40 | −0.30 | −0.07 | 0.05 | 0.06 | 0.21 | −0.16 | 0.12 | −0.16 | −0.07 | −0.04 | – | 0.29 | −0.04 |
| 0.45 | 0.58 | 0.02 | 0.14 | 0.10 | <0.01 | 0.03 | 0.62 | 0.74 | 0.66 | 0.15 | 0.26 | 0.41 | 0.26 | 0.65 | 0.78 | – | 0.04 | 0.77 | |
| Symptom satisfactionp | 0.05 | −0.03 | 0.36 | 0.30 | 0.11 | 0.47 | −0.38 | −0.39 | 0.09 | −0.37 | −0.09 | 0.01 | 0.14 | 0.01 | −0.02 | −0.03 | 0.29 | – | −0.04 |
| 0.76 | 0.86 | 0.01 | 0.03 | 0.46 | <0.01 | <0.01 | <0.01 | 0.54 | <0.01 | 0.52 | 0.94 | 0.34 | 0.94 | 0.88 | 0.86 | 0.04 | – | 0.77 | |
| Expectations on treatmentq | 0.12 | −0.01 | −0.21 | −0.05 | 0.08 | <0.01 | <0.01 | 0.09 | 0.22 | 0.24 | 0.09 | 0.06 | −0.11 | 0.06 | 0.01 | 0.34 | −0.04 | −0.20 | – |
| 0.42 | 0.97 | 0.14 | 0.74 | 0.57 | 0.98 | 0.98 | 0.53 | 0.13 | 0.09 | 0.55 | 0.69 | 0.46 | 0.69 | 0.97 | 0.02 | 0.77 | 0.17 | – |
n = 50; Pearson’s rank correlation coefficient on the first row and P values on the second rows for each variable. Significance level = 0.05
ODI Oswestry Disability Index, VAS visual analogue scale, ASLR test active straight leg raise test, SUI stress urinary incontinence
aWell-being: 1–6: very well, well, fairly well, fairly bad, bad, very bad
bPain intensity: VAS 0–100 mm, where 0 represents no pain
cPain location: number of cm2
dODI: 0–100 % where 0 represents no disability
eEQ-5D: 1– (−0.59) in which −0.59 is the lowest health-related quality of life
fEQ-VAS: 0–100 mm, where 0 represents worst perceived health
gGait speed: m/s
hHip extensor strength: Newton
iTrunk flexor endurance: 0–120 s
jTwo categories: 0 = no problems and 1 = mild to severe problems with load transfer between the trunk and legs
kLumbar component in addition to pelvic girdle pain, two categories: yes and no
lTreated with specific stabilizing exercises, two categories: yes and no
mSUI, two categories: yes and no
nActivity level last 3 months, two categories: manage all household, including gardening, light physical activity and the activities mentioned before + exercises at increasing intensity
oTwo categories: always to several times per week and occasionally to never
pSymptom satisfaction: two categories: delighted to mostly satisfied and mixed feelings to terrible feelings
qExpectations on treatment, two categories: completely restored to quite improved and not improved but some relief to no expectation
The multiple linear regression model was executed in two steps. Step 1: a forward stepwise procedure was used to identify two-way interaction effects after entering all main effects in the regression model. A significance level of 1 % was used. Two interaction effects were identified. Step 2: the two interaction effects and their main effects were entered in the regression model, and a forward stepwise procedure was used on the remaining 14 factors. A significance level of 5 % was used. No significant predictors were found in addition to the two interaction effects identified in Step 1.
The Wilcoxon signed-rank test was used to detect differences over time for the ODI. The statistical software package SPSS was used (version 17.0; SPSS, Inc., Chicago, IL, USA).
Results
Eighty-eight women with persistent postpartum PGP and PGP in combination with lumbar pain were included in a randomized controlled study 3 months postpartum (Gutke et al. 2010) and 58 women (66 %) completed the 12-month follow-up (15 months postpartum) (Fig. 1). The regression model included 50 cases, 37 with PGP and 13 with combined pain at 3 months postpartum. Descriptive data and comparisons between the women included and not included in the regression model are listed in Table 3. Compared with the 50 participants who were included in the regression analysis the 38 excluded women had a higher disability (ODI 20 vs. 16 %, P = 0.012).
Fig. 1.
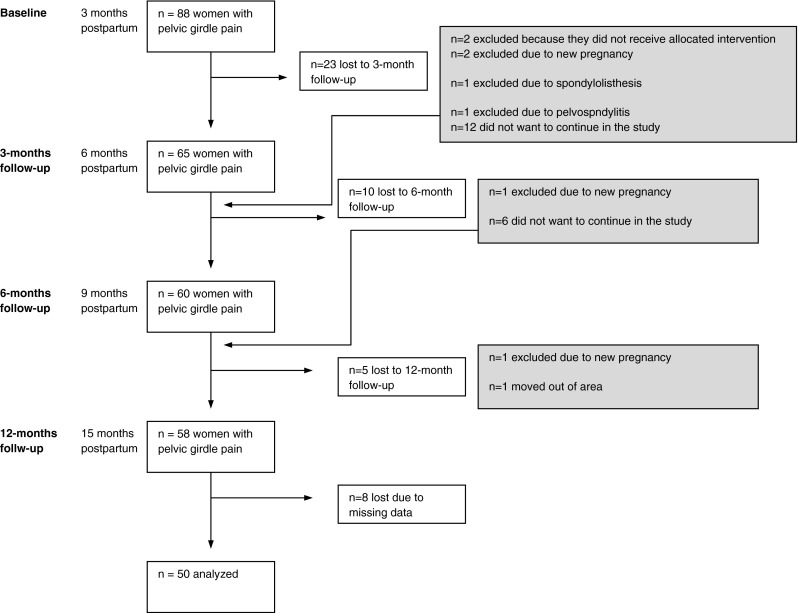
Enrolment at evaluations 3 months postpartum and the 12 month follow-up
Table 3.
Comparisons of the characteristics 3 months postpartum between the women included in the multiple linear regression analysis and the women that were excluded/dropped-out
| Variables | Women not included in the regression model (n = 38) | Women included in the regression model (n = 50) | P |
|---|---|---|---|
| Age (years) median (25th, 75th percentile) | 32 (29, 35) | 31 (28, 33) | 0.126 |
| EuroQol 5D scorea, median (25th, 75th percentile) | 0.76 (0.73, 0.80) | 0.80 (0.73, 0.80) | 0.941 |
| EQ VAS (mm)b, median (25th, 75th percentile) | 75 (67, 80) | 80 (70, 90) | 0.186 |
| Oswestry Disability Index (%) median (25th, 75th percentile) | 20 (14, 30) | 16 (10, 24) | 0.012 |
| Pain intensity at the moment measured with VAS (mm)c, median (25th, 75th percentile) | 35 (22, 48) | 31 (13, 52) | 0.452 |
| Pain localization measured with pain drawings (no. of cm2), median (25th, 75th percentile) | 9 (5, 13) | 9 (6, 12) | 0.739 |
| Gait speed (m/s), mean (SD) | 1.3 (0.2) | 1.3 (0.2) | 0.798 |
| Hip extensor strength (Newton)d, mean (SD) | 265 (103) | 265 (96) | 0.982 |
| Trunk flexor endurance (s), mean (SD) | 31 (25) | 33 (31) | 0.775 |
| Urinary leakage, n (%) | |||
| Yes | 9 (25) | 14 (28) | 0.757 |
| No | 27 (75) | 36 (72) | |
| Activity level last 3 months n (%) | |||
| Manage all household duties, including gardening and light physical activity | 29 (83) | 39 (78) | 0.582 |
| The afore-mentioned activities + exercises at increasing intensity | 6 (17) | 11 (22) | |
| ASLR testen (%) | |||
| 0 | 20 (56) | 32 (64) | 0.624 |
| ≥1 | 16 (44) | 18 (36) | |
| Group n (%) | |||
| SSE | 16 (42) | 18 (36) | 0.560 |
| Reference | 22 (58) | 32 (64) | |
| Symptom satisfaction | |||
| Delighted to mostly satisfied n (%) | 11 (31) | 17 (34) | 0.737 |
| Mixed feelings to terrible | 25 (69) | 33 (66) | |
| Expectations on treatment n (%) | |||
| Completely restored or quite improved | 34 (97) | 43 (86) | 0.083 |
| Not improved but get some relief of the symptoms or no expectations of being restored | 1 (3) | 7 (14) | |
| Well-being n (%) | |||
| Very well to well | 25 (69) | 37 (74) | 0.642 |
| Fairly well good to very bad | 11 (31) | 13 (26) | |
| Pain frequency n (%) | |||
| Always, daily to several times/week | 34 (94) | 38 (76) | 0.022 |
| Occasionally to never | 2 (6) | 12 (24) | |
VAS visual analogue scale, SSE specific stabilizing exercises, ASLRtest active straight leg raise test
a1–(−0.59), in which −0.59 is the lowest health-related quality of life
b0–100 in which 0 is the lowest thinkable health
c0–100 mm, in which 100 is the highest thinkable pain
dThe highest value for either the left or right leg is presented
e0 = no problems with performing the ASLR test; 1–6 = problems of various degree to perform the ASLR test
fCombined pain = pelvic girdle pain in combination with lumbar pain
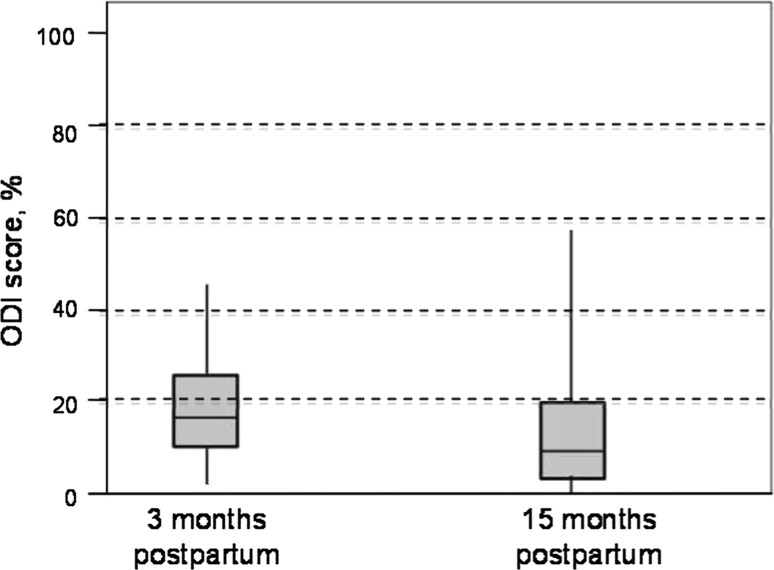
At 15 months postpartum, 28 women were classified as having PGP or combined pain, three women with lumbar pain only, and 19 women with no lumbopelvic pain. There was a significant improvement in the ODI between 3 and 15 months postpartum (Fig. 2). The disability level had increased, or was the same as 3 months postpartum in 34 % of the women; in 66 % of the women the disability level had decreased.
Fig. 2.
Distribution of Oswestry (ODI) score at approximately 3 months postpartum (baseline) and 15 months postpartum. Median values given by horizontal line, boxes show the interquartiles and whiskars the range. P < 0.01; n = 50; scale range from 0 to 100 %, 0–20 % = minimal disability; 21–40 % = moderate disability; 41–60 % = severe disability; 61–80 % = crippled; 81–100 % = bedbound or exaggerating the symptoms
Two two-way interaction effects were significantly associated with long-term disability 15 months postpartum: (a) age + trunk flexor endurance, and (b) disability + hip extensor strength. The final model explained 52 % of the variation in the ODI at 15 months postpartum (Table 4). Pain intensity, number of pain sites, combined pain, well-being, health-related quality of life, occurrence of stress urinary incontinence, treatment with SSE, expectations regarding treatment, symptom satisfaction, gait speed, and the ASLR test were not found to be significant predictors of long-term disability at 15 months postpartum (P > 0.05).
Table 4.
Predictive factors for disability measured with Oswestry Disability Index in women with persistent pelvic girdle pain at 15 months postpartum
| Independent variables | Dependent variable: continues Oswestry Disability Index at 15 months postpartum | |||
|---|---|---|---|---|
| β-Coefficient | 95 % CI | t | P | |
| Constant | 58.49 | |||
| Age | −1.36 | −2.11 to −0.60 | −3.63 | <0.0008 |
| Trunk flexor endurancea | −1.23 | −1.83 to −0.62 | −4.10 | <0.0002 |
| Oswestry Disability Indexb | −1.14 | −2.04 to −0.24 | −2.56 | <0.0140 |
| Hip extensor strengthc | −0.05 | −0.11 to 0.01 | −1.74 | <0.0885 |
| Interaction: age and trunk flexor enduranced | 0.04 | 0.02 to 0.06 | 4.15 | <0.0002 |
| Interaction: disability and hip extensor strengthe | 0.01 | <0.01 to 0.01 | 3.83 | <0.0004 |
Multiple linear regression analysis with Oswestry Disability Index (ODI) at 15 months postpartum as dependent variable. The independent variables were collected at baseline evaluation 3 months postpartum
Unstandardized β-coefficient, 95 % CI = 95 % confidence interval and P value given for β-coefficients. Adjusted R2 was 0.52. n = 50
aTrunk flexor endurance 0–120 s
bODI: 0–100 %: high value indicating high disability
cHip extensor strength measured in Newtons
dInteraction between age and trunk flexor endurance
eInteraction between disability measured with the ODI and hip extensor strength
Discussion
There was a significant decrease in disability measured with the ODI between 3 and 15 months postpartum; however, 62 % of the women included in the multiple linear regression model still reported lumbopelvic pain and had clinically identifiable signs of PGP when examined at 15 months postpartum. Women who experience persistent postpartum lumbopelvic pain have a substantial risk for new episodes or long-term lumbopelvic pain [23]. Our results are in line with this finding, since all the women included in the present study experienced persistent PGP 3 months postpartum and were therefore likely to have a built-in risk for long-term disability. Despite the risk for long-term disability, we identified two two-way interaction effects that were predictive of disability 15 months postpartum. The main effects of the identified interaction effects can be categorized as biological (age), physical functioning (trunk flexor endurance and hip extensor strength), and self-rated function (disability).
Previous studies found age to be a predictor for postpartum lumbopelvic pain. However, age has been proposed to be a bimodal factor due to conflicting results [1]. Younger age [24], as well as older age [2], has been reported to be a risk factor for postpartum lumbopelvic pain. Our finding that age comprises an interaction effect together with trunk flexor endurance, and the previous finding that age is a bimodal factor, indicates that it might be important not to interpret age alone as a predictor for long-term disability.
Disability measured with ODI was also one of the main effects in one of the two interaction effects. ODI is a subjective measure of physical capacity. The change in ODI from 3 to 15 months postpartum is rather low and could be considered not clinically relevant. In addition, a relevant goal for healthy, young women should probably be 0 % on the ODI. Enthoven et al. [25] found that higher disability measured with the ODI was a predictor of disability at both 1- and 5-year follow-ups for primary care patients with LBP.
The two two-way interaction effects indicate that muscle function was predictive of long-term disability. This provides further evidence of an association between muscle dysfunction and pregnancy-related PGP. One can argue that the low beta values might not be clinically relevant; however, it is important to remember that the women included in the present study are at risk for more long-term problems since their pain has not disappeared at 3 months postpartum [23]. Therefore, it is possible that we cannot expect larger changes without an effective intervention. Low endurance of the trunk flexor muscles early in pregnancy has been found to be a predictor for PGP at 3 months postpartum [2]. In addition, Vollestad and Stuge demonstrated that treatment with SSE is the most significant predictor of improved long-term disability and evening pain in women with persistent postpartum PGP [10]. Since the objective of SSE is to improve muscle function, Vollestad and Stuge’s results might suggest to some degree that muscle function is important for predicting long-term disability.
The ASLR test performed postpartum has also been found to be predictive of disability and evening pain in women with persistent PGP 12 months postpartum [10]. However, when the test was performed during pregnancy, it could not predict disability or pain 3 months postpartum [8] or late in pregnancy [9] in women with PGP. One can speculate that long-term disability in women with postpartum PGP arises from mechanisms different from those involved in short-term outcomes. It may be important not to rely only on measurements collected during pregnancy when predicting long-term disability in women with persistent postpartum PGP. In addition to elapsed time from pregnancy, it may be important to take interaction effects into account when looking for predictors of long-term disability in women with persistent postpartum PGP. Significant interaction effects have not been identified from measurements collected during pregnancy [8, 9].
The present study has several strengths, including a prospective design, multivariable statistics, and the implementation of clinical risk factors. A limitation is the limited number of women included in the study. For multiple linear regression analysis, it is crucial that all subjects respond to all questions and undergo all physical tests that are included in the analysis. These restrictions reduced this model to 50 cases. Studies are dependent on the willingness of the participants to participate and to conduct the follow-ups. Since the participants in this study are women that recently have become mothers they are likely to have to adapt to a new life in many perspectives. This might somehow explain the dropouts and it is possible that the loss of women from the inclusion in the RCT and the 12-month follow-up might have influenced the results to some degree. The women that were not included in the regression model rated their disability somewhat higher than those women that were included.
Conclusion
More than 60 % of the women in our study reported persistent PGP at 15 months postpartum and had clinically identifiable signs. Age, muscle function, and disability seem to influence the long-term outcome on disability in women with persistent postpartum PGP. It may be important to consider the possibility of different variables’ impact on each other when predicting long-term disability including physical functioning and self-rated factors. Further studies are needed to investigate the impact of interaction effects on long-term disability in women with persistent postpartum PGP.
Acknowledgments
The authors thank Henrik Magnusson for statistical support. This study was supported by grants from the Swedish Research Council, the Vardal Foundation, Foundation of the Region Västra Götaland, Trygg Hansa Research Foundation, the Rehabilitation and Medical Research Foundation, and Linköping University, Sweden.
Conflict of interest
None.
References
- 1.Wu WH, Meijer OG, Uegaki K, Mens JM, van Dieen JH, Wuisman PI, Ostgaard HC. Pregnancy-related pelvic girdle pain (PPP), I: terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13(7):575–589. doi: 10.1007/s00586-003-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutke A, Ostgaard HC, Oberg B. Predicting persistent pregnancy-related low back pain. Spine (Phila Pa.1976) 2008;33(12):E386–E393. doi: 10.1097/BRS.0b013e31817331a4. [DOI] [PubMed] [Google Scholar]
- 3.Brynhildsen J, Hansson A, Persson A, Hammar M. Follow-up of patients with low back pain during pregnancy. Obstet Gynecol. 1998;91(2):182–186. doi: 10.1016/S0029-7844(97)00630-3. [DOI] [PubMed] [Google Scholar]
- 4.Svensson HO, Andersson GB, Hagstad A, Jansson PO. The relationship of low-back pain to pregnancy and gynecologic factors. Spine (Phila Pa.1976) 1990;15(5):371–375. doi: 10.1097/00007632-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Biering-Sorensen F. A prospective study of low back pain in a general population. I. Occurrence, recurrence and aetiology. Scand J Rehabil Med. 1983;15(2):71–79. [PubMed] [Google Scholar]
- 6.Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogren IM. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur Spine J. 2006;15(7):1093–1102. doi: 10.1007/s00586-005-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson HS, Mengshoel AM, Veierod MB, Vollestad N. Pelvic girdle pain: potential risk factors in pregnancy in relation to disability and pain intensity three months postpartum. Man Ther. 2010;15(6):522–528. doi: 10.1016/j.math.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Robinson HS, Veierod MB, Mengshoel AM, Vollestad NK (2010) Pelvic girdle pain—associations between risk factors in early pregnancy and disability or pain intensity in late pregnancy: a prospective cohort study. BMC Musculoskelet Disord doi:10.1186/1471-2474-11-91 [DOI] [PMC free article] [PubMed]
- 10.Vollestad NK, Stuge B. Prognostic factors for recovery from postpartum pelvic girdle pain. Eur Spine J. 2009;18(5):718–726. doi: 10.1007/s00586-009-0911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutke A, Ostgaard HC, Oberg B. Association between muscle function and low back pain in relation to pregnancy. J Rehabil Med. 2008;40(4):304–311. doi: 10.2340/16501977-0170. [DOI] [PubMed] [Google Scholar]
- 12.Noren L, Ostgaard S, Johansson G, Ostgaard HC. Lumbar back and posterior pelvic pain during pregnancy: a 3-year follow-up. Eur Spine J. 2002;11(3):267–271. doi: 10.1007/s00586-001-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutke A, Sjodahl J, Oberg B. Specific muscle stabilizing as home exercises for persistent pelvic girdle pain after pregnancy: a randomized, controlled clinical trial. J Rehabil Med. 2010;42(10):929–935. doi: 10.2340/16501977-0615. [DOI] [PubMed] [Google Scholar]
- 14.Frändin K. Physical activity and functional performance in a population studied longitudinally from 70 to 76 years of age. Sweden: Dissertation, Göteborg University; 1995. [Google Scholar]
- 15.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 16.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 17.Cherkin DC, Deyo RA, Street JH, Barlow W. Predicting poor outcomes for back pain seen in primary care using patients’ own criteria. Spine (Phila Pa.1976) 1996;21(24):2900–2907. doi: 10.1097/00007632-199612150-00023. [DOI] [PubMed] [Google Scholar]
- 18.Gutke A, Kjellby-Wendt G, Oberg B. The inter-rater reliability of a standardised classification system for pregnancy-related lumbopelvic pain. Man Ther. 2010;15(1):13–18. doi: 10.1016/j.math.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Mens JM, Vleeming A, Snijders CJ, Stam HJ, Ginai AZ. The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8(6):468–473. doi: 10.1007/s005860050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert HB, Godskesen M, Korsholm L, Westergaard JG. Risk factors in developing pregnancy-related pelvic girdle pain. Acta Obstet Gynecol Scand. 2006;85(5):539–544. doi: 10.1080/00016340600578415. [DOI] [PubMed] [Google Scholar]
- 21.Bastiaanssen JM, de Bie RA, Bastiaenen CH, Essed GG, van den Brandt PA. A historical perspective on pregnancy-related low back and/or pelvic girdle pain. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):3–14. doi: 10.1016/j.ejogrb.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell-Andrews AA, Kain ZN. Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obstet Gynecol. 2004;104(1):65–70. doi: 10.1097/01.AOG.0000129403.54061.0e. [DOI] [PubMed] [Google Scholar]
- 23.Ostgaard HC, Zetherstrom G, Roos-Hansson E. Back pain in relation to pregnancy: a 6-year follow-up. Spine (Phila Pa.1976) 1997;22(24):2945–2950. doi: 10.1097/00007632-199712150-00018. [DOI] [PubMed] [Google Scholar]
- 24.Turgut F, Turgut M, Cetinsahin M. A prospective study of persistent back pain after pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;80(1):45–48. doi: 10.1016/S0301-2115(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 25.Enthoven P, Skargren E, Carstensen J, Oberg B. Predictive factors for 1- and 5-year outcome for disability in a working population of patients with low back pain treated in primary care. Pain. 2006;122(1–2):137–144. doi: 10.1016/j.pain.2006.01.022. [DOI] [PubMed] [Google Scholar]